Abstract
Neuronostatin, derived from the somatostatin preprohormone, is a recently described peptide that is produced by several tissues involved in cardiovascular regulation and metabolism, including the hypothalamus. Injection of neuronostatin into the lateral cerebroventricle led to a dose-related increase in mean arterial pressure (MAP) in rats. Any attempt to inhibit the production of neuronostatin would alter somatostatin production as well, making determination of the physiological relevance of the peptide's pharmacologic effects by compromise of production approaches impossible. Therefore, we employed an alternative approach to identify and compromise the production of the neuronostatin receptor. Because neuronostatin was shown to signal via a PKA-dependent mechanism, we hypothesized that the neuronostatin receptor was a G protein-coupled receptor (GPCR), in particular, one of the orphan GPCRs for which the ligand is unknown. Therefore, we screened neuronostatin-responsive tissues, including hypothalamus, heart, pancreatic α-cells, and the gastric tumor cell line KATOIII, for expression of orphan GPCRs. Four orphan GPCRs were expressed by all cell types, including GPR56 and GPR107. Knockdown of GPR107, but not GPR56 or GPR146, led to a loss of responsiveness to neuronostatin by KATOIII cells. Rats injected with siRNA directed against GPR107 (2 μg/day for 2 days) into the lateral cerebroventricle did not exhibit an increase in MAP in response to neuronostatin treatment. Rats with compromised GPR107 expression also displayed blunted reactivity in a baroreflex sensitivity test, indicating that GPR107 and neuronostatin may be important regulators of cardiovascular function. Thus, GPR107 is a promising candidate receptor for neuronostatin, and neuronostatin, interacting with GPR107, may play an important role in the central control of cardiovascular function.
Keywords: neuronostatin, orphan G protein-coupled receptor, central control of blood pressure
neuronostatin, a product of the somatostatin preprohormone, is a recently described 13-amino acid peptide hormone that exerts multiple cardiovascular and metabolic actions in a variety of tissues (10, 15, 20). When injected into the lateral cerebroventricle (intracerebroventricularly) of adult, male rats, neuronostatin induced a significant, dose-related, and biphasic increase in mean arterial pressure (MAP) (15, 20). This elevation in MAP was found to be dependent upon two separate mechanisms: activation of the sympathetic nervous system and stimulation of vasopressin secretion from the posterior pituitary gland, since the first phase was blocked by pretreatment with phentolamine, while the second phase was reversed by pretreatment with a vasopressin receptor antagonist (20). In addition, neuronostatin directly altered the contractility of isolated cardiomyocytes and whole heart preparations via PKA and/or JNK-dependent mechanisms (10, 19). Because neuronostatin is derived from the somatostatin preprohormone, any attempt to compromise the production of neuronostatin would result in loss of somatostatin as well. Therefore, we chose an alternative approach to determine the physiological relevance of the central, cardiovascular effects of neuronostatin—identification and compromise of the neuronostatin receptor.
Neuronostatin directly initiates specific signaling cascades in at least four different cell types: hypothalamic neurons (15), cardiomyocytes (10, 19), pancreatic α-cells (15), and a gastric tumor cell line, KATOIII (15). We reasoned that because these cell types responded to direct application of neuronostatin in vitro, they must express a neuronostatin receptor(s). Furthermore, neuronostatin signaled via a PKA-dependent mechanism (3), suggesting that the neuronostatin receptor(s) was a G protein-coupled receptor (1, 4). Therefore, we hypothesized that the neuronostatin receptor was one of the remaining orphan G protein-coupled receptors, for which the ligand is currently unknown (8, 16). We identified by a bioinformatics approach (2) those orphan GPCRs that belonged to receptor families bearing sequence homology to GPCRs that interact with small peptides (3). We then developed an mRNA-screeening approach to narrow the pool of potential receptors, identified a candidate neuronostatin receptor, GPR107, and provide here evidence for a physiologically relevant action of neuronostatin in brain, acting via GPR107.
MATERIALS AND METHODS
Cell culture.
The human gastric tumor cell line, KATOIII [American Type Culture Collection (ATCC), Manassas, VA] was cultured in Iscove's Modified Dulbecco Medium (IMDM) supplemented with 20% FBS and 1% penicillin/streptomyocin, as recommended by ATCC. Cells were incubated at 37°C with 5% CO2. This semiadherent cell line was grown to 80% confluency before passage or plating for experimentation. Culture reagents were purchased from ATCC.
PCR.
Total RNA was collected from two cell lines (KATOIII and the mouse pancreatic alpha cell line, αTC-1) and rat tissues (dissected following death by rapid decapitation) using the RNeasy Kit (Qiagen), according to the kit directions. RNA was used as a template to produce cDNA using oligo d(T) (Invitrogen) and MML-V reverse transcriptase (Promega). Expression of orphan GPCRs was determined by PCR using GoTaqGreen MasterMix (Promega), according to the manufacturer's instructions (Applied Biosystems, GeneAmp PCR System 2400) (94°C for 3 min, 94°C for 30 s, 56°C for 45 s, 72°C for 45 s, GOTO Step 2 × 25 cycles, 72°C for 10 min, and 4°C forever). Primers were designed using PrimerQuest software (Integrated DNA Technologies), and specificity was confirmed using PrimerBLAST (NCBI). Primer sequences are reported in Tables 1 and 2. PCR products were separated on a 1% agarose gel and visualized and photographed (FujiFilm LAS3000 Luminescent Image Analyzer, Fuji Life Sciences). Brightness and contrast of the entire gels were adjusted using Apple iPhoto, and gels were assembled and annotated using Apple Keynote. Reactions were repeated at least three times.
Table 1.
Human primer sequences
| Target Name | RefSeq ID | Forward Primer | Reverse Primer | Tm (F, R) | Amplicon Size | Location on Sequence (F, R) |
|---|---|---|---|---|---|---|
| LPAR5 | NM_020400.5 | AGGGCAATGCTTCCTCTTCACTCT | AGGCTGTACAGACATGGTCCCAAA | 58, 58 | 332 | 19, 350 |
| OXGR1 | NM_080818.3 | CCACGAACGGGTAATCCTGATGAA | GTCTAGTGGCTCATTCATGGTTGTC | 57, 56 | 153 | 110, 262 |
| SUCNR1 | NM_033050.4 | TGGCAGAGTTCCTGTCAAGGGAT | AGGACTCCCACAACGAACTCAATC | 57, 57 | 199 | 23, 221 |
| GPR15 | NM_005290.1 | AGGGAGCTCACGCTGATTGATGAT | TATTGAAGGGCAGCCAGGAGACAA | 58, 58 | 262 | 514, 775 |
| GPR17 | NM_001161415.1 | TCAGGATGTCCAAACGGAGTTGGT | CAGAGCCAGGGTATTGCCAACTAA | 58, 57 | 248 | 607, 854 |
| GPR18 | NM_005292.3 | ACAGGAGGTGCTGCTTTCTGTGAA | ACGTCCTGCCGTGAAGGAGATTAT | 58, 58 | 891 | 269, 1159 |
| GPR19 | NM_006143.2 | TCTGCCAAGCCAATACCTGATGGA | CATCAAAGATCCACGATGCCGCAA | 58, 58 | 473 | 457, 929 |
| GPR22 | NM_005295.2 | ACCAGGGAATAGGTCTACATTACTGA | GTCATCAATGTCATCTCGCACTGT | 55, 56 | 898 | 524, 1421 |
| GPR26 | NM_153442.3 | AACTGTGGCACATGCTCTTTCTGC | TCACCCTGGAGTGAAGTCAAGCAA | 60, 60 | 535 | 3309, 3843 |
| GPR31 | NM_005299.2 | TAAGGTCAACCTGCTGTCTCCTCA | CATTGCAGAACACGATGAGGCCAA | 57, 58 | 230 | 363, 592 |
| GPR32 | NM_001506.1 | TCCTGACACGTGATCGCTCTTGTT | AATGGGCAGAGACAGTGAGAGCAT | 58, 58 | 239 | 53, 291 |
| GPR55 | NM_005683.3 | TCCTTAAGAACAGGTGGCCCGATT | TGTTTCTCACCAGGAACTGCAGGA | 58, 58 | 621 | 336, 956 |
| GPR56 | NM_005682 | CCAGCACCAGCTACAGCCGAAGAA | TGGAGCAGAGGTAGGCGGCAAT | 61, 60 | 287 | 1291, 1577 |
| GPR63 | NM_030784.2 | ATGGTCTTCTCGGCAGTGTTGACT | TAAGCCTGGTAGCCTGGATTGGTT | 58, 58 | 725 | 361, 1085 |
| GPR82 | NM_080817.4 | GGTGTCATGCAAGAATCTATTCACAC | TGCAATCTGAGAGATCATGGCTCC | 55, 57 | 842 | 5, 846 |
| GPR83 | NM_016540.3 | ACCTTCTCCGACTGGCAGAACTTT | TGGCAGATAGCATGTGGGAGTGAA | 58, 58 | 482 | 314, 795 |
| GPR85 | NM_001146265.1 | TCAGGCTTTGTGGTGTCTGTGGTA | TCAGAAAGGCTGTTAGAGGCGAGA | 58, 57 | 400 | 294, 694 |
| GPR107 (PCR) | NM_001136557.1 | AGTGGAAGTGGCTCTACCAGCTC | TCACTGTCGGTGGTGCCAATAGTT | 58, 58 | 350 | 1792, 2141 |
| GPR107 (RT-PCR) | NM_001136557.1 | TGGCTTAGAAGGGTCAGGCAGAAA | ATGGGACAGGGAGAACAACCATGT | 58, 58 | 112 | 3485, 3596 |
| GPR146 | NM_138445.2 | ACTTTGTCAACATGGCAGTGGCAG | TGGACACATGGCTGCAGATGTAGA | 58, 58 | 324 | 205, 528 |
| GPR160 | NM_014373.2 | TTGCGCAAATGTCTCCGAGCTTAC | AGCATTCTGTGCCTTCAGGCTTTG | 58, 58 | 716 | 382, 1097 |
| GPR176 | NM_007223.1 | TCCTCAGCTTCCCTGCTATTGCTT | AGGGCACGCTACACAAGATGAAGA | 58, 58 | 450 | 1261, 1710 |
| GPR183 (EB12) | NM_004951.4 | ACTCACAGCTACTACACAGAGACC | CCAGTCAAAGCCCATTGCATAGTAGG | 55, 58 | 351 | 115, 465 |
| GAPDH | NM_002046.3 | TCACTGCCACTCAGAAGACTGT | CGTTCAGCTCTAGGATGACCTT | 58, 56 | 135 | 644, 778 |
| cFos | NM_005252.3 | GGACTCAAGTCCTTACCTCTTCC | CCTGGCTCAACATGCTACTAACT | 56, 57 | 106 | 1524, 1629 |
Table 2.
Rodent primer sequences
| Target Name | Mouse RefSeq ID | Rat RefSeq ID | Forward Primer | Reverse Primer | Tm (F,R) | Amplicon Length | Location (F, R) |
|---|---|---|---|---|---|---|---|
| LPAR5 | NM_001163268.1 | XM_001063300.2 | TCTTCCTGCGTGTACTGCGCGTA | ACAGTTCATCGCTGAAGCTCTCGAA | 61, 59 | 414 | 423, 836 (M) |
| OXGR1 | NM_001001490 | NM_207588 | TGACGGACTTGCTGTATCTGACCA | AAGTCATGGGCATGACAGCTACCA | 58, 58 | 279 | 753, 1031 (M) |
| SUCNR1 | NM_032400 | NM_001001518 | ACCTCTTCTGCATGAAGAACTGGA | GCACTGTTCAGAAAGGCCAGAGG | 56, 58 | 721 | 188, 908 (M) |
| GPR15 | NM_001162955 | NM_001105890 | CAAGGAGGCTGTGTGCTCATTACC | TGGCCCGGCGGATATAGCTGTCAA | 58, 62 | 282 | 656, 937 |
| GPR17 | NM_001025381 | NM_001071777 | ACCTGGCCTGCGCCTTCCTGTG | GGCCAGAACCATAGCAATCATGCG | 63, 59 | 281 | 629, 909 (M); 446, 726 (Rat) |
| GPR18 | NM_182806 | NM_001079710 | GGTGCTACGTGGTCATCATTCACAG | TTCATGAGGAAGGTGGTGAAGGCT | 58, 58 | 214 | 746, 959 (M); 835, 1048 (Rat) |
| GPR19 | NM_008157 | NM_080579 | TCCATGGCGTGTGCTGACCTTCTC | ATGCAGATGGAGAGCAGCACGTAG | 61, 59 | 167 | 469, 635 (Rat) |
| GPR22 | NM_175191 | NM_001106722 | AGGCTCTTAACATCCGCATAGGCA | TCGCCGGAGGGCAATTATTACAGA | 58, 58 | 179 | 2150, 2328 (M) |
| GPR26 | NM_173410 | NM_138841 | TGTACCGTGGTCAACATGCCACTA | AGTCAATGGGTGCTGTGGAGAAGA | 58, 58 | 655 | 631, 1285 (M) |
| GPR31 | NM_001013832.2 | NM_001169132.1 | AAGTCCTGCTCTTCCTGCTGACAT | TCCTGATGAGCTCACTGTTGCAGA | 58, 58 | 345 | 263, 607 (Rat) |
| GPR32 | TCCTGACACGTGATCGCTCTTGTT | AATGGGCAGAGACAGTGAGAGCAT | 58, 58 | 239 | |||
| GPR55 | NM_001033290 | AF100789.1 | ACAACATGTCGGATGTCACCTGGA | ACAACTGCAGGAACAAGCTGATGC | 58, 58 | 336 (M); 309 (Rat) | 735, 1070 (M) |
| GPR56 | NM_018882 | NM_152242 | ACTTCCTTCCAAGGCTTCCTCATC | AGCTAGGTATCTACCTACCAGAGCA | 56, 56 | 310 (M); 317 (Rat) | 2241, 2550 (M) |
| GPR63 | NM_030733 | NM_001106640 | CTCATTGCAGTCTCCTGGGCAAC | AGGGTCTCTGTAGACTCATGAGAC | 58, 55 | 307 | 1027, 1333 (Rat) |
| GPR82 | NM_175669 | AAGCAGACCACTGTGACAACGAGA | ATTGTTCTGGCAAAGTTGGGCTGG | 58, 58 | 526 | 217, 742 (M) | |
| GPR83 | NM_010287 | NM_080411 | TTGCTGTCATCTGGGTCATGGCTA | ACCACAAGCACCAGCATCTTCACG | 58, 60 | 325 | 849, 1173 (M) |
| GPR85 | NM_145066 | NM_022254 | TGGGCTTCCATCAGCATATTCCA | TGAGCAGCACAGATCCAGCAGGA | 56, 60 | 389 | 928, 1316 (M) |
| GPR107 (Mouse) | NM_178760.4 | AGGCTATCACAGAGCGATCC | GAACAAGGCCATGGAAACAT | 56, 54 | 257 | 600, 856 | |
| GPR107 (Rat) | NM_001107828.1 | TCCAGAAGTGTTCTCCAGTTGGCT | TTAGAGACTACCCTTTCTGTCCTCGG | 58, 57 | 154 | 2421, 2574 | |
| GPR146 | NM_030258 | NM_001109062 | GTGACACTGCTCATCCTGTTCAAC | AGGTCCTTAGCAACCTGTAGGATG | 56, 56 | 530 | 479, 1008 (M) |
| GPR160 | NM_001134385 | NM_001025147 | ACTGGTGTAATCTGTCCAGAGCCA | AGGATGAGGACCTGAAGTGCTACA | 57, 57 | 430 | 577, 1006 (Rat) |
| GPR176 | NM_201367 | XM_001080723 | ACGATGCTCTTCTGCAAGGTGCT | TTCCTCATCCTCCGAGGGCTTAA | 59, 57 | 828 | 566, 1393 (M) |
| GPR183 (EB12) | NM_183031 | NM_001109386.1 | TGCACCCTCTGCGCTACAACAAGA | AGTGGGTTCTGCTTGGCAGTCCT | 61, 60 | 295 | 469, 763 (M) |
| HPRT-1 | NM_013556.2 | NM_012583.2 | AGTCCCAGCGTCGTGATTAGTGAT | CTCGAGCAAGTCTTTCAGTCCTGT | 58, 57 | 142 | 79, 220 (Rat) |
siRNA-mediated compromise of receptor expression.
KATOIII cells were plated onto 12-well plates in a volume of 1 ml/well in IMDM with 20% FBS, and incubated overnight at 37°C. The following day, cells were transfected with either vehicle alone (Lipofectamine 2000 in OPTI-MEM media, Invitrogen), GPR107 siRNA (0.1, 1.0, or 10 nM), GPR56 siRNA (0.1, 1.0, 10, or 100 nM), GPR146 siRNA (1, 10, or 100 nM), or eGFP siRNA (1 nM) using Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions, and cells were incubated at 37°C. Knockdown of the three candidate receptors was performed in separate experiments. Each of the three experiments included vehicle- and eGFP siRNA-transfected control cells. Five hours later, the medium was changed to serum-free IMDM (1 ml/well), and cells were incubated at 37°C overnight. On the following day, medium was removed, and cells were exposed to fresh medium (1 ml IMDM) alone or containing 30 nM neuronostatin (1) or 20% FBS (positive control). Cells were incubated for one additional hour and then lysed, and RNA was collected (RNeasy Kit, Qiagen). First-strand cDNA synthesis was accomplished using Oligo d(T) (Invitrogen) and MML-V reverse transcriptase (Promega), according to the manufacturers' instructions. Experiments were performed in triplicate and repeated at least 4 times. Sequences of siRNA constructs are reported in Table 3.
Table 3.
siRNA constructs
| Target | Species | RefSeq ID | Sequence | Assay |
|---|---|---|---|---|
| GPR107 | Human | NM_001136557.1 | 5′-rCrCrUrArGrArCrCrGrUrArCrArArArGrArA-3′ 5′-rGrCrCrArUrCrArUrUrCrUrUrUrGrUrArCrG-3′ | In Vitro |
| GPR56 | Human | NM_005682 | [5′-rArGrArUrUrArCrArUrCrUrUrCrUrCrUrArUrGrGrCrArAGC-3′ 5′-rGrCrUrUrGrCrCrArUrArGrArGrArArGrArUrGrUrArArUrCrUrCrC-3′] [5′-rGrArArCrArCrCrArArArGrUrArGrCrCrArArCrCrUrCrACG-3′; 5′-rCrG rUrGrArGrGrUrUrGrGrCrUrArCrUrUrUrGrGrUrGrUrUrCrUrG-3′] | In Vitro |
| GPR146 | Human | NM_138445.2 | [5′-rArGrCrUrArUrUrCrArArUrArGrCrArGrUrGrArCrGrCrGCT-3′ 5′-rArGrCrGrCrGrUrCrArCrUrGrCrUrArUrUrGrArArUrArGrCrUrCrC-3′] [5′-rArCrCrGrCrUrArCrArUrGrArArCrCrArGrArGrCrUrUrCCC-3′ 5′-rGr GrGrArArGrCrUrCrUrGrGrUrUrCrArUrGrUrArGrCrGrGrUrArG-3′] 5′-[rGrGrArCrGrCrCrArCrArCrUrArUrCrUrGrArUrCrCrUrGCT-3′ 5′-rArGrCrArGrGrArUrCrArGrArUrArGrUrGrUrGrGrCrGrUrCrCrArG-3′] | In Vitro |
| GRP107 | Rat | NM_001107828.1 | [5′-rArGrArCrUrArUrArGrCrArGrArGrArArArUrUrCrUrUrCTC-3′; 5′-rGrArGrArArGrArArUrUrUrCrUrCrUrGrCrUrArUrArGrUrCrUrUrU-3′] [5′-rGrGrArGrUrGrUrCrGrArGrGrArGrArCrArArGrGrUrGrUTA-3′; 5′-rUrArArCrArCrCrUrUrGrUrCrUrCrCrUrCrGrArCrArCrUrCrCrCrU-3′] [5′-rGrUrCrUrCrUrArArArCrArArArCrArCrUrUrArGrGrUrUAA-3′; 5′-rUrUrArArCrCrUrArArGrUrGrUrUrUrGrUrUrUrArGrArGrArCrUrA-3′] | In Vivo |
| eGFP | Heteractis magnifica | AY157666.1 | 5′-rArCrCrCrUrGrArArGrUrUrCrArUrCrUrGrCrArCrCrArCrCrG-3′ 5′-rCrGrGrUrGrGrUrGrCrArGrArUrGrArArCrUrUrCrArGrGrGrUrCrA-3′ | In Vitro, In Vivo |
Real-time PCR.
Changes in the expression of GPR56, GPR107, GPR146, and c-Fos in KATOIII cells were determined using iQ SYBR Green Master Mix (Bio-Rad) and normalized to GAPDH using the delta delta C(t) method (see below). Reactions were performed with a Bio-Rad CFX96 RT-PCR machine, using the following program: 95°C for 5 min, 94°C for 15 s, 60°C for 1 min, plate read, GOTO Step 2 × 30 cycles, 72°C for 10 min, and melt curve from 65°C to 96°C interval 1°C for 0.01 s. Primer sets are listed in Table 1. Data are presented as percent of control, normalized to vehicle-transfected, vehicle-treated cells. Relative C(t) values were calculated as relative C(t)sample = C(t)cFos/GPR107/GPR56 − C(t)GAPDH. Changes in expression were calculated as fold change = 2[Rel C(t)Control − Rel C(t)Exp], where Rel C(t)Control is the relative C(t) of the vehicle-transfected, vehicle-treated cells, and Rel C(t)Exp is the relative C(t) of the treatment group. For each replicate, fold change was converted to percent change, and means were calculated as the average percent change for each treatment group across experiments (at least 12 replicates for each treatment group).
Animals.
All procedures and protocols have been approved by the St. Louis University Animal Care and Use Committee. Adult, male rats (250–300 g, Harlan Laboratories) were maintained under controlled conditions (lights on 0600–1800; 23–25°C) with free access to standard lab chow and tap water. Rats were anesthetized with a mixture of ketamine (Ketaset, Fort Dodge Animal Health) and xylazine (TranquiVed, Vedco) injected intraperitoneally (70 mg/kg ketamine and 9 mg/kg xylazine, administered in 0.1 ml/100 gram body wt) and a stainless-steel cannula (23 gauge, 17 mm) was implanted into the right lateral cerebroventricle (intracerebroventricularly) using a stereotaxic device, as previously described (22). Buprenorphine (0.05 mg/kg, given at 0.1 ml/100 gram body wt sc) was administered subcutaneously for analgesia, and 10 ml sterile saline (0.9% NaCl sc) was injected to compensate for anticipated fluid loss. Animals were observed for a minimum of 5 days until recovery of presurgical weights prior to the implantation of a polyethylene (PE) cannula (PE-50) into the left carotid artery, as previously described (22). Animals that were used for Baroreflex Testing received an additional cannula implanted into the right jugular vein, as previously described (9). Following surgery, animals were housed singly.
Blood pressure.
Prior to experimentation, rats received two injections, 24 h apart, of 2 μg siRNA in 2 μl sterile saline directed against rat GPR107 or eGFP, administered intracerebroventricularly. The second injection was administered on the same day as carotid cannulation. On the day of experimentation (24 h following the last injection of siRNA), rats were habituated to a quiet recording room for at least 2 h. The carotid cannula was flushed with heparinized saline (200 U/ml 0.9% sterile saline) and connected to a pressure transducer (DigiMed, Micromed). Baseline mean arterial pressure (MAP) and heart rate (HR) were recorded at 1-min intervals for at least 30 min. Rats were injected then intracerebroventricularly with three subsequent substances, each injection spaced at a minimum of 1 h apart: saline vehicle, 300 pmol of neuronostatin in sterile saline (15, 20), and 50 pmol of ANG II in sterile saline (22). MAP and HR were recorded at 1-min intervals. Data are represented as area under the curve of change from preinjection baseline (defined as the average of MAP or HR for 10 min prior to injection), or as change from preinjection baseline.
Baroreflex testing.
Rats were injected with 2 μg of siRNA in 2 μl sterile saline directed against either eGFP or GPR107 once a day for two consecutive days. Carotid and jugular cannulae were implanted on the day of the second injection, and experimentation was conducted 24 h later. On the day of the experiment, animals were habituated to a quiet testing room for at least 2 h. Carotid cannulae were connected to a pressure transducer (DigiMed, Micromed), and jugular cannulae were attached to an extension line through which drugs could be administered. Both cannulas were flushed with heparinized saline (200 U/ml sterile saline), and baseline MAP and HR were recorded for at least 30 min at 1-s intervals. Recording intervals were shortened to 1 s to increase the resolution for detection of changes in MAP and subsequently HR in response to manipulation of arterial pressure. Animals were injected then with increasing doses of a vasodilator (sodium nitroprusside, 7.5, 15, and 30 mg/kg; Sigma-Aldrich) (17), followed by an injection of sterile saline, and then increasing doses of a vasoconstrictor (phenylephrine HCl, 5, 25, 50 mg/kg; Research Biomedicals International) (17). All injections were administered in a volume of 0.2 ml, followed by a 0.1-ml flush with heparinized saline. Injections were staggered in 5-min intervals to allow recovery to baseline MAP. Data are represented as peak change in MAP vs. the peak change in HR following each injection. Peak changes were determined by comparison from the preinjection baseline for each injection (average of MAP or HR for 10 s prior to injection). Each animal is represented by 6 data points (17). The time delay between the peak change in MAP and the peak change in HR was calculated as the number of seconds between the peaks.
Confirmation of receptor mRNA compromise in vivo.
Animals that had received siRNA intracerebroventricularly on two subsequent days were killed by rapid decapitation on the day after the last injection, and brains were removed. Hypothalami were dissected and immediately homogenized in lysis buffer (buffer RLT from RNeasy Kit, Qiagen). Total RNA was collected (RNeasy Kit, Qiagen), and cDNA was generated using Oligo d(T) (Invitrogen) and MML-V reverse transcriptase (Promega). PCR was performed using primers specific for GPR107 and the housekeeping gene, HPRT-1 (12). Amplicons were separated on a 1% agarose gel, and imaged using a FujiFilm Luminescent Image Analyzer (LAS-3000). Band density was measured using GelEval (FrogDance Software, University of Dundee, Scotland). GPR107 band density for each hypothalami was normalized to the corresponding HPRT-1 band, and percent knockdown was calculated by setting the average GPR107 expression from control animals (eGFP siRNA treated) to 100%.
Materials.
All primers and siRNA constructs were designed with the assistance of and purchased from Integrated DNA Technologies. Sequences of human primers, rodent primers, and siRNA constructs are reported in Tables 1, 2, and 3, respectively. Peptides were purchased from Phoenix Pharmaceuticals.
Statistics.
All molecular data were analyzed using a Student's t-test. Knockdown experiments were analyzed further using ANOVA to determine concentration dependence. A nonparametric test (Mann-Whitney U) was used to analyze blood pressure data, since the data were coded to account for the natural variability in baseline mean arterial pressure between animals (23). Baroreflex data were analyzed using linear regression analysis (comparison of slopes). Differences between groups were considered significant if P ≤ 0.05.
RESULTS
Approach.
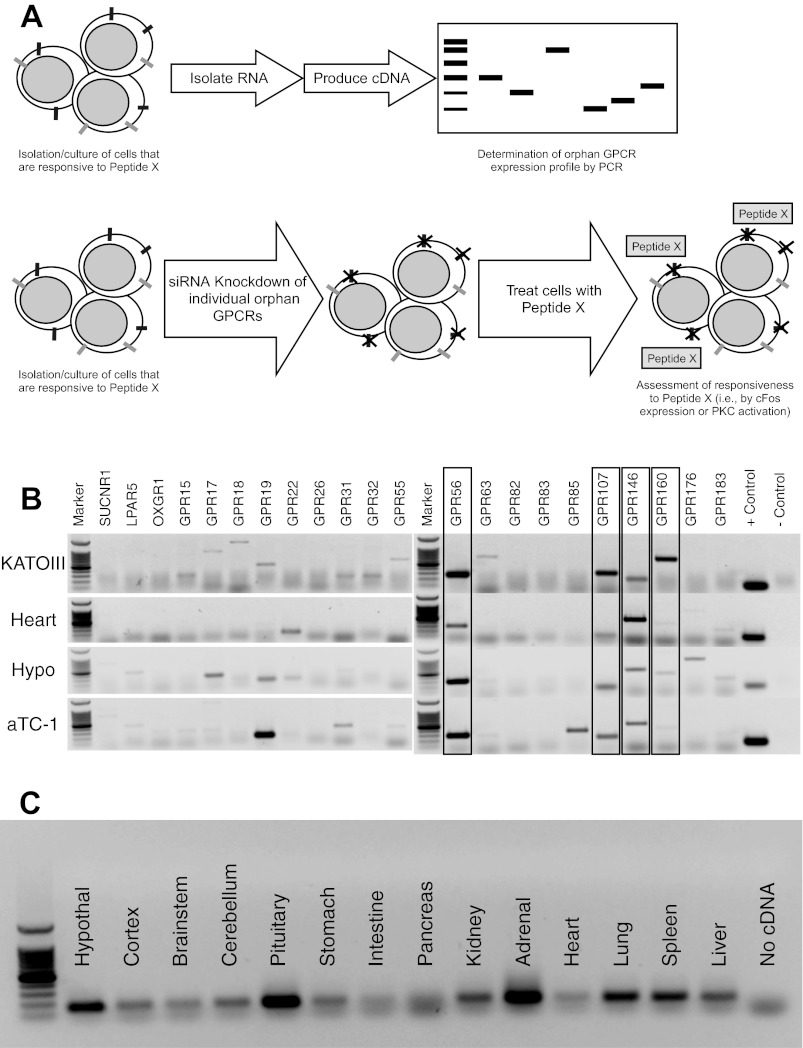
The approach used in this study is illustrated in Fig. 1A. Briefly, neuronostatin-responsive tissues were screened for the expression of orphan GPCRs using PCR. Orphan GPCRs that were detected in all responsive cell/tissue types were then compromised using siRNA in a reporter cell (KATOIII), and those cells were assessed for sensitivity to neuronostatin using c-Fos as an indicator of neuronostatin action.
Fig. 1.
A: identification of candidate receptors. Our approach for matching peptides with their receptor(s) is shown. Four orphan G protein-coupled receptors (GPCRs), GPR56, GPR107, GPR146, and GPR160, were expressed by all four neuronostatin-responsive tissue/cell types (B), as determined by PCR. C: expression of GPR107 by rat tissues.
Identification of GPR107 as a candidate receptor for neuronostatin.
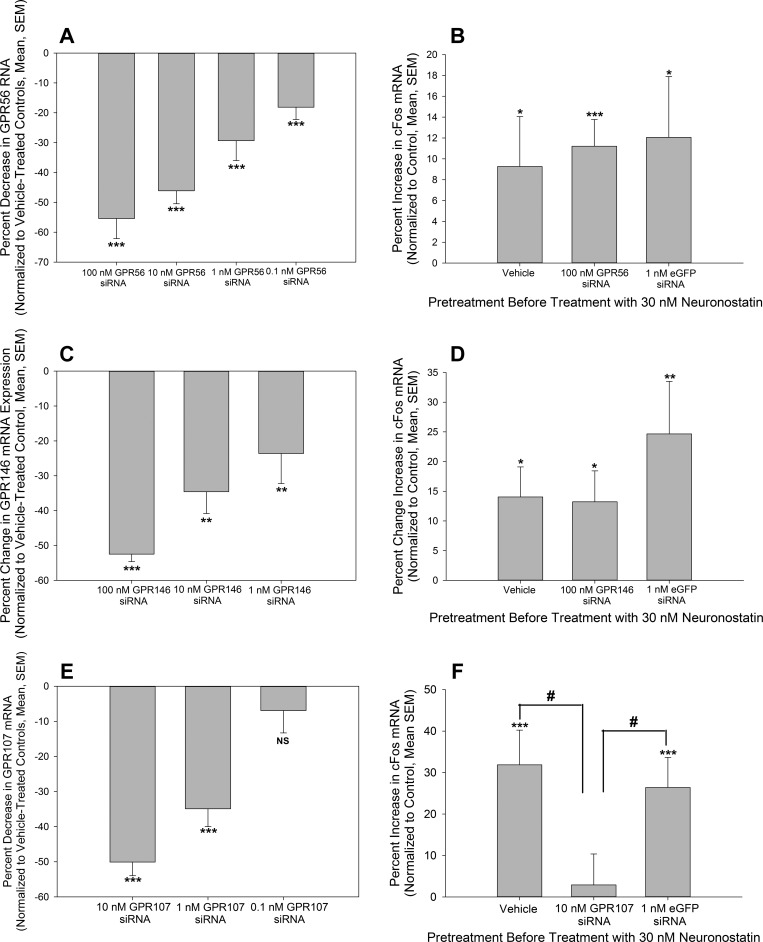
Neuronostatin activates KATOIII cells (15), cardiomyoctes (10, 19), hypothalamic neurons (15), and pancreatic α-cells (15). Therefore, we screened these cell types for the candidate orphan GPCRs using PCR (Fig. 1B). Of the 22 orphan GPCRs, only four were expressed by all four cell/tissue types: GPR56, GPR107, GPR146, and GPR160. We reasoned that those four orphan GPCRs were the best candidates for a neuronostatin receptor, and thus, we began compromising individual receptors in KATOIII cells using siRNA (sequences for primer sets and siRNA are listed in Tables 1, 2, and 3). Combinations of siRNA constructs were validated and selected in preliminary experiments (data not shown) based on the ability of the constructs to consistently compromise production of the receptor. We attained >50% knockdown using a concentration of 100 nM siRNA directed against human GPR56, the recently deorphanized receptor of collagen III (11), in KATOIII cells (Fig. 2A). This concentration of siRNA was selected for further experimentation since lower concentrations of GPR56 siRNA did not provide adequate compromise of GPR56 production (Fig. 2A). However, when analyzed by ANOVA, between-group differences attained significance (P < 0.001). KATOIII cells were transfected then with vehicle (Lipofectamine 2000 in treatment media), 100 nM GPR56 siRNA, or with siRNA directed against enhanced green fluorescent protein (eGFP) to serve as a siRNA-transfection control, and treated with 30 nM neuronostatin for 1 h (15). In all three groups, neuronostatin induced small but significant increases in c-Fos expression compared with vehicle/vehicle-treated controls, as determined by real-time PCR. No statistical differences were observed in c-Fos expression between neuronostatin-treated cells (Fig. 2B).
Fig. 2.
Compromise of GPR107, but not GPR56, in KATOIII cells results in a loss of neuronostatin-induced expression of c-Fos. A: transfection of KATOIII cells with siRNA directed against GPR56 led to a concentration-related decrease in GPR56 expression, as determined by RT-PCR. B: treatment of KATOIII cells transfected with GPR56 siRNA resulted in a significant increase in c-Fos expression similar to that observed in vehicle-transfected cells. C: transfection of KATOIII cells with siRNA directed against GPR146 led to a concentration-related decrease in GPR146 mRNA levels. D: knockdown of endogenous GPR146 in KATOIII cells did not affect neuronostatin-induced c-Fos expression. E: Transfection of KATOIII cells with siRNA directed against GPR107 led to a concentration-related decrease in GPR107 RNA levels. F: transfection with siRNA directed against GPR107 abolished neuronostatin-induced c-Fos expression in KATOIII cells. Data are presented as percent of control (vehicle-transfected, vehicle-treated cells). *P < 0.05, **P < 0.01, ***P < 0.001 vs. control. #P < 0.05 vs. GPR107 siRNA-transfected, neuronostatin-treated cells.
Transfection of KATOIII cells with siRNA directed against human GPR146 led to significant decreases in expression of GPR146, with 100 nM siRNA yielding the greatest inhibition of GPR146 expression (>50%) (Fig. 2C). Analysis using ANOVA revealed significant between-group differences (P < 0.001). KATOIII cells were transfected then with either vehicle, 100 nM GPR146 siRNA, or 1 nM eGFP siRNA, and the following day, cells were treated with 30 nM neuronostatin for 1 h. Exposure to neuronostatin led to significant increases in c-Fos expression in all three transfection groups compared with vehicle-treated control cells (Fig. 2D). Although eGFP siRNA transfected cells appeared to respond to neuronostatin with a greater increase in c-Fos expression than vehicle- or GPR146 siRNA-treated cells, this effect was not statistically significant (no differences in the c-Fos response between vehicle-, GPR146 siRNA-, or eGFP siRNA-treated groups) (Fig. 2D).
KATOIII cells transfected with siRNA-directed against human GPR107-exhibited decreases in GPR107 expression, with 10 nM siRNA yielding the greatest knockdown of consistently >50% (Fig. 2E). Using ANOVA, differences in knockdown were found to be statistically significant (P < 0.001). KATOIII cells then were transfected with vehicle, GPR107 siRNA, or eGFP siRNA, followed by treatment with 30 nM neurononstatin. KATOIII cells that were transfected with either vehicle or the control siRNA-directed against eGFP exhibited significant increases in c-Fos expression, compared with vehicle/vehicle-treated cells. However, an increase in c-Fos expression was not observed in cells that were transfected with siRNA-directed against GPR107 (Fig. 2F). The expression of c-Fos in response to neuronostatin by GPR107 siRNA-transfected cells was found to be significantly different from vehicle- and eGFP siRNA-transfected cells (P < 0.05 using t-tests).
To identify additional sites of potential neuronostatin action via GPR107, we assessed expression of GPR107 in rat tissues by PCR. GPR107 mRNA was detected in almost all tissue types tested, excluding intestine (duodenum) and exocrine pancreas (Fig. 1C).
In vivo compromise of GPR107 expression.
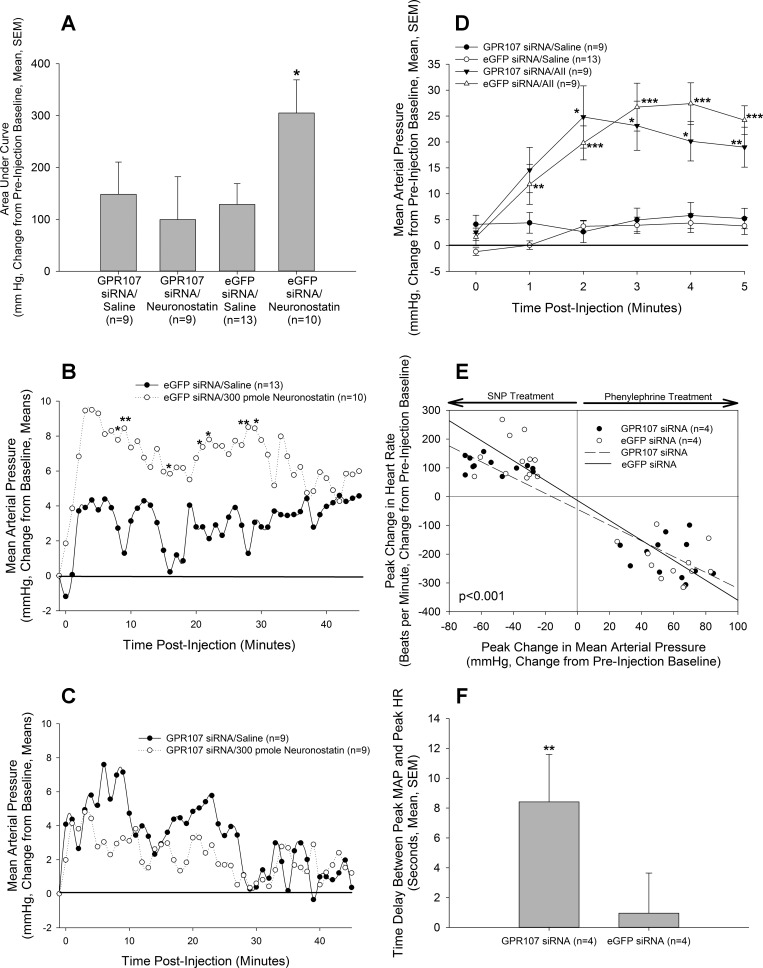
In rats treated with siRNA directed against eGFP, we observed that central administration of neuronostatin induced a significant increase in MAP when the data were analyzed as area under the curve for the total observation time of 45 min (Fig. 3, A and B) (15, 20), compared with eGFP siRNA-treated, saline-injected controls (Fig. 3B). However, rats that were treated with GPR107 siRNA were no longer able to respond to neuronostatin with an increase in MAP (Fig. 3, A and C). Treatment with GPR107 siRNA did not interfere with the pressor response (Fig. 3D) to a dose of ANG II (50 pmol) (20).
Fig. 3.
In vivo compromise of GPR107 production. Rats bearing intracerebroventricular and carotid cannulae were injected on two consecutive days with 2 μg siRNA, directed against either GPR107 or eGFP, in 2 μl of sterile saline. A and C: rats injected with GPR107 siRNA did not exhibit an elevation in MAP following treatment with 300-pmol neuronostatin, while control eGFP siRNA-injected rats responded with a biphasic increase in MAP (B). However, a pressor dose (50 pmol) of angiotensin II (AII) (D) significantly increased MAP in both GPR56 siRNA- and GPR107 siRNA-injected animals. Compromise of GPR107 production blunted (E) and delayed (F) the cardiovascular responses of conscious rats in a test of baroreflex sensitivity, compared with eGFP siRNA-injected controls. MAP and HR data are presented as a change from preinjection baseline of 10 recording intervals (1 min for A–D; 1 s for E and F). *P < 0.05, **P < 0.01, ***P < 0.001 vs. controls (In A–D: eGFP siRNA/saline vs. eGFP siRNA/neuronostatin or AII; GPR107 siRNA/saline vs. GPR107 siRNA/neuronostatin or AII. E and F: eGFP siRNA vs. GPR107 siRNA).
Preliminary evidence from our laboratory suggests that compromise of neuronostatin action in brain by passive immunoneutralization alters baroreflex sensitivity in rats (Yosten GLC and Samson WK, submitted for publication), indicating that brain-derived neuronostatin may be an important regulator of cardiovascular function. We, therefore, tested baroreflex sensitivity in rats treated with siRNA against GPR107 or eGFP. Linear regression analysis (comparison of slopes) of the responses to changes in arterial pressures (Fig. 3E) revealed significant differences between animals treated with siRNA against GPR107 vs. controls (eGFP siRNA-treated animals). These differences appeared to be more prominent when animals were injected with the vasodilator, since animals that received GPR107 siRNA could not mount the same increase in HR in response to a decrease in MAP, as eGFP siRNA-treated rats. Furthermore, rats that were treated with GPR107 siRNA exhibited a delay in peak HR response compared with eGFP siRNA -treated animals (Fig. 3F). Knockdown of GPR107 expression in siRNA-treated rats was confirmed by PCR [GPR107 expression (% of control): eGFP siRNA = 100.0 ± 9.4; GPR107 siRNA = 80.7 ± 3.6; P < 0.05].
DISCUSSION
Here, we describe a novel approach for matching peptide hormones with their cognate receptors based on a directed screening method that takes advantage of a peptide's known pharmacological actions. Using this approach, we identified four orphan GPCRs that were expressed by all four known neuronostatin-responsive cell/tissue types, including GPR56 and GPR107. During the completion of this study, another group reported the deorphanization of GPR56 as a receptor for collagen III (11). Thus, GPR56 was a convenient and important control in our studies. In addition to expression in all four neuronostatin-responsive cell types, GPR107 is also expressed in a wide variety of tissues. Thus far, the only tissue types in which we were unable to detect GPR107 were exocrine pancreas and small intestine (duodenum). Our data suggest that compromise of GPR107 in vitro and in vivo abrogates the actions of neuronostatin to increase c-Fos mRNA expression and elevate MAP, respectively. These data suggest that neuronostatin interacts with GPR107 to exert these activities.
Very little has been reported on the function of GPR107. It is known that the receptor is highly conserved across species, with homologs of GPR107 expressed in human, chimpanzee, rat, mouse, cow, chicken, zebrafish, fruit fly, mosquito, snails (7), C. elegans, and even by some plant species (rice and A. thaliana) (NCBI Gene, National Center for Biotechnology Information, U.S. National Library of Medicine). Because of this high degree of evolutionary conservation, GPR107 and neuronostatin may have important, as yet undetermined, roles in normal physiology or development in addition to its reported cardiovascular and metabolic activities. GPR107 was originally cloned from human lung cDNA (6), and, thus, is referred to also as LUSTR1 (lung seven transmembrane receptor 1). The LUSTR family of proteins comprises a small family of seven transmembrane domain receptors, which includes GPR107 and GPR108. Members of the LUSTR family share similar structure in the third intracellular loop (6). However, it is likely that GPR107 and GPR108 do not bind the same ligand(s), since alignment of the two proteins reveals only weak sequence homology (6).
In these studies, we demonstrate that a 20% reduction in endogenous GPR107 expression in vivo completely abolished the hypertensive effect of centrally injected neuronostatin. In vivo compromise of production technologies have had varying efficacies, depending upon the gene of interest, target tissue, and methodology used. Different groups have reported efficacies ranging from 20 to 90% (5, 13, 14, 18, 21). In our experience (21) and as shown by others (14), knockdown of 20–30% is sufficient to observe measurable differences in a physiological process in vivo.
Although compromise of GPR107 levels, as monitored by mRNA levels, resulted in the disruption of neuronostatin's actions both in vitro and in vivo, it is possible that GPR107 is not a cognate receptor of neuronostatin, but instead is a coreceptor or downstream effector protein. Thus, it will be essential to validate GPR107 as a cognate receptor of neuronostatin in future cell expression studies. These essential confirmatory experiments should include binding studies in a null cell line that is overexpressing GPR107, as well as rescue experiments, in which siRNA-treated KATOIII cells are transfected with a plasmid encoding an siRNA-insensitive sequence for GPR107 and tested for recovery of responsiveness to neuronostatin. Binding studies using iodinated neuronostatin will be difficult, since the endogenous neuronostatin peptide does not contain a tyrosine (15). Although we have succeeded in synthesizing an N-tyrosylated [neuronostatin-13 (Tyr0)] neuronostatin molecule, this modification appears to interfere with neuronostatin action, since we have found that N-Tyr-neuronostatin does not increase c-Fos message in KATOIII cells (Yosten GLC and Samson, WK, unpublished data). Furthermore, neuronostatin is COOH-terminally amidated (15), and this posttranslational modification is essential for neuronostatin signaling, since nonamidated neuronostatin does not alter mean arterial pressure in rats (20); thus, tyrosylation of the COOH terminus is not an option. Despite these difficulties, it is imperative to seek alternative methodologies to confirm the binding of neuronostatin to GPR107.
In conclusion, our in vitro studies point to the necessary involvement of GPR107 in the neuronostatin's action to induce early gene expression. The in vivo results indicate that GPR107 may be an essential participant in the central action of neuronostatin to raise MAP in conscious rats. Whether GPR107 is the initial receptor for neuronostatin in this model or is a receptor expressed in downstream neuronal circuits activated by neuronostatin cannot be determined by these studies; however, a combination of the in vitro and the in vivo results point promisingly to the identification of GPR107 as the cognate receptor for this recently discovered peptide hormone. This experimental approach can now be employed to study the potential physiological relevance of the additional actions of neuronostatin in brain (20), heart (10, 19), and pancreas (15), studies that were not possible prior to identification of the potential neuronostatin receptor.
Perspectives and Significance
Neuronostatin is produced in many peripheral tissues, including the pancreas, spleen, and heart (15), and thus, they may exert additional physiologically relevant actions in tissues besides the central nervous system. In particular, neuronostatin was detected in somatostatin-producing delta cells of the endocrine pancreas (15). Intraperitoneal injection of neuronostatin into rodents led to an accumulation of cJun in pancreatic α-cells (15), suggesting that neuronostatin may directly influence α-cell function. Centrally injected neuronostatin increases sympathetic nervous system activity (20), which could influence glucose homeostasis through catecholamine-induced stimulation of glucagon release and inhibition of insulin secretion. If neuronostatin is found to similarly alter pancreatic hormone secretion, then it would be reasonable to hypothesize that neuronostatin acts in a coordinated fashion in central and peripheral sites to influence glucose homeostasis.
GRANTS
This work was supported by National Institutes of Health Grant HL-06623 to W. K. Samson.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Aaron Hsueh (Stanford University) for his continued support and input, especially for offering his expertise in bioinformatics to aid in the identification of orphan GPCR targets. The authors would like also to thank Dr. Jaw-Kang Chang and Phoenix Pharmaceuticals for their ongoing collaboration and for synthesizing and providing neuronostatin-13 (Tyr0).
REFERENCES
- 1.Appert-Collin A, Baisamy L, Diviani D. Regulation of G protein-coupled receptor signaling by a-kinase anchoring proteins. J Recept Signal Transduct Res 26: 631–646, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Ben-Shlomo I, Rauch R, Avsian-Kretchmer O, Hsueh AJ. Matching receptome genes with their ligands for surveying paracrine/autocrine signaling systems. Mol Endocrinol 21: 2009–2014, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Ben-Shlomo I, Yu Hsu S, Rauch R, Kowalski HW, Hsueh AJ. Signaling receptome: a genomic and evolutionary perspective of plasma membrane receptors involved in signal transduction. Sci STKE 2003: RE9, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Brinks HL, Eckhart AD. Regulation of GPCR signaling in hypertension. Biochem Biophys Acta 1802: 1268–1275, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czech MP, Aouadi M, Tesz GJ. RNAi-based therapeutic strategies for metabolic disease. Nat Rev Endocrinol 7: 473–484, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Edgar AJ. Human GPR107 and murine Gpr108 are members of the LUSTR family of proteins found in both plants and animals, having similar topology to G protein-coupled receptors. DNA Seq 18: 235–241, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Gorbushin AM, Klimovich AV, Iakovleva NV. Himasthla elongate: effect of infection on expression of the LUSTR-like receptor mRNA in common periwinkle haemocytes. Exp Parasitol 123: 24–30, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Harmar AJ, Hills RA, Rosser EM, Jones M, Buneman OP, Dunbar DR, Greenhill SD, Hale VA, Sharman JL, Bonner TI, Catterall WA, Davenport AP, Delagrange P, Dollery CT, Foord SM, Gutman GA, Laudet V, Neubig RR, Ohlstein EH, Olsen RW, Peters J, Pin JP, Ruffolo RR, Searls DB, Wright MW, Spedding M. IUPHAR-DB: the IUPHAR database of G protein-coupled receptors and ion channels. Nucl Acids Res 37: D680–D685, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harms PG, Ojeda SR. A rapid and simple procedure for chronic cannulation of the rat jugular vein. J Appl Physiol 36: 391–392, 1974 [DOI] [PubMed] [Google Scholar]
- 10.Hua Y, Ma H, Samson WK, Ren J. Neuronostatin inhibits cardiac contractile function via a protein kinase A- and JNK-dependent mechanism in murine hearts. Am J Physiol Regul Integr Comp Physiol 297: R682–R689, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo R, Jeong SJ, Jin Z, Strokes N, Li S, Piao X. G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination. Proc Natl Acad Sci USA 108: 12925–12930, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martino A, Cabiati M, Campan M, Prescimone T, Minocci D, Caselli C, Rossi AM, Giannessi D, Del Ry S. Selection of reference genes for normalization of real-time PCR data in minipig heart failure model and evaluation of TNF-α mRNA expression. J Biotechnol 153: 92–99, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443: 709–712, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Ojano-Dirain C, Gluchakova LG, Zhong L, Zolotukhin S, Muzyczka N, Srivastava A, Stacpoole PW. An animal model of PDH deficiency using AAV8-siRNA vector-mediated knockdown of pyruvate dehydrogenase E1α. Mol Genet Metab 101: 183–191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samson WK, Zhang JV, Avsian-Kretchmer O, Cui K, Yosten GLC, Klein C, Lyu RM, Wang YX, Chen XQ, Yang J, Price CJ, Hoyda TD, Ferguson AV, Yuan XB, Chang JK, Hsueh AJ. Neuronostatin encoded by the somatostatin gene regulates neuronal, cardiovascular, and metabolic functions. J Biol Chem 283: 31949–31959, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharman JL, Mpamhanga CP, Spedding M, Germain P, Staels B, Dacquet C, Laudet V, Harmar AJ, NC IUPHAR. IUPHAR-DB: new receptors and tools for easy searching and visualization of pharmacological data. Nucl Acids Res 39:D534–D538, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor MM, Keown CA, Samson WK. Involvement of the central adrenomedullin peptides in the baroreflex. Regul Pept 112: 87–93, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Thakker DR, Natt F, Husken D, van der Putten H, Maier R, Hoyer D, Cryan JF. siRNA-mediated knockdown of the serotonin transporter in the adult mouse brain. Mol Psychiatry 10: 782–789, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Vainio L, Perjes A, Ryti N, Magga J, Alakoski T, Serpi R, Kaikkonen L, Piuhola J, Szokodi I, Ruskoaho H, Kerkela R. Neuronostatin, a novel peptide encoded by somatostatin gene, regulates cardiac contractile function and cardiomyocyte survival. J Biol Chem 287: 4572–4580, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yosten GLC, Pate AT, Samson WK. Neuronostatin acts in brain to biphasically increase mean arterial pressure through sympatho-activation followed by vasopressin secretion: the role of melanocortin receptors. Am J Physiol Regul Integr Comp Physiol 300: R1194–R1199, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yosten GLC, Redlinger LJ, Samson WK. Evidence for a role of endogenous nesfatin-1 in the control of water drinking. J Neuroendocrinol 24: 1078–1084, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Yosten GLC, Samson WK. Nesfatin-1 exerts cardiovascular actions in brain: possible interaction with the central melanocortin system. Am J Physiol Regul Integr Comp Physiol 297: R330–R336, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zar JH. Biostatistical Analysis. Englewood Cliffs, NJ: Prentice-Hall, 1984 [Google Scholar]