Abstract
Introduction
In 1998, the first Japanese practice guidelines on osteoporosis was published. It has been updated several times, with the most recent being the full-scale 2011 edition and its abridged edition. The present guidelines provide information for the managements of primary osteoporosis in postmenopausal women and men over 50 years old, a summary of the evidence for the treatment of secondary osteoporosis, and a summary of the evidence for the prevention of osteoporosis in younger people.
Method
The present Executive Summary is primarily based on the content of the 2011 Japanese abridged edition. One of the key changes is revision of the criteria for initiation of pharmacological treatment, along with an introduction of the fracture risk factors used in FRAX®. Key figures and tables were selected from the Japanese abridged edition and a reference list was added.
Result and conclusions
The essential points of the Japanese practice guidelines on osteoporosis were translated into English for the first time. It is hoped that the content of the guidelines becomes known throughout the world.
Keywords: Criteria for initiation of pharmacological treatment, Diagnosis of osteoporosis, Fracture risk assessment, Prevention of osteoporosis, Secondary osteoporosis, Treatment of osteoporosis
Preamble
In 1998, we published the “Guidelines for (Pharmacological) Treatment of Osteoporosis 1998” under the name of the Working Group for Developing Guidelines for Osteoporosis in the Osteoporosis Research Project supported by the Ministry of Health and Welfare (present-day Ministry of Health, Labor, and Welfare) of Japan. Although they were the first Japanese guidelines for the diagnosis and treatment of osteoporosis and also set a precedent for evidence-based practice guidelines in Japan, there were few effective therapeutic agents for osteoporosis available in Japan at that time. The 1998 edition was updated in 2002.
There has been tremendous change in the field of osteoporosis inside and outside Japan since that update. Addressing osteoporosis has become a more urgent issue also in Japan because of its fast-aging society. Therefore, we published the comprehensive “Guidelines for Prevention and Treatment of Osteoporosis 2006” under the name of the Committee for Developing Guidelines for Prevention and Treatment of Osteoporosis 2006, an ad hoc organization comprising the Japan Osteoporosis Society, Japanese Society for Bone and Mineral Research, and Japan Osteoporosis Foundation. Emphasizing prevention, covering secondary osteoporosis, presenting the criteria for initiation of pharmacological treatment, and grading the recommendation for each therapeutic agent, these guidelines were highly rated in the medical and clinical arenas. Immediately thereafter we published an abridged edition to disseminate the content of the 2006 Guidelines to a greater number of doctors and healthcare professionals.
In late 2011, the 2006 Guidelines and its abridged edition were updated. Key changes are as follows: profile of the research progress on bone quality, revision of the criteria for initiation of pharmacological treatment (associated with the re-examination of the risk factors for fracture and introducing FRAX®), more detailed descriptions about secondary osteoporosis (including new information on the relationship between lifestyle-related diseases and fracture risk), evaluation of new therapeutic agents, and bone metabolic markers covered by public insurance. The present Executive Summary is primarily based on the content of the updated 2011 Japanese abridged edition. Only the most key figures and tables were selected from the Japanese abridged edition and a reference list was added. We hope this Executive Summary contributes to the advancement of medical care for osteoporosis in Asia and the world.
In developing the guidelines, a systematic literature search of MEDLINE, EMBSE, Cochrane Library, and PubMed was conducted. The treatment recommendations in these clinical guidelines were determined by the consensus of the committee. The draft guidelines were available for physician comments at the annual meetings of the Japan Osteoporosis Society in 2010 and 2011.
The funding for all costs to produce the guidelines and this position paper was obtained from the Japan Osteoporosis Society, Japanese Society for Bone and Mineral Research, and Japan Osteoporosis Foundation. All of the authors state they have no conflict of interest related to the guidelines or this position paper.
Definition, epidemiology, and etiology
Definition
The United States National Institutes of Health (NIH) Consensus Development Conference on Osteoporosis Prevention, Diagnosis, and Therapy held in 2000 proposed a new definition of osteoporosis as follows: Osteoporosis is defined as a skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fractures. Further, it was stated that bone strength reflects the integration of two main features: bone mineral density (BMD), which accounts for almost 70 % of bone strength, and bone quality, which accounts for the remaining 30 %.
Risk factors for fractures vary among individuals, and include presence or absence of fragility fractures, family history, lifestyle factors, as well as BMD. Therefore, in clinical practice, the risk of fracture should be comprehensively evaluated based on these clinical risk factors for each individual.
Recently, some algorithms have been developed to quantitatively estimate an individual’s fracture risk by integrating multiple risk factors (see “Risk factors for fracture” for FRAX®).
Epidemiology
The estimated number of osteoporotic patients aged 40 or over in Japan is 12,800,000 (3,000,000 men and 9,800,000 women), based on the result of a survey of the prevalence of osteoporosis (diagnosed with BMD at the lumbar vertebrae or proximal femur) stratified by age in the general population (Fig. 1) [1] and the population structure stratified by age groups in 2005. Furthermore, the estimated annual incidence of osteoporosis, based on the BMD at the lumbar vertebrae in the population aged between 40 and 79 years, is 0.6 % in men and 2.3 % in women.
Fig. 1.
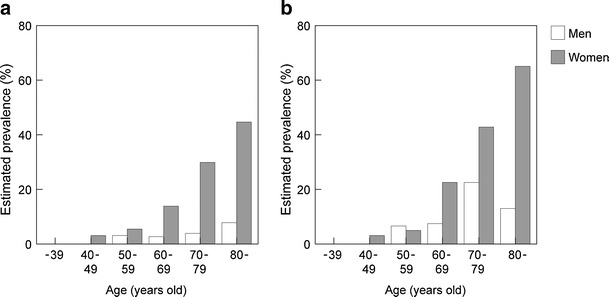
Estimated prevalence of osteoporosis in Japan. Osteoporosis was diagnosed from BMD at vertebrae L2–4 (a) and proximal femur (b). Data from Yoshimura [1] (Copyright© 2009 Springer Science + Business Media BV)
The estimated incidence of proximal femoral fractures due to osteoporosis in Japan was 148,100 (31,300 men and 116,800 women) in 2007 [2]. A follow-up study targeting a rural population revealed that the 10-year cumulative incidence of vertebral fractures was 5.1 and 14 % for men and women in their 60s, respectively, and 10.8 and 22.2 % among men and women in their 70s, respectively [3]. However, a long-term trend shows that a later year of birth is associated with a lower incidence of vertebral fractures.
The incidence of proximal femoral fractures was found to be higher in western Japan than in eastern Japan. As compared to reports from Western countries, the incidence of proximal femoral fractures is lower and that of vertebral fractures is similar or higher in Japan.
Etiology
From middle-age onward, BMD decreases and bone quality deteriorates with advancing age, resulting in loss of bone strength. Especially in women, BMD decreases sharply in the perimenopausal period and for several years thereafter. In addition to this natural course, genetic factors, nutritional deficiency since childhood and puberty, lack of exercise, and unhealthy lifestyle also cause loss of bone strength. Primary osteoporosis is the clinical condition in which these factors have caused a significant loss of bone strength.
Bone remodeling consists of bone resorption by osteoclasts and bone formation by osteoblasts, a mechanism to maintain bone strength. If bone resorption increases with advancing age and menopause and exceeds the rate of bone formation, BMD will begin to decrease. Low BMD is caused by activation of osteoclasts due to estrogen deficiency associated with menopause, and by inadequate secondary mineralization, microarchitecture deterioration, and a decrease in capacity for absorbing calcium associated with advancing age, among other factors (Fig. 2).
Fig. 2.
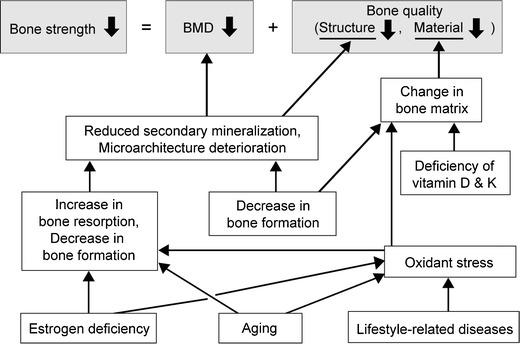
Factors causing deterioration of bone strength
Inadequate secondary mineralization and microarchitecture deterioration result in deterioration of bone quality, which is, however, also affected by the cell function of synthesizing bone matrix, conditions surrounding bone matrix (i.e., levels of oxidation and glycation), and levels of vitamins D and K. When oxidative stress and glycation increase in association with aging and lifestyle-related diseases, the non-enzymatic (nonphysiological) cross-links (see “Prevention of falls”) increase between collagen molecules in the bone matrix, resulting in a loss of bone strength (Fig. 2).
Prognosis
Fractures associated with osteoporosis, in particular proximal femoral fractures, lead to impairment in mobility and vital functions and an increase in mortality. The relative risk of overall mortality is high in older women with a low BMD and vertebral deformity, and the greater the number of vertebral fractures, the higher the risk of mortality. Decreased BMD at the proximal femur increases the long-term mortality risk, regardless of the presence or absence of vertebral fracture.
According to a survey on quality of life (QOL), patients with osteoporosis score lower on factors related to posture/body shape and falls/psychological in a self-assessment of QOL than persons in the general population who have undergone an osteoporosis screening.
Low BMD is strongly related to the Certification of Needed Long-Term Care for the public nursing-care insurance system in Japan. That is, osteoporosis or low BMD is one of the most significant factors for becoming fragile/immobilized or even becoming bedridden or institutionalized. Therefore, prevention of osteoporotic fractures is likely to prevent reduced mobility or immobilization.
Diagnosis
Diagnostic procedures
The procedures for diagnosis of osteoporosis are shown in Fig. 3 [4].
Fig. 3.
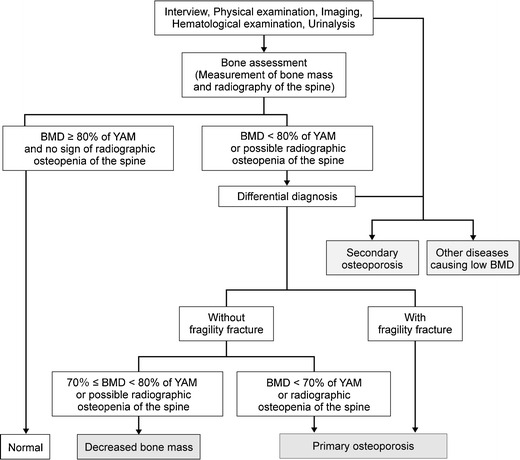
Procedure for the diagnosis of osteoporosis. YAM young adult mean (20 to 44 years of age). Adapted from Orimo [4] (Copyright© 2001 Springer Science + Business Media BV)
For the diagnosis of osteoporosis, a medical interview, physical examination, diagnostic imaging, and blood and urine examinations (including measurement of bone metabolic markers) should be conducted first. Then, bone assessment must be conducted with bone mass measurement and spinal radiography. Based on this information, diseases causing low bone mass or secondary osteoporosis should be excluded, and then an accurate diagnosis of primary osteoporosis should be made based on the diagnostic criteria (see “Diagnostic criteria for primary osteoporosis”).
Information obtained in the diagnostic process about factors that could contribute to osteoporosis and the risk factors for fractures (e.g., family history, prevalent fractures, and bone metabolic markers) should be used to evaluate the severity of osteoporosis and the fracture risk. This information will also be useful to provide guidance about lifestyle modification and to select the optimal therapeutic strategy.
Clinical presentation
In the absence of a fracture, osteoporosis is nearly asymptomatic. However, patients with osteoporosis are predisposed to the development of fractures due to loss of bone strength, and the occurrence of fractures will severely impair their QOL (Fig. 4). Osteoporotic fracture is also called fragility fracture.
Fig. 4.
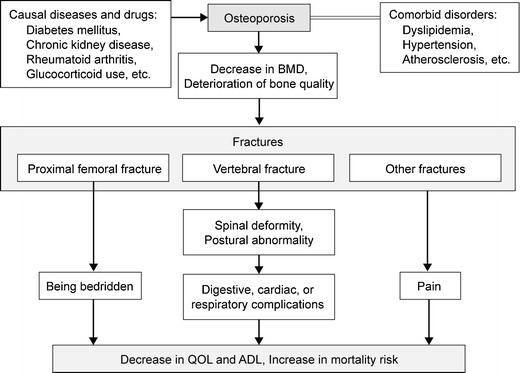
Clinical presentation and prognosis of osteoporosis
Proximal femoral fractures directly lead to decreases in the activities of daily living (ADL) and can lead to patients being bedridden, resulting in poor prognosis.
The estimated prevalence of vertebral fractures in Japanese in their early 70s is 25 % and is 43 % in person over 80 years old. The occurrence of vertebral fractures often leads to subsequent vertebral fractures. Since a vertebral deformity persists after the fracture heals, accumulation of vertebral fractures in multiple sites causes kyphosis (round back). Progressive kyphosis leads to deterioration of QOL due to significantly limited ADL and lumbar backache, and can cause functional declines or disorders of the digestive, respiratory, and cardiac systems.
Some lifestyle-related diseases which cause atherosclerosis such as diabetes mellitus (DM), hypertension, dyslipidemia, and chronic kidney diseases (CKD) have attracted attention in relation to osteoporosis. In particular, DM and CKD predispose patients to osteoporosis, and increase their fracture risk (see “Prevention of falls”). The possibility of hidden osteoporosis always should be considered during medical care of patients with lifestyle-related diseases.
Medical interview and physical examination
The objectives of the medical interview and physical examination are to assess the presence and symptoms of osteoporotic fractures, risk factors for osteoporosis and fractures, and to obtain information for the differential diagnosis.
Family history of proximal femoral fractures (in either or both parents), loss of height (4 cm or more relative to the height at 25 years of age), current smoking, and excessive alcohol consumption (3 units/day or more, 1 unit = 8–10 g ethanol) are particularly important risk factors for osteoporotic fractures. Therefore, taking a careful history including these factors is needed. History of glucocorticoids use, rheumatoid arthritis, and lifestyle-related diseases such as diabetes mellitus are important information for the differential diagnosis.
In regard to the physical findings, a rounded back, fewer than 20 teeth, and a value of less than −4 on the Female Osteoporosis Self-Assessment Tool for Asians are key factors that strongly suggest osteoporosis.
Bone assessment
It is recommended that BMDs of the lumbar spine and/or proximal femur are measured by dual-energy X-ray absorptiometry (DXA). When there is a fracture or deformity in the lumbar vertebrae that increases the influence of an artifact on spine BMD, the data of lumbar spine should not be used. If the measurement at either of these sites is not successful (because of bilateral hip surgery, multiple fractures of the lumbar vertebra, severe vertebral deformity, or excessive obesity, etc.), another choice is forearm bone.
Microdensitometry has been developed in Japan to radiologically assess BMD, mainly of cortical bone in the second metacarpal.
The speed of sound and broadband ultrasound attenuation through bone are measured with quantitative ultrasound (QUS). This is a non-invasive measurement technique and may provide reliable information on bone quality along with the BMD. However, it is easily affected by measurement conditions, among other factors. The parameters used in QUS were standardized by the QUS Standardization Committee of the Japan Osteoporosis Society in 2010 [5].
Fracture evaluation
Radiography of the thoracic and lumbar vertebrae are essential for assessment of fracture, deformity, or change in the vertebrae, and for exclusion of other similar disorders that present with lower back pain, round back, or low bone mass. In the Japanese diagnostic criteria, the presence of fragility fractures alone confirms the diagnosis of osteoporosis (see “Diagnostic criteria for primary osteoporosis”). Since most of the prevalent fragility fractures, however, are vertebral fractures, usually without pain, radiography is fundamental for their proper diagnosis. Either semiquantitative assessment or quantitative morphometry is used. The lateral DXA images for vertebral fracture assessment can be used, but more clinical experience in Japan is needed to make a recommendation.
If used during the early period after a fracture has occurred (within 2 weeks), MRI provides a better diagnostic yield than plain radiography. MRI is helpful particularly for fresh vertebral fractures, because the height of the vertebral body often does not decrease in the early period. Since it is, however, impractical to diagnose all the cases with MRI, MRI is recommended when it is necessary to distinguish osteoporotic fractures including non-vertebral fractures from those caused by other diseases, or for a detailed examination regarding complicating diseases.
Bone metabolic markers
The increase of bone metabolic markers is a BMD-independent predictor of fractures, and bone metabolic markers are one of the indices of fracture risk. There are two types of bone metabolic markers: bone resorption markers and bone formation markers. Examinations of blood or urine for these bone metabolic markers easily provide information on the bone metabolic state (Fig. 5) [6].
Fig. 5.
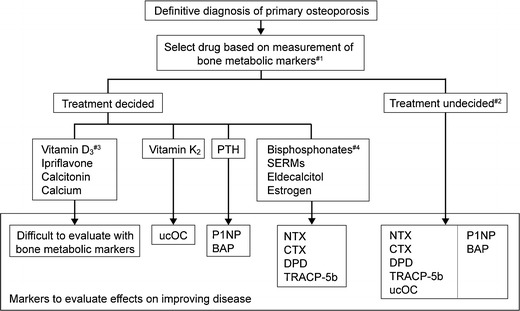
Measurement of bone metabolic markers in drug treatment of osteoporosis. #1: in patients taking bisphosphonates, measure after stopping drug for at least 6 months, and in patients taking other osteoporosis drugs, measure after stopping drug for at least 1 month. #2: measure one type each of a resorption marker and formation marker. #3: excluding eldecalcitol. #4: in patients expected to be on long-term bisphosphonate therapy, measure bone resorption markers and BAP or P1NP. Nishizawa [6] (Copyright© 2012 Springer Science + Business Media BV)
Bone metabolic markers are useful particularly for the following situations. (1) The patient has little understanding of the need for treatment. (2) The patient is scheduled to receive pharmacotherapy. (3) It is difficult to decide what drug to choose. (4) You want to adopt an appropriate treatment for the patient’s pathological condition. Bone metabolic markers are also useful for evaluation of the response to treatment. Thus, it is recommended to measure them at the time of diagnosis if possible.
Among bone metabolic markers, undercarboxylated osteocalcin (ucOC) can be used as an index of vitamin K deficiency in the bones.
When the values of bone resorption markers are abnormally high, the presence of other metabolic bone diseases is suspected.
Differential diagnosis
The targets of differentiation from primary osteoporosis are secondary osteoporosis and other bone-related diseases. Secondary osteoporosis is caused by other diseases or treatments, but its clinical state can seem similar to that of primary osteoporosis, while other bone-related diseases display a clinical state that is different from that of primary osteoporosis. Some instances of secondary osteoporosis and other bone-related diseases are critical or require immediate medical attention. Further, most types of secondary osteoporosis require a therapeutic strategy different from that for primary osteoporosis, and the appropriate treatment of the causative diseases may lead to a dramatic improvement in secondary osteoporosis. Therefore, the differential diagnosis is an extremely important process, despite the prevalence of secondary osteoporosis being low. The probability of secondary osteoporosis is relatively high among premenopausal women and men.
Information for the differential diagnosis can be obtained in every step of the diagnostic process. In the medical interview, thorough medical and surgical histories are needed, including current medications. Radiography may be useful for exclusion of osteomalacia and bone metastases of malignant tumors. Various causative states of secondary osteoporosis may be suspected by the results of blood and urine examinations, for example, hypercalcemia, hypocalcemia, elevated alkaline phosphatase level, and proteinuria.
It is usually considered that patients who visit specialized medical institutes, such as university hospitals, are likely to have secondary osteoporosis due to endocrine diseases and others.
Diagnostic criteria for primary osteoporosis
After excluding both the presence of other diseases characterized by low bone mass and the possibility of secondary osteoporosis, primary osteoporosis should be diagnosed by a two-step approach: (1) presence or absence of fragility fractures and (2) BMD or assessment of osteopenia on spinal radiography (Fig. 6) [4].
Fig. 6.
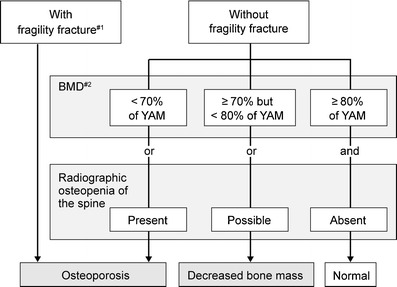
Diagnostic criteria for primary osteoporosis (updated in 2000). Primary osteoporosis is diagnosed according to these criteria in the absence of diseases causing low bone mass or secondary osteoporosis. #1: fragility fracture is a nontraumatic bone fracture that is caused by slight external force to a bone with low BMD (BMD less then 80 % of YAM). Sites of fracture include the spine, proximal femur, and the distal end of the radius. #2: BMD usually refers to lumbar BMD. However, when the measurement is inappropriate for reasons such as spinal deformity, the proximal femur BMD should be used. When measurement at those sites is difficult, BMD of the radius, second metacarpal bone, or calcaneus will be used. Revision of additional T-scores is under consideration. Adapted from Orimo [4] (Copyright © 2001 Springer Science + Business Media BV)
Primary osteoporosis is diagnosed on the presence of any fragility fractures (defined as a nontraumatic bone fracture caused by slight external force to a bone with low bone mass, which correlates to a BMD < 80 % of young adult mean (YAM) or radiographic osteopenia of the spine) at sites including spine, proximal femur, and the distal end of radius. If there is no fragility fracture, the BMD level is used to diagnose the patient as “normal”, “decreased bone mass”, or “osteoporosis”. Evaluation of osteopenia based on spinal radiography should be used as supplementary means, and quantitative bone densitometry is preferable for bone assessment.
The T-score to YAM of BMD, not the percentage, is used as diagnostic criteria internationally. A T-score of −1.5 represents a value of −1.5 standard deviation of the YAM and is approximately equivalent to 80 % of the YAM in Japan. A T-score of −2.5 is approximately equivalent to 70 % of the YAM. Internationally, the proximal femur is considered to be the standard measurement site for BMD.
Risk factors
Risk factors for fracture
Major risk factors for osteoporotic fractures are female gender, advanced age, low BMD, and prevalent fractures. In addition, many other factors affect fracture risk directly or indirectly. Although a poor intake of calcium increases fracture risk via low BMD, other risk factors for fractures such as age, prevalent fracture, family history of fractures, smoking, and drinking are independent of BMD. Low body weight also is a BMD-independent risk factor, but only for proximal femoral fractures.
The FRAX® (Fracture Risk Assessment Tool) was developed to estimate the 10-year probability of fractures in individual patients by the World Health Organization (WHO) in 2008 based on 11 risk factors identified from worldwide data in ten cohorts. FRAX is a convenient tool to easily identify a person at high risk for fractures, and therefore has been incorporated into the criteria for initiation of pharmacological treatment in the present guidelines (see “Criteria for initiation of pharmacological treatment”).
Prevention
Primary prevention of osteoporosis
The most important measure for primary prevention of osteoporosis is education appropriate to each age group: in early life to acquire as high a peak bone mass (PBM) as possible, to maintain acquired PBM through exercise thereafter, and to minimize its decrease after menopause.
A study on the age-specific distribution of bone mass in Japanese women revealed that PBM is achieved at 18 years of age [7]. Thus, before age 18 is the most effective time for physicians to encourage young people to increase PBM to its maximal level. Guidance on maintenance of adequate weight, active intake of calcium, and weight-bearing exercise is effective.
For middle-aged and older persons, guidance on maintenance of adequate weight, aerobic exercises especially walking, and weight-bearing exercise is effective. Smoking cessation and limiting alcohol intake to less than 3 units/day (1 unit = 8–10 g ethanol) is likely to decrease the fracture risk.
Prevention of falls
Most proximal femoral fractures in elderly people occur because of a fall. Risk factors for proximal femoral fractures are a past history of falls and the number of falls, and fall-related factors including generalized weakness, paralysis, muscular weakness, use of sleep-inducing drugs, and decreased vision.
Approaches to prevent falls include (1) exercise interventions (e.g., training to increase strength of muscle, balance, walking ability, and flexibility); (2) non-exercise interventions (e.g., instruction about medication, diet, and environment, along with education and guidance for behavior modification); and (3) multifactorial intervention (e.g., in addition to 1 and 2, an individualized approach based on the physical and mental functioning, environment, and medical assessment of a patient).
In elderly people, vitamin D deficiency increases the risk of falls, and administration of vitamin D can reduce the frequency of falls.
Wearing a hip protector is effective for the prevention of proximal femoral fractures; especially in high-risk groups in elderly care facilities.
Osteoporosis screening
Osteoporosis screening is spreading as a part of the Elderly Health Services (currently as a project under the Health Promotion Law) in Japan, and is performed every 5 years in women from 40 to 70 years old. The screening rate (the percentage of women who underwent osteoporosis screening against the entire target female population) was 4.6 % in 2005.
Osteoporosis screening for people of middle and older age is aimed at early detection of asymptomatic osteoporotic patients and persons at risk of osteoporosis to prevent future fractures. Persons at risk of osteoporosis should be given guidance on diet and exercises, and asymptomatic patients should be targets for early intervention (secondary prevention).
In screening, persons should be classified as either “Complete examination required”, “Guidance required”, or “No apparent abnormality” based on the results of the medical interview and bone mass measurement (Fig. 7) [8]. The criteria for requiring a complete examination is a bone mass of less than 80 % of YAM; this is different from the diagnostic criteria for osteoporosis (i.e., when BMD is less than 70 % of YAM in the absence of fragility fracture). In addition, bone mass measurement at the calcaneus (including QUS), which is not used to diagnose osteoporosis, is also permitted in the screening. The reason for these differences is that screening should identify the persons requiring the full diagnostic assessment for osteoporosis.
Fig. 7.
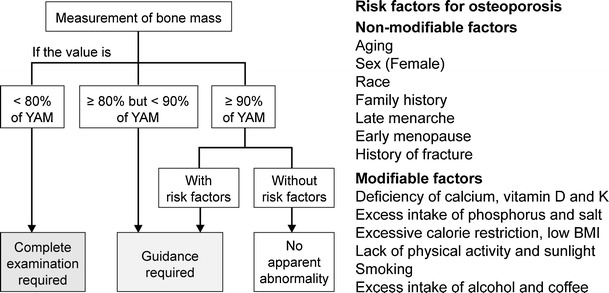
Criteria for osteoporosis screening. Risk factors for osteoporosis: non-modifiable factors: aging, sex (female), race, family history, late menarche, early menopause, and history of fracture. Modifiable factors: deficiency of calcium, vitamin D, and K; excess intake of phosphorus and salt; excessive calorie restriction; low BMI; lack of physical activity and sunlight; smoking; and excess intake of alcohol and coffee Orimo [8]
FRAX® will become suitable for osteoporosis screening after the cutoff values for fracture probability are established for complete examination and for guidance.
Treatment
Criteria for initiation of pharmacological treatment
The goals of osteoporosis treatment are prevention of fracture as a complication and maintenance of good skeletal health. Important strategies to reduce the fracture risk in osteoporotic patients are treatment with a bone resorption inhibitor or bone formation stimulant and guidance to establish a lifestyle that leads to maintenance and enhancement of bone strength and to avoid risk factors for fractures, such as a fall, that are independent of a decrease in bone strength.
The risk factors for fracture include low BMD, factors that contribute to a decrease in BMD, and deterioration of bone matrix, including lifestyle-related diseases. A prevalent fragility fracture is the most important among all these factors with the exception of low BMD. Family history of proximal femoral fractures significantly increases the fracture risk even in persons without a fragility fracture who have a “low bone mass” based on their BMD.
Based on this new knowledge about risk factors and the consideration about using FRAX® (see “Risk factors for fracture”), the criteria for initiating pharmacological treatment to prevent fragility fracture was established as shown in Fig. 8. In these criteria, FRAX® is used to consider whether or not to initiate pharmacological treatment in persons without a fragility fracture who have a low bone mass. This is because persons with a fracture risk comparable to patients with osteoporosis possibly could be included in this group and need other measures to assess the magnitude of the fracture risk other than low BMD. Considering that the 10-year probability of major osteoporotic fractures in the patients receiving pharmacological treatment was observed around 15 % in Japanese clinical settings, we adopted 15 % as a treatment threshold for the persons with low bone mass. In the guidelines, FRAX® is not used in the first-line screening to determine the persons who need further examination such as bone densitometry. As stated earlier, the cutoff value for the screening in Japan is being studied. The cutoff value of a 15 % 10-year probability is used for women and men younger than 75 years old, because almost all of the persons of this age group have a value above 15 % and thus its power as a cutoff value is too weak.
Fig. 8.
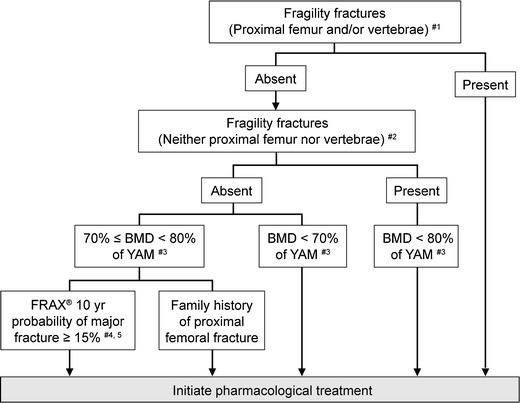
Criteria for initiation of pharmacological treatment. #1: this means proximal femoral fracture and/or vertebral fracture caused by slight external force after menopause in women and after age 50 in men. #2: this means distal forearm, proximal humerus, pelvis, lower leg and/or rib fracture caused by slight external force after menopause in women and after age 50 in men. #3: revision of additional T-scores is under consideration for some measurement sites. #4: this should be applied in persons <75 years. Additionally, a lower cutoff value does not include all young persons in and around their 50s for whom pharmacological treatment is recommended based on the present diagnostic criteria. #5: as these criteria refer to primary osteoporosis, they should not be applied to persons whose FRAX® risk factors are “glucocorticoid”, “rheumatoid arthritis”, or “secondary osteoporosis”. That is, these criteria should be applied only in persons who answer “No” to each of these items
Evaluation of response to treatment
The optimal method for bone mass measurement to evaluate the therapeutic effect is DXA at the lumbar vertebrae on the anteroposterior direction, because it is sensitive enough to detect changes in bone mass. If the bone mass cannot be measured precisely at the lumbar vertebrae, measurement at the total hip is recommended. The timing of measurement should be determined based on the least significant change of each method.
The efficacy of drugs with significant effects on bone metabolism can be evaluated by measuring bone metabolic markers. It is beneficial to measure bone resorption markers at 3 to 6 months after the initiation of treatment and bone formation markers every 6 to 12 months. Attention should be paid to the minimum significant change of each marker.
Plain radiography is useful for detection of incident vertebral fractures after the initiation of treatment. CT, MRI, and bone scintigraphy are sometimes required for confirmation of minor fractures, incomplete fractures, and unapparent fractures, and for differentiation from other clinical conditions including tumors.
QOL assessment using the Japanese Osteoporosis Quality of Life Questionnaire (JOQOL) is useful also for evaluation of therapeutic effects.
Basic treatments (non-pharmacological treatment)
A daily intake of calcium (700 to 800 mg) is recommended to optimize the effect of pharmacological treatment. It has been reported that calcium derivatives and calcium supplements may increase the risk of cardiovascular diseases. However, dietary intake of the same amount of calcium has not been shown to increase cardiovascular risk. Moreover, those adverse findings were reported from outside Japan, where calcium intake, serum lipid levels, and BMI are different from those in Japan. At this time, calcium as a medicine or supplement should not exceed 500 mg per dose.
Vitamin D (recommended daily intake, 10 to 20 μg) and vitamin K (250 to 300 μg) are also essential, and they should be prescribed to be taken as a medicine when it is difficult for the patient to obtain a sufficient amount from dietary sources. Hyperhomocysteinemia due to vitamin deficiency (vitamins B6, B12, and folic acid) involved in homocysteine metabolism has been shown to be a BMD-independent risk factor for fracture. It is recommended to warn patients not to consume excessive amounts of phosphorus, salt content, caffeine, and alcohol.
It has been demonstrated that high-impact activities, resistance exercises, back muscle exercises, stretching exercises, aerobic exercises, walking, and balance training can increase BMD and prevent vertebral fractures and falls in patients with osteoporosis.
In terms of pain relief, few data from randomized controlled trials are available about the effects of various physical therapies, nerve blocks, and surgeries; however, the efficacy of some drugs has been demonstrated.
Pharmacological treatment
These Guidelines detail the effect of each therapeutic agent used in Japan on BMD and the risk of vertebral fracture, non-vertebral fracture, and proximal femoral fracture, based on evidence from Japan and abroad. Each recommendation is also graded (Table 1). In regard to some therapeutic agents, the effect on QOL is also described. Table 2 shows the prescription drugs covered by the public health insurance in Japan.
Table 1.
Grading of recommendation of therapeutic agents for osteoporosis in Japan
| Therapeutic agent | BMD | Vertebral fracture | Non-vertebral fracture | Proximal femoral fracture | |
|---|---|---|---|---|---|
| Calcium | Calcium L-aspartate hydrate | C | C | C | C |
| Dibasic calcium phosphate hydrate | C | C | C | C | |
| Estrogen | Estriol | C | C | C | C |
| Conjugated estrogensa | A | A | A | A | |
| Estradiol | A | C | C | C | |
| Active vitamin D3 | Alfacalcidol | B | B | B | C |
| Calcitriol | B | B | B | C | |
| Eldecalcitol | A | A | B | C | |
| Vitamin K2 | Menatetrenone | B | B | B | C |
| Bisphosphonate | Etidronate disodium | A | B | C | C |
| Alendronate sodium hydrate | A | A | A | A | |
| Sodium risedronate hydrate | A | A | A | A | |
| Minodronic acid hydrate | A | A | C | C | |
| SERM | Raloxifene hydrochloride | A | A | B | C |
| Bazedoxifene acetate | A | A | B | C | |
| Calcitoninb | Elcatonin | B | B | C | C |
| Calcitonin (Salmon) | B | B | C | C | |
| PTH | Teriparatide (genetical recombination) | A | A | A | C |
| Other drugs | Ipriflavone | C | C | C | C |
| Nandrolone decanoate | C | C | C | C | |
A strongly recommended to use, B recommended to use, C not enough evidence to recommend use, D recommended not to use
aAdministration of conjugated estrogen for osteoporosis is not covered by the public health insurance in Japan
bCalcitonin has an analgesic effect, and reduces pain due to osteoporosis (grade A)
Table 2.
Prescriptions of anti-osteoporotic agents covered by the public health insurance in Japan (as of September 2011)
| Generic name | Launched | Prescription for osteoporosis |
|---|---|---|
| Calcium L-aspartate hydrate | 1968 | 1.2 mg/day, p.o. |
| Dibasic calcium phosphate hydrate | 1985 | 3 g/day, p.o. |
| Estriol | 1969 | 1 mg/day, p.o. |
| Conjugated estrogens | 1999 | Not covered by the public insurance |
| Estradiol | 2008 | 1 mg/day, p.o. |
| Alfacalcidol a | 1981 | 0.5 or 1 μg/day, p.o. (adult) |
| Calcitriol | 1986 | 0.5 μg/day, p.o. |
| Eldecalcitola | 2011 | 0.75 or 0.5 μg/day, p.o. |
| Menatetrenonea | 1995 | 45 mg/day, p.o. |
| Etidronate disodium | 1990 | 200 or 400 mg/day, p.o. (intermittent) |
| Alendronate sodium hydrate | 2001 | 5 mg/day or 35 mg/w, p.o. |
| Sodium risedronate hydrate | 2002 | 2.5 mg/day or 17.5 mg/w, p.o. |
| Minodronic acid hydrate a | 2009 | 1 mg/day or 50 mg/4w, p.o. |
| Raloxifene hydrochloride | 2004 | 60 mg/day, p.o. |
| Bazedoxifene acetate | 2010 | 20 mg/day, p.o. |
| Elcatonina | 1982 | 20 IU/w, i.m. |
| Calcitonin (Salmon) | 1990 | 20 IU/w, i.m. |
| Teriparatide (genetical recombination) | 2010 | 24 μg/day, s.c. (up to 24 months) |
| Ipriflavone | 1988 | 200 mg/day, p.o. |
| Nandrolone decanoate | 1984 | 25 or 50 mg/3 w, i.m. |
Teriparatide acetate, a new drug developed in Japan, came to market in November 2011. Prescription is 56.5 μg/w, s.c., up to 72 weeks
aAgents developed in Japan
For the selection of therapeutic agents, the full range of drug-related information must be considered: the efficacy of each medicine on BMD, fracture risk, QOL including pain, bone metabolic markers, risk of fall, as well as safety, including effects other than those on bone metabolism per se and adverse effects. Further, the patient’s clinical state must be considered.
The systematic review published by MacLean and colleagues indicated that bisphosphonates (alendronate and risedronate) are a first-line agent for patients at high risk of vertebral, non-vertebral, or proximal femoral fracture [9]. Parathyroid hormone derivatives are first-line agents for patients at high risk of vertebral or non-vertebral fracture. Selective estrogen receptor modulators (SERMs) are first-line agents for patients at high risk of vertebral fracture. Minodronic acid, a bisphosphonate developed in Japan, is expected to be used for the high-risk group for vertebral fracture. Eldecalcitol, an active vitamin D3 derivative developed in Japan, is expected to be used for the high-risk group for vertebral or non-vertebral fracture. However, more data are required for these new agents.
Estrogen derivatives
A postmenopausal decrease in bone mass is caused by estrogen deficiency. Therefore, estrogen replacement has been considered to be an effective treatment option for osteoporosis since early times. Estrogen replacement is useful also for prevention and treatment of other diseases and symptoms caused by estrogen deficiency. Administration of estrogen to young amenorrheic women or relatively young postmenopausal women can prevent osteoporosis. Estrogen is also useful for treatment of osteoporosis in women with climacteric symptoms in relatively early stage of postmenopause. Conjugated estrogen, estradiol, and estriol are the approved estrogen derivatives in Japan.
Although conjugated estrogen increases BMD and prevents vertebral, non-vertebral, and proximal femoral fracture, it is not covered by the public health insurance in Japan for the treatment of osteoporosis.
Estradiol increases BMD, but there is little evidence that it prevents fractures.
There is almost no evidence about the effects of estriol.
Alfacalcidol and calcitriol (active vitamin D3 derivatives)
Alfacalcidol and calcitriol are active vitamin D3 derivatives. Alfacalcidol, developed in Japan, is a prodrug requiring hydroxylation in the liver for activation. Because these derivatives were approved for the treatment of osteoporosis in 1983 and 1989, respectively, there is insufficient large clinical trial data. However, several reports suggested these agents maintain lumbar BMD at a significantly higher level as compared to placebo, or reduce the risk of vertebral and non-vertebral fractures (not statistically significant; Fig. 9a) [10].
Fig. 9.
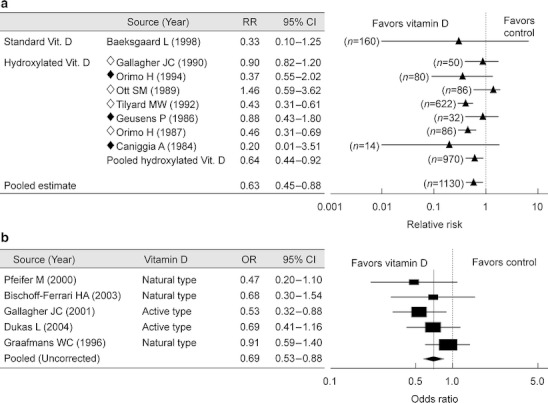
Meta-analyses on the efficacy of vitamin D. a Relative risk for vertebral fractures after treatment with vitamin D. RR relative risk, CI confidence interval. Open rhombus indicates using calcitriol and closed rhombus using alfacalcidol. Adapted from Papadimitropoulos [10] (Copyright© 2002 The Endocrine Society). b Compared risk of falling between vitamin D-treated group and control group. OR odds ratio, CI confidence interval. Adapted from Bischoff-Ferrari [11] (Copyright© 2004 American Medical Association)
It has been reported also that vitamin D deficiency causes atrophy of the type II muscle fibers, and that vitamin D supplementation improves trunk imbalance. Active vitamin D3 derivatives (alfacalcidol and calcitriol) reduce falls among the elderly (Fig. 9b) [11]. These active vitamin D3 derivatives have been confirmed to be safe, even for long-term use, and they are recommended for the elderly (see “Combination therapy” for combination with bisphosphonate).
Eldecalcitol (active vitamin D3 derivative)
Although the conventional active vitamin D3 derivatives have been reported to be effective for preventing fractures, they have not been shown to increase BMD significantly. Various vitamin D3 derivatives have been investigated; of these eldecalcitol was developed in Japan. Eldecalcitol showed superior efficacy to alfacalcidol to increase BMD (Fig. 10a) [12], while its effect on calcium absorption was nearly unchanged. Eldecalcitol may exert its actions by promoting calcium absorption from the small intestine, similar to the conventional active vitamin D3 derivatives, and prevent bone resorption by inhibiting osteoclastic function.
Fig. 10.
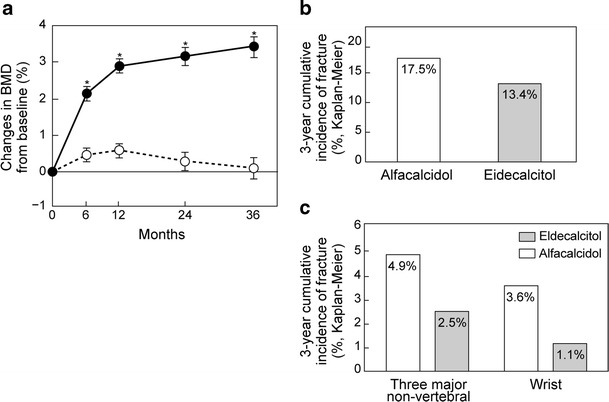
Effect of eldecalcitol compared with alfacalcidol. a Change in lumbar BMD. Data are mean ± SE, *p < 0.001 vs alfacalcidol group by Student t test (Matsumoto [12] (Copyright© 2011 Elsevier)). b Incidence of vertebral fracture. Hazard ratio (HR) is 0.74 and 95 % confidence interval (CI) is 0.56–0.97. Data from Matsumoto [12] (Copyright© 2011 Elsevier). c Incidence of non-vertebral fractures. HR for three major non-vertebral fractures is 0.52 and 95 % CI is 0.29–0.93, p = 0.031. Three major non-vertebral sites mean humerus, wrist, and hip, i.e., the three sites of major non-vertebral fractures recognized as osteoporotic fractures in FRAX®. HR for wrist fractures is 0.29 and 95 % CI is 0.11–0.77, p = 0.005. Data from Matsumoto [12] (Copyright© 2011 Elsevier) and the website of Pharmaceuticals and Medical Devices Agency (in Japanese)
In a comparative study of eldecalcitol and alfacalcidol, the incidence of vertebral fractures was found to be significantly lower in the eldecalcitol group (Fig. 10b) [12]. While there was no significant difference in the overall incidence of non-vertebral fractures between the eldecalcitol and alfacalcidol groups, there was a trend towards a greater decrease in the incidence of non-vertebral fractures at the three major sites (humerus, wrist, and hip) in the eldecalcitol group than in the alfacalcidol group (Fig. 10c) [12]. Of note, the incidence of wrist fractures was significantly reduced in the eldecalcitol group.
Clinical trials of eldecalcitol have been conducted in patients over a wide range of age and severity, and this agent can be used across the entire spectrum of patients with osteoporosis.
Menatetrenone (vitamin K2 derivative)
In elderly women and patients with osteoporosis being treated with a bisphosphonate, insufficient intake of vitamin K is a BMD-independent risk factor for fractures. Menatetrenone, a vitamin K2 derivative, promotes carboxylation of osteocalcin, and thereby it reduces the serum level of ucOC, an index of vitamin K deficiency.
Menatetrenone slightly increases lumbar BMD and reduces vertebral and non-vertebral fractures (Fig. 11) [13]. Menatetrenone is considered to exert its fracture-reducing effect via a mechanism of action other than increasing BMD.
Fig. 11.
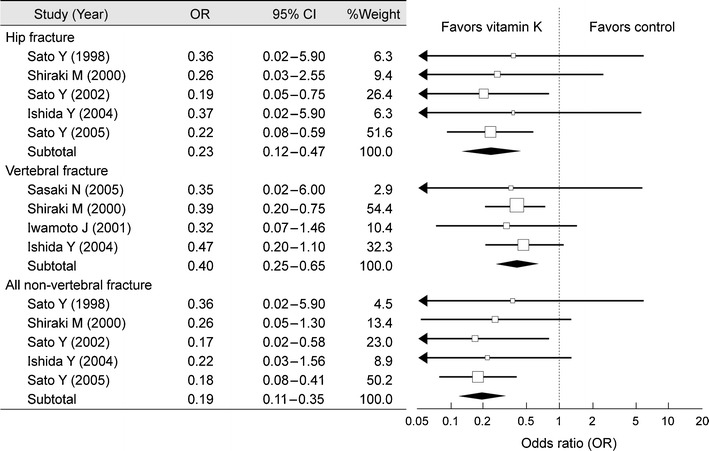
Meta-analysis on the efficacy of menatetrenone on fractures. OR odds ratio, CI confidence interval (Cockayne [13] (Copyright© 2006 American Medical Association))
Etidronate (bisphosphonate)
Notably, for etidronate, a first-generation bisphosphonate, there is a small margin between its serum level for the onset of its inhibitory actions on bone resorption and the serum level for its inhibitory effects on bone formation. Close attention must be paid to its narrow safety range. Thus, a cyclical intermittent treatment strategy (200 to 400 mg/day once daily for 2 weeks, followed by a rest period of 10 to 12 weeks) is essential.
Because etidronate reduces bone resorption, it is effective particularly for high-turnover osteoporosis, and it maintains bone mass even in low-turnover osteoporosis. Etidronate reduces blood and urine levels of bone metabolic markers. Etidronate reduces incident vertebral fractures in patients who have vertebral fractures. There is no clear evidence about whether or not etidronate reduces non-vertebral fractures.
Alendronate (bisphosphonate)
Alendronate, a second-generation bisphosphonate, has a very wide safety range. Its inhibitory effect on bone resorption is exerted at a much smaller dose than the dose for its inhibitory effect on bone formation (approximately 1/6,000).
Many clinical trials and meta-analyses have shown that alendronate increases BMD, reduces fractures at the vertebra/non-vertebra, proximal femur, and distal end of the forearm; and improves the bone metabolic marker profile. Alendronate has been reported to reduce vertebral fracture and increase lumbar BMD also in men with osteoporosis.
In terms of QOL, a decrease in the duration of bed rest for low back pain, a decrease in the days of activity restriction, and improvement of arthralgia and pain-related QOL scores after treatment with alendronate have been reported (see “Combination therapy” for the combination with active vitamin D3 derivatives).
A once-weekly dose of alendronate (35 mg), compared to a daily dose of alendronate (5 mg) was shown to have a similar effect on lumbar BMD and urinary levels of type I collagen cross-linked N-telopeptides (NTX); the incidence of adverse reactions and drug discontinuation was lower in the once-weekly group.
Risedronate (bisphosphonate)
Risedronate, a third-generation bisphosphonate, has a strong inhibitory effect on bone resorption.
Many clinical trials and meta-analyses have shown that risedronate increases BMD and reduces fractures at the vertebra/non-vertebra and proximal femur in postmenopausal women. Risedronate was reported to increase lumbar BMD also in men with osteoporosis. Large-scale clinical trials in North America, Europe, and Australia have shown preventive effects with risedronate against incident vertebral fracture from the first year of treatment. In Japan, it was reported that risedronate improved scores for body pain, vitality, and social functioning in QOL assessment using the SF-36 scale.
Once-weekly risedronate (35 mg), compared to daily risedronate (5 mg), was shown to increase BMD at the femoral neck and trochanter to the same degree in a study in the USA. In a Japanese clinical trial, once-weekly risedronate (17.5 mg), compared to daily risedronate (2.5 mg), increased lumbar BMD to the same degree at 48 weeks.
Minodronic acid (bisphosphonate)
Minodronic acid is the only domestically developed bisphosphonate for osteoporosis, and the only bisphosphonate which has been investigated for its inhibitory effect on fracture in Japanese patients at doses approved in Japan. Minodronic acid has the strongest inhibitory effect on bone resorption among the bisphosphonates currently available in Japan.
The efficacy of minodronic acid on BMD at the lumbar spine and total hip is equivalent to alendronate (Fig. 12a) [14]. In addition, minodronic acid significantly increased BMD in patients who had a poor response to other bisphosphonates.
Fig. 12.
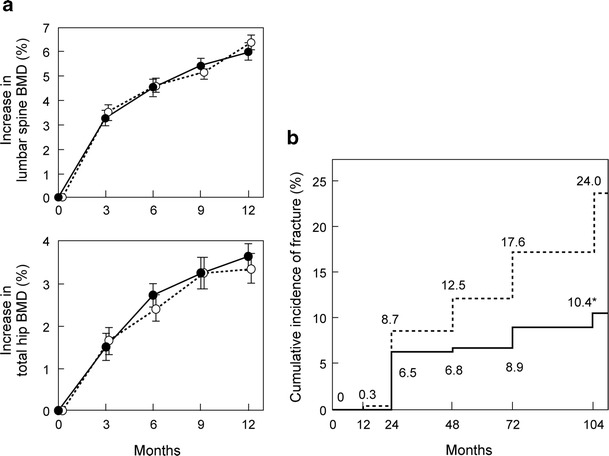
Effect of minodronic acid on BMD and vertebral fracture. a Percent change in lumbar spine and total hip BMD. Solid line is minodronic acid 1 mg (n = 134) and broken line is alendronate 5 mg (n = 135). Data are mean ± SE (Hagino [14] (Copyright© 2009 Elsevier)). b Incidence of vertebral fracture. Solid line is minodronic acid (n = 339) and broken line is placebo (n = 328). Relative risk is 0.411 (95 % confidence interval 0.267–0.634) by Cox regression model. *p < 0.0001 by log-rank test between the groups (Matsumoto [15] (Copyright© 2009 Springer Science + Business Media BV))
Minodronic acid reduced vertebral fracture risk by 59 % in Japanese patients with osteoporosis (Fig. 12b) [15], and no difference was observed in the effect between patients above and below 75 years of age. No clinical trial to determine the effect of minodronic acid on non-vertebral fracture or proximal femoral fracture has been conducted. The results of the ongoing Japanese Osteoporosis Intervention Trial (JOINT)-04 initiated in 2011 by the Adequate Treatment of Osteoporosis (A-TOP) Research Group (see “Combination therapy”) are greatly anticipated to answer these questions. Minodronic acid is available for daily use (1 mg) and once every 4 weeks (50 mg).
Raloxifene (SERM)
Raloxifene, a selective estrogen receptor modulator, binds to the estrogen receptor (ER) with an affinity equivalent to estrogen and induces a conformational change at the helix 12 in the C-terminal part of ER; this conformational change produced by raloxifene is different from that produced by estrogen. Thus, raloxifene has a tissue-selective pharmacological action: it shows estrogen-like effects on bone, but not on the breast or uterus.
The Multiple Outcomes of Raloxifene Evaluation, a large-scale randomized controlled trial with 7,705 patients in 25 countries, demonstrated that raloxifene increased BMD and reduced incident vertebral fractures, regardless of the presence or absence of prevalent vertebral fractures and even in subjects with low bone mass (osteopenia). Additionally, raloxifene significantly reduced the incidence of non-vertebral fractures in patients with severe vertebral fractures. In Japan, a 3-year post-marketing surveillance demonstrated that the overall incidence of clinical fractures was as low as 1.2 %.
Many observational studies from Japan and abroad demonstrated the effect of raloxifene on QOL, including pain relief. A meta-analysis revealed that raloxifene decreases the overall mortality by 10 %.
Venous thromboembolism is one of the clinically important adverse events of SERMs. The incidence of venous thromboembolism in patients treated with raloxifene is 0.2 %, stated in the drug package insert, based on the results of a 3-year post-marketing surveillance conducted in 7,557 Japanese patients.
Bazedoxifene (SERM)
Bazedoxifene, a SERM, has an estrogen-like action selectively on bone metabolism and lipid metabolism, but not on the breast or uterus.
An international multi-center clinical trial demonstrated that bazedoxifene increases BMD and reduces vertebral fractures, similar to raloxifene. Although no overall reduction on non-vertebral fractures was observed with bazedoxifene, the incidence of non-vertebral fracture in postmenopausal women at a higher risk of fracture was significantly reduced by bazedoxifene as compared to placebo and raloxifene. Additionally, the higher the FRAX® score, the more effectively bazedoxifene reduced osteoporotic fractures. Bazedoxifene was also reported to improve the profile of bone metabolic markers. The effect of bazedoxifene on proximal femoral fracture has not been studied yet.
A significant decrease in the incidence of vertebral fractures and the safety of the drug were consistently observed during the 5-year treatment with bazedoxifene.
Calcitonin derivatives
Calcitonin is a bone resorption inhibitor acting directly on osteoclasts and pre-osteoclasts to control their functions. Calcitonin also relieves pain via the central serotoninergic system, and therefore its derivatives may be the first choice to obtain pain relief and improves QOL in the early phase after the occurrence of osteoporotic fractures or in patients with postural distortion associated with vertebral fractures.
There are some reports on the effect of calcitonin derivatives on BMD and vertebral fracture (Fig. 13a) [16], but none on non-vertebral or proximal femoral fractures.
Fig. 13.
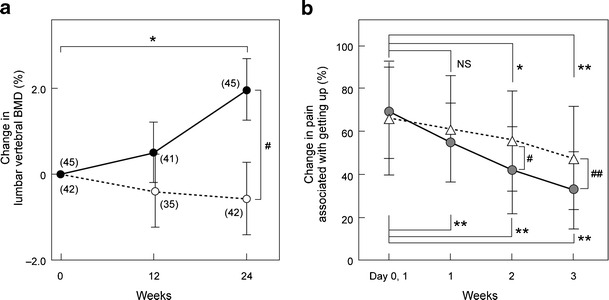
Effect of elcatonin on BMD and pain associated with vertebral fracture. a Percent change in lumbar spine BMD. Solid line is elcatonin (20 units per week) with 0.6 g calcium lactate and broken line is control (calcium lactate only). Data are mean ± SE. Numerals in parentheses denote number of patients. Comparison within groups: Student’s paired t-test, #p < 0.05; between groups: Student’s unpaired t-test, *p < 0.05. Orimo H [16] (Copyright© 1996 Springer Science + Business Media BV). b Percent change in pain associated with getting up evaluated with visual analog scale (VAS). Solid line is elcatonin (20 units per week, n = 44) and broken line is control (untreated, n = 42). Two-way repeated-measures ANOVA, *p < 0.05, **p < 0.01, NS not significant. Mann–Whitney U test, #p < 0.05, ##p < 0.01 (Nakano [17])
Some randomized clinical trials and systematic reviews revealed significant reductions in the severity of pain associated with ADLs 1 to 4 weeks after calcitonin was started (Fig. 13b) [17]. In terms of QOL, improvement in SF-36 scores, pain relief, and improved ADLs, and an enhanced effect of rehabilitation in patients who had a total hip replacement after proximal femoral fracture was reported.
Outside of Japan, intra-nasal formulations of calcitonin derivatives are used primarily, and a preventive effect on fractures and beneficial effect on pain was observed. However, the increased risk of cancer was reported from the European Medical Association (EMA) in patients treated with calcitonin and intra-nasal calcitonin was withdrawn from the European market.
Although antibodies might be produced after injection of calcitonin derivatives, they do not influence the effect of calcitonin and are not involved in the side effects of calcitonin derivatives. Therefore, patient monitoring is not needed.
Teriparatide (recombinant human parathyroid hormone)
Unlike bone-resorption inhibitors, intermittent administration of teriparatide (a recombinant form) as a daily subcutaneous injection specifically increases serum P1NP, a bone formation marker, indicating promotion of bone remodeling followed by the formation of bone tissue.
Teriparatide, given as a daily subcutaneous injection, is recommended in patients at high risk of fractures such as patients who have had a fracture(s) while being treated with a bisphosphonate or SERM, elderly patients with multiple vertebral fractures or proximal femoral fractures, or patients with significantly reduced BMD. The combination of teriparatide with an oral bisphosphonate is not recommended.
Teriparatide increases BMD at the lumbar vertebrae and proximal femur, and reduces vertebral and non-vertebral fracture. The incidence of a radial fracture is reduced with teriparatide, while the apparent BMD of the radius is slightly decreased in association with the formation of new bone matrix, and the external diameter of the radius is increased. A meta-analysis revealed that teriparatide reduces low back pain.
Teriparatide (a recombinant form) approved in Japan is self-injected daily at home, after instruction by physicians or nurses. The total dosing period is limited to 24 months. After 24 months of treatment with teriparatide, adequate treatment with a bone-resorption inhibitor is recommended to maintain the bone strength.
Combination therapy
Osteoporosis is a multifactorial disease, thus combination therapy with agents with different mechanisms of action is considered reasonable. However, the efficacy of combination therapy lacks evidence at this time.
The Adequate Treatment of Osteoporosis (A-TOP) Research Group was authorized in the year 2000 by the Japan Society of Osteoporosis and assisted by the Public Health Research Foundation to obtain clinical evidence regarding osteoporosis treatment. It conducted a clinical trial comparing monotherapy with alendronate, a new bisphosphonate at the time, and combination therapy with alendronate and alfacalcidol, an active vitamin D3 derivative developed in Japan (Japanese Osteoporosis Intervention Trial: JOINT-02). The incidence of vertebral fracture was significantly reduced in the combination therapy group during the first 6 months of treatment, and in both subgroups of patients with multiple vertebral fractures and grade 3 vertebral fractures by semiquantitative assessment during the 2-year treatment period (Fig. 14) [18]. The incidence of non-vertebral fracture (weight-bearing bones) was also significantly reduced in the combination therapy group. Based on these results, combination therapy with alendronate and an active vitamin D3 derivative is recommended for the prevention of incident vertebral and non-vertebral fracture in patients at a high risk of fracture.
Fig. 14.
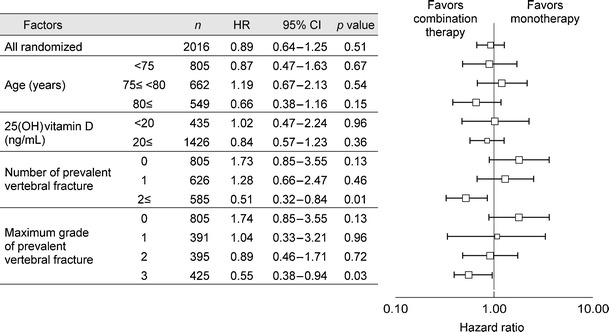
Efficacy of combination therapy with alendronate and active vitamin D3 on vertebral fracture. HR hazard ratio of incident vertebral fracture, CI confidence interval (Orimo [18] (Copyright© 2011 Informa Plc.))
Secondary osteoporosis
Osteoporosis secondary to other diseases
Secondary osteoporosis is defined as decreased BMD and deteriorated bone quality (pathologic state specific to osteoporosis) having one or more causes in addition to genetic factors, lifestyle, menopause, and aging. Secondary osteoporosis that is caused by a disease, such as hyperparathyroidism, can be improved by treating the underlying disease.
Hyperparathyroidism can be classified into either primary hyperparathyroidism, a disorder of the parathyroid itself, or secondary hyperparathyroidism, a pathological state secondary to other disorders, such as chronic kidney disease or vitamin D deficiency/depletion. In both types of hyperparathyroidism, excessively secreted parathyroid hormone promotes bone turnover and consequently decreases the BMD, resulting in an increased fracture risk. However, the therapeutic strategies employed for each type are entirely different. Primary hyperparathyroidism is treated mainly by parathyroidectomy, and there is no evidence regarding pharmacologic treatment. Secondary hyperparathyroidism improves with treatment of its underlying disease. Hyperparathyroidism secondary to CKD should be treated in accordance with the Japanese Evidence-based Practice Guideline for the Treatment of CKD.
In rheumatoid arthritis, bone resorption increases and BMD decreases because of several factors, including activation of inflammatory cytokines, immobility, and use of glucocorticoids. Consequently, the fracture risk increases. Infliximab, an anti-TNF agent used to treat rheumatoid arthritis, increases BMD in patients with osteoporosis secondary to rheumatoid arthritis. Among the useful therapeutic medications for osteoporosis, bisphosphonates reduce fracture risk.
Osteoporosis secondary to lifestyle-related diseases
In recent years, it was demonstrated that bone metabolism is influenced by some atherosclerosis-inducing disorders such as diabetes mellitus, dyslipidemia, hypertension, and chronic kidney disease. In particular, osteoporosis caused by diabetes mellitus or CKD is established as “osteoporosis secondary to lifestyle-related diseases”, bringing it special attention within secondary osteoporosis. A vigorous assessment for osteoporosis is recommended in patients with these diseases.
Osteoporosis secondary to lifestyle-related diseases is mainly associated with deterioration in bone quality, whereas BMD is relatively well-preserved in most cases. Therefore, therapeutic intervention in patients with diabetes mellitus or CKD should be started as soon as “decreased bone mass” is identified, in accordance with the diagnostic criteria of osteoporosis.
The main cause of deterioration in bone quality in these patients is thought to be altered cross-links among the collagen molecules in bone tissue (nonphysiological collagen cross-links, i.e., advanced glycation endproducts) due to an increase in oxidative stress and acceleration of glycation.
While the therapeutic modality has not been established yet, the benefit of alendronate, risedronate, raloxifene, and parathyroid hormone derivatives has been reported in large clinical trials. Pentosidine is likely to be a marker for bone quality and is expected to be an index of the fracture risk.
Treatment-related osteoporosis
Glucocorticoid agents and sex hormone lowering therapy are important causes of treatment-related osteoporosis.
Systemically administrated glucocorticoid decreases bone mass and increases fracture risk, thus 50 % of patients under long-term treatment with glucocorticoids suffer from osteoporosis. In general, patients taking glucocorticoids at doses of 5 mg (prednisolone equivalent) or more per day for 3 months or more should be assessed for bone mass and the need for osteoporosis treatment. Moreover, it is recommended to start treatment at higher BMD values than those used in the criteria for treatment of primary osteoporosis. In Japan, a revision of the 2004 “Guidelines on the management and treatment of corticosteroid-induced osteoporosis” is being developed.
Even though guidelines currently recommend bisphosphonates for the treatment of glucocorticoid-induced osteoporosis, generally they are not recommended for women intending to become pregnant. Although teriparatide is expected to increase bone mass, it is indicated only for “osteoporosis with a high risk of fractures”.
Endocrine therapy (sex hormone lowering therapy) for breast cancer and prostate cancer decreases BMD. Bisphosphonates can improve BMD in these patients, but there is no evidence yet about its ability to reduce fracture risk.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Conflicts of interest
None.
Footnotes
A Report of the Committee for Developing Guidelines for Prevention and Treatment of Osteoporosis: Japan Osteoporosis Society, Japanese Society for Bone and Mineral Research, and Japan Osteoporosis Foundation
References
- 1.Yoshimura N, Muraki S, Oka H, et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab. 2009;27:620–628. doi: 10.1007/s00774-009-0080-8. [DOI] [PubMed] [Google Scholar]
- 2.Orimo H, Yaegashi Y, Onoda T, et al. Hip fracture incidence in Japan: estimates of new patients in 2007 and 20-year trends. Arch Osteoporos. 2009;4:71–77. doi: 10.1007/s11657-009-0031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshimura N, Kinoshita H, Oka H et al (2006) Cumulative incidence and changes in prevalence of vertebral fractures in a rural Japanese community: a 10-year follow-up of the Miyama cohort. Arch Osteoporos. doi:10.1007/s11657-006-0007-0
- 4.Orimo H, Hayashi Y, Fukunaga M, et al. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab. 2001;19:331–337. doi: 10.1007/s007740170001. [DOI] [PubMed] [Google Scholar]
- 5.You K. Osteoporos Jpn. 2011;19:626. [Google Scholar]
- 6.Nishizawa Y, Ohta h, Miura M, et al. Guidelines for the use of bone metabolic markers in the diagnosis and treatment of osteoporosis (2012 Edition). J Bone Miner Metab. doi:10.1007/s00774-012-0392-y [DOI] [PubMed]
- 7.Orito S, Kuroda T, Onoe Y, et al. Age-related distribution of bone and skeletal parameters in 1322 Japanese young women. J Bone Miner Metab. 2009;27:698–704. doi: 10.1007/s00774-009-0094-2. [DOI] [PubMed] [Google Scholar]
- 8.Orimo H, Hosoi T, Fukunaga M, editors. Manual for osteoporosis screening and health guidance. Tokyo: Life Science Publishing; 2009. [Google Scholar]
- 9.MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148:197–213. doi: 10.7326/0003-4819-148-3-200802050-00198. [DOI] [PubMed] [Google Scholar]
- 10.Papadimitropoulos E, Wells G, Shea B, et al. Meta-analysis of the efficacy of vitamin D treatment in preventing osteoporosis in postmenopausal women. Endocr Rev. 2002;23:560–569. doi: 10.1210/er.2001-8002. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls: a meta-analysis. JAMA. 2004;291:1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto T, Ito M, Hayashi Y, et al. A new active vitamin D3 analog, eldecalcitol, prevents the risk of osteoporotic fractures: a randomized, active comparator, double-blind study. Bone. 2011;49:605–612. doi: 10.1016/j.bone.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2009;166:1256–1261. doi: 10.1001/archinte.166.12.1256. [DOI] [PubMed] [Google Scholar]
- 14.Hagino H, Nishizawa Y, Sone T, et al. A double-blinded head-to-head trial of minodronate and alendronate in women with postmenopausal osteoporosis. Bone. 2009;44:1078–1084. doi: 10.1016/j.bone.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto T, Hagino H, Shiraki M, et al. Effect of daily oral minodronate on vertebral fractures in Japanese postmenopausal women with established osteoporosis: a randomized placebo-controlled double-blind study. Osteoporos Int. 2009;20:1429–1437. doi: 10.1007/s00198-008-0816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orimo H, Morii H, Inoue T, et al. Effect of elcatonin on involutional osteoporosis. J Bone Miner Metab. 1996;14:73–78. doi: 10.1007/BF01768835. [DOI] [Google Scholar]
- 17.Nakano T. Clinical effect of calcitonin (elcatonin) on acute pain for osteoporotic vertebral fracture: semi blind randomized controlled trial. Osteoporos Jpn. 2011;19:279. [Google Scholar]
- 18.Orimo H, Nakamura T, Fukunaga M, et al. Effects of alendronate plus alfacalcidol in osteoporosis patients with a high risk of fracture: the Japanese Osteoporosis Intervention Trial (JOINT)–02. Curr Med Res Opin. 2011;27:1273–1284. doi: 10.1185/03007995.2011.580341. [DOI] [PubMed] [Google Scholar]


