Abstract
One of the key regulators of acute steroid hormone biosynthesis in steroidogenic tissues is the steroidogenic acute regulatory (STAR) protein. Acute regulation of STAR production on the transcriptional level is mainly achieved through a cAMP-dependent mechanism, which is well understood. However, less is known about the posttranscriptional regulation of STAR synthesis, specifically the factors influencing the destiny of the Star mRNA after it leaves the nucleus. Here, we show that the 3′-untranslated region of Star mRNA interacts with the heterogeneous nuclear ribonucleoprotein K-homology (KH) motif of the mitochondrial scaffold A-kinase anchoring protein 1 (AKAP1) in vitro with a moderate affinity as measured by EMSAs. A mutation that mimics the phosphorylation state of the KH motif at a specific serine either did not alter, or had a negative impact on, protein-RNA binding under these conditions. The KH motif of AKAP1 binds short pyrimidine-rich RNA molecules with a stable hairpin structure as demonstrated by in vitro selection. AKAP1 also interacts with STAR mRNA in a dibutyryl-cAMP-stimulated human steroidogenic adrenocortical carcinoma cell line in vivo. Therefore, we propose a model in which AKAP1 anchors Star mRNA at the mitochondria, thus stabilizing the translational complex at this organelle, a situation that might affect STAR production and steroidogenesis. In addition, we suggest that the last 216 amino acid residues of AKAP1 might participate in the degradation of STAR and other nuclear-encoded mitochondrial mRNAs through interaction with a RNA-induced silencing complex, specifically with the argonaute 2 protein.
Steroid hormones regulate essential physiological processes such as reproduction, carbohydrate metabolism, and electrolyte homeostasis and are mainly produced in the gonads and adrenal glands. Deficiency in the biosynthesis of all steroid hormones results in a life-threatening condition known as lipoid congenital adrenal hyperplasia, most cases of which are caused by mutations in the steroidogenic acute regulatory (STAR) protein gene (1). STAR's activity facilitates the transfer of cholesterol between mitochondrial membranes to provide cholesterol substrate to the cytochrome P450 side-chain cleavage enzyme (P450scc; CYP11A1). Inside the mitochondria, cytochrome P450 side-chain cleavage enzyme converts cholesterol to pregnenolone, which is the first steroid formed in the production of all steroids (2, 3). STAR gene expression in mammals is tightly and acutely regulated by the trophic hormones of the pituitary and is mediated through cAMP-dependent mechanisms (4–6). In addition, proper function of STAR requires type II protein kinase A (PKA)-mediated phosphorylation (7, 8), which appears to occur in the close vicinity of the mitochondria. As part of this mechanism, mitochondrial levels of PKA are elevated through its interaction with the mitochondrial scaffold A-kinase anchoring protein 1 (AKAP1, D-AKAP1) (7–11).
Proteins, like AKAP1, possessing single or multiple K-homology (KH) motifs are known to be involved in diverse cellular actions including the synthesis of coding and noncoding RNA molecules, with the KH motif directly binding to the RNA (12–16). The RNA sequences, which usually bind with micromolar affinity to the KH motif, are comprised of several unpaired low-complexity nucleotides that interact with the hydrophobic binding pocket of the polypeptide. Specifically, the KH motif of AKAP1 has been shown to bind to the nuclear-encoded MnSOD and Fo-f mRNAs, the products of which are located in the mitochondria (17).
The regulation of STAR gene expression has been studied extensively (4, 5). However, little is known about the regulation of translation of Star mRNA or its interaction with regulatory RNA-binding proteins. Recently, it was shown that tetradecandoyl phorbol acetate-inducible sequence 11b (TIS11b), a zinc finger protein with affinity for AU-rich RNA sequences, facilitated the turnover of STAR mRNA in a cAMP-dependent manner (18). In addition, small interfering RNA-mediated knockdown of AKAP1 reduced STAR protein levels without affecting the steady-state levels of the Star mRNA (7). These findings suggest that AKAP1 might be involved in targeting the Star mRNA to the mitochondria and modulating the synthesis of STAR protein at its point of action. Therefore, as a first step in investigating the role of the KH motif of AKAP1 in steroidogenesis, we sought to determine whether Star mRNA binds to the KH motif of AKAP1 in vivo and in vitro.
In the current study, we demonstrated that the Star mRNA associates with AKAP1 in vivo in a cAMP analog stimulated H295R human adrenocortical carcinoma cell line. We determined that the KH motif of AKAP1 interacts in vitro with the 3′-untranslated region (UTR) of the mouse Star mRNA with micromolar affinity. We also identified, by in vitro selection, unpaired pyrimidine-rich RNA sequences as the best candidates for binding to the KH motif of AKAP1. We found several of the pyrimidine-rich sequences within the 3′-UTR of the Star mRNA. In addition, we found that AKAP1, through its Tudor domain, interacts with argonaute 2 (Ago2) protein, which is part of the RNA-induced silencing complex (RISC). These findings support a model in which STAR expression might be regulated on the translational level at the mitochondrial membrane and that the localization of the Star mRNA at the mitochondria is mediated by AKAP1.
Materials and Methods
Cell culture maintenance and indirect fluorescent immunolabeling
The human adrenocortical carcinoma cell line H295R (American Type Culture Collection, Manassas, VA) was cultured using the conditions that were described previously (19). Indirect immunofluorescent labeling was performed on H295R cells that were grown on size 1½ glass coverslips (Fisher, Pittsburgh, PA) as described previously (20). The cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA)/1× PBS for 20 min and washed twice with 1× PBS for 5 min. Subsequently, the cells were permeabilized with 1% Triton X-100/1× PBS and washed with 1× PBS, and the nonspecific binding sites were blocked with 0.5% nonfat milk (NFM)/1× PBS for 15 min. The primary rabbit antigen-purified polyclonal antihuman AKAP1 antibody (1:200 dilution; ProteinTech Inc., Chicago, IL) and monoclonal anti-translocase of outer mitochondrial membrane 20 homolog (TOMM20) antibody (1:200 dilution; Abnova, Taipei City, Taiwan) were applied in 0.5% NFM/1× PBS for 2 h. Cells were washed three times with 1× PBS, and secondary antimouse Cy3-conjugated and antirabbit DyLight 488-conjugated antibodies, both developed in donkey (Jackson ImmunoResearch Inc., West Grove, PA), were applied in 0.5% NFM/1× PBS for 40 min. Subsequently, the cells were washed twice with 1× PBS for 5 min, stained with 4′,6-diamidino-2-phenylindole/1× PBS for 5 min, washed once with 1× PBS, and embedded in ProLong Gold antifade reagent (Invitrogen, Carlsbad, CA). Coverslips were cured overnight and sealed with nail polish to prevent further drying. Fluorescent and differential interference contrast images were acquired on an inverted Nikon microscope equipped with an Andor iXon EMCCD camera (Andor Technology, Belfast, UK). Images were processed for brightness and contrast using Photoshop CS2 (Adobe, San Jose, CA). Colocalization coefficients were calculated using a software plug-in for ImageJ.
Construction of the expression plasmids and recombinant protein purification
Maltose-binding protein (MBP)-wild-type (WT) KH-Tudor [pPG82, the last 325 amino acid residues from the mouse AKAP1 (7), which were fused at the amino-terminal end to MBP in pPG80, which is a derivate of pMal-c possessing a PreScission protease cleavage site, a multiple cloning site, a TEV protease cleavage site, and a hexahistidine tag at the carboxy end]; MBP-S585D KH-Tudor (pPG83), similar to the WT KH-Tudor, but bearing a mutation that changes Ser585 to an aspartic acid residue (S585D); and MBP-WT KH (pPG102) and MBP-S585D KH (pPG103) spanned amino acid residues 532–645 and were created by site-directed mutagenesis using as a template pPG82 and pPG83, respectively. MBP-Tudor (pPG104) was created by site-directed mutagenesis from pPG82, removing amino acid residues from 532–642. All clones were confirmed by sequencing the corresponding plasmids.
Expression and purification of recombinant proteins was performed as described previously (20, 21) with some modifications outlined below. The expression of the recombinant proteins was induced with 0.1 mm isopropyl β-D-1-thiogalctopyranoside (IPTG) in the Rosetta 2(DE3)pLysS (EMD4Biosciences, Darmstadt, Germany) bacterial strain. The induction of MBP-WT KH-Tudor and MBP-S585D KH-Tudor was carried out for 16 h at 18 C and of MBP-WT KH, MBP-S585D, and MBP-Tudor for 4 h at 37 C. The bacterial pellets (∼3 g wet weight) were suspended in 50 ml buffer S3 [1.5 m NaCl, 25 mm Tris-HCl (pH 7.4), 1 mm EDTA, 0.05% (wt/vol) NaN3, and 1 mm dithiothreitol freshly added] supplemented with protease inhibitors (Sigma Chemical Co., St. Louis, MO), and the bacterial cells were disrupted by 15 cycles of sonication at 4 C. The insoluble material was removed by centrifugation at 15,000 × g for 20 min at 4 C. The cleared supernatants were loaded over an amylose resin (New England Biolabs, Beverly, MA) packed in a chromatographic column and preequilibrated with buffer S3. The column was washed with five column volumes (cv) of the buffer S3, followed by a wash with five cv of buffer S1 [0.3 m NaCl, 25 mm Tris-HCl (pH 7.4), 1 mm EDTA, 0.05% (wt/vol) NaN3, and 1 mm dithiothreitol freshly added]. Finally, the column was washed with five cv of HEPES buffer [0.3 m NaCl, 20 mm HEPES (pH 7.6), 5 mm imidazole, 0.05% (wt/vol) NaN3] and eluted by applying five cv of HEPES buffer supplemented with 10 mm maltose. Usually, the majority of the recombinant proteins was recovered in the first half of the elution volume. Recombinant proteins were further purified over a TALON (Clontech, Palo Alto, CA) resin packed in a chromatographic column, eluted in HEPES buffer supplemented with 200 mm imidazole. The purified proteins were concentrated using filter units with a proper membrane size (Millipore, Billerica, MA), and the buffer was exchanged to 50 mm NaCl, 10 mm Tris-HCl (pH 7.4), 0.05% (wt/vol) NaN3. Subsequently, the proteins were concentrated again and the molarity was determined by spectrophotometry at 280 nm using the predicted molecular weight and the extinction coefficient assuming that all cysteines were reduced as calculated on the EXPASY web server (22). The molar concentration of the proteins was in the 100- to 200-μm range, except MBP-Tudor, which was 46 μm. The identity of MBP-WT KH-Tudor and MBP-S585D KH-Tudor was confirmed by a mass spectrometry. Before each EMSA experiment, sufficient amounts of proteins were diluted to 20 μm (the highest concentration used) with serial dilutions down to 2.4 and 4.8 nm.
In vitro RNA synthesis and 3′-end fluorescein labeling
The RNAs used in the study were in vitro transcribed using a MEGAshortscript T7 kit (Ambion, Austin, TX). The DNA templates for the proximal 261 nucleotides (nt) and the distal 263 nt of the 3′-UTR of Star mRNA (23), and the systematic evolution of ligands by exponential enrichment (SELEX) clones 8-1 and 8-4 were generated using PCR technology with a Pfu polymerase. The primers corresponding to the sense strand of the templates possessed a T7 RNA polymerase promoter. DNA templates were cleaned up using QIAquick PCR purification kit (QIAGEN, Valencia, CA) and ethanol precipitated. Usually, approximately 8–10 μg of the DNA templates was used for the in vitro transcription reaction, which yielded 1–2 nmol RNA. Subsequently, the RNAs longer than 100 nt were purified using a MEGAclear kit (Ambion); RNAs shorter than 100 nt were purified using RNeasy mini kit (QIAGEN), and 0.5 nmol of the purified RNAs were dephosphorylated using a combination of T4 polynucleotide kinase (New England Biolabs) and calf intestinal alkaline phosphatase (New England Biolabs), followed by phenol/chloroform extraction and ethanol precipitation.
The 3′-end of the RNA molecules were oxidized using sodium periodate, and fluorescein-5-thiosemicarbazide (Invitrogen) was used to label the RNAs with a single fluorescein dye at the 3′-end (21, 24). Labeled RNAs were cleaned up over G25 Sephadex columns (Roche Applied Science, Indianapolis, IN) to remove the unincorporated dye. The labeling efficiency and the yield were determined by measuring the absorption at 260 and 491 nm. The efficiency of the labeling was above 80%. The labeled RNA probes were diluted in water to 100 nm before the EMSA experiments.
EMSA and determination of apparent dissociation constants (Kd,app)
For the experiments presented in Fig. 2, 300 pm 3′-end fluorescein-labeled RNA molecules were equilibrated with the serial dilution of the recombinant proteins for 3 h at room temperature in buffer containing 10 mm Tris-HCl (pH 7.4), 50 mm NaCl, 1 mm MgCl2, 0.01% (vol/vol) IGEPAL CA630, 5 μg/ml heparin, 10 U RNaseOUT (Invitrogen), and 10 μg/ml yeast tRNA (21). After the completion of the incubation time, the reactions were supplemented with 6% glycerol and were loaded on a 6% native vertical polyacrylamide gel in 0.5× Tris-borate/EDTA buffer at 4 C. Experiments presented in Fig. 3 were performed under slightly different conditions: incubation time was shortened to 10 min at room temperature and the buffer was modified to 10 mm Tris-HCl (pH7.4), 200 mm NaCl, 0.01% (vol/vol) IGEPAL CA630, 10 μg/ml yeast tRNA, 5% glycerol. The protein-RNA complexes were resolved from the free RNA on 6% native slab polyacrylamide gels in 0.5× Tris-borate/EDTA buffer at 4 C. No difference in the affinities was observed using either condition. The protein-RNA complexes and free RNAs were visualized using a fluorescent gel imager (Pharos FX scanner; Bio-Rad, Hercules, CA) as recommended by the manufacturer, and the images were quantified using ImageJ software. The bound fraction of the RNA was calculated as a fraction of the bound and free RNA or as a disappearance of the free RNA and plotted toward the protein concentration. The apparent dissociation constants were calculated by fitting the data to a modified version of the Hill equation (24, 25) using GraphPad Prism version 5.2 software for Windows (GraphPad Software, San Diego, CA). Unpaired t test was also performed using GraphPad Prism.
Fig. 2.
EMSA of the proximal 261 nt of Star mRNA interacting with regions of the mouse AKAP1 within the last 325 amino acid residues of the protein. A, the last 325 amino acid residues of AKAP1 (WT KH-Tudor) and with a mutation S585D (S585D KH-Tudor); amino acid residues 532–645 (WT KH) and with a mutation S585D (S585D KH); and amino acid residues 642 to 857 (Tudor domain) as purified recombinant proteins fused to MBP resolved on a SDS-PAGE and stained with Coomassie Brilliant Blue. B, Representative EMSA results for the recombinant either WT or S585D mutant of the last 325 amino acid residues of the mouse AKAP1 fused to MBP binding to the proximal 261 nt of Star mRNA. The triangles represent 2-fold serial dilutions. *, Free RNA. The graph on the right represents a quantification of three independent EMSA experiments and fit of the data to the Hill equation to establish the apparent dissociation constant and the Hill coefficient. C, same as B, but the recombinant proteins represent either WT or S585D mutant of the KH motif of AKAP1 fused to MBP. D, Representative EMSA experiment with the recombinant Tudor domain, covering amino acid residues from 642–857 of the mouse AKAP1 as an amino-terminal fusion to MBP and the proximal 261 nt of the mouse Star mRNA. The asterisk in the table indicates apparent dissociation constants that are significantly different from each other (P < 0.05).
Fig. 3.
Results from the SELEX. A, Representative EMSA experiments of the initial RNA pool (SR106-0) and the 8-fold-enriched RNA pool (SR106-8) binding to the WT KH of AKAP1 fusion with MBP. The graph on the right represents measurements of the bound fraction of the RNA plotted toward the protein concentration of WT KH and fitted to the Hill equation from three independent experiments. B, Thirty-three clones were analyzed; shown are the clones that align in two motifs: RNA motifs I and II. Clone names are located in front of the RNA sequence. Lowercase letters represent the flanking 21 and 19 fixed sequences. The most common unpaired low-complexity sequence is in bold uppercase letters. C, A representative secondary structure of clones 8-4, E06, and F08 as determined by mfold. Bold letters represent the seven-base consensus motif. D, Representative EMSAs of clone 8-4 binding to the WT KH motif of AKAP1 fused to MBP. On the right, bound RNA is plotted vs. the concentration of the WT KH motif of AKAP1 fused to MBP fitted to the Hill equation. The data were obtained from three independent experiments.
SELEX technology
SELEX technology (21, 26), with some modifications as outlined below, was used to determine high-affinity binding RNA sequences to the recombinant MBP-WT KH motif of AKAP1. To construct the initial library, 600 pmol single-stranded DNA oligonucleotide was used [N106, 5′-GCGTCAAGTCTGCAGTGAA(N)30TCGTAGATGTGAGATCCATTCCC-3′], which gave approximately 4 × 1014 different molecules. To convert the DNA oligonucleotide to double-stranded DNA, six cycles of PCR were performed with primers N105 (5′-GATAATACGACTCACTATAGGGAATGGATCTCACATCTACGA-3′) and N107 (5′-GCGTCAAGTCTGCAGTGAA-3′). The PCR product was used as a template in an in vitro transcription reaction using MEGAshortscript T7 kit (Ambion). Eight cycles of positive selection were performed. In the first two cycles approximately 60 pmol, in the second two approximately 30 pmol, in the third two approximately 15 pmol, and in the last two approximately 30 pmol of the recombinant MBP-WT KH protein coupled to amylose beads (New England BioLabs) were incubated with approximately 0.8–1 nmol of the initial and selected RNA pools. The binding reaction was carried out at room temperature for 30 min (initial four cycles) and 10 min (last four cycles) in a binding buffer [10 mm Tris-HCl (pH 7.4), 200 mm NaCl, 1 mm MgCl2, 0.01% (vol/vol) IGEPAL CA630, and 50 μg/ml yeast tRNA]. The free RNAs were separated from protein-RNA complexes by a low-speed centrifugation over a 45-μm pore-size spin column (Millipore). The protein-RNA complexes were washed four times with binding buffer without IGEPAL CA630. During the last two selection cycles, salt concentration of washes two and three was increased to 500 mm NaCl. The protein-RNA complexes were eluted from the beads in the binding buffer supplemented with 10 mm maltose. The RNA was phenol/chloroform extracted and ethanol precipitated. The recovered RNA was converted to cDNA using primer N107 and Superscript III reverse transcriptase (Invitrogen). Ten cycles of PCR were performed using primers N105 and N107 and the cDNA as a template to generate a new DNA template for the next cycle of in vitro transcription, followed by selection. After the eighth cycle, a negative selection was performed, leaving out the recombinant protein, thus eliminating the RNA molecules that would be nonspecifically bound. After the negative selection, the RNA recovered from the flow-through was converted to a cDNA. The subsequent double-stranded PCR product was cloned in the pCR4-TOPO vector using the TOPO TA cloning kit (Invitrogen).
UV cross-linking, immunoprecipitation of AKAP1, Western blot, RNA purification, and RT-PCR analysis
At 80–90% confluence, three 10-cm dishes of H295R cells were stimulated with freshly prepared 1 mm dibutyryl-cAMP (Sigma) in culture media for 4 h. After completion of the incubation time, the treated and the same number of control cells were rinsed with 1× PBS and irradiated for 200 mJ/cm2 (UVP UV Crosslinker CL-1000 at 254 nm; UVP, Upland, CA). Cells were washed with 1× PBS, scraped in 1 ml 1× PBS, collected by low-speed centrifugation at 4 C, and frozen. The treated and control cells were lysed in 1.5 ml RIPA buffer [1× PBS, 0.5% (vol/vol) IGEPAL CA 300, 0.5% (wt/vol) sodium salt of deoxycholic acid, 0.1% (wt/vol) sodium dodecyl sulfate (SDS), 0.05% (wt/vol) NaN3] supplemented with SUPERaseIn (Ambion) and protease inhibitors (Sigma). The cell lysate was incubated on ice for 10 min followed by centrifugation at 20,300 × g for 20 min at 4 C. Cleared lysate was added to protein A magnetic beads (Dynabeads; Invitrogen) precoupled with 4 μg polyclonal AKAP1 antibody (ProteinTech) or 4 μg antibody against the goat IgG, matching the isotype of the AKAP1 antibody (Sigma). Immunoprecipitation was carried out for 1 h at 4 C. Magnetic beads were collected on the magnetic separator and were washed twice with RIPA buffer, followed by three washes with RIPA buffer containing five times more PBS (5× PBS), followed by two washes with RIPA buffer. To recover the RNA associated with AKAP1, the immunoprecipitated AKAP1 protein was digested with 2 mg/ml proteinase K in a buffer containing 3 m urea, 0.5% (wt/vol) SDS for 30 min at 45 C, followed by phenol/chloroform extraction and 2-propanol precipitation in the presence of 0.3 m sodium acetate. Protein samples were collected for Western blot analysis at each step of the protocol. To maximize conversion of the RNA associated with the AKAP to cDNA, a reverse transcriptase reaction was performed with a combination of random hexanucleotides and an oligo-deoxythymidine primer and Superscript III reverse transcriptase (Invitrogen). Primer pairs specific for the mRNA being tested were as follows: STAR mRNA (accession number NM_000349, product size 180 bp), primers N143 (5′-CTACTCGGTTCTCGGCTGGAAGAG-3′) and N144 (5′-GCCCACATCTGGGACCACTTTACT-3′); Fo-f mRNA (accession number NM_004889, product size 274 bp), primers N151 (5′-CAGTTGGTGAGTGTCCGGCCC-3′) and N152 (5′-TGGTATTTGCGGAGCCGCTCG-3′); MNSOD mRNA (accession number NM_000636, product size 209 bp), primers N149 (5′-ACCAGGAGGCGTTGGCCAAG-3′) and N150 (5′-TGCAGCCGTCAGCTTCTCCT-3′); GAPDH mRNA (accession number NM_002046, product size 234 bp) primers N157 (5′-TCTTTTGCGTCGCCAGCCGAG-3′) and N158 (5′-CCCGTTCTCAGCCTTGACGGT-3′); and MLN64 mRNA (accession number NM_006804, product size 315 bp) primers N163 (5′-CCCAGGTTGCTGTTGCCCGT-3′) and N164 (5′-GACAGGGCAGGAAGGTCTTCAGGA-3′). PCRs were performed with EmeraldAmp GT PCR Master Mix (Clontech). Between 1/10 and one fifth of the PCRs were analyzed on 2% agarose gels stained with ethidium bromide.
For immunoprecipitation, cell lysates were prepared in immunoprecipitation buffer [20 mm HEPES (pH 7.4), 150 mm NaCl, 1% (vol/vol) Triton X-100, 5% (vol/vol) glycerol] supplemented with SUPERaseIn (Ambion) and protease inhibitors (Sigma) as previously described (27). Cleared cell lysate was added to either 4 μg isotype control antibody against the goat IgG or 4 μg antibody against AKAP1 coupled to protein A Dynabeads (Invitrogen). Immunoprecipitations with antibody against AKAP1 were either untreated or treated with ribonuclease (RNase) or RNase-free deoxyribonuclease (DNase) I for 5 min at room temperature followed by incubation at 4 C 1 h.
Western blots were performed using the NuPAGE SDS-PAGE gel system (Invitrogen). Primary polyclonal antibodies against AKAP1 (ProteinTech), rabbit monoclonal antibody against Ago2 (Cell Signaling Technology, Danvers, MA), mouse monoclonal anti-actin (Ambion), and secondary antirabbit and antimouse horseradish peroxidase-conjugated antibody (Thermo Scientific, Pittsburgh, PA) were consecutively applied as recommended by the suppliers. In the Western blot shown in Fig. 4A, the membrane was initially probed with anti-AKAP1 antibody, appropriate secondary horseradish peroxidase-conjugated antibody, and developed with enhanced chemiluminescence (PerkinElmer, Norwalk, CT). Subsequently, the same membrane was stripped in 0.1 m glycine (pH 2.6) and reprobed with anti-actin antibody.
Fig. 4.
STAR mRNA associates with AKAP1 in vivo in the human adrenocortical carcinoma cell line H295R as determined by UV cross-linking, followed by immunoprecipitation and RT-PCR. A, Representative Western blot with antibodies against AKAP1 or β-actin of cell lysates obtained from control and 1 mm dibutyryl-cAMP-treated UV-irradiated H295R cells as indicated. Lanes 1 and 2, Input, approximately 1/100 of the total amount of protein was loaded on the gel; lanes 3 and 4, the flow-through from the input samples incubated with antibody against AKAP1 (Fl AK); lanes 5 and 6, the flow-through from the input samples incubated with the background control IgG (Fl IgG); lanes 7 and 8, 1/30 of the immunoprecipitated samples with antibody against AKAP1 (IP AK); lane 9, 1/30 of the immunoprecipitated sample with background control IgG (IP IgG). B, Representative agarose gel electrophoresis of RT-PCR analysis of the indicated human genes. C, AKAP1 interacts with Ago2 protein. A representative Western blot with antibodies against AKAP1 or Ago2 proteins of cell lysates obtained from H295R cells as indicated. Lane 1, Input, approximately 1/200 of the total amount of protein was loaded on the gel; lanes 3–5, the flow-through from the input samples incubated with antibodies either against isotype control IgG (Fl IgG) or AKAP1 (Fl AK); lane 6, one fourth of the immunoprecipitated sample with the background control IgG (IP IgG); lanes 7–9, one fourth of the immunoprecipitated samples with antibody against AKAP1 (IP AK); lane 7, no treatment; lane 8, treatment with RNase; lane 9, treatment with RNase-free DNase I. Representative Western blots are shown of three biological replicates. D, Plot of lanes 6 and 7 of the Western blot present on C showing the intensity and distribution of Ago2 protein.
Homology modeling and BLAST comparison
Primary protein sequence of the KH motif of AKAP1 (amino acid residues 559–637) was aligned manually to the primary sequence of the KH3 of Nova-2 protein (pdb accession number 1EC6, amino acid residues 1–81) as shown on Fig. 5A in SWISS-PdbViewer (28, 29). The alignment was submitted for a computational modeling on the SWISS-MODEL Workplace (30, 31). The homology model of the KH motif of AKAP1 on the KH3 of Nova-2 structure had a Z-score QMEAN of −0.82. The S585D mutation was introduced in the SWISS-PdbViewer, and all homology models were rendered in three dimensions. Phosphate addition to the serine 585 in the homology model was kindly introduced by R. Bryan Sutton (Texas Tech University Health Sciences Center). The KH3 of Nova-2 protein demonstrated the highest percent identity to the KH motif of AKAP1 in the protein bank database.
Fig. 5.
Homology modeling of the KH motif on the Nova-2 KH3 crystal structure (pdb number 1EC6). A, Alignment used for the homology modeling with a schematic representation of the α-helices and β-sheets as determined by the modeling. The black dot represents the position of the serine 585 in the mouse KH motif of AKAP1. The crossed sticks represent the invariant GXXG loop in the KH motifs. B, The homology model of the KH motif of AKAP1 with either serine 585 (WT, space-filled representation), the mutated serine 585 to aspartic acid residue (S585D, space-filled representation), or phosphorylated state of the serine 585 (WT-Phos, space-filled representation) in relation to the RNA from the original crystal structure spanning nucleotides 5′-AUCACC-3′ (represented as sticks). Aspartic acid and phosphorylated state of the serine 585 (space-filled representation) are rotated to be in the closest proximity to the nucleic acid.
Results
AKAP1 protein localizes to mitochondria in the human adrenocortical carcinoma cell line H295R
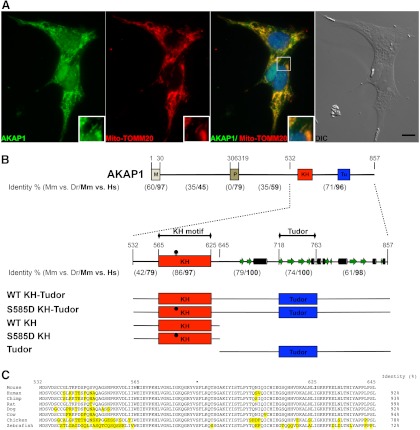
As shown previously by us (7) and others (32, 33), AKAP1 is localized to the mitochondria. To confirm the subcellular distribution of AKAP1 in the H259R human steroidogenic adrenocortical carcinoma cell line, double indirect immunofluorescent labeling was performed. Visual analysis of the labeling signal produced by the AKAP1 antibody showed that AKAP1 closely resembled a mitochondrial network (Fig. 1A, left) with the majority of the signal overlapping with the signal produced from the anti-TOMM20 antibody, which delineates the mitochondria (34) (Fig. 1A). Colocalization of both signals was confirmed with a Pearson's coefficient of 0.835, Mander's overlap coefficient of 0.814, and an AKAP1 to TOMM20 channel pixel ratio of 0.967. We conclude that the majority of AKAP1 protein is associated with mitochondria in the H295R adrenocortical carcinoma cell line within approximately 200 nm, the colocalization detection limit of light microscopy.
Fig. 1.
Subcellular localization and conserved domain organization of AKAP1. A, Wide-field fluorescent and differential interference contrast (DIC) micrographs of the human adrenocortical carcinoma cell line H295R labeled by indirect immunofluorescence with a polyclonal antibody against AKAP1 (green) and monoclonal antibody toward TOMM20 (red). In the composite image, blue represents 4′,6-diamidino-2-phenylindole-stained nuclei. Inset, A single mitochondrion. Bar in DIC image, 10 μm. B, Schematic representation of the conserved domain organization of AKAP1 as annotated on the mouse protein (GenBank accession number NP_033778). Rectangles represent the domains/motifs of the mouse AKPA1. The line represents interdomain regions. KH, Heterogeneous nuclear ribonucleoprotein KH motif; M, mitochondrial targeting sequence; P, amino acid residues that interact with PKA; Tu, Tudor domain. Below is shown the percent identity as determined by BLAST pair alignment comparison of the domains/motifs and interdomain regions between mouse (Mm) and zebrafish (Dr) and between mouse (Mm) and human (Hs) (values in bold). The secondary structure of amino acid residues 642–857 is shown as predicted by PSIPRED. Underneath is the position of the recombinant proteins used in the subsequent experiments. Black dots represent the position of the Serine 585 on the mouse KH motif and subsequent S585D mutant in the recombinant proteins. C, Amino acid residues from 532–645 of the mouse AKAP1 aligned to several vertebrate species. The amino acid residues differing from the mouse sequence are highlighted in yellow. The dot represents serine 585 in the mouse protein.
The last 325 amino acid residues from AKAP1 protein are highly conserved
BLAST algorithm comparison of the primary sequence of AKAP1 for the separate domains and interdomain regions showed that the last 325 amino acid residues are more conserved than the rest of the protein among vertebrate species. A more detailed analysis of the last 325 amino acid residues revealed that the most conserved region of AKAP1 was the KH motif with 86% identity between mouse and zebrafish and 97% between mouse and human (Fig. 1, B and C).
Secondary structure prediction performed on amino acid residues 642–857 with PSIPRED (35) revealed that the Tudor domain and the flanking sequences of AKAP1 closely resembled a Tudor staphylococcal nuclease-like (TSN) domain, similar to the human P100 protein (36, 37) (Fig. 1B).
The last 325 amino acid residues and the KH motif of mouse AKAP1 bind to the 3′-UTR of mouse Star mRNA in vitro
Our hypothesis suggested that AKAP1, through its KH motif, might assist in the association of Star mRNA to the outer mitochondrial membrane. To examine the ability of AKAP1 to interact with the Star mRNA in vitro, the following recombinant proteins, WT KH-Tudor, Tudor domain, and WT KH motif were expressed as MBP fusions and purified from bacteria. In addition, serine 585 located within the KH motif was mutated to aspartic acid (Fig. 1B, black dots; and Fig. 2, A–C, S585D KH-Tudor and S585D KH), and the corresponding recombinant proteins fused to MBP were also expressed and purified from bacteria. The substitution (S585D) is believed to mimic the phosphorylation state of the KH motif. This substitution was performed because of recent reports (17, 38) that the phosphorylated KH motif at Ser585 and/or the mutant (S585D) affected the affinity of the protein for RNA. Therefore, the impact of the mutation (S585D) on the affinity of the KH motif for the Star mRNA under our assay and binding conditions was tested.
All the recombinant fusion proteins, except for the MBP-Tudor domain, were at least 97% pure and homogeneous as determined by Coomassie Brilliant Blue-stained SDS-PAGE (Fig. 2A). To distinguish between protein-bound and free RNA, an EMSA (24, 25) was used. The amount of bound RNA was plotted as a function of the protein concentration. Affinities of the WT KH-Tudor and WT KH and the S585D mutants (S585D KH-Tudor and S585D KH) for the Star 3′-UTR RNA were calculated by fitting the data to the Hill equation (24, 25) (Fig. 2, B and C). All the proteins possessing either WT or mutated (S585D) KH motif bound to the Star 3′-UTR RNA with affinities in the 0.38- to 2.44-μm range (see Fig. 2). We did not observe complex formation between the Tudor domain and the 3′-UTR of Star mRNA (Fig. 2D). Therefore, an apparent dissociation constant and a Hill coefficient for the Tudor domain were not calculated. Also, the recombinant proteins containing the Tudor domains degraded the RNA when present in high concentrations (Fig. 2, B and D). The difference in the apparent dissociation constants of WT KH vs. S585D KH was significant (P < 0.05).
The affinities of all recombinant proteins to the distal 263 nt of the 3′-UTR of Star mRNA were also determined. The values for the apparent dissociation constants (data not shown) were similar to those calculated for the proximal 261 nt of the 3′-UTR of Star mRNA. As expected, the Tudor domain did not bind to the RNA and showed slight RNase activity. Therefore, we concluded that the KH motif of AKAP1 binds to the 3′-UTR of Star mRNA with an affinity in the nanomolar to micromolar range.
RNA selection for WT KH motif of AKAP1
Hill coefficients for affinity interactions (Fig. 2) indicated that the binding of the KH motif to the RNA is a cooperative event. As a result, we hypothesized that there were multiple binding sequences within the 3′-UTR of the Star mRNA with a similar or different affinity for the KH motif of AKAP1. Therefore, an RNA selection experiment using a 30-nt random library was performed, and the requirements for the RNA molecule to bind to the KH motif of AKAP1 were determined.
To determine short RNA sequences that could bind with a high affinity to the WT KH motif of AKAP1, SELEX (26) was performed. Before selection, an apparent dissociation constant of 3.33 ± 1 μm and a Hill coefficient of 1.0 for the initial RNA pool (SR106-0) was measured using EMSA (Fig. 3A). After eight rounds of selection, the enriched RNA pool (SR106-8) increased its affinity for the WT KH motif by approximately 9-fold (Kd,app = 0.38 ± 0.03 μm, Hill coefficient = 0.7, Fig. 3A). At this point, the RNA pool was converted to cDNA and cloned, and individual clones were sequenced. Analysis of the sequences showed that 24% of the clones (eight of 33) could be assembled in a separate group (RNA motif I, Fig. 3B). Several of the clones in the group were represented more than once, suggesting that the SELEX enriched for RNA molecules with a high affinity for the WT KH motif. A second group (seven of 33) of sequences could also be aligned (RNA motif II), representing 21% of the clones.
Analysis of the sequences in RNA motifs I and II using the mfold RNA secondary prediction software (39) indicated that both motifs could form distinct stem-loop structured within the RNA molecule (up to four different structures per clone) with various low-complexity sequences unpaired (Fig. 3B). All the RNA molecules in the RNA motif I were organized in stable secondary structures with free energies ranging from −18.30 to −7.35 kcal/mol as predicted by mfold. Analysis of the structures demonstrated that the seven-base 5′-AGCAUUC-3′ consensus sequence was unpaired in the majority of the cases (Fig. 3C). Additional uridine-rich sequences were also unpaired (not shown), but a uniform consensus longer than three nt for these sequences could not be determined.
We did not find multiple clones with identical sequences in RNA motif II. However, the hairpin structures (free energy ranging from −15.10 to −7.63 kcal/mol) were organized in such a way that the unique five-base (5′-UCUUA-3′) consensus sequence was unpaired. Additional four- to seven-nt-long, uridine-rich sequences were also unpaired within the same structure (not shown). The five-base consensus sequence was preceded by a guanosine, which was invariably engaged in formation of a stem structure. In the majority of the clones, the five-base consensus sequence was also followed by a guanosine, which in some of the clones was involved in a formation of stems (clone H12 in all of the structures) or was unpaired (clone H04 in all of the structures).
Analysis of the enriched RNA pool (SR106-8) showed that the free RNA existed in two discrete bands (Fig. 3A, bottom, lane 0). The top band appeared to have a stronger interaction with the WT KH recombinant protein. Therefore, we suggest that the RNA molecules in the upper band form a strong secondary structure that promotes short, low-complexity sequences to remain unpaired, thus manifesting high affinity for the KH motif of AKAP1.
Next, representative clones were tested for protein-RNA binding by EMSA and the affinity for the WT KH motif of AKAP1 was determined. Both clones 8-4 (RNA motif I) and 8-1 (RNA motif II) showed similar binding patterns (Fig. 3D and data not shown). Clone 8-4 was represented three times (clones 8-4, E06, and F08) and displayed a notable apparent dissociation constant of 0.14 ± 0.04 μm. Such a Kd,app is well below the reported affinity for a single KH motif (13, 16).
RNA motif I and II sequences are present in the 3′-UTR of Star mRNA
Next, the distribution of the consensus RNA motifs, as determined by SELEX, which demonstrated a high affinity to the WT KH recombinant protein, were examined in the mouse and human STAR mRNA (Table 1). Query sequences were derived from the unpaired oligonucleotides 5′-AGCAUUCCUC-3′ and 5′-UCUUA-3′, representing RNA motif I and the complete RNA motif II (Table 1). Subsequently, 24 mouse and 11 human unique sequences were mapped to the mouse and human STAR mRNA (Table 1). Analysis of the location of the mapped sequences revealed that the majority, 22 and seven, were located at the 3′-UTR of the mouse and human mRNA, respectively. Three human sequences and one mouse sequence were positioned within the short interspersed elements and the exact positions determined by the RepeatMasker web-based software (Smit, A. F. A., R. Hubley, and P. Green, RepeatMasker Open-3.0, 1996–2010, http://www.repeatmasker.org). The majority of the mRNA sequences that were mapped were between five and six nt. Only one seven-nt-long sequence (5′-CAUUCCU-3′) was mapped in the Star mRNA. Most of the sequences were pyrimidine rich. In addition, 60% of the flanking nucleotides were pyrimidines.
Table 1.
Consensus sequences determined through SELEX found in mouse and human STAR mRNA
| Length (nt) | Sequences (5′→3′) | Species |
||
|---|---|---|---|---|
| Mouse | Human | |||
| Length of mRNA (nt) | 4007 | 2695 | ||
| Accession number | NM_011485 | NM_000349 | ||
| ORF span | 57-911 | 265-1122 | ||
| Proximal 261 nt | 912-1172 | NA | ||
| Interspersed repeats | 1599-1726 (SINE/Alu) | 1792-2089 (SINE/Alu) | ||
| 1731-1943 (SINE/B2) | 2585-2695 (SINE/Alu) | |||
| 1949-2061 (SINE/Alu) | ||||
| 2364-2503 (SINE/Alu) | ||||
| 2531-2614 (SINE/B4) | ||||
| 2768-2918 (SINE/B4)/C | ||||
| 3438-3543 (SINE/Alu)/C | ||||
| 3544-3570 (SINE/B4)/C | ||||
| 3834-3930 (SINE/Alu) | ||||
| RNA motif I | ||||
| 10 | AGCAUUCCUC | ND | ND | |
| 9 | AGCAUUCCU | ND | ND | |
| 8 | AGCAUUCC | ND | ND | |
| 7 | AGCAUUC | ND | ND | |
| 6 | AGCAUU | ND | ND | |
| 5 | AGCAU | (C)54-58(G) | (C)994-998(C) | |
| (C)990-994(C)b | (G)1024-1028(C) | |||
| (C)1800-1804(C)a | (C)1823-1827(U)a | |||
| (C)2098-2102(C) | ||||
| (A)2745-2749(G) | ||||
| 9 | GCAUUCCUC | ND | ND | |
| 8 | CAUUCCUC | ND | ND | |
| 7 | AUUCCUC | ND | ND | |
| 6 | UUCCUC | (G)60-65 (G) | ND | |
| 5 | UCCUC | (U)61-65 (G) | (C)1573-1577(C) | |
| (C)1297-1301(U) | ||||
| 8 | GCAUUCCU | ND | ND | |
| 7 | GCAUUCC | ND | ND | |
| 6 | GCAUUC | (G)3323-3328(U) | ND | |
| 5 | GCAUU | (G)3323-3327(C) | (A)1824-1828(U)a | |
| (G)3562-3566(U)a | ||||
| (U)3786-3790(A) | ||||
| 7 | CAUUCCU | (A)2314-2319(G) | ND | |
| 6 | CAUUCC | (A)2313-2318(U) | ND | |
| 5 | CAUUC | (A)2313-2317(C) | (A)278-282(A) | |
| (U)2738-2742(U) | (A)755-759 (A) | |||
| (G)3324-3328(U) | (U)1336-1340(A) | |||
| (A)3387-3391(A) | ||||
| (U)3467-3471(U)a | ||||
| (U)3746-3750(U) | ||||
| (U)4003-4007 | ||||
| 6 | AUUCCU | (C)2315-2319(G) | ND | |
| (A)2686-2691(U) | ||||
| 5 | AUUCC | (G)181-185(A) | (A)2438-2442(A) | |
| (U)1354-1358(A) | (A)2550-2554(C) | |||
| (C)2314-2318(U) | ||||
| (A)2686-2690(U) | ||||
| 5 | UUCCU | (G)60-64(C) | (C)34-38(U) | |
| (U)1571-1575(A) | ||||
| (C)2238-2242(U) | ||||
| (A)2316-2319(G) | ||||
| (A)2687-2691(U) | ||||
| RNA motif II | ||||
| 5 | UCUUA | (G)1216-1220(C) | (U)2275-2279(A) | |
| (G)3019-3023(A) | ||||
| (A)3292-3296(U) | ||||
Sequences derived from the clone 8-4 and RNA motif II are shown in uppercase letters. In the interspersed repeats, C represents the sequence corresponding to the complementary consensus sequence of the interspersed repeat. The positions of the unique sequences are in bold. Overlapping sequences are shown in normal text. Sequences within the open reading frame (ORF) of STAR mRNA are underlined. Nucleotides in front of and following the consensus in mouse and human STAR mRNA are shown in parentheses. NA, Not applicable; ND, not determined; SINE, short interspersed elements.
Sequences within the short interspersed elements.
Sequence presented in the proximal 261 nt of mouse Star mRNA.
AKAP1 interacts with the STAR mRNA in vivo
Having established that the KH motif of AKAP1 interacted with the Star mRNA in vitro, we wanted to determine whether such an interaction occurs in vivo. To test this possible interaction, RNAs associated with AKAP1 were UV cross-linked to the protein. Subsequently, AKAP1 and associated RNAs were recovered by immunoprecipitation of the AKAP1 under stringent washing conditions permitting only the RNA molecules with covalent bonds to the protein to be recovered. This experiment was performed in the steroidogenic H295R human cell line with and without treatment with 1 mm dibutyryl-cAMP, which stimulates the expression of STAR (19). Western blot analysis, using an antibody against AKAP1, of a total cell lysate from UV-irradiated H295R cells revealed two bands with an apparent molecular mass of approximately 140 and 52 kDa (Fig. 4A, lanes 1 and 2, input). The 140-kDa band was likely AKAP1 (9). However, the identity of the approximately 52-kDa band was not clear, because the band was not present in total protein samples from human HeLa cells (data not shown). Stimulation of the H295R cells for 4 h with 1 mm dibutyryl-cAMP slightly elevated the amount of AKAP1 over the basal level (Fig. 4A, lane 1 vs. 2). Immunoprecipitation with the anti-AKAP1 antibody almost completely depleted AKAP1 from the flow-through, but it did not change the amount of the unidentified approximately 52-kDa protein (compare lanes 1 and 2 vs. lanes 3 and 4; Fl AK). The majority of AKAP1 was observed in the immunoprecipitated samples (lanes 7 and 8). A control antibody did not precipitate AKAP1 or the unidentified protein (lane 9). These results suggested that the anti-AKAP1 antibody efficiently precipitated AKAP1 from H295R cell line.
After the immunoprecipitation, RT-PCR was performed. The STAR mRNA associated with AKAP1 was consistently detected above the background upon stimulation with 1 mm dibutyryl-cAMP (Fig. 4B, lanes IP AK +), was not detected in the H295R cells that were not treated with 1 mm dibutyryl-cAMP (lane IP AK −). The mRNAs of the manganese superoxide dismutase (MNSOD) and F2 subunit of mitochondrial ATP synthase (ATP5J2 and Fo-f) were also consistently detected. These mRNAs have been previously shown to be nuclear-encoded, integral mitochondrial proteins that interact with the KH motif of AKAP1 in vitro (17). The amount of the PCR products for the MNSOD and ATP5J2 were almost identical in both dibutyryl-cAMP-treated and control samples. Two mRNAs that have not been shown to interact with the AKAP1, the metastatic lymph node 64 protein (MLN64) mRNA and the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA were also identified. This suggests a direct interaction between AKAP1 and the STAR mRNA in vivo.
AKAP1 interacts with Ago2 protein in the H295R cell line
We established that the AKAP1 possesses a Tudor/TSN domain using secondary structure prediction software (Fig. 1B). Therefore, we tested whether Ago2 protein, part of the RISC reported to be associated with the mitochondria (40), interacts with AKAP1 using an immunoprecipitation protocol against the endogenous proteins.
Cleared cell lysates from H295R cells were subjected to immunoprecipitation with either isotype control antibody against goat IgG or AKAP1 antibody (Fig. 4C). As expected, the control IgG did not deplete the AKAP1 from the cell lysate (Fig. 4C, lane 2). However, the antibody against AKAP1 completely removes the AKAP1 protein from the cell lysate (Fig. 4C, lanes 3–5). Similarly, the immunoprecipitated samples revealed that the isotype control antibody does not immunoprecipitate, but the AKAP1 antibody does precipitate the AKAP1 (Fig. 4C, lanes 6–9). In addition, because both AKAP1 and Ago2 protein interact with RNA, the role of nucleic acid, which may mediate the interaction between the proteins was evaluated. Samples were treated with RNase (Fig. 4C, lanes 4 and 8), and RNase-free DNase I (Fig. 4C, lanes 5 and 9). The treatment with either enzyme does not change the profile of the immunoprecipitated AKAP1 (Fig. 4C, lanes 7–9). The same set of samples was probed with an antibody toward Ago2 protein (Fig. 4, C and D). The control background antibody (Fig. 4C, lane 6, and Fig. 4D) does not pull down the Ago2 protein. However, AKAP1 antibody efficiently pulls down the Ago2 protein with or without treatment with RNase or DNase I (Fig. 4C, lanes 7–9, and Fig. 4D). However, the flow-through fractions depleted of AKAP1 do not show diminished levels of Ago2 protein, suggesting that the AKAP1 interacts only with a small fraction of Ago2 pool of proteins (Fig. 4C, lanes 2–5).
Discussion
One of the objectives of the current studies was to determine whether there existed an interaction between STAR mRNA and the KH motif of AKAP1 in vitro and in vivo. A second objective was, if such an interaction occurs, to identify the RNA sequences that bind the KH motif. Establishing such an interaction would support a model for the localization and translation of nuclear-encoded STAR mRNA and other integral mitochondrial proteins in both general and specific cellular processes, in this case, the initial steps of steroidogenesis.
Our data demonstrate that the KH motif and the last 325 amino acid residues of AKAP1 bind to the 3′-UTR of STAR mRNA in vitro with binding affinities that are comparable to those previously reported for single KH motifs (13, 16). We determined that unpaired pyrimidine-rich RNA sequences bind to the KH motif of AKAP1. However, the primary RNA sequences are not sufficient for optimal binding, and a stable secondary structure is required. Paradoxically, most of the sequences within the 3′-UTR of STAR mRNA identified as potential binding sites for the KH motif of AKAP1 (Table 1) are not unpaired but are involved in the formation of the stem segment of the secondary structure that may hinder the interaction. However, the five-nt-long 5′-UCUUA-3′ sequence located at 3019–3023 of the mouse 3′-UTR of Star mRNA is unpaired. This sequence is located in close vicinity to the AU-rich element previously identified to be involved in the down-regulation of Star mRNA expression through its association with TIS11b (18). These findings suggest that multiple protein factors may participate in the modulation of the binding of Star mRNA to the KH motif of AKAP1 in vivo either through direct interaction with the binding sites or through modulation of the secondary structure of the mRNA.
It has been reported (17, 38) that the phosphorylation of Ser585 located at the consensus PKA binding site (R582YVS585) within the KH motif of AKAP1 increases the affinity of the motif for RNA. However, our results do not support this observation (Fig. 2). Homology modeling of the KH motif of AKAP1 on the crystal structure of the KH3 motif of Nova-2 protein (14) (Fig. 5) agrees with our experimental observation that the S585D mutant has a negative or inconsequential impact on RNA binding. The homology model reveals that Ser585 is located in the middle of the second α-helix of the motif and is likely involved in the formation of the nucleic acid-binding hydrophobic groove. The phosphorylation of the Ser585 would 1) alter the electrostatic potential of the binding pocket, which would hinder the interactions with the negatively charged nucleic acid, and 2) impose steric constraints on the binding of the nucleic acid (Fig. 5B). Similar conclusions, albeit to a smaller extent, are also valid for the S585D mutant of the KH motif (Fig. 5B). However, we do not exclude the possibility that S585D substitution does not act as a phosphomimetic mutant, which might explain the similar binding affinity for the WT and S585D mutant proteins.
We also determined that AKAP1, through its Tudor/TSN domain and Ago2, which is part of a complex known as RISC, form a direct protein-protein interaction (Fig. 4C). This interaction is not mediated by nucleic acid (Figs. 4, C and D). AKAP1 may either interact with cellular P-bodies, reported recently to be transiently associated with mitochondria (41), or with the Ago2 protein, which is directly associated with the outer mitochondrial membrane (40), or both. This finding further supports the bioinformatic observation that the Tudor domain of AKAP1 is likely organized as a TSN domain. The EMSA results using the purified MBP-Tudor domain of AKAP1 also indicated that the Tudor/TSN domain possessed weak RNase activity (Fig. 2, B and D), as previously observed for an Escherichia coli-expressed and purified TSN domain (42). In addition, a recent article demonstrates that natural antisense transcripts of the Star mRNA are involved in the regulation of STAR expression (43). Therefore, degradation of Star mRNA by the RISC coupled with the involvement of the Tudor/TSN domain of AKAP1 would ensure that a single mRNA is translated only once and is immediately degraded. By these actions, an acute and precise regulation of the synthesis of steroid hormones would be achieved and would agree with many previous observations, including those that demonstrated the need for ongoing transcription and translation in the maintenance of steroidogenesis. The exact nature of such a process would require further experimentation.
We would like to propose the following cotranslational import model for STAR expression, a scheme that has been described for other nuclear-encoded mitochondrial protein mRNAs as reported by other investigators including Ahmed and Fisher (44), Saint-Georges et al. (45), Garcia et al. (46, 47), and Eliyahu et al. (48). Those investigators presented models in which nuclear-encoded mitochondrial proteins could be either posttranslationally or cotranslationally imported into mitochondria. Slightly more than half of nuclear-encoded mitochondrial proteins are cotranslationally imported. Using the cotranslational model, nuclear-encoded mRNA having proteins localized to the mitochondria can be segregated into two different groups: 1) mRNAs localizing to the close vicinity of the mitochondria and requiring interaction with mitochondrial anchored RNA-binding protein and 2) mRNAs colocalizing at the mitochondria without requiring mediation by an RNA-binding protein. In this study, we show that the KH motif of AKAP1 interacts with pyrimidine-rich sequences and possesses affinity for the 3′-UTR of Star mRNA in vitro and interacts with it in vivo. Previous observations from our laboratory demonstrate that small interfering RNA-mediated knockdown of AKAP1 abrogated STAR protein synthesis (7) but did not alter the steady-state level of the Star mRNA. This suggests that one of AKAP1's functions is to aid in the efficient expression of the STAR protein. Because the steady-state level of Star mRNA does not change after AKAP1 knockdown, it is plausible that AKAP1, through its KH motif, tethers Star mRNA at the outer mitochondrial membrane. More than one AKAP1 protein may be involved in this action, because the KH motif recognizes low-complexity RNA sequences. Given our current results, we favor the cotranslational mechanism for STAR protein import that involves the use of AKAP1 as a mitochondrial scaffold protein anchoring the STAR mRNA to the outer mitochondrial membrane.
However, we are aware that at present we lack observations that unequivocally demonstrate the localization of the Star mRNA at the mitochondria under conditions that preserve the morphology of the cell, e.g. fluorescent in situ hybridization. To our knowledge, this relationship between Star mRNA and mitochondria has not yet been shown. Our own attempts to measure the distance between Star mRNA and mitochondria using double fluorescent in situ hybridization were not successful, despite using a previously published protocol (49) designed for making such measurements.
This proposed model provides an additional, and somewhat unique, mechanism for controlling the acute synthesis of steroid hormones, with that control being at the level of translation of the STAR protein in the vicinity of the mitochondria. In general, it is believed that once nuclear-encoded mitochondrial protein mRNAs are transcribed, they will quickly and automatically be translated and the proteins imported into the mitochondria. Several reports (48, 50–52) demonstrate that more than half of the nuclear-encoded mitochondrial mRNAs in yeast are localized in the close proximity of the outer mitochondrial membrane. This strongly suggests that an additional posttranscriptional step regulating the expression of mitochondrial proteins exists. This process may involve the translocase complex of the mitochondria as well as additional RNA-binding proteins that have been shown to further influence the localization, stability, and translation of the transcripts of the majority of the nuclear-encoded mitochondrial mRNAs at the mitochondria (53). It is possible that STAR expression, and thus steroidogenesis, is also influenced by this mechanism.
Acknowledgments
We thank Drs. Jeffrey A. Chao, Matthew T. Dyson, Luchezar K. Karagyozov, Steven R. King, Clinton C. MacDonald, and R. Bryan Sutton for helpful discussions. In addition, we thank Drs. Chao and MacDonald for valuable suggestions on the manuscript. We also thank Kerry Fuson and Kamakshi Nayak for technical support in the protein buffer exchange. We thank Dr. Barry Maurer for the use of the Pharos FX scanner and Dr. Sutton for introducing the phosphate group at the Ser585 of the homology model of the KH motif. We also thank Dr. Raul Martínez-Zaguilán for help with the microscopic imaging.
This work was supported by National Institutes of Health Grant HD-17481 and with funds from the Robert A. Welch Foundation (Grant B1-0028).
Disclosure Summary: The authors have no disclosures to make.
Footnotes
- Ago2
- Argonaute 2
- AKAP1
- A-kinase anchoring protein 1
- cv
- column volume
- DNase
- deoxyribonuclease
- KH
- K-homology
- IPTG
- isopropyl-β-D-1-thiogalctopyranoside
- MBP
- maltose-binding protein
- NFM
- nonfat milk
- nt
- nucleotides
- PKA
- protein kinase A
- RISC
- RNA-induced silencing complex
- RNase
- ribonuclease
- SDS
- sodium dodecyl sulfate
- SELEX
- systematic evolution of ligands by exponential enrichment
- STAR
- steroidogenic acute regulatory
- TIS11b
- tetradecandoyl phorbol acetate-inducible sequence 11b
- TOMM20
- translocase of outer mitochondrial membrane 20 homolog
- TSN
- Tudor staphylococcal nuclease-like
- UTR
- untranslated region.
References
- 1. King SR, Bhangoo A, Stocco DM. 2011. Functional and physiological consequences of StAR deficiency: role in lipoid congenital adrenal hyperplasia. Endocr Dev 20:47–53 [DOI] [PubMed] [Google Scholar]
- 2. Miller WL, Bose HS. 2011. Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res 52:2111–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stocco DM, Clark BJ. 1996. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev 17:221–244 [DOI] [PubMed] [Google Scholar]
- 4. Lavoie HA, King SR. 2009. Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B. Exp Biol Med (Maywood) 234:880–907 [DOI] [PubMed] [Google Scholar]
- 5. Stocco DM, Wang X, Jo Y, Manna PR. 2005. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol 19:2647–2659 [DOI] [PubMed] [Google Scholar]
- 6. Ascoli M, Fanelli F, Segaloff DL. 2002. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev 23:141–174 [DOI] [PubMed] [Google Scholar]
- 7. Dyson MT, Jones JK, Kowalewski MP, Manna PR, Alonso M, Gottesman ME, Stocco DM. 2008. Mitochondrial A-kinase anchoring protein 121 binds type II protein kinase A and enhances steroidogenic acute regulatory protein-mediated steroidogenesis in MA-10 mouse Leydig tumor cells. Biol Reprod 78:267–277 [DOI] [PubMed] [Google Scholar]
- 8. Dyson MT, Kowalewski MP, Manna PR, Stocco DM. 2009. The differential regulation of steroidogenic acute regulatory protein-mediated steroidogenesis by type I and type II PKA in MA-10 cells. Mol Cell Endocrinol 300:94–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Q, Lin RY, Rubin CS. 1997. Organelle-specific targeting of protein kinase AII (PKAII). Molecular and in situ characterization of murine A kinase anchor proteins that recruit regulatory subunits of PKAII to the cytoplasmic surface of mitochondria. J Biol Chem 272:15247–15257 [DOI] [PubMed] [Google Scholar]
- 10. Huang LJ, Durick K, Weiner JA, Chun J, Taylor SS. 1997. Identification of a novel protein kinase A anchoring protein that binds both type I and type II regulatory subunits. J Biol Chem 272:8057–8064 [DOI] [PubMed] [Google Scholar]
- 11. Carlucci A, Lignitto L, Feliciello A. 2008. Control of mitochondria dynamics and oxidative metabolism by cAMP, AKAPs and the proteasome. Trends Cell Biol 18:604–613 [DOI] [PubMed] [Google Scholar]
- 12. Du Z, Lee JK, Fenn S, Tjhen R, Stroud RM, James TL. 2007. X-ray crystallographic and NMR studies of protein-protein and protein-nucleic acid interactions involving the KH domains from human poly(C)-binding protein-2. RNA 13:1043–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jensen KB, Musunuru K, Lewis HA, Burley SK, Darnell RB. 2000. The tetranucleotide UCAY directs the specific recognition of RNA by the Nova K-homology 3 domain. Proc Natl Acad Sci USA 97:5740–5745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lewis HA, Musunuru K, Jensen KB, Edo C, Chen H, Darnell RB, Burley SK. 2000. Sequence-specific RNA binding by a Nova KH domain: implications for paraneoplastic disease and the fragile X syndrome. Cell 100:323–332 [DOI] [PubMed] [Google Scholar]
- 15. Teplova M, Malinina L, Darnell JC, Song J, Lu M, Abagyan R, Musunuru K, Teplov A, Burley SK, Darnell RB, Patel DJ. 2011. Protein-RNA and protein-protein recognition by dual KH1/2 domains of the neuronal splicing factor Nova-1. Structure 19:930–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Valverde R, Edwards L, Regan L. 2008. Structure and function of KH domains. FEBS J 275:2712–2726 [DOI] [PubMed] [Google Scholar]
- 17. Ginsberg MD, Feliciello A, Jones JK, Avvedimento EV, Gottesman ME. 2003. PKA-dependent binding of mRNA to the mitochondrial AKAP121 protein. J Mol Biol 327:885–897 [DOI] [PubMed] [Google Scholar]
- 18. Duan H, Cherradi N, Feige JJ, Jefcoate C. 2009. cAMP-dependent posttranscriptional regulation of steroidogenic acute regulatory (STAR) protein by the zinc finger protein ZFP36L1/TIS11b. Mol Endocrinol 23:497–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clark BJ, Pezzi V, Stocco DM, Rainey WE. 1995. The steroidogenic acute regulatory protein is induced by angiotensin II and K+ in H295R adrenocortical cells. Mol Cell Endocrinol 115:215–219 [DOI] [PubMed] [Google Scholar]
- 20. Grozdanov PN, Roy S, Kittur N, Meier UT. 2009. SHQ1 is required prior to NAF1 for assembly of H/ACA small nucleolar and telomerase RNPs. RNA 15:1188–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chao JA, Patskovsky Y, Patel V, Levy M, Almo SC, Singer RH. 2010. ZBP1 recognition of β-actin zipcode induces RNA looping. Genes Dev 24:148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. 2003. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31:3784–3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clark BJ, Wells J, King SR, Stocco DM. 1994. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 269:28314–28322 [PubMed] [Google Scholar]
- 24. Pagano JM, Clingman CC, Ryder SP. 2011. Quantitative approaches to monitor protein-nucleic acid interactions using fluorescent probes. RNA 17:14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ryder SP, Recht MI, Williamson JR. 2008. Quantitative analysis of protein-RNA interactions by gel mobility shift. Methods Mol Biol 488:99–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tuerk C, Gold L. 1990. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510 [DOI] [PubMed] [Google Scholar]
- 27. Grozdanov PN, Petkov PM, Karagyozov LK, Dabeva MD. 2006. Expression and localization of PCSK9 in rat hepatic cells. Biochem Cell Biol 84:80–92 [DOI] [PubMed] [Google Scholar]
- 28. Guex N, Peitsch MC, Schwede T. 2009. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis 30(Suppl 1):S162–S173 [DOI] [PubMed] [Google Scholar]
- 29. Guex N, Peitsch MC. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723 [DOI] [PubMed] [Google Scholar]
- 30. Bordoli L, Kiefer F, Arnold K, Benkert P, Battey J, Schwede T. 2009. Protein structure homology modeling using SWISS-MODEL workspace. Nat Protoc 4:1–13 [DOI] [PubMed] [Google Scholar]
- 31. Arnold K, Bordoli L, Kopp J, Schwede T. 2006. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201 [DOI] [PubMed] [Google Scholar]
- 32. Cardone L, de Cristofaro T, Affaitati A, Garbi C, Ginsberg MD, Saviano M, Varrone S, Rubin CS, Gottesman ME, Avvedimento EV, Feliciello A. 2002. A-kinase anchor protein 84/121 are targeted to mitochondria and mitotic spindles by overlapping amino-terminal motifs. J Mol Biol 320:663–675 [DOI] [PubMed] [Google Scholar]
- 33. Ma Y, Taylor SS. 2008. A molecular switch for targeting between endoplasmic reticulum (ER) and mitochondria: conversion of a mitochondria-targeting element into an ER-targeting signal in DAKAP1. J Biol Chem 283:11743–11751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bolender N, Sickmann A, Wagner R, Meisinger C, Pfanner N. 2008. Multiple pathways for sorting mitochondrial precursor proteins. EMBO Rep 9:42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bryson K, McGuffin LJ, Marsden RL, Ward JJ, Sodhi JS, Jones DT. 2005. Protein structure prediction servers at University College London. Nucleic Acids Res 33:W36–W38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Friberg A, Corsini L, Mourão A, Sattler M. 2009. Structure and ligand binding of the extended Tudor domain of D. melanogaster Tudor-SN. J Mol Biol 387:921–934 [DOI] [PubMed] [Google Scholar]
- 37. Liu H, Wang JY, Huang Y, Li Z, Gong W, Lehmann R, Xu RM. 2010. Structural basis for methylarginine-dependent recognition of Aubergine by Tudor. Genes Dev 24:1876–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rogne M, Stokka AJ, Taskén K, Collas P, Küntziger T. 2009. Mutually exclusive binding of PP1 and RNA to AKAP149 affects the mitochondrial network. Human molecular genetics 18:978–987 [DOI] [PubMed] [Google Scholar]
- 39. Zuker M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31:3406–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bandiera S, Rüberg S, Girard M, Cagnard N, Hanein S, Chrétien D, Munnich A, Lyonnet S, Henrion-Caude A. 2011. Nuclear outsourcing of RNA interference components to human mitochondria. PloS one 6:e20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang L, Mollet S, Souquere S, Le Roy F, Ernoult-Lange M, Pierron G, Dautry F, Weil D. 2011. Mitochondria associate with P-bodies and modulate microRNA-mediated RNA interference. J Biol Chem 286:24219–24230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li CL, Yang WZ, Chen YP, Yuan HS. 2008. Structural and functional insights into human Tudor-SN, a key component linking RNA interference and editing. Nucleic Acids Res 36:3579–3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Castillo AF, Fan J, Papadopoulos V, Podestá EJ. 2011. Hormone-dependent expression of a steroidogenic acute regulatory protein natural antisense transcript in MA-10 mouse tumor Leydig cells. PloS one 6:e22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ahmed AU, Fisher PR. 2009. Import of nuclear-encoded mitochondrial proteins: a cotranslational perspective. Int Rev Cell Mol Biol 273:49–68 [DOI] [PubMed] [Google Scholar]
- 45. Saint-Georges Y, Garcia M, Delaveau T, Jourdren L, Le Crom S, Lemoine S, Tanty V, Devaux F, Jacq C. 2008. Yeast mitochondrial biogenesis: a role for the PUF RNA-binding protein Puf3p in mRNA localization. PloS one 3:e2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Garcia M, Darzacq X, Delaveau T, Jourdren L, Singer RH, Jacq C. 2007. Mitochondria-associated yeast mRNAs and the biogenesis of molecular complexes. Mol Biol Cell 18:362–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garcia M, Delaveau T, Goussard S, Jacq C. 2010. Mitochondrial presequence and open reading frame mediate asymmetric localization of messenger RNA. EMBO Rep 11:285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eliyahu E, Pnueli L, Melamed D, Scherrer T, Gerber AP, Pines O, Rapaport D, Arava Y. 2010. Tom20 mediates localization of mRNAs to mitochondria in a translation-dependent manner. Mol Cell Biol 30:284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jourdren L, Delaveau T, Marquenet E, Jacq C, Garcia M. 2010. CORSEN, a new software dedicated to microscope-based 3D distance measurements: mRNA-mitochondria distance, from single-cell to population analyses. RNA 16:1301–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Devaux F, Lelandais G, Garcia M, Goussard S, Jacq C. 2010. Posttranscriptional control of mitochondrial biogenesis: spatio-temporal regulation of the protein import process. FEBS Lett 584:4273–4279 [DOI] [PubMed] [Google Scholar]
- 51. Eliyahu E, Melamed D, Arava Y. 2011. Genome-wide analysis of RNA extracted from isolated mitochondria. Methods Mol Biol 714:287–299 [DOI] [PubMed] [Google Scholar]
- 52. Gadir N, Haim-Vilmovsky L, Kraut-Cohen J, Gerst JE. 2011. Localization of mRNAs coding for mitochondrial proteins in the yeast Saccharomyces cerevisiae. RNA 17:1551–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Quenault T, Lithgow T, Traven A. 2011. PUF proteins: repression, activation and mRNA localization. Trends Cell Biol 21:104–112 [DOI] [PubMed] [Google Scholar]