Abstract
AIMS
Interleukin-1β (IL-1β) is a cytokine that is known to activate the stress axis and suppress the reproductive axis. Different brain areas are involved in the regulation of these two axes. However, they are both under the stimulatory control of the catecholamine, norepinephrine (NE). Here, we hypothesized that IL-1β differentially affects these two axes by modulating NE levels in specific brain regions.
METHODS
Female Sprague-Dawley rats in proestrus were injected intraperitoneally with either PBS-1.0% BSA (control) or 5 µg of IL-1β at 1 pm. Groups of rats were sacrificed at 1, 3, and 5 pm and their brains were collected. Brain areas associated with reproduction as well as areas associated with stress axis activity were isolated and analyzed for NE concentrations using HPLC-EC. Trunk blood was analyzed for IL-1β, corticosterone and luteinizing hormone levels.
KEY FINDINGS
As a general trend, treatment with IL-1β significantly decreased NE levels (p<0.05) in the areas controlling reproductive functions when compared to the control group. In contrast, NE levels increased significantly (p<0.05) in the stress associated areas. LH levels were markedly decreased with IL-1β treatment while corticosterone levels increased dramatically.
SIGNIFICANCE
The ability of IL-1β to produce differential effects on the stress and reproductive axis could be explained by modulation of NE levels in specific brain areas that are associated with these functions. This differential regulation of NE may be an adaptive phenomenon in response to a systemic immune challenge.
Keywords: Norepinephrine, luteinizing hormone, corticosterone, stress axis, reproductive axis
INTRODUCTION
Interleukins are proteins that are released by activated macrophages during a systemic inflammatory response [1]. Interleukin-1β (IL-1β), in particular, has been shown to activate the hypothalamo-pituitary-adrenal (HPA) axis and suppress the hypothalamo-pituitary-gonadal (HPG) axis [2–5]. These neuroendocrine effects constitute an essential homeostatic adaptation during an immune challenge [6]. To produce these effects, IL-1β has to act on specific brain regions that are involved in the regulation of the HPA and HPG axes.
The HPG or reproductive axis is comprised of gonadotropin releasing hormone (GnRH) neurons that are localized in distinct areas of the brain [7–9]. GnRH neurons are regulated by several neurotransmitters, of which norepinephrine (NE) plays a crucial stimulatory role [10]. Stimulation of GnRH secretion by NE results in the release of luteinizing hormone (LH) from the anterior pituitary, a critical event for ovulation. Cyclical increases in LH levels and ovulation contribute to estrous cyclicity that is essential for female reproduction [11]. Both central and peripheral administration of IL-1β can inhibit reproductive functions. Specifically, it interferes with the transcription and translation of GnRH culminating in reduced GnRH secretion in the hypothalamus [3, 12, 13]. IL-1β also suppresses the preovulatory LH surge and thereby, blocks ovulation [4, 13].
The HPA axis or stress axis on the other hand, is regulated by certain brain areas that are rich in corticotrophin releasing hormone (CRH) neurons and are responsive to immune stimulation [14]. Like GnRH neurons, CRH neurons are also stimulated by NE [10]. IL-1β activates the HPA axis by stimulating CRH neurons, increasing CRH secretion from the hypothalamus, adrenocorticotropin (ACTH) from the pituitary, culminating in an increase in corticosterone secretion from the adrenal gland [2, 5, 15, 16]. Corticosterone helps the animal respond appropriately to an immune challenge by mobilizing and utilizing energy stores [17]. The mechanism by which IL-1β stimulates CRH secretion is unclear. There is some indication that increases in NE levels in the paraventricular nucleus (PVN) may contribute to this phenomenon [18–21]. However, it is not clear if NE levels increase in other CRH containing areas such as the bed nucleus of the stria terminalis (BNST) and the central amygdala (CeA) as well.
Since both the HPA and HPG axes are under the stimulatory control of NE, we wanted to investigate the simultaneous effects of IL-1β on NE levels in the different brain sites that regulate these axes. We have previously shown that the noradrenergic nuclei in the brain stem that innervate brain areas that regulate the HPA and HPG axes are influenced differentially by IL-1β[22]. Since IL-1β activates the HPA axis while simultaneously suppressing the HPG axis, we hypothesized that IL-1β would increase NE levels in brain regions that contain CRH neurons, but would decrease NE levels in brain regions that contain GnRH neurons. Although there is some evidence to indicate that IL-1β could affect NE levels in hypothalamic areas in the context of the HPA or the HPG axes, there are no studies that have examined the effects of IL-1β on these axes simultaneously, in the same animal. Therefore, the present study was designed to explore IL-1β’s modulation of noradrenergic neurons, both temporally and across various brain areas controlling stress and reproductive functions.
Materials and Methods
Animals
Three-month-old female Sprague Dawley rats were obtained from Harlan Laboratories (Indianapolis, IN). They were housed in temperature- (23 ± 2°C) and light-controlled (lights on from 0500 to 1900 h) animal rooms and provided with rat chow and water ad libitum. Animal use protocols were approved by the IACUC at Michigan State University.
Treatment
Estrous cyclicity in rats was monitored by daily vaginal cytology and rats that showed regular estrous cycles were selected. On the day of proestrus, they were randomly subjected to one of the two treatments, control (PBS-1.0% BSA) or IL-1β 5µg, injected i.p. at 1300 hrs. They were sacrificed in groups of 8 at three time points: 1300 (1 pm), 1500 (3 pm) and 1700 (5 pm) hrs by rapid decapitation. Trunk blood was collected and serum separated for LH, corticosterone and IL-1β analysis. Brains were removed, snap frozen in liquid nitrogen and stored at −70°C.
Palkovits’ microdissection of discrete brain areas
Serial coronal sections (300 µm thick) of the brain were obtained using a cryostat (Slee Mainz, London, UK) maintained at −10°C. The sections were transferred to pre-cleaned microscopic slides and placed on a cold stage at −10°C. The following areas were microdissected using a 500-µm-diameter punch: GnRH rich areas such as the diagonal band of Broca (DBB), organum vasculosum lamina terminalis (OVLT), and parts of the hypothalamus including the medial preoptic area (MPA), suprachiasmatic nucleus (SCN) and the arcuate nucleus (Arc), and CRH rich areas such as the central amygdala (CeA), bed nucleus of the stria terminalis (BNST) and the paraventricular nucleus (PVN). The rat brain stereotaxic atlas was used as a reference [23]. Tissue samples were obtained bilaterally, and all the subdivisions of the areas were included. The samples were kept on dry ice before they were used for neurotransmitter detection by high performance liquid chromatography, followed by electrochemical detection (HPLC-EC).
HPLC – EC
The HPLC-EC procedure for determination of NE in tissue samples has been described previously[24]. Briefly, the HPLC-EC apparatus consisted of a LC-10 AT/VP pump (Shimadzu, Columbia, MD), a phase II 5-µm ODS reverse-phase C-18 column (Phenomenex, Torrance, CA), a glassy carbon electrode (Bioanalytical Systems, West Lafayette, IN), a model CTO-10 AT/VP column oven (Shimadzu) maintained at 37°C, and an LC-4C amperometric detector (Bioanalytical Systems, West Lafayette, IN). The data were integrated using a computer with the Class-VP chromatography Laboratory Automated Software system (version 7.3 SP1, Shimadzu, Columbia, MD). At the time of analysis, the samples were homogenized in 0.1M perchloric acid and the supernatant was collected after centrifugation at 1000 g for 5 minutes. 60 µl of supernatant and 30 µl of the internal standard (0.05 M dihydroxy benzylamine; Sigma Chemical Co., St. Louis, MO) were mixed together and loaded into an autosampler (model SIL-10AF, Shimadzu, Columbia, MD) with a sample cooler maintaining samples at 4°C. The autosampler injected 75µl of the mixture into the HPLC system. NE concentrations in the chromatograms were determined using the Class VP software. The sensitivity of the system was less than 1 pg.
LH radioimmunoassay
Double antibody RIA was used to determine LH levels in the serum samples as described before [25, 26]. The reference preparation for LH was NIDDK rLH-RP-3. The primary antibody, anti rLH-S11 was used at a dilution of 1:184,000. LH standards, iodination quality LH protein and LH primary antibody were obtained from Dr A.F. Parlow, NIDDK. LH was iodinated by Dr. Robert Speth, Peptide Radioiodination Service, University of Mississippi. Serum samples (50–75µl) were assayed in duplicates. The assay had a sensitivity of <10 pg. The inter-assay variability was 10.4% and the intra-assay variability was 3.8%.
Corticosterone radioimmunoassay
Double antibody RIA was used to measure corticosterone levels in the serum as described previously [27]. Corticosterone standards and the tracer were obtained from Diagnostic Products Inc. (Los Angeles, CA). The primary and secondary antibodies were generated in our laboratory and used at a dilution of 1:17500 and 1:11000 respectively. The sensitivity of the corticosterone assay was 0.2 ng/ml. The intra- and inter-assay variability were 4.5% and 6.4%, respectively.
Serum IL-1β-ELISA
Serum IL-1β levels were measured in duplicate using a commercial ELISA kit (TiterZyme Kits, Assay Design, Ann Arbor, MI). The assay was carried out according to the manufacturers’ instructions. The sensitivity of the kit was <12 pg/ml. All data are expressed as ng cytokine per ml serum.
Statistical analysis
All statistical procedures were performed using SAS software (Cary, NC). Changes in NE concentrations in brain areas at various time points were analyzed by two way ANOVA followed by Fisher’s LSD test. The differences in the levels of corticosterone, LH and IL-1β were also analyzed by two-way ANOVA followed by Fisher’s LSD test. p<0.05 was considered to be significant.
RESULTS
Effect of systemic IL-1β on circulating IL-1β levels
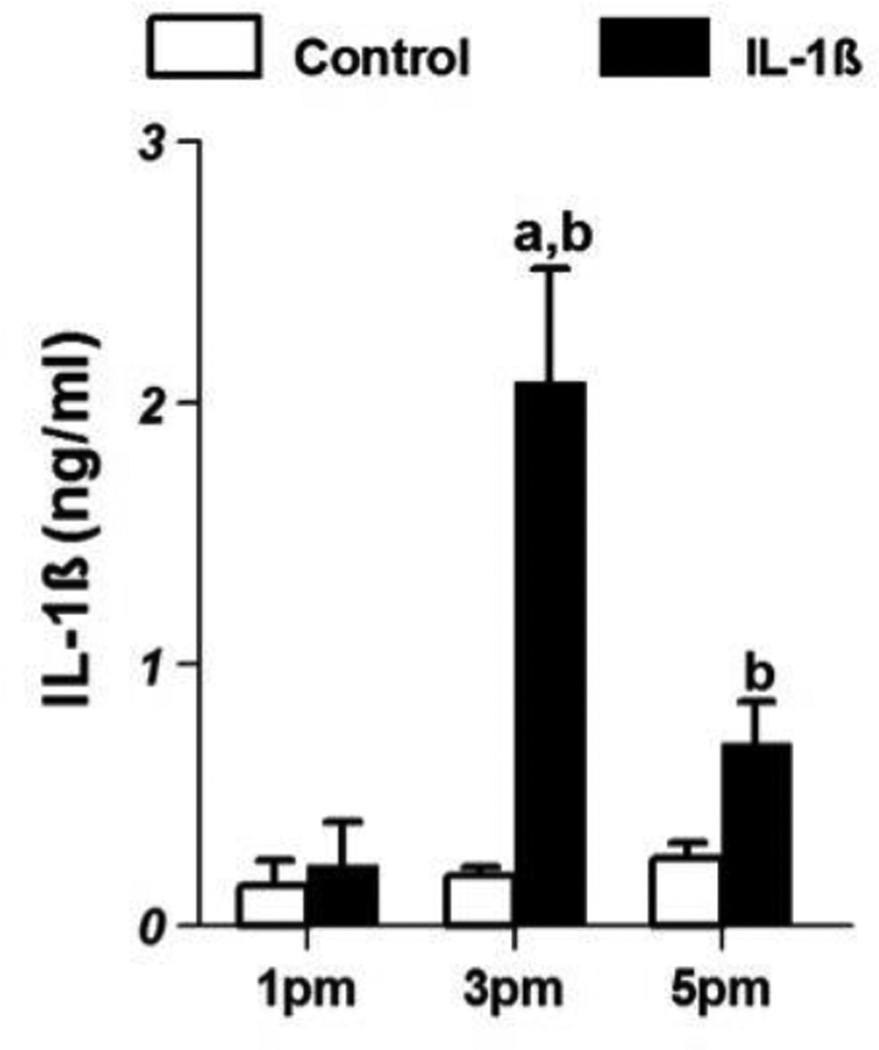
The patterns of serum IL-1β (ng/ml; mean±SE) in control and IL-1β treated animals are shown in Fig 1. The levels of IL-1β in the control animals at 1 pm were 0.15 ± 0.1 and remained at the same level at 3 pm and 5 pm. However, in the IL-1β-treated animals they increased to 2.08 ± 0.44 at 3 pm, which were significantly different from levels at 1 pm (0.23 ± 0.16) as well as control levels at 3 pm (0.19 ± 0.03; p<0.05). At 5 pm, the levels dropped to 0.69 ± 0.16 which were also higher than control values (0.26 ± 0.06) at that time point.
Figure 1.
Serum IL-1β levels (mean±SE;ng/ml) in control (n=8/group) and IL-1β-treated female rats(n=8/group) on proestrus. ‘a’ indicates significant difference (p<0.05) from levels at 1 pm and ‘b’ indicates difference (p<0.05) from levels in control animals.
Effect of IL-1β on serum corticosterone levels
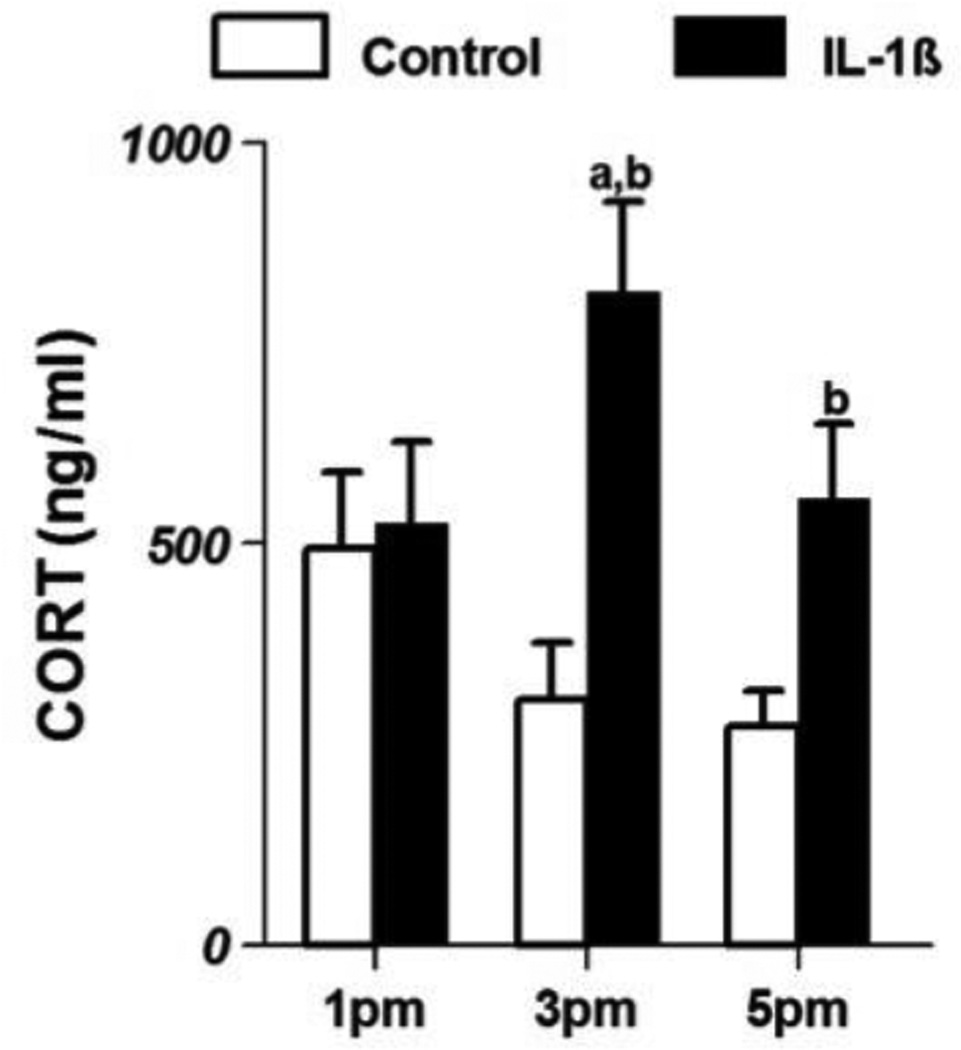
Changes in serum corticosterone in control and IL-1β-treated animals are shown in Fig 2. At 1 pm, corticosterone levels (ng/ml; mean ±SE) in control animals (492.87±96.9) were not different from those in IL-1β-treated animals (524.9±102.9). However, at 3 and 5 pm, IL-1β treatment increased corticosterone levels to 812.18±112.7 and 556.0±93.7 respectively. These levels were significantly higher (p<0.05) than the levels in control animals (305.26±70.9, 271.92±43.3 at 3 and 5 pm respectively).
Figure 2.
Serum corticosterone levels (mean±SE;ng/ml) in control (n=8/group) and IL-1β-treated female rats(n=8/group) on proestrus. ‘a’ indicates significant difference (p<0.05) from levels at 1 pm and ‘b’ indicates difference (p<0.05) from levels in control animals.
Effect of IL-1β on serum LH levels
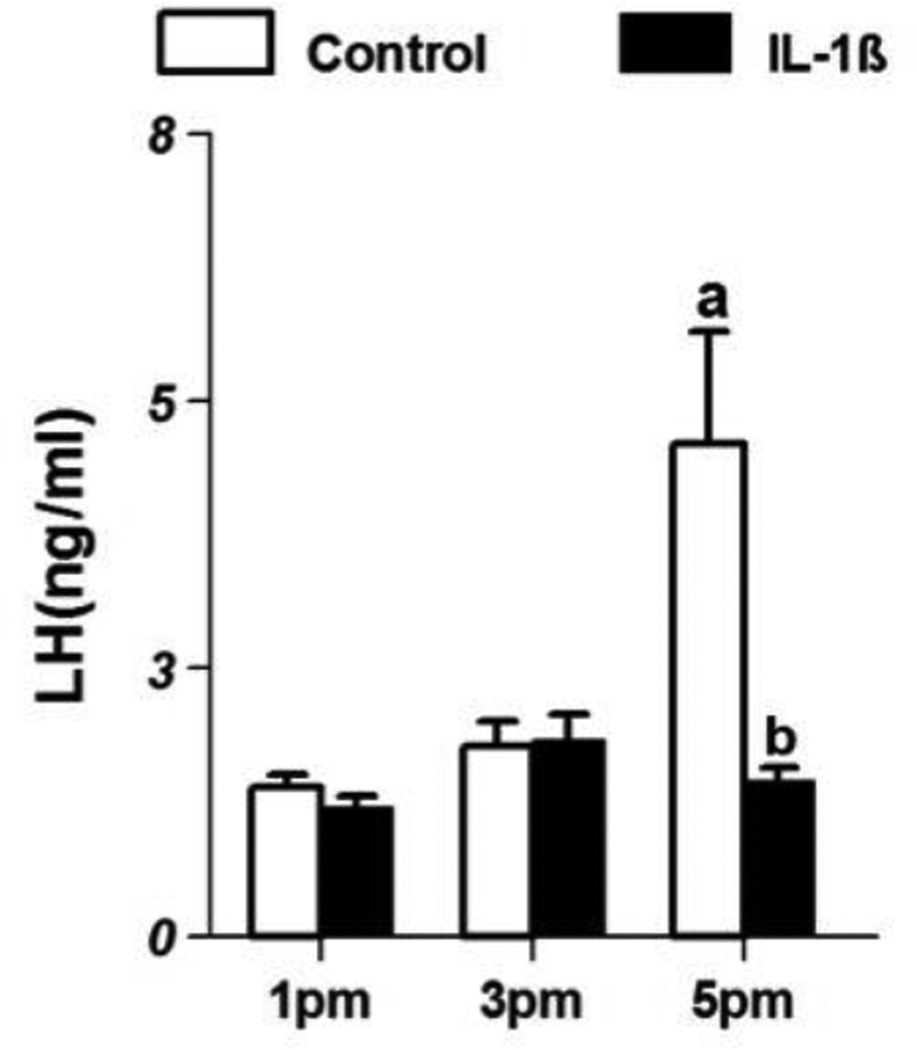
LH levels in control and IL-1β-treated animals are shown in Fig 3. In control animals, LH levels (ng/ml; mean ±SE) at 1 pm were 1.48±0.1 and increased modestly to 1.89±0.2 in the 3 pm group and increased significantly in the 5 pm group (4.91±1.1, p<0.05). However in IL-1β-treated animals, LH levels at 1 pm were 1.29±0.1 and remained at the same level in the groups sacrificed at 3 and 5 pm. Treatment with IL-1β significantly reduced LH levels when compared to control animals in the 5 pm group (p<0.05).
Figure 3.
Serum luteinizing hormone levels (mean±SE;ng/ml) in control (n=8/group) and IL-1β- treated female rats(n=8/group) on proestrus. ‘a’ indicates significant difference (p<0.05) from levels at 1 pm and ‘b’ indicates difference (p<0.05) from levels in control animals.
Effect of IL-1β on NE levels in brain regions involved in HPA regulation
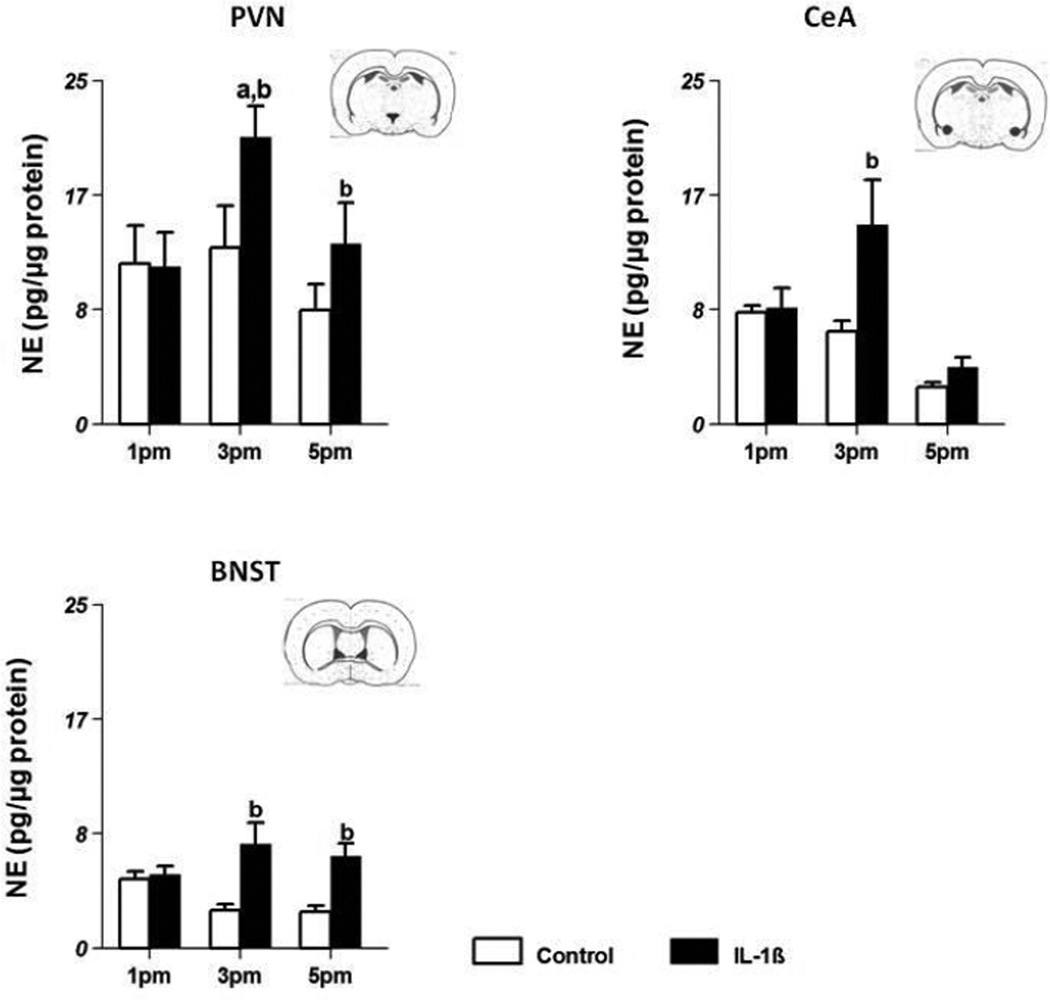
NE levels in control and IL-1β-treated animals in three areas involved in stress axis regulation are shown in Fig 4.
Figure 4.
Effect of systemic injections of PBS (control, n=8/group) and 5µg IL-1β (n=8/group) on NE concentrations (mean±SE;pg/µg protein) in the paraventricular nucleus (PVN) (Fig 4A), bed nucleus of the stria terminalis (BNST) (Fig 4B) and central amygdala (CeA) (Fig 4C). ‘a’ indicates significant difference (p<0.05) from levels at 1 pm and ‘b’ indicates difference (p<0.05) from levels in control animals. Insets contain representative coronal sections of the brain depicting the location of individual nuclei.
PVN
NE levels (pg/µg protein; mean ±SE) in control animals at 1 pm were 11.67±2.8 (Fig 4a) and remained at that level at 3 pm and 5 pm. In IL-1β-treated animals, NE levels at 1 pm were 11.48±2.5, increased significantly to 20.9±2.2 (p<0.05) at 3 pm and returned to 13.14±2.9 at 5 pm. NE levels in IL-1β-treated animals were significantly higher at 3 and 5 pm (p<0.05) than the levels in control animals.
BNST
In control animals, NE levels were 5.03±0.5 at 1 pm and were lower at 3 and 5 pm (2.73±0.5, 2.66±0.4 respectively). In IL-1β-treated animals, NE levels at 1 pm were comparable to those in control animals (5.36±0.6) but increased at 3 pm (7.57±1.6) and at 5 pm (6.71±0.9) compared to control animals (Fig 4b) (p<0.05).
CeA
In control animals, NE levels remained about the same at all three time points. After IL-1β treatment, NE levels were higher (14.50±3.3) than the control group (6.70±0.7) only at 3 pm. (Fig 4c; p<0.05).
In summary IL-1β treatment produced a marked increase in NE levels at 3 pm and 5 pm compared to the control group only in the PVN and the BNST. In the CeA, NE levels increased only at 3 pm after IL-1β treatment and declined to baseline by 5 pm.
Effect of IL-1β on NE levels in HPG axis-related areas
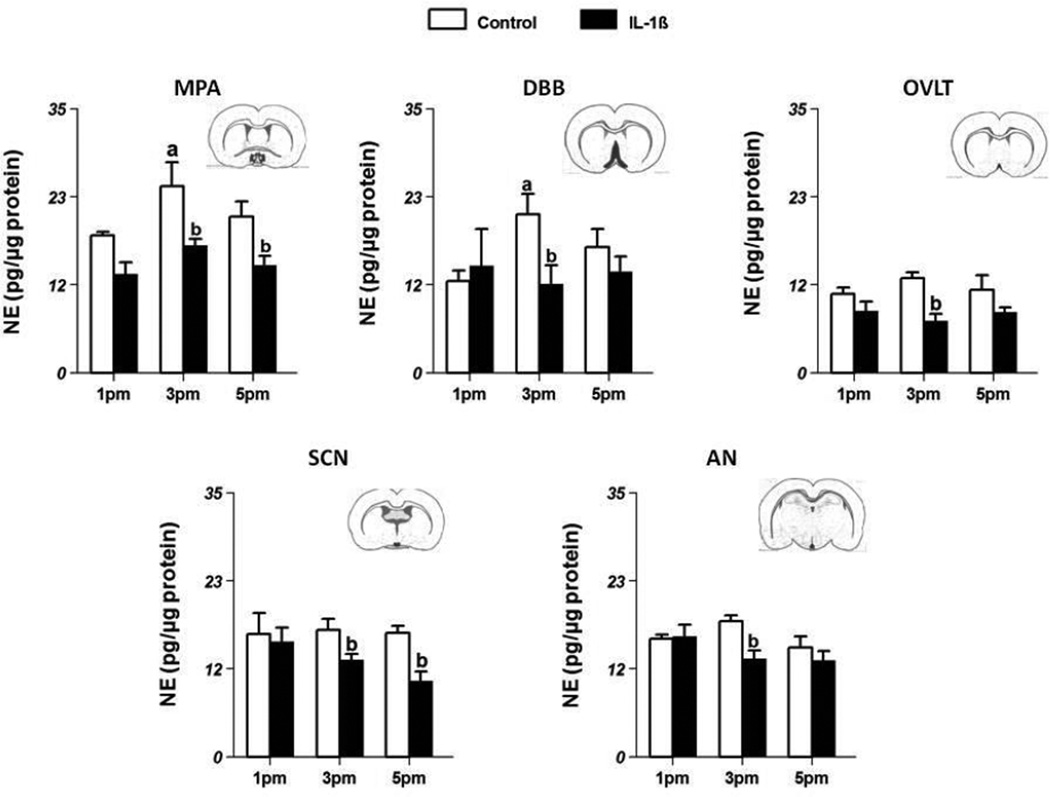
NE levels in control and IL-1β-treated animals in various areas related to the HPG axis are shown in Fig 5.
Figure 5.
Effect of systemic injections of PBS (control, n=8/group) and 5µg IL-1β (n=8/group) on NE concentrations (mean±SE;pg/µg protein) in the organum vasculosum lamina terminals (OVLT) (Fig 5A), diagonal band of Broca (DBB; Fig 5B), suprachiasmatic nucleus (SCN; Fig 5C),medial preoptic area (MPA; Fig 5D), and arcuate nucleus (AN; Fig 5E). ‘a’ indicates significant difference (p<0.05) from levels at 1 pm and ‘b’ indicates difference (p<0.05) from levels in control animals. Insets contain representative coronal sections of the brain depicting the location of individual nuclei.
OVLT
NE levels (pg/µg protein; mean ±SE) in control animals at 1 pm were 10.47±0.8 (Fig 5a) and remained about the same at 3 pm (12.51±0.8) and 5 pm (10.96±1.9). In IL-1β-treated animals also, NE levels were similar at 1 pm (8.22±1.2), 3 pm (6.92±0.9) and 5 pm (8.05±0.7). However, at 3 pm, NE levels in IL-1β-treated animals were significantly lower than those in control animals (p<0.05).
DBB
In control animals, NE levels at 1 pm were 12.15±1.4, and significantly increased to 20.98±1.4 at 3 pm (p<0.05; Fig 5b). This increase at 3 pm was blocked by treatment with IL-1β (11.76±2.5; p<0.05). The levels of NE in the IL-1β-treated animals did not change with time.
SCN
NE levels in control animals at 1 pm were 16.26±2.8 (Fig 5c) and remained at similar levels at 3 pm and 5 pm. In the IL-1β-treated animals, NE levels at 1 pm were 15.26±1.8 that were not different from the levels in control animals. However at 3 pm and 5 pm, NE levels in IL-1β-treated animals (12.83±0.8 and 10.03±1.3 respectively) were significantly lower (p<0.05) compared to the levels in control animals (16.85±1.5 and 16.39±0.9 respectively).
MPA
In control animals NE levels at 1 pm were 18.22±0.5, and increased significantly to 24.70±3.2 at 3 pm (p<0.05; Fig 5d). In IL-1β-treated animals, NE levels at 1 pm were 13.07±1.6 and remained at about the same level at 3 pm and 5 pm. However, when compared to the corresponding control groups at each time point, treatment with IL-1β significantly decreased (p<0.05) NE levels at 3 pm (16.89±0.9) as well as 5 pm (14.26±1.26).
Arc
NE levels in the Arc remained stable throughout the afternoon of proestrus (Fig. 5e). IL-1β-treatment decreased NE levels at 3 pm (13.01±1.1) compared to the corresponding control group (17.96 ±0.8). There were no changes in NE levels with IL-1β treatment at 1 pm or 5 pm.
In summary, NE levels increased at 3 pm in control animals in the DBB and the MPA. This increase corresponds well with the LH surge seen at 5 pm. Treatment with IL-1β blocked this increase in NE levels in these areas. Treatment with IL-1β also decreased NE levels from control values in the OVLT, SCN and Arc at 3 pm.
DISCUSSION
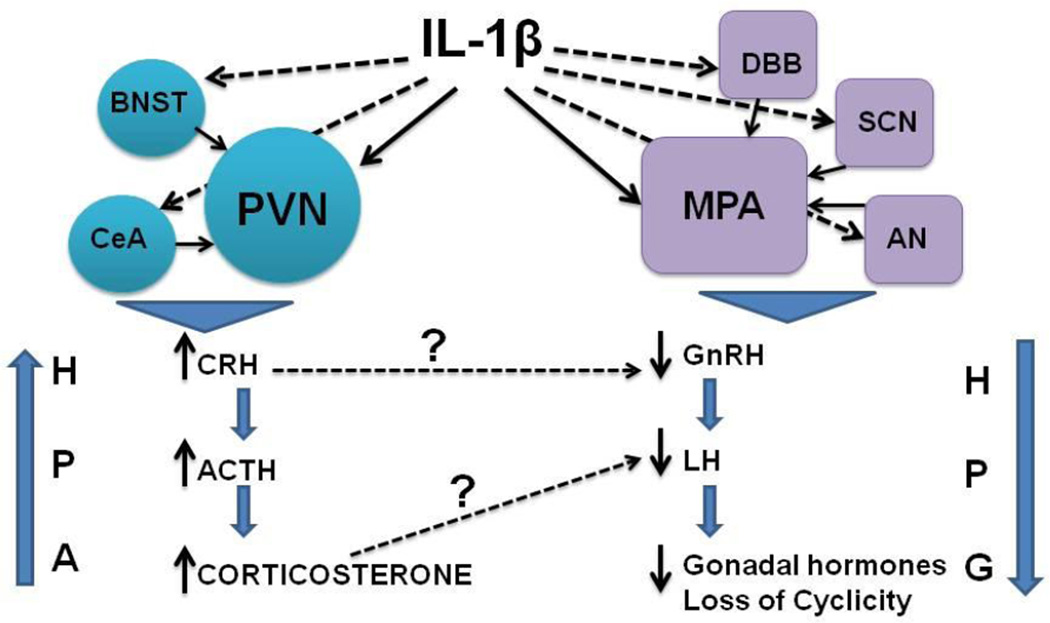
The present study provides the first evidence for the involvement of NE in both the activation and inhibition of the stress and reproductive axis respectively as a result of IL-1β treatment. More interestingly, as a general trend, there was suppression of noradrenergic activity in the HPG axis-related areas while NE levels were increased in HPA-related areas after IL-1β treatment. These results suggest that NE is a common mediator for IL-1β’s effects on both these axes, but IL-1β’s effects on NE are specific to each axis (Fig 6).
Figure 6.
Schematic representation of the effect of IL-1β on the HPA and HPG axes. Dashed arrows indicate milder effects on specific nuclei. Bold arrows indicate strong effects on specific nuclei. Effects on hypothalamic nuclei involved in stress axis regulation (circles) ultimately result in increased corticosterone, while effects on nuclei involved in regulation of reproductive functions (squares) results in suppression of LH secretion and loss of cyclicity.
Simultaneous changes in NE levels in the context of HPA activation and HPG inhibition have not been studied before. NE is stimulatory to both CRH as well as GnRH neurons [10]. However, it was not clear how IL-1β treatment would alter NE levels to produce diametrically opposite effects on the HPA and HPG axes. We chose female rats in proestrus as our subjects because NE levels during proestrus have been well characterized in female rats [25, 28]. NE levels increase gradually during the afternoon reaching a peak just before the LH surge in these animals. In the present study, NE levels increased in control animals at 3 pm in two of the five areas studied, viz., the DBB and the MPA. This increase occurred before the increase in LH levels in these animals corroborating well with earlier studies [24, 29]. We have also reported that treatment with IL-1β suppresses NE release specifically in the MPA and this is accompanied by a blockade of the LH surge [30, 31]. In the present study, we have performed a more comprehensive analysis of GnRH-rich brain areas such as the DBB, Arc, SCN and the OVLT that have been implicated in LH regulation [7–9]. Although NE levels did not increase in the Arc, SCN and OVLT in control animals before the LH surge, treatment with IL-1β was able to suppress NE levels in these areas at 3 and 5 pm compared to the control group. This probably suggests that noradrenergic tone in these three areas is important, but as seen in control rats, an increase in NE levels in the MPA and DBB appears to be critical for the LH surge.
The exact mechanism by which IL-1β decreases NE levels in HPG-related areas is not clear. IL-1β can produce this effect by two possible mechanisms: 1. By decreasing NE synthesis through its direct effects on brainstem noradrenergic neurons or 2. By decreasing NE release from noradrenergic terminals. NE synthesis involves a rate-limiting step where L-tyrosine is converted to L-DOPA by the enzyme tyrosine hydroxylase (TH) [32]. Bypassing this step by treating animals with L-DOPA is capable of partially reversing the IL-1β-induced reduction in NE levels in the MPA [31]. This suggests that IL-1β is indeed capable of affecting NE synthesis as stated in the first possibility. On the other hand, NE release in the MPA can also influenced by a variety of neurontransmitters [33, 34]. One of these, gamma amino butyric acid (GABA), is believed to inhibit NE release. In fact, GABA levels decrease in the MPA during the afternoon of proestrus to facilitate the rise in NE levels [35]. We have found that IL-1β can increase GABA levels and this most likely contributes to the reduction in NE levels in the MPA [36]. Similar phenomena may be in operation in other HPG-related areas as well. This needs further investigation.
In contrast to its effects on the HPG axis, IL-1β treatment stimulates HPA activity. The effect of IL-1β on HPA activity in female rats has not been studied in detail, however the few studies that have examined this effect report that female rats have a robust increase in plasma ACTH levels in response to IL-1β treatment [37]. The reason for the marked increases in ACTH is not clear. However, studies in male rats suggest that increases in NE levels in the PVN may be a main contributing factor [19, 21]. In the present study, there was a significant increase in corticosterone levels in the serum and NE levels in the PVN at 3 pm compared to levels at 1 pm with IL-1β treatment. This suggests that IL-1β produces a marked stimulation of noradrenergic activity, more so in the PVN than in the other two areas because in the BNST and CeA, NE levels at 3 pm were no different from those at 1 pm. However, NE levels in the BNST and CeA of IL-1β treated animals were higher than that in control animals at 3 pm suggesting that noradrenergic tone is important in these areas for IL-1β’s effects. All these three areas are believed to play an important role in integrating the stress response to IL-1β [38]. Results from the present study indicate that NE in these three regions could be a potential mediator of the stress response induced by IL-1β.
Since the present study was conducted in female rats in proestrus, it is likely that the ovarian steroids in circulation favored a strong HPA response to IL-1β treatment. In fact, an exaggerated HPA response has been demonstrated in female animals during systemic immune challenge compared to their male counterparts [39]. Moreover, immune stimulation with bacterial lipopolysaccharide produces a marked activation of PVN neurons during proestrus rather than diestrus [40]. All these suggest that the ovarian hormonal profile during proestrus might have influenced the outcome of the HPA response to IL-1β.
Treatment with IL-1β increased circulating IL-1β levels by 3 pm and the levels subsided by 5 pm. Most changes in NE levels in various nuclei (both suppression in HPG areas and elevations in HPA areas) occur in parallel to the rise in IL-1β levels. Circulating IL-1β can act on these different nuclei by crossing the blood brain barrier[41], activating the vagus[42] or by increasing IL-1 receptor expression in NE neurons [43].
It is fascinating that IL-1β could increase NE levels in areas related to HPA regulation while simultaneously decreasing NE levels in other areas that regulate reproductive functions. This requires activating or inhibiting specific neuronal populations in the brain stem that provide noradrenergic innervation to these areas. There is considerable overlap in noradrenergic innervation to the brain regions that were studied here. The MPA appears to receive noradrenergic innervations from the A2 and A6 noradrenergic nuclei [44], while the DBB receives innervation from A6 [45] and the OVLT from A1 [46]. The PVN receives noradrenergic input from the A1 and A2 regions [47], the BNST from A1 [46] and the CeA from A2 region [48]. There are also bidirectional connections between these different nuclei and the areas under investigation making noradrenergic interactions highly complex [49]. In a recent study, we examined the effect of IL-1β on the expression of several genes in the A1, A2 and A6 noradrenergic neuronal groups. There were dramatic increases in the mRNA for monocyte chemoattractant protein-1, and prostaglandin synthase in all three areas, but there were site specific changes in cytokine mRNA levels indicating that specific neuroendocrine pathways may be differentially activated in response to IL-1β treatment [43]. This has also been suggested by previous studies using different stress paradigms [50].
CONCLUSIONS
Taken together, results from the present study lead us to conclude that IL-1β activates specific noradrenergic pathways to modulate NE levels in specific brain regions to differentially regulate the stress and reproductive axes (Fig 6). This phenomenon may be critical for animals to mount an appropriate homeostatic response during a systemic immune challenge.
Acknowledgements
This work was supported by NSF IBN 0236385, NIH AG027697 and the Charles Cowham Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Roitt IM, Delves PJ. Roitt's essential immunology. Oxford ; Malden, MA: Blackwell Science; 2001. [Google Scholar]
- 2.Berkenbosch F, Vanoers J, Delrey A, Tilders F, Besedovsky H. Corticotrophin-releasing factor producing neurons in the rat activated by interleukin-1. Science. 1987;238(4826):524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- 3.Kalra PS, Sahu A, Kalra SP. Interleukin-1 inhibits the ovarian steroid-induced luteinizing hormone surge and release of hypothalamic luteinizing hormone-releasing hormone in rats. Endocrinology. 1990;126(4):2145–2152. doi: 10.1210/endo-126-4-2145. [DOI] [PubMed] [Google Scholar]
- 4.Rivier C, Vale W. Cytokines act within the brain to inhibit luteinizing hormone secretion and ovulation in the rat. Endocrinology. 1990;127(2):849–856. doi: 10.1210/endo-127-2-849. [DOI] [PubMed] [Google Scholar]
- 5.Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238(4826):522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- 6.O'Connor TM, O'Halloran DJ, Shanahan F. The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia. Qjm. 2000;93(6):323–333. doi: 10.1093/qjmed/93.6.323. [DOI] [PubMed] [Google Scholar]
- 7.Barraclough CA, Wise PM. The role of brain catecholamines in the regulation of pituitary luteinizing hormone and follicle stimulating hormone secretion. Endocrine Reviews. 1984;3:91–119. doi: 10.1210/edrv-3-1-91. [DOI] [PubMed] [Google Scholar]
- 8.Herbison AE. Physiology of Gonadotrophin-Releasing hormone neuronal network. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. San Diego: Academic Press; 2006. [Google Scholar]
- 9.Hiatt ES, Brunetta PG, Seiler GR, Barney SA, Selles WD, Wooledge KH, King JC. Subgroups of luteinizing hormone-releasing hormone perikarya defined by computer analyses in the basal forebrain of intact female rats. Endocrinology. 1992;130(2):1030–1043. doi: 10.1210/endo.130.2.1733705. [DOI] [PubMed] [Google Scholar]
- 10.Mueller E, Nistico G. Brain Messengers and the Pituitary. San Diego: Academic Press; 1989. [Google Scholar]
- 11.Everett JW. Neurobiology of Reproduction in the Female Rat. Berlin Heidelberg: Springer-Verlag; 1989. [PubMed] [Google Scholar]
- 12.Kang SS, Kim SR, Leonhardt S, Jarry H, Wuttke W, Kim K. Effect of interleukin-1beta on gonadotropin-releasing hormone (GnRH) and GnRH receptor gene expression in castrated male rats. J Neuroendocrinol. 2000;12(5):421–429. doi: 10.1046/j.1365-2826.2000.00466.x. [DOI] [PubMed] [Google Scholar]
- 13.Rivier C, Rivest S. Mechanisms mediating the effects of cytokines on neuroendocrine functions in the rat. Ciba Found Symp. 1993;172:204–220. doi: 10.1002/9780470514368.ch10. discussion 20-5. [DOI] [PubMed] [Google Scholar]
- 14.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Besedovsky H, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233(4764):652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- 16.Brady LS, Lynn AB, Herkenham M, Gottesfeld Z. Systemic interleukin-1 induces early and late patterns of c-fos mRNA expression in brain. J Neurosci. 1994;14(8):4951–4964. doi: 10.1523/JNEUROSCI.14-08-04951.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guijarro A, Laviano A, Meguid MM. Hypothalamic integration of immune function and metabolism. Prog Brain Res. 2006;153:367–405. doi: 10.1016/S0079-6123(06)53022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeKeyser FG, Leker RR, Weidenfeld J. Activation of the adrenocortical axis by surgical stress: involvement of central norepinephrine and interleukin-1. Neuroimmunomodulation. 2000;7(4):182–188. doi: 10.1159/000026437. [DOI] [PubMed] [Google Scholar]
- 19.MohanKumar PS, Quadri SK. Systemic administration of interleukin-1 stimulates norepinephrine release in the paraventricular nucleus. Life Sci. 1993;52(24):1961–1967. doi: 10.1016/0024-3205(93)90637-i. [DOI] [PubMed] [Google Scholar]
- 20.Jansen AS, Schmidt ED, Voorn P, Tilders FJ. Substance induced plasticity in noradrenergic innervation of the paraventricular hypothalamic nucleus. Eur J Neurosci. 2003;17(2):298–306. doi: 10.1046/j.1460-9568.2003.02453.x. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt ED, Schoffelmeer AN, De Vries TJ, Wardeh G, Dogterom G, Bol JG, Binnekade R, Tilders FJ. A single administration of interleukin-1 or amphetamine induces long-lasting increases in evoked noradrenaline release in the hypothalamus and sensitization of ACTH and corticosterone responses in rats. Eur J Neurosci. 2001;13(10):1923–1930. doi: 10.1046/j.0953-816x.2001.01569.x. [DOI] [PubMed] [Google Scholar]
- 22.Sirivelu MP, MohanKumar PS, MohanKumar SM. Differential effects of systemic interleukin-1beta on gene expression in brainstem noradrenergic nuclei. Life Sci. 2012;90(1–2):77–81. doi: 10.1016/j.lfs.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C. The rat brain in stereotaxic co-ordinates. New York: Academic Press; 1986. [Google Scholar]
- 24.Kasturi BS, MohanKumar SM, Sirivelu MP, MohanKumar PS. Chronic exposure to low levels of oestradiol-17beta affects oestrous cyclicity, hypothalamic norepinephrine and serum luteinising hormone in young intact rats. J Neuroendocrinol. 2009;21(6):568–577. doi: 10.1111/j.1365-2826.2009.01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MohanKumar PS, Thyagarajan S, Quadri SK. Correlations of catecholamine release in the medial preoptic area with proestrous surges of luteinizing hormone and prolactin: effects of aging. Endocrinology. 1994;135(1):119–126. doi: 10.1210/endo.135.1.8013343. [DOI] [PubMed] [Google Scholar]
- 26.MohanKumar SM, MohanKumar PS. Effects of interleukin-1 beta on the steroid-induced luteinizing hormone surge: role of norepinephrine in the medial preoptic area. Brain Res Bull. 2002;58(4):405–409. doi: 10.1016/s0361-9230(02)00809-2. [DOI] [PubMed] [Google Scholar]
- 27.Francis J, MohanKumar SM, MohanKumar PS. Correlations of norepinephrine release in the paraventricular nucleus with plasma corticosterone and leptin after systemic lipopolysaccharide: blockade by soluble IL-1 receptor. Brain Res. 2000;867(1–2):180–187. doi: 10.1016/s0006-8993(00)02311-8. [DOI] [PubMed] [Google Scholar]
- 28.Szawka RE, Franci CR, Anselmo-Franci JA. Noradrenaline release in the medial preoptic area during the rat oestrous cycle: temporal relationship with plasma secretory surges of prolactin and luteinising hormone. J Neuroendocrinol. 2007;19(5):374–382. doi: 10.1111/j.1365-2826.2007.01542.x. [DOI] [PubMed] [Google Scholar]
- 29.Mohankumar PS, Thyagarajan S, Quadri SK. Cyclic and age-related changes in norepinephrine concentrations in the medial preoptic area and arcuate nucleus. Brain Res Bull. 1995;38(6):561–564. doi: 10.1016/0361-9230(95)02031-4. [DOI] [PubMed] [Google Scholar]
- 30.MohanKumar SM, MohanKumar PS. Aging alters norepinephrine release in the medial preoptic area in response to steroid priming in ovariectomized rats. Brain Res. 2004;1023(1):24–30. doi: 10.1016/j.brainres.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 31.Sirivelu MP, Shin AC, Perez GI, MohanKumar PS, MohanKumar SM. Effect of L-dopa on interleukin-1 beta-induced suppression of luteinizing hormone secretion in intact female rats. Hum Reprod. 2009;24(3):718–725. doi: 10.1093/humrep/den434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagatsu T, Levitt M, Udenfriend S. Tyrosine Hydroxylase. the Initial Step in Norepinephrine Biosynthesis. J Biol Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- 33.Dobson H, Ghuman S, Prabhakar S, Smith R. A conceptual model of the influence of stress on female reproduction. Reproduction. 2003;125(2):151–163. doi: 10.1530/rep.0.1250151. [DOI] [PubMed] [Google Scholar]
- 34.Ushigome A, Nomura M, Tanaka J. Modulation of noradrenaline release in the median preoptic area by GABAergic inputs from the organum vasculosum of the lamina terminalis in the rat. Neurochem Int. 2004;44(3):139–144. doi: 10.1016/s0197-0186(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 35.Robinson JE, Kendrick KM, Lambart CE. Changes in the release of gamma-aminobutyric acid and catecholamines in the preoptic/septal area prior to and during the preovulatory surge of luteinizing hormone in the ewe. J Neuroendocrinol. 1991;3(4):393–399. doi: 10.1111/j.1365-2826.1991.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 36.Sirivelu MP, Burnett R, Shin AC, Kim C, MohanKumar PS, MohanKumar SM. Interaction between GABA and norepinephrine in interleukin-1beta-induced suppression of the luteinizing hormone surge. Brain Res. 2009;1248:107–114. doi: 10.1016/j.brainres.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rivier C. Stimulatory effect of interleukin-1 beta on the hypothalamic-pituitary-adrenal axis of the rat: influence of age, gender and circulating sex steroids. J Endocrinol. 1994;140(3):365–372. doi: 10.1677/joe.0.1400365. [DOI] [PubMed] [Google Scholar]
- 38.Crane JW, Buller KM, Day TA. Evidence that the bed nucleus of the stria terminalis contributes to the modulation of hypophysiotropic corticotropin-releasing factor cell responses to systemic interleukin-1beta. J Comp Neurol. 2003;467(2):232–242. doi: 10.1002/cne.10918. [DOI] [PubMed] [Google Scholar]
- 39.Rivier C. Female rats release more corticosterone than males in response to alcohol: influence of circulating sex steroids and possible consequences for blood alcohol levels. Alcohol Clin Exp Res. 1993;17(4):854–859. doi: 10.1111/j.1530-0277.1993.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 40.Nappi RE, Bonneau MJ, Rivest S. Influence of the estrous cycle on c-fos and CRH gene transcription in the brain of endotoxin-challenged female rats. Neuroendocrinology. 1997;65(1):29–46. doi: 10.1159/000127162. [DOI] [PubMed] [Google Scholar]
- 41.Banks WA, Kastin AJ, Durham DA. Bidirectional transport of interleukin-1 alpha across the blood-brain barrier. Brain Res Bull. 1989;23(6):433–437. doi: 10.1016/0361-9230(89)90185-8. [DOI] [PubMed] [Google Scholar]
- 42.Goehler LE, Relton JK, Dripps D, Kiechle R, Tartaglia N, Maier SF, Watkins LR. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res Bull. 1997;43(3):357–364. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 43.Sirivelu MP, MohanKumar PS, MohanKumar SM. Differential effects of systemic interleukin-1beta on gene expression in brainstem noradrenergic nuclei. Life Sci. 90(1–2):77–81. doi: 10.1016/j.lfs.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell RE, Herbison AE. Definition of brainstem afferents to gonadotropin-releasing hormone neurons in the mouse using conditional viral tract tracing. Endocrinology. 2007;148(12):5884–5890. doi: 10.1210/en.2007-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haghdoost-Yazdi H, Pasbakhsh P, Vatanparast J, Rajaei F, Behzadi G. Topographical and quantitative distribution of the projecting neurons to main divisions of the septal area. Neurol Res. 2009;31(5):503–513. doi: 10.1179/174313208X353712. [DOI] [PubMed] [Google Scholar]
- 46.Woulfe JM, Flumerfelt BA, Hrycyshyn AW. Efferent connections of the A1 noradrenergic cell group: a DBH immunohistochemical and PHA-L anterograde tracing study. Exp Neurol. 1990;109(3):308–322. doi: 10.1016/s0014-4886(05)80022-6. [DOI] [PubMed] [Google Scholar]
- 47.Larsen PJ, Mikkelsen JD. Functional identification of central afferent projections conveying information of acute"stress" to the hypothalamic paraventricular nucleus. J Neurosci. 1995;15(4):2609–2627. doi: 10.1523/JNEUROSCI.15-04-02609.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zardetto-Smith AM, Gray TS. Organization of peptidergic and catecholaminergic efferents from the nucleus of the solitary tract to the rat amygdala. Brain Res Bull. 1990;25(6):875–887. doi: 10.1016/0361-9230(90)90183-z. [DOI] [PubMed] [Google Scholar]
- 49.Sved AF, Cano G, Card JP. Neuroanatomical specificity of the circuits controlling sympathetic outflow to different targets. Clin Exp Pharmacol Physiol. 2001;28(1–2):115–119. doi: 10.1046/j.1440-1681.2001.03403.x. [DOI] [PubMed] [Google Scholar]
- 50.Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev. 2001;22(4):502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]