Abstract
The mechanisms by which the cervix remains closed during the massive uterine expansion of pregnancy are unknown. IL-8 is important for recruitment of immune cells into the cervical stroma, matrix remodeling, and dilation of the cervix during labor. Previously, we have shown that several cytokine genes transcriptionally repressed in the cervix during gestation are activated during cervical ripening and dilation. IL-8 gene expression is repressed in cervical stromal cells during pregnancy by the transcription factor microphthalmia-associated transcription factor (MiTF-CX). Here, we tested the hypothesis that hypoxia and the transcription factor hypoxia inducible factor-1α (HIF-1α) may regulate MiTF-CX and cervical ripening. Using tissues from women during pregnancy before and after cervical ripening, we show that, during cervical ripening, HIF-1α was stabilized and relocalized to the nucleus. Further, we found that hypoxia and two hypoxia mimetics that stabilize HIF-1α activated the transcriptional repressor differentiated embryo chondrocyte-expressed gene 1, which bound to sites in the MiTF-CX promoter crucial for its positive autoregulation. Ectopic overexpression of MiTF-CX abrogated hypoxia-induced up-regulation of IL-8 gene expression. We also show that activation of HIF-1α induced cyclooxygenase-2 and that prostaglandin E2 repressed MiTF-CX. We conclude that hypoxia and stabilization of the transcription factor HIF-1α result in up-regulation of differentiated embryo chondrocyte-expressed gene 1, loss of MiTF, and absence of MiTF binding to the IL-8 promoter, which in turn leads to up-regulation of IL-8 gene expression. Hypoxia also up-regulated cyclooxygenase-2, leading to prostaglandin E2-mediated loss of MiTF in cervical stromal cells. The results support a pivotal role for hypoxia and HIF-1α in the cervical ripening process during pregnancy.
The uterine cervix is a rigid closed structure during early gestation that undergoes progressive matrix remodeling during pregnancy. During most of gestation, the cervix, although soft, remains closed maintaining pregnancy until term. During late gestation, it undergoes a significant remodeling process termed cervical ripening, characterized by disorganization and dispersion of the collagen matrix and an increase in infiltration of immune cells (1, 2). In species other than humans, old world monkeys, and guinea pigs, cervical ripening and dilation are preceded by progesterone withdrawal. Nevertheless, in all species, progesterone levels in blood or tissue remain greater than the dissociation constant (Kd) of progesterone for its nuclear hormone receptors. In women, progesterone levels do not fall. Thus, it has been difficult to ascertain the cellular and molecular mechanisms that lead to cervical ripening and dilation. This is particularly important, because preterm shortening and ripening of the cervix is a major risk factor for preterm birth (3).
Previous work indicates that cervical ripening is brought about by a complex hormone signaling cascade that culminates in 1) increased expression of prostaglandin endoperoxide synthase 2 [cyclooxygenase-2 (COX-2)] (4); 2) simultaneous decreases in 15-hydroxyprostaglandin-dehydrogenase (PGDH), thereby leading to increased prostaglandin E2 (PGE2) synthesis (5, 6); and 3) increased number and activation of infiltrating immune cells, which, in humans, are believed to be mediated predominantly by the proinflammatory cytokine, IL-8. The cellular and molecular mechanisms preceding infiltration of immune cells and increased prostaglandin biosynthesis are unknown. Previously, we identified and cloned a transcription factor expressed in human cervical stromal cells [microphthalmia-associated transcription factor (MiTF-CX)] that suppresses mRNA and protein synthesis of IL-8 (7). In the cervix, MiTF-CX positively regulates its own gene expression but suppresses transcription of IL-8 by binding to specific E-boxes in the promoter regions of respective genes (7). The dramatic loss of cervical MiTF during human cervical ripening and dilation thereby leads to increased expression of IL-8 mRNA and protein. The molecular mechanisms that initiate loss of this important progestational transcription factor in the cervix are not known.
Isoforms of MiTF differ in their amino terminus and are expressed in a tissue-specific manner due to alternative promoters. The melanoma-associated isoform of MiTF and other isoforms expressed in mast cells are negatively regulated by hypoxia and hypoxia mimetics through differentiated embryo chondrocyte-expressed gene 1 (DEC1), a known hypoxia inducible factor-1α (HIF-1α)-induced transcriptional repressor (8). Here, we tested the hypothesis that hypoxia, or two hypoxia mimetics that activate HIF-1α [i.e. cobalt(II) chloride hexahydrate (CoCl2) (9) and deferoxamine mesylate (DFO)], regulates MiTF-CX in cervical stromal cells. Our results indicate that stabilization and nuclear relocation of HIF-1α led to decreased MiTF-CX mRNA and protein levels, which was required for hypoxia-induced up-regulation of IL-8 gene expression. Gene expression analysis and immunohistochemistry data confirmed activation of HIF-1α in cervical stromal tissues obtained from women in labor together with increases in DEC1. Further, hypoxia-increased COX-2 gene expression and treatment of stromal cells with PGE2 also resulted in loss of MiTF-CX. Hypoxia-induced up-regulation of COX-2 was not crucial for its effect on MiTF-CX or IL-8 gene expression. Overall, these studies indicate that HIF-1α may play an important role in the initiation of a cascade of gene regulatory events leading to cervical ripening and dilation at term.
Results
Hypoxia suppresses MiTF-CX gene expression
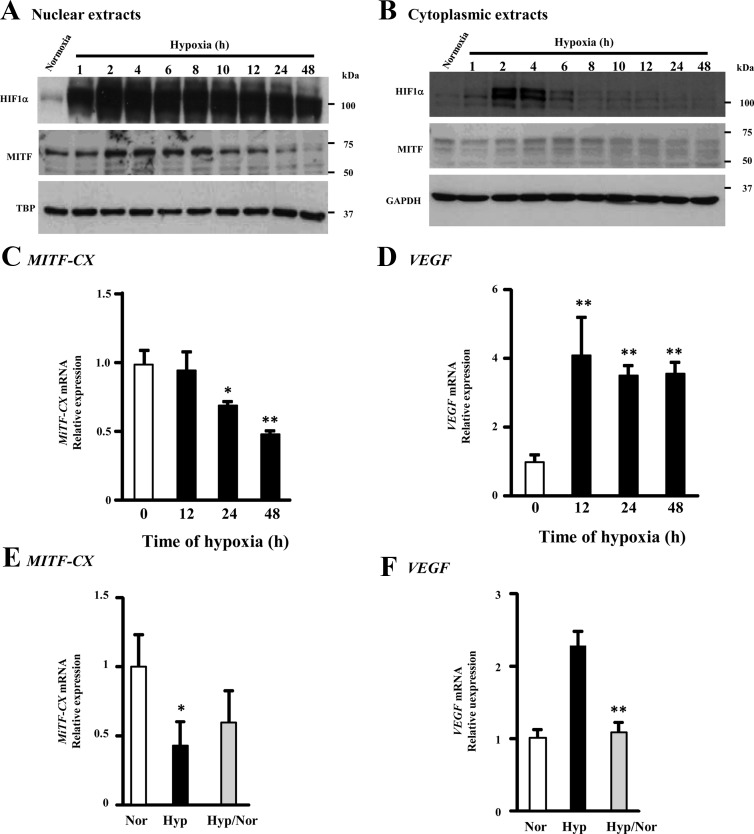
Previously, we found that MiTF-CX gene expression is decreased significantly in cervical stromal cells during labor (7). To determine whether MiTF-CX was regulated by hypoxia in the cervix, cervical stromal cells were incubated under hypoxic conditions (2% O2) for various times, and MiTF-CX and HIF-1α were quantified in nuclear and cytoplasmic extracts (Fig. 1). As expected, HIF-1α levels increased in the nucleus compared with normoxic conditions (Fig. 1A). In contrast, nuclear MiTF-CX decreased approximately 80% by 48 h compared with normoxia (Fig. 1A). Western blot analysis of cytoplasmic extracts revealed little change in MiTF-CX protein levels (Fig. 1B). To determine whether hypoxia-induced down-regulation of MiTF-CX was accompanied by loss of MiTF-CX mRNA, MiTF-CX transcripts were quantified in stromal cells under normoxic and hypoxic conditions for various times (Fig. 1C). MiTF-CX mRNA levels decreased time dependently after initiation of hypoxia with 50% reduction by 48 h. As a positive control, we also quantified mRNA levels of the hypoxia-responsive gene, VEGF, which is known to be up-regulated under hypoxic condition. VEGF gene expression was up-regulated 4-fold under hypoxic conditions (Fig. 1D). To determine whether this altered gene expression profile is reversible, cervical stromal cells were incubated under hypoxic conditions for 36 h followed by incubation under normoxic conditions for 12 h. Thereafter, mRNA levels of MiTF-CX and vascular endothelial growth factor (VEGF) were quantified and compared with that in cells incubated either under normoxic conditions or hypoxic conditions throughout the experiment. Results indicated that hypoxia-induced changes in VEGF gene expression were reversible but that hypoxia-induced changes in MiTF-CX were not as sensitive to reversal by reoxygenation (Fig. 1, E and F).
Fig. 1.
Hypoxia down-regulates MiTF-CX gene expression. A and B, Cervical stromal cells were incubated in either 20% O2 (normoxia) or 2% O2 (hypoxia) for 1–48 h. Nuclear and cytoplasmic extracts were analyzed for HIF-1α and MiTF-CX expression levels by immunoblot analysis. TBP and GAPDH were used as loading controls for nuclear and cytoplasmic extracts, respectively. C and D, Cervical stromal cells were incubated under normoxic (0) or hypoxic conditions for 12, 24, or 48 h. Thereafter, mRNA levels of MiTF-CX (C) and VEGF (D) were quantified using qPCR. Data were normalized to GAPDH and represent mean ± sd of three samples in each group. *, P < 0.01; **, P < 0.001 compared with 0-h time point. E and F, Cervical stromal cells were incubated in hypoxic conditions for 36 h followed by incubation under normoxic conditions for 12 h (Hyp/Nor). Thereafter, mRNA levels of MiTF-CX (E) or VEGF (F) were quantified and compared with cells either incubated under normoxic (Nor) or hypoxic (Hyp) conditions. Data were normalized to GAPDH and represent mean ± sd of three samples in each group. *, P = 0.03; **, P < 0.001 compared with Nor.
Hypoxia mimetics that activate HIF-1α down-regulate MiTF-CX gene expression
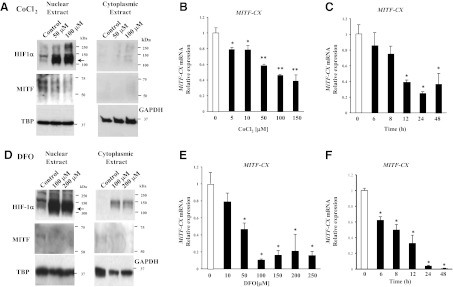
To determine the mechanism of hypoxia-induced down-regulation of MiTF-CX, we tested the hypothesis that the transcription factor, HIF-1α, mediates loss of MiTF-CX in cervical stromal cells. Stromal cells were treated with vehicle or the hypoxia mimetics, CoCl2, and DFO to activate HIF-1α in cells even in the presence of oxygen (9). Cytoplasmic and nuclear extracts from stromal cells treated with either vehicle or CoCl2 (50 or 100 μm) or DFO (100 or 200 μm) for 24 h were analyzed for HIF-1α and MiTF-CX using immunoblotting. As expected, CoCl2 and DFO treatment resulted in activation and nuclear localization of HIF-1α (Fig. 2, A and D). Concomitantly, MiTF-CX protein levels were reduced significantly by CoCl2/DFO treatment (Fig. 2, A and D). CoCl2/DFO treatment also resulted in dose-dependent (Fig. 2, B and E) and time-dependent (Fig. 2, C and F) decreases in MiTF-CX mRNA.
Fig. 2.
Hypoxia mimetics down-regulates MiTF-CX gene expression. A, Cervical stromal cells were treated with either vehicle (control) or CoCl2 (50 or 100 μm) for 24 h. Nuclear and cytoplasmic extracts were prepared analyzed for HIF-1α and MiTF-CX using immunoblot analysis. TBP and GAPDH were used as loading controls for nuclear and cytoplasmic extracts, respectively. B and C, Cervical stromal cells were treated with increasing concentrations of CoCl2 from 5 to 150 μm for 24 h (B) or 150 μm as a function of time (C). MiTF-CX mRNA levels were quantified and normalized to GAPDH. Data represent mean ± sd of three samples in each group and represent three experiments conducted in three-cell preps with consistent results. *, P < 0.005; **, P < 0.0001 compared with untreated cells. D, Cervical stromal cells were treated with either vehicle (control) or DFO (100 or 200 μm) for 24 h. Nuclear and cytoplasmic extracts were prepared analyzed for HIF-1α and MiTF-CX using immunoblot analysis. E and F, Cervical stromal cells were treated with increasing concentrations of DFO from 10 to 250 μm for 24 h (E) or 200 μm as a function of time (F). MiTF-CX mRNA levels were quantified and normalized to GAPDH. Data represent mean ± sd of three samples in each group and represent three experiments conducted in three-cell preps with consistent results. *, P < 0.0001 compared with untreated cells.
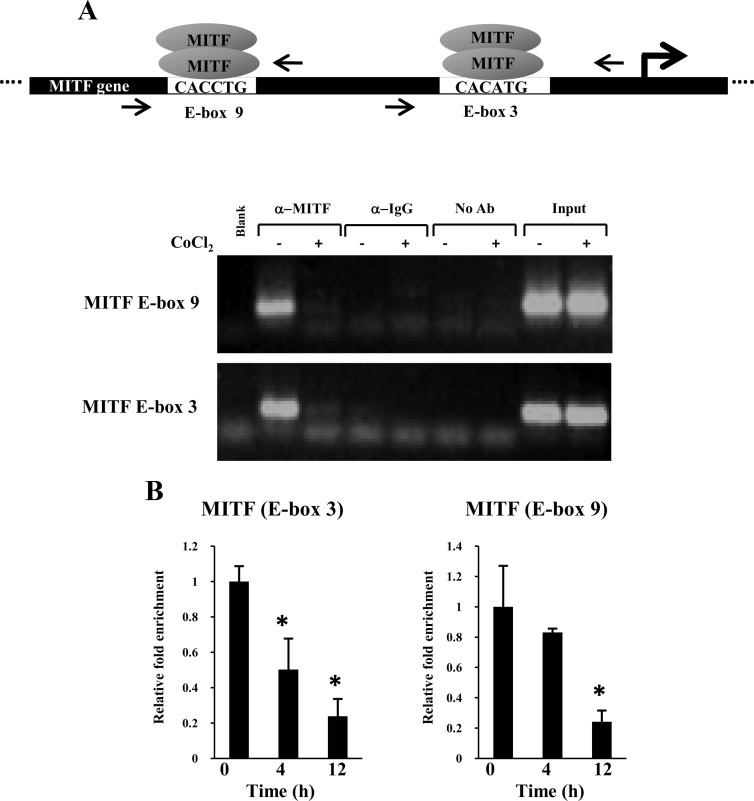
Previously, we reported that the MiTF-CX gene harbors multiple MiTF-CX binding sites (E-boxes) in its promoter and that MiTF-CX positively regulates its own gene expression through two E-box motifs, 3 (−323 to −316) and 9 (−977 to −972). To gain insight into the mechanisms by which hypoxia negatively regulates MiTF-CX, cervical stromal cells were treated with vehicle or CoCl2 (200 μm) for 48 h. Thereafter, MiTF-CX binding to its own promoter was analyzed using chromatin immunoprecipitation (ChIP) analysis. Sonicated chromatin extracts were immunoprecipitated with antibodies against MiTF-CX. Thereafter, sequence-specific amplification (conventional PCR) of regions that include MiTF binding sites (i.e. E-boxes 3 and 9) were compared (Fig. 3A). Although both E-boxes were amplified in control resting cells, amplification was absent in cells immunoprecipitated with either IgG or no antibody controls (Fig. 3A), indicating MiTF antibody specificity. Sequence-specific amplification was absent in sonicated chromatin extracts from CoCl2-treated samples. Thus, CoCl2-induced pseudohypoxia resulted in reduced occupancy of MiTF-CX to its own promoter and thereby would reduce MiTF-CX-induced expression of the MiTF-CX gene. Loss of MiTF-CX was not observed until 12–24 h after induction of hypoxia. To determine whether decreased binding of MiTF-CX occurred before loss of the transcription factor in cells, real-time ChIP assays were conducted at 4 and 24 h (Fig. 3B). Although binding to E-box 9 was reduced marginally, binding of MiTF-CX to E-box 3 was decreased substantially at 4 h (Fig. 3B). MiTF-CX binding to both E-boxes was reduced 75% after 24 h of treatment with CoCl2. These results suggest that E-box 3 may be more sensitive to hypoxia than E-box 9 and that hypoxia mediates decreased expression of MiTF-CX first by decreasing binding to its own promoter, which thereby leads to decreased expression through loss of its positive autoregulatory loop.
Fig. 3.
CoCl2 decreases MiTF binding to its own promoter. A, ChIP analysis show that MiTF-CX was associated with MiTF-CX E-box 9 and E-box 3 in untreated cells but not in cervical stromal cells treated with 200 μm CoCl2 for 24 h. Equal input of DNA from cells treated with and without CoCl2 was ensured by PCR amplification of DNA extracted from 1/100th of chromatin used for immunoprecipitation of transcription factor-bound DNA (input). Amplification was absent if MiTF antibodies were substituted with IgG or no antibody. B, Cervical stromal cells were treated with CoCl2 (250 μm) for either 4 or 12 h followed by ChIP analysis and real-time quantitative PCR. *, P < 0.05 compared with time 0.
Hypoxia induces the MiTF repressor protein DEC1
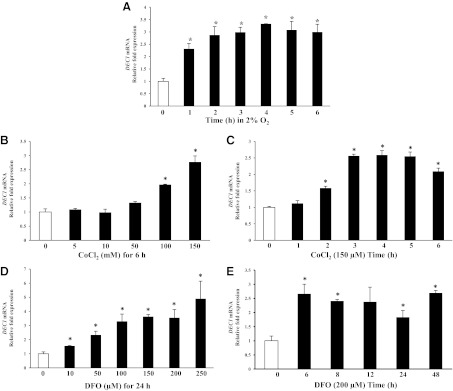
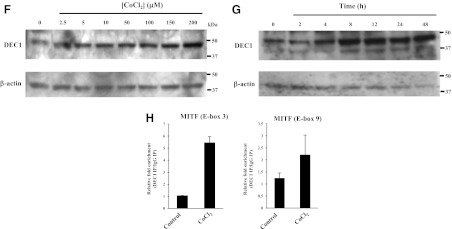
To determine whether loss of MiTF-CX binding to its own promoter was due to HIF-1α-MiTF binding interactions, MiTF-CX-expressing cervical stromal cells were immunoprecipitated with MiTF antibodies and thereafter examined for HIF-1α protein. Using a variety of conditions, direct HIF-1α-MiTF interactions were not detected (data not shown). This finding is not surprising, because MiTF has been shown to dimerize only with members of its own gene family restricted to transcription factor for immunoglobulin heavy-chain enhancer (TFE)3, TFEb, and TFEc. Previous studies have shown that a transcriptional repressor, DEC1, negatively regulates MiTF in various cell lines under hypoxic conditions (8). To determine whether DEC1 is activated by HIF-1α in cervical stromal cells, DEC1 gene expression was quantified after incubating cells under hypoxic condition or treatment with either CoCl2 or DFO. DEC1 mRNA was increased significantly upon exposure to hypoxic conditions (Fig. 4A) or after treatment with the hypoxia mimetics, CoCl2 and DFO, in a dose-dependent (Fig. 4, B and D) and time-dependent (Fig. 4, C and E) manner. Protein levels of DEC1 were also increased after treatment with increasing concentrations of CoCl2 in a time-dependent manner (Fig. 4, F and G). Because DEC1 DNA binding sites are similar to those of MiTF, real-time ChIP analysis was conducted to determine whether DEC1 binds to the same sites as MiTF (E-boxes 3 and 9). Results show that treatment of cervical stromal cells with CoCl2 for 24 h resulted in increased DEC1 binding to MiTF E-box 3 (5.5-fold compared with untreated cells). Binding of DEC1 to MiTF E-box 9 was not detected (Fig. 4H).
Fig. 4.
Hypoxia and hypoxia mimetics induce DEC1 gene expression in cervical stromal cells. A, Cervical stromal cells were incubated under normoxic (0) or hypoxic conditions for different time points. Thereafter, mRNA levels of DEC1 were quantified using qPCR. Data were normalized to GAPDH and represent mean ± sd of three samples in each group. *, P < 0.02 compared with 0-h time point. B and D, Cervical stromal cells were treated with increasing concentrations of CoCl2 from 5 to 150 μm or DFO from 10 to 250 μm for 24 h. DEC1 mRNA levels were quantified and normalized to GAPDH. Data represent mean ± sd of three samples in each group. *, P < 0.009 compared with untreated cells. C and E, Cervical stromal cells were treated with CoCl2 (100 μm) or DFO (200 μm) for different times as indicated. Thereafter, DEC1 mRNA was quantified using qPCR and normalized to GAPDH. Data represent mean ± sd of three samples in each group. *, P < 0.008 compared with untreated cells. F, Whole-cell protein extracts from cervical stromal cells treated with different concentrations of CoCl2 for 24 h were analyzed using immunoblotting with DEC1 antibodies. β-Actin served as a loading control. G, Whole-cell protein lysates prepared from cervical stromal cells treated with CoCl2 (150 μm) for various times were analyzed by Western blotting using DEC1 and β-actin antibodies. H, ChIP analysis of DEC1 binding to MiTF-CX regulatory E-boxes. Cells were treated with control or 200 μm CoCl2 for 24 h. Sonicated chromatin was immunoprecipitated with DEC1 antibody or IgG. Results represent relative fold enrichment of DEC1/IgG normalized by input used of three different comparisons. IP, Immuno pull-down.
HIF-1α is induced and relocalized to the nucleus in cervical stromal, epithelial, and endothelial cells during labor in vivo
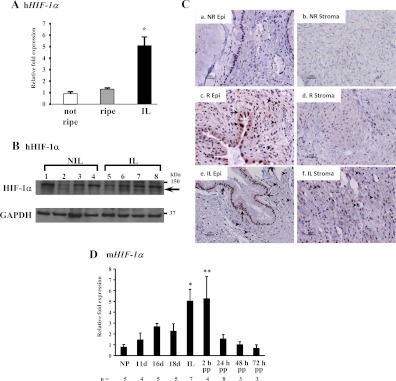
After establishing that hypoxia mediates repression of MiTF-CX gene expression in cervical stromal cells in culture, next, we quantified mRNA levels and examined nuclear localization of HIF-1α in cervical stromal tissues from women before and after cervical ripening and dilation during labor. Interestingly, HIF-1α mRNA was increased marginally in stromal tissues during ripening but before dilation and labor. In contrast, HIF-1α gene expression was increased significantly (5-fold) in tissues obtained from women in labor (Fig. 5A). Although immunoblot analysis of tissue lysates revealed moderate increases in HIF-1α protein (Fig. 5B), immunohistochemistry revealed dramatic changes in localization of HIF-1α in cervical epithelial, stromal, and endothelial cells in tissue sections from women before and after cervical ripening (Fig. 5C). HIF-1α immunostaining was absent in glandular epithelial and stromal cells in the unripe cervix before labor (Fig. 5C, a and b). Immunostaining for HIF-1α was heterogeneous in the ripened cervix before labor. Nuclear staining was evident in many, but not all, endocervical epithelial cells, some of which demonstrated specific cytoplasmic, but not nuclear, staining (Fig. 5C, c). Interestingly, nuclear staining was also present in stromal cells surrounding the epithelial cells that line the endocervix but absent in stromal cells throughout the cervical matrix remote from the endocervix (Fig. 5C, d). These data indicate that although mRNA levels of HIF-1α do not increase in cervical tissues from women during ripening before labor, HIF-1α is stabilized in a number of endocervical epithelial and stromal cells adjacent to the endocervix. In tissues from women in labor, striking intense nuclear staining was evident in both epithelial and stromal cells throughout the cervical stroma (Fig. 5C, e and f). To determine whether up-regulation of HIF-1α was unique to human pregnancy, we quantified HIF-1α mRNA in the mouse cervix at various stages of gestation (Fig. 5D). Like humans, HIF-1α mRNA increased significantly in the mouse cervix as a function of gestational age with maximum levels during labor and immediately postpartum (Fig. 5D). Interestingly, however, in contrast with humans, HIF-1α increased progressively during pregnancy and cervical ripening, perhaps corresponding to the progressive increases in cervical distensibility observed in rodents (10, 11). Interestingly, mRNA levels of the HIF-1α-responsive gene, DEC1, which is known to repress MiTF gene expression, was also increased significantly in cervical stroma during labor (data not shown).
Fig. 5.
HIF-1α is regulated during cervical ripening in humans and mice. A, HIF-1α mRNA levels were quantified in cervical stroma obtained from women not in labor before (not ripe, n = 9) and after (ripe, n = 7) cervical ripening and in labor with cervical dilation (IL, n = 6). Data were normalized to GAPDH and represent mean ± sem. *, P ≤ 0.01 compared with not ripe or ripe. B, Tissue lysates of cervical stroma from women not in labor before cervical ripening (NIL, lanes 1–4) and in labor (IL, lanes 5–8) were analyzed by Western blotting using a HIF-1α and GAPDH antibodies as a loading control. Specific HIF-1α signals are indicated with an arrow. C, Immunolocalization of HIF-1α in endocervical (a, c, and e) and stromal (b, d, and f) tissues from women before ripening (a and b), after ripening before labor (c and d), and in labor with cervical dilation (e and f). Immunostaining for nuclear HIF-1α in endocervical columnar epithelial cells is denoted by arrows, whereas arrowheads denote nuclear staining in stroma. Note cytoplasmic staining in some epithelial cells in the ripe cervix before labor, whereas nuclear staining is evident uniformly epithelial cells after labor. Results were consistent among three samples in each group. Staining was absent in tissue sections incubated without primary antibody (data not shown). D, HIF-1α mRNA was quantified in the cervix obtained from nonpregnant (NP) and pregnant mice during gestation and the postpartum (PP) time period. Data were normalized to β-2 microglobulin (B2M) and represent mean ± sem. Number of samples in each group are noted. *, P = 0.007; **, P < 0.05 compared with NP group. h, Human; R, ripe; NR, not ripe; Epi, epithelium.
COX-2 gene expression is induced by hypoxia in cervical stromal cells
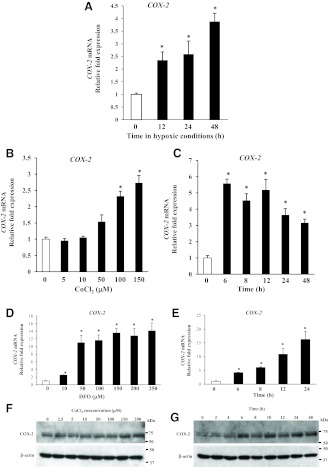
Other HIF-1α-responsive genes in the cervix were investigated in the context of cervical ripening. COX-2 levels are elevated during cervical ripening in cervical stromal and endothelial cells (12). To determine whether hypoxia regulated COX-2 gene expression in human cervical stromal cells, time-dependent accumulation of COX-2 mRNA in stromal cells was quantified after incubation under hypoxic conditions (Fig. 6A). Specifically, COX-2 gene expression was increased 4-fold after 48 h in hypoxic conditions. CoCl2/DFO treatment also induced COX-2 gene expression in a dose-dependent manner (Fig. 6, B and D), indicating that HIF-1α plays a major role in hypoxia-induced up-regulation of COX-2 gene expression. Treatment with CoCl2 resulted in 5.5-fold increases in COX-2 mRNA within 6 h, and levels remained elevated for 48 h (Fig. 6C). DFO treatment resulted in time-dependent increases in COX-2 mRNA levels reaching 16-fold by 24 h (Fig. 6E). Further, immunoblotting with COX-2-specific antibodies demonstrated dose- and time-dependent increases in COX-2 protein in cervical stromal cells after treatment with CoCl2 (Fig. 6, F and G).
Fig. 6.
Hypoxia and hypoxia mimetics induce COX-2 gene expression in cervical stromal cells. A, Cervical stromal cells were incubated under hypoxic conditions (2% O2) for 0, 12, 24, and 48 h, and mRNA levels of COX-2 were quantified. Data were normalized to GAPDH and represent mean ± sd (n = 3). *, P < 0.003 compared with 0 h. B and D, Cervical stromal cells were treated with control or increasing concentrations of either CoCl2 (5–150 μm) or DFO (10–250 μm) for 24 h. Data were normalized to GAPDH and represent mean ± sd (n = 3). *, P < 0.008 compared with control. C and E, Cervical stromal cells were treated with either CoCl2 (150 μm) or DFO (200 μm), and levels of COX-2 mRNA (normalized to GAPDH) were quantified as a function of time. Data represent mean ± sd (n = 3). *, P < 0.007 compared with 0 h. F, Whole-cell protein extracts from cervical stromal cells treated with different concentrations of CoCl2 for 24 h were analyzed using immunoblotting with COX-2 and β-actin antibodies. Results represent two experiments from two different cell preps with identical results. G, Whole-cell protein lysates prepared from cervical stromal cells treated with CoCl2 (150 μm) for various times were analyzed by Western blotting using COX-2 and β-actin antibodies.
Dinoprostone (PGE2) represses MiTF-CX gene expression
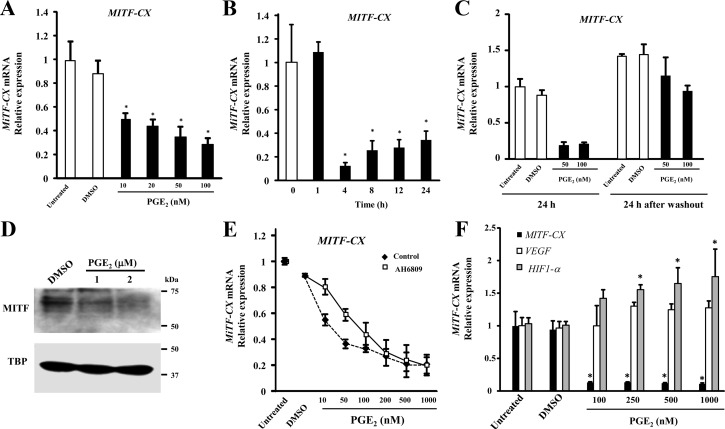
Because increased COX-2 enzyme leads to increased synthesis of prostaglandins, we were intrigued to study the effect of prostaglandins on MiTF-CX gene expression. Dinoprostone was used because it is known to effect cervical ripening in women and has strong abortive activity (13). Surprisingly, treatment of cervical stromal cells with PGE2 resulted in dose-dependent decreases in MiTF-CX mRNA (Fig. 7A) with greatly reduced levels within 4 h (Fig. 7B). After 4 h, MiTF-CX increased modestly, an effect that may be due to metabolism of PGE2 by the cells, suggesting that MiTF-CX gene repression by PGE2 may be reversible. To test this hypothesis, we treated stromal cells with either dimethylsulfoxide (DMSO) or PGE2 (50–100 nm) for 24 h, PGE2-containing media were removed, and cells were further incubated with fresh medium without PGE2. Results of this experiment indicated that MiTF-CX suppression by PGE2 was reversible (Fig. 7C). MiTF-CX protein levels were also decreased significantly by treatment with increasing concentrations of PGE2 (Fig. 7D). The specificity of PGE2 effects on MiTF-CX were tested with the prostaglandin E receptor 2, EP2 subtype antagonist AH6809 (14). Cervical stromal cells were pretreated with DMSO or AH6809 (10 μm) for 1 h. Thereafter, cells were treated with either DMSO or increasing concentrations of PGE2 (10–1000 nm) for 23 h (Fig. 7E). The IC50 for PGE2-induced repression of MiTF-CX gene expression was 15.8 ± 1.9 nm. Treatment with AH6809 (10 μm) resulted in significant increases in the IC50 for PGE2 (80.3 ± 12 nm, P = 0.006). The inhibitory effect of PGE2 on MiTF-CX was relatively specific. Although VEGF mRNA levels were not altered, PGE2 increased expression of HIF-1α (Fig. 7F), suggesting a feed-forward regulatory loop.
Fig. 7.
PGE2 represses MiTF-CX gene expression. A, Cervical stromal cells were treated with increasing concentrations of PGE2 from 0 to 150 μm for 24 h followed by quantification of mRNA levels of MiTF-CX. Data represent relative fold expression over GAPDH mRNA levels ± sd (n = 3). *, P < 0.006 compared with DMSO treatment B, Cervical stromal cells were treated with PGE2 (100 nm) for different time points as indicated followed by quantification of mRNA levels of MiTF-CX. Data represent mean ± sd (n = 3). *, P < 0.03 compared with 0 h. DMSO treatment served as a vehicle control. C, Cervical stromal cells were treated with either DMSO or PGE2 (50–100 nm) for 24 h followed RNA extraction. Similarly treated cells were washed twice with DMEM to remove PGE2 and further incubated for 24 h followed by RNA extraction and quantification of MiTF-CX expression levels in all the samples. Data represent relative fold expression over GAPDH mRNA levels ± sd (n = 3). As a control, cervical stromal cells were treated with DMSO. D, Cervical stromal cells were treated either with DMSO or 1 or 2 μm concentration of PGE2 for 24 h followed by extraction of nuclear proteins and subjected to immunoblotting using specific antibodies against MiTF-CX. TBP served as a loading control. E, AH6809 is a prostaglandin antagonist. Cervical stromal cells were treated with AH6809 (10 μm) for 1 h followed by treatment with either DMSO or increasing concentrations of PGE2 from 10 to 1000 nm for 24 h. Data represent relative fold expression over GAPDH mRNA levels ± sd (n = 3). F, Gene expression levels of MiTF-CX (solid bar), VEGF (open bar), or HIF-1α (gray bar) were quantified in control stromal cells or cells treated with DMSO or PGE2 (100–1000 nm) for 24 h.
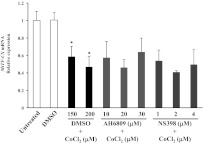
To determine whether hypoxia-induced up-regulation of COX-2-derived prostaglandins was obligatory for hypoxia-induced loss of MiTF, cells were treated with either the COX-2 inhibitor, NS398 (1–4 μm), or the PGE2 receptor antagonist, AH6809 (10–30 μm), in combination with CoCl2 for 24 h. Inhibition of COX-2 or the EP2 receptor did not alter hypoxia-induced down-regulation of MiTF-CX (Fig. 8). These results suggest that although PGE2, in sufficient doses, may reduce MiTF-CX in cervical stromal cells and may thereby contribute to hypoxia-induced effects, activation of COX-2 is not required.
Fig. 8.
Effect of PGE2 receptor blockade or COX-2 inhibition on CoCl2-induced repression of MiTF-CX. Cervical stromal cells were treated with CoCl2 with or without AH6809 (10–30 μm) or NS398 (1–4 μm) for 24 h. Thereafter, mRNA levels of MiTF-CX were quantified. *, P < 0.05 compared with control. Levels of MiTF-CX mRNA after treatment with CoCl2 +AH6809 or +NS398 were not statistically different from those treated with CoCl2 + DMSO.
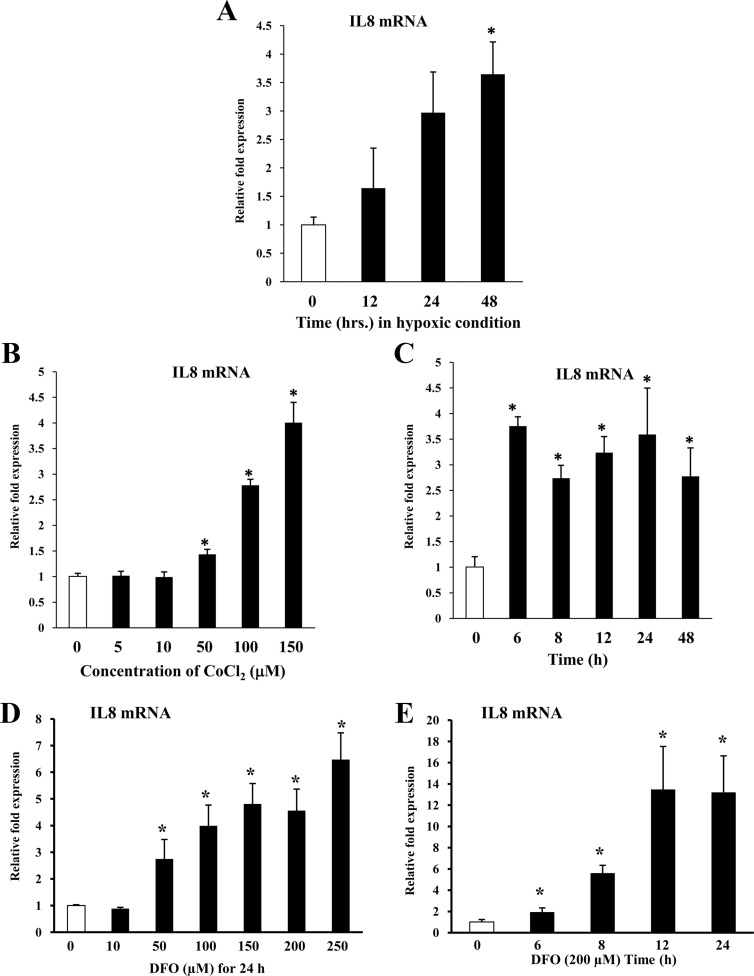
Hypoxia induces IL-8 gene expression in cervical stromal cells
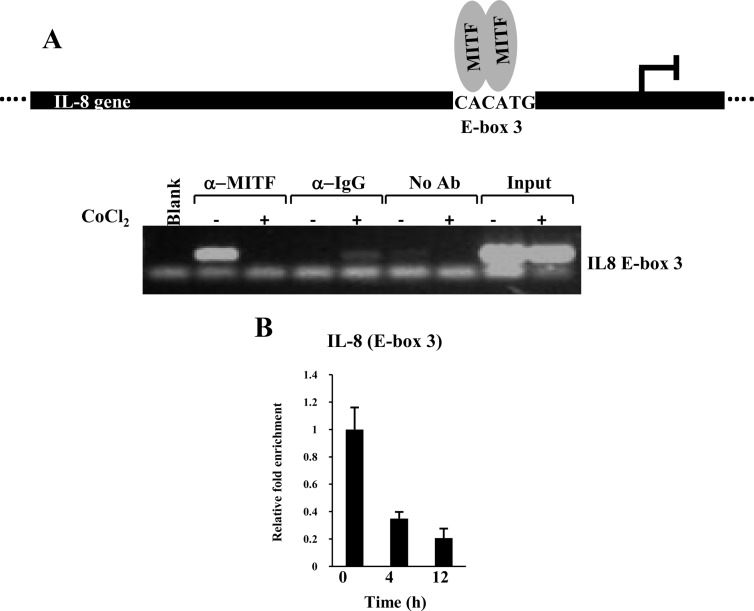
It is well established that IL-8 gene expression is increased significantly during cervical ripening and is important for the attraction of immune cells to the cervix during cervical ripening and dilation. MiTF-CX negatively regulates IL-8 gene expression in the cervix during gestation (7). In endothelial cells, HIF-1α induces IL-8 gene expression (15). These observations led us to ascertain whether HIF-1α induces IL-8 gene expression in cervical stromal cells and whether MiTF-CX could suppress this action. Hypoxia resulted in time-dependent increases in IL-8 mRNA in cervical stromal cells (Fig. 9A). Further, treatment with increasing concentrations of CoCl2 or DFO resulted in dose-dependent (Fig. 9, B and D) and time-dependent (Fig. 9, C and E) increases in IL-8 mRNA. MiTF-CX suppresses IL-8 gene expression by binding to E-box 3 (−424 to −419) in the IL-8 promoter. Thus, we assessed the effect of hypoxia on recruitment of MiTF-CX to the IL-8 promoter using ChIP assays. Occupancy of MiTF-CX to its regulatory element in the IL-8 promoter was reduced drastically after treatment with CoCl2 for 24 h (Fig. 10A). Further, real-time ChIP analysis showed that binding was partially reduced at 4 h, before loss of MiTF-CX protein in cells (Fig. 10B). These results indicate that hypoxia not only decreased MiTF-CX by inhibition of MITF-CX binding to its own promoter but also reduced binding of MiTF-CX to its regulatory site in the IL-8 promoter.
Fig. 9.
Hypoxia and hypoxia mimetics induce IL-8 gene expression in CSC. A, Cervical stromal cells were incubated under hypoxic conditions for 0, 12, 24, and 48 h followed by quantification of mRNA levels of IL-8. Data represent mean relative mRNA levels normalized to GAPDH ± sd (n = 3). *, P < 0.001 compared with control. B and D, Cervical stromal cells were treated with increasing concentrations of either CoCl2 or DFO for 24 h followed by quantification of mRNA levels of IL-8. Data represent mean ± sd (n = 3). *, P < 0.03 compared with controls. C and E, Cervical stromal cells were treated with CoCl2 (150 μm) or DFO (200 μm) for indicated time points followed by quantification of IL-8 mRNA levels. Data represent mean ± sd (n = 3). *, P < 0.03 compared with controls.
Fig. 10.
ChIP analysis of MiTF-CX association with E-box 3 in the IL-8 promoter. A, MiTF-CX was bound to E-box 3 in untreated cells but not cells treated with CoCl2 for 24 h. Normalization of input was shown by PCR amplification of DNA extracted from 1/100th of chromatin used for immune pull-down. Amplification is absent in chromatin immunoprecipitated with IgG or no antibody. B, Recruitment of MiTF-CX to the IL-8 promoter. Binding of MiTF-CX to E-box 3 of the IL-8 promoter decreased within 4 h of treatment with CoCl2. Data represent relative fold enrichment of IL-8 promoter after normalizing with input for each sample n = 3 in each group. *, P < 0.05 compared with 0 time. Ab, Antibody.
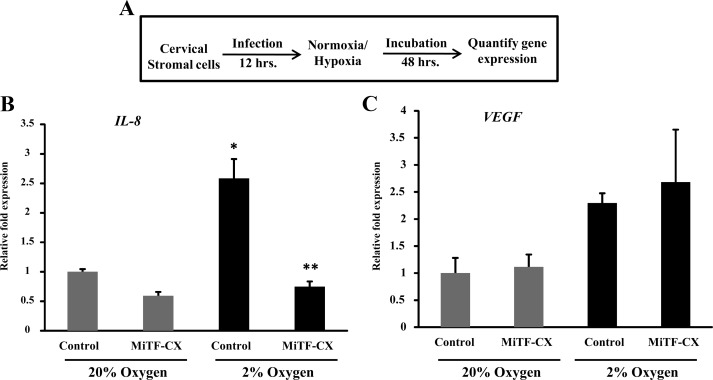
Ectopic overexpression of MiTF-CX abrogates hypoxia-mediated induction of IL-8 gene expression
Having established that hypoxia and hypoxia mimetics led to loss of MiTF-CX and up-regulation of IL-8 gene expression, we sought to determine whether rescue of hypoxia-induced loss of MiTF-CX could block activation of IL-8 gene expression in cervical stromal cells. Stromal cells were treated with either control adenovirus or adenovirus expressing MiTF-CX for 12 h. Thereafter, cells were incubated under normoxic or hypoxic conditions for 48 h (Fig. 11A). As expected, ectopic overexpression of MiTF-CX resulted in decreased IL-8 mRNA compared with control virus (Fig. 11B). Hypoxia-induced up-regulation of IL-8 gene expression was abrogated by ectopic overexpression of MiTF-CX (Fig. 11B). To determine the specificity of this effect, gene expression levels of VEGF were also quantified (Fig. 11C). Ectopic overexpression of MiTF-CX did not affect basal expression of VEGF under normoxic conditions nor did it affect hypoxia-induced up-regulation of VEGF (Fig. 11C). These results suggest that, in cervical stromal cells, hypoxia leads to increased expression of IL-8, primarily through loss of its negative regulator, MiTF-CX.
Fig. 11.
MiTF-CX abrogates hypoxia-mediated overexpression of IL-8 gene expression. A, Scheme of experimental conditions. Cervical stromal cells were infected with adenovirus expressing MITF-CX isoform or control adenovirus for 12 h followed by incubation either under normoxic or hypoxic condition for 48 h. B, mRNA levels of IL-8 gene were quantified in cervical stromal cells after infection and incubation as shown in A. Data represent relative fold expression over GAPDH mRNA levels ± sd (n = 3). C, After infection and incubation, mRNA levels of VEGF were quantified. Data represent relative fold expression over GAPDH mRNA levels ± sd (n = 3). Gray bars represent 20% O2 (normoxic), and black bars represent 2% O2 (hypoxic). *, P < 0.01 compared with Control 20%; **, P < 0.01 compared with Control 2%.
Discussion
Several cytokine genes are transcriptionally repressed in the cervix during gestation only to be activated during cervical ripening and dilation. Recently, we identified a novel isoform of MiTF-CX in human cervical stromal cells that positively regulates its own transcriptional activation but negatively regulates IL-8 gene expression (7). Although we cloned and characterized the upstream regulatory region of the MiTF-CX gene, mechanisms of regulation other that autoregulation were unknown. The major findings of the current study indicate that 1) hypoxia and two hypoxia mimetics that stabilize HIF-1α activate a transcriptional repressor DEC1 that binds to the MiTF-CX promoter, 2) hypoxia down-regulates MiTF-CX gene expression in cervical stromal cells, 3) HIF-1α is activated during human cervical ripening in vivo, 4) hypoxia and hypoxia mimetics up-regulate COX-2 and IL-8 gene expression in these cells, and 5) hypoxia-mediated repression of MiTF-CX is a prerequisite for induction of IL-8 gene expression. Further, we demonstrated that hypoxia induced COX-2 in human cervical stromal cells and that PGE2 also repressed MiTF-CX gene expression. Although up-regulation of COX-2 was not crucial for hypoxia-mediated down-regulation of MiTF-CX, the results may explain the widely used successful use of exogenously applied PGE2 for cervical ripening in the clinical setting.
MiTF-CX suppression was not immediate but significant after 10 h, suggesting that the rapid induction of HIF-1α by hypoxia may not directly suppress MiTF gene expression. The cause for the transient up-regulation of MiTF after hypoxia is unknown. Hypoxia activates the transcriptional repressor, DEC1, through HIF-1α. Here, we found that DEC1 was bound to MiTF-CX promoter E-box 3 after treatment with hypoxia mimetics in cervical stromal cells. Also, hypoxia increased IL-8 gene expression through loss of MiTF binding to the IL-8 promoter. Taken together, under physiologic in vivo conditions, HIF-1α appears to initiate a cascade of events by causing a net increase in DEC1 protein levels by 4 h, which decreases binding of MiTF-CX to both its own and IL-8 promoters, thereby leading to decreased cellular levels of MiTF-CX and loss of its repressor activity on IL-8 gene expression (Fig. 12).
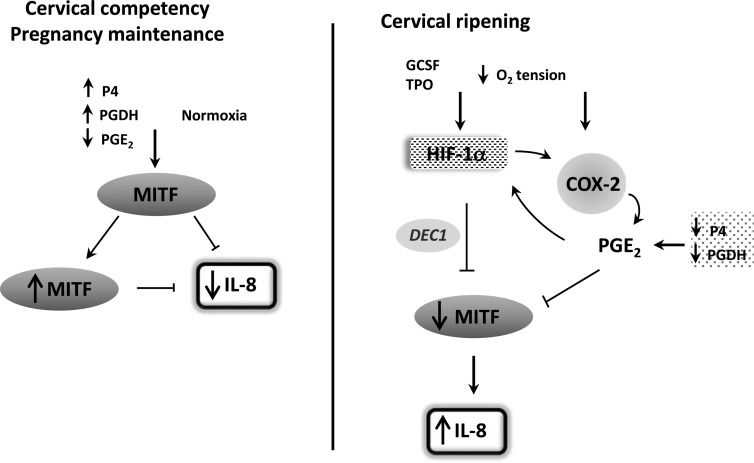
Fig. 12.
Model in which hypoxia and HIF-1α play important roles in cervical ripening. During most of gestation, the cervix is under normoxic conditions and serves to protect and maintain pregnancy. Increased progesterone (P4) up-regulates PGDH to decrease PGE2 levels in the cervix. MiTF-CX levels are increased and maintained in an autoregulatory feedback loop. MiTF-CX represses IL-8 gene expression in cervical stromal cells. During cervical ripening before labor, HIF-1α is stabilized in cervical stromal cells either through decreased O2 tension or increases in pseudohypoxia, factors such as GCSF and TPO. HIF-1α activates DEC1, leading to loss of MiTF-CX. Hypoxia and hypoxia mimetics that stabilize HIF-1α also result in induction of COX-2 and prostaglandin production, which further compromises MiTF-CX gene expression. Local progesterone metabolism and decreased PGDH contribute to up-regulated levels of PGE2. As in other cell types, PGE2 may also lead to further stabilization of HIF-1α. Ultimately, repression of IL-8 is lost leading to increased IL-8 production and infiltration of immune cells that further amplify IL-8 through IL-8 receptors on cervical stromal cells, neutrophils, monocytes, and macrophages.
Hypoxia-induced regulation of COX-2
Our experimental results indicated that hypoxia and hypoxia mimetics induced expression of COX-2, and PGE2 suppressed MiTF-CX gene expression through PGE2 receptors in cervical stromal cells. Increased prostaglandin production by COX-2, however, was not essential for hypoxia-induced effects on MiTF-CX and IL-8. Nonetheless, COX-2 induction may contribute to other mechanisms of cervical ripening independent of MiTF-CX. The mechanisms by which hypoxia alters MiTF-CX appear more complex and may not be reversible. Hypoxia-induced increased expression of VEGF and PGE2-induced repression of MiTF-CX were completely reversible. Thus, multiple mechanisms may be involved regarding the effect of hypoxia on gene expression in cervical stromal cells.
Regulation of cervical ripening by hypoxia?
It is widely known that cervical dilation of more than or equal to 3 cm is an ominous sign for preterm birth and that prevention of preterm birth is virtually impossible once the cervix is dilated to this extent. Results from this investigation provide some insight for this observation. Our data indicate that, during labor in vivo, HIF-1α is induced and relocalized to the nuclei of cervical stromal cells. It is tempting to speculate that, at term, massive growth of the uterus is sufficient to impede blood supply to the cervix, which is predominantly derived from cervical branches of the uterine artery bilaterally. Our data obtained from the ripe cervix before labor support this concept. Specifically, HIF-1α was localized to the nucleus in endocervical epithelial and stroma cells near the endocervix (i.e. the region most distal to arterial blood supply) before increased expression of HIF-1α mRNA. HIF-1α was not induced in stromal cells more radial to the endocervical canal. Hence, HIF-1α may be instrumental in initiation of cervical ripening in epithelial and inner stromal cells, leading to COX-2 gene expression and prostaglandin production (16). Local progesterone metabolism (17), decreased PGDH, and stretch-mediated increases in local prostaglandin production may also contribute to down-regulation of MITF-CX, up-regulation of IL-8, recruitment of immune cells, and cervical dilation, a cascade of events difficult to reverse. Previous studies demonstrating up-regulation of VEGF, a well-established HIF-1α target, during cervical ripening (18) and our data indicating HIF-1α stabilization and up-regulation of COX-2 before labor support this concept. Other reports indicating that the micro RNA, miR 34c, is up-regulated in cervical biopsies from women in labor (19) also support the role of hypoxia in cervical dilation, because miR 34c is known to be associated with oxidative stress (20).
HIF-1α protein may be induced by factors other than low oxygen tension in a phenomenon termed pseudohypoxia. For example, HIF-1α activation may be induced by IL-1β, insulin (21), thrombopoetin (TPO) (22), granulocyte colony-stimulating factor (GCSF) (23), angiotensin II (24), and thrombin (25). Interestingly, TPO and GCSF are also up-regulated in cervical stromal tissues during labor (our unpublished data) (26). These factors, therefore, may contribute to induction and stabilization of HIF-1α in the cervix during labor. Importantly, TPO and GCSF are known to be induced through bacterially derived lipopolysaccharide-induced up-regulation of nuclear factor κB. In other systems, these pathways induce and stabilize HIF-1α (27). It is possible, therefore, that HIF-1α plays a central role in physiologic cervical ripening at term predominantly through decreased oxygen tension but through infection and inflammation during pathophysiologic cervical dilation during preterm labor.
Materials and Methods
Human cervical tissues
Human cervical tissues were obtained from pregnant women undergoing cesarean hysterectomy using a protocol approved by the Institutional Review Board at the University of Texas Southwestern Medical Center. Full-thickness cervical tissues were obtained at the level of the internal cervical os. Thereafter, cervical stromal tissues were separated from the cervical epithelium, snap frozen in liquid N2, and stored at −80 C until use. Cervical ripening was ascertained using a modified Bishop's score in vivo before hysterectomy except in cases of placenta previa, in which cervical status was assessed ex vivo. Cervical specimens were obtained from specimens in which the cervix appeared normal, both grossly and histologically. Tissues from women with clinical or histological chorioamnionitis, rupture of the membranes more than 16 h, abnormal vaginal discharge, or positive cervical cultures for streptococcus, gonorrhea, trichomonas, or syphilis were not included.
Cell culture and treatments
Cervical stroma was obtained from nonpregnant women undergoing hysterectomy for benign gynecological conditions unrelated to cervical disease under a protocol approved by the Institutional Review Board at the University of Texas Southwestern Medical Center. To obtain primary cell cultures (P0), stroma from human cervix was minced into tiny pieces and incubated in DMEM with 10% fetal bovine serum for 20–25 d. Thereafter, cells were subcultured (P1) for various experiments. Hypoxia conditions were established by incubation of cells in a hypoxic chamber with 2% O2 (Coy Laboratory Products, Inc., Grass Lake, MI) or treated with different concentrations of the hypoxia mimetics, CoCl2 (catalog no. C8661; Sigma, St. Louis, MO) or DFO (catalog no. sc-203331; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Stock solutions of CoCl2 and DFO were prepared in DMEM and further diluted in DMEM before adding to the culture. PGE2 and PGE2 antagonist AH6809 (catalog nos. 14010 and 14050; Cayman Chemical, Ann Arbor, MI) were dissolved in DMSO and further diluted in DMSO for treatment of cells. For overexpression of MiTF-CX, cervical stromal cells were infected with either control adenovirus or MiTF-CX-expressing adenovirus for indicated time points (7).
Polyacrylamide gel electrophoresis and immunoblotting
Protein extracts from tissues, whole-cell extracts, or nuclear extracts from cells were resolved using 12% polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane (catalog no. 162-0115; Bio-Rad Laboratories, Inc., Munich, Germany) overnight. The membranes were blocked with 5% skim milk solution in PBS for 2 h at 21 C followed with incubation with specific antibodies against either glyceraldehyde phosphate dehydrogenase (GAPDH), TATA binding protein (TBP), MITF-CX, COX-2, HIF-1α, DEC1, or β-actin in 2.5% skim milk solution in PBS over night at 4 C followed by three washes with PBS containing 0.1% Tween 20 and further incubation with horseradish peroxidase-conjugated antimouse/rabbit secondary antibodies (dilution of 1:5000) for 1 h at room temperature. Blots were washed three times with PBS containing Tween 20 and developed using ECL Plus Western blotting detection system (catalog no. RPN2132; GE Healthcare UK Ltd., Buckinghamshire, UK). Immunoreactive signals were detected by exposing the membranes to Hyperfilm (catalog no. 28906836; Amersham Hyperfilm ECL; GE Healthcare UK Ltd.) For stripping, blots were incubated in stripping buffer [62.5 mm Tris-HCl (pH 7.5), 2% SDS, and 100 mm 2-Mercaptoethanol] for 1 h at 37 C with shaking followed by washes with PBS and used for probing with different antibodies.
Nuclear extract preparation
Nuclear extracts were prepared as described elsewhere (28). Briefly, cells were harvested from 100 mm cell culture dishes and washed with 5 ml of cold 2× PBS. Cells were resuspended gently in 800 μl of cold buffer A [10 mm Tris-HCl (pH 7.9), 10 mm KCl, 0.1 mm EDTA, and 0.1 mm EGTA] for 30 min. Nonidet P-40 (10%, 25 μl) was added to each tube, vortexed for 5 sec, and centrifuged at 13,000 rpm for 30 sec at 4 C to pellet nuclei. Supernatants (cytoplasmic extracts) were separated and the nuclei further lysed in 150 μl of buffer B [20 mm Tris-HCl (pH 7.9), 400 mm NaCl, 1 mm EDTA, and 1 mm EGTA] by rocking for 1 h at 4 C. Lysates were further clarified by centrifugation at 13,000 rpm for 5 min at 4 C.
RNA extraction and quantitative real-time PCR
For RNA extraction from human cervix, the tissues were pulverized in liquid nitrogen, and guanidine hydrochloride extraction method was used as described elsewhere (29). RNA extraction from cells was done using RNA extraction kit from Ambion (catalog no. AM1914; Ambion, Austin, TX). cDNA synthesis was performed using Superscript III from Invitrogen according to supplier's protocol (catalog no. 18080-044; Invitrogen, Carlsbad, CA). Quantitative PCR (qPCR) was done using iTaq SYBR Green Supermix from Bio-Rad Laboratories, Inc. (catalog no. 172-5850) in an ABI 7900HT Fast Real-Time PCR system. Gene-specific oligonucleotides (see Table 1) were designed as described previously (29).
Table 1.
Oligonucleotides used in qPCR to quantify mRNA levels
| Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) |
|---|---|---|
| IL-8 | GGCAGCCTTCCTGATTTCTG | TGCACTGACATCTAAGTTCTTTAGCA |
| GAPDH | GGAGTCAACGGATTTGGTCGTA | CAACAATATCCACTTTACCAGAGTTA |
| VEGF | CATGCAGATTATGCGGATCAA | TTTGTTGTGCTGTAGGAAGCTCAT |
| MiTF-CX | TTGGGAGGAGGACCAGGTTT | GAGCTTATCGGAGGCTTGGA |
| Probe FAMCTCAATGCAGTTCCGCCGAG | ||
| hHIF-1α | ff;12GCCCCAGATTCAGGATCAGA | TGGGACTATTAGGCTCAGGTGAAC |
| mHIF-1α | GAACATCAAGTCAGCAACGTG | TTTGACGGATGAGGAATGGG |
| COX-2 | GAATCATTCACCAGGCAAATT | TCTGTACTGCGGGTGGAACA |
| Probe FAMTCCTACCACCAGCAACCCTGC |
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissues were sectioned at 5 μm and mounted on slides. Tissue sections from positive and negative control sections were mounted on the same slide. Sections were immunostained with antibodies to HIF1-α (catalog no. ab2185; Abcam) or IgG (negative controls) as described previously (7).
ChIP assay
ChIP assays were conducted as described previously (7). ChIP was done either with anti-MITF-antibodies or anti-DEC1 antibodies (catalog no. A300-649A; Bethyl Laboratories, Montgomery, TX). Cervical stromal cells were seeded overnight to 85% confluency in 150-mm dishes and maintained in DMEM with 10% fetal bovine serum. Cells were treated with 200 μm CoCl2 for 4 or 24 h. Specific primers used for conventional PCR amplification are shown in Table 2, and real-time quantification are shown in Table 3.
Table 2.
Oligonucleotides used to amplify specific regions in IL-8 and MiTF-CX promoters (conventional PCR)
| Region | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) |
|---|---|---|
| IL-8 promoter | ||
| E-box 3 (−431 to −145) | GTTCTAACACCTGCCACTCT | GCACCCTCATCTTTTCATTAT |
| MiTF-CX promoter | ||
| E-box 3 (−416 to −206) | CCTTTGAGTTCTGCAGTGC | TCCTAATACTGCCAATCCTG |
| E-box 9 (−1081 to −883) | TGTTAGACATCTTGGAGGGC | AAAGCAGCGACCACAGACAG |
Table 3.
Oligonucleotides used to amplify specific regions in IL-8 and MiTF-CX promoters (real-time quantitative PCR)
| Region | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) |
|---|---|---|
| IL-8 promoter | ||
| E-box 3 (−343 to −213) | TCACCAAATTGTGGAGCTTCAGT | GCAAACCTGAGTCATCACACTTCC |
| MiTF-CX promoter | ||
| E-box 3 (−401 to −288) | GTGCAGTTTGCACAGGCTGATGTT | CACTGAGGTCCTATTCCTGACAGT |
| E-box 9 (−1075 to −976) | ACATCTTGGAGGGCATGGTCCTAA | GCCTGGAGACCACAAGAAAGAGCTTA |
Acknowledgments
We thank Dr. Carole Mendelson (University of Texas Southwestern Medical Center) for use of the hypoxia facility and Mr. Patrick Keller for technical assistance. We also thank the Human Tissue and Biological Core Laboratory for tissue acquisition.
This work was supported by National Institutes of Health Grant P01HD11149 and March of Dimes Grant 6FY06337 (to R.A.W.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ChIP
- Chromatin immunoprecipitation
- CoCl2
- cobalt(II) chloride hexahydrate
- COX-2
- cyclooxygenase-2
- DEC1
- differentiated embryo chondrocyte-expressed gene 1
- DFO
- deferoxamine mesylate
- DMSO
- dimethylsulfoxide
- GAPDH
- glyceraldehyde phosphate dehydrogenase
- GCSF
- granulocyte colony-stimulating factor
- HIF-1α
- hypoxia inducible factor-1α
- MiTF-CX
- microphthalmia-associated transcription factor
- PGDH
- 15-hydroxyprostaglandin-dehydrogenase
- PGE2
- prostaglandin E2
- qPCR
- quantitative PCR
- TBP
- TATA binding protein
- TFE
- transcription factor for immunoglobulin heavy-chain enhancer
- TPO
- thrombopoetin
- VEGF
- vascular endothelial growth factor.
References
- 1. Read CP, Word RA, Ruscheinsky MA, Timmons BC, Mahendroo MS. 2007. Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction 134:327–340 [DOI] [PubMed] [Google Scholar]
- 2. Word RA, Li XH, Hnat M, Carrick K. 2007. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med 25:69–79 [DOI] [PubMed] [Google Scholar]
- 3. Goldenberg RL, Iams JD, Mercer BM, Meis PJ, Moawad AH, Copper RL, Das A, Thom E, Johnson F, McNellis D, Miodovnik M, Van Dorsten JP, Caritis SN, Thurnau GR, Bottoms SF. 1998. The preterm prediction study: the value of new vs standard risk factors in predicting early and all spontaneous preterm births. NICHD MFMU network. Am J Public Health 88:233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stjernholm-Vladic Y, Stygar D, Mansson C, Masironi B, Akerberg S, Wang H, Ekman-Ordeberg G, Sahlin L. 2004. Factors involved in the inflammatory events of cervical ripening in humans. Reprod Biol Endocrinol 2:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelly RW. 2002. Inflammatory mediators and cervical ripening. J Reprod Immunol 57:217–224 [DOI] [PubMed] [Google Scholar]
- 6. Denison FC, Calder AA, Kelly RW. 1999. The action of prostaglandin E2 on the human cervix: stimulation of interleukin 8 and inhibition of secretory leukocyte protease inhibitor. Am J Obstet Gynecol 180:614–620 [DOI] [PubMed] [Google Scholar]
- 7. Li XH, Kishore AH, Dao D, Zheng W, Roman CA, Word RA. 2010. A novel isoform of micropthalmia-associated transcription factor inhibits IL-8 gene expression in human cervical stromal cells. Mol Endocrinol 24:1512–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feige E, Yokoyama S, Levy C, Khaled M, Igras V, Lin RJ, Lee S, Widlund HR, Granter SR, Kung AL, Fisher DE. 2011. Hypoxia-induced transcriptional repression of the melanoma-associated oncogene MITF. Proc Natl Acad Sci USA 108:E924–E933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yuan Y, Hilliard G, Ferguson T, Millhorn DE. 2003. Cobalt inhibits the interaction between hypoxia-inducible factor-α and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-α. J Biol Chem 278:15911–15916 [DOI] [PubMed] [Google Scholar]
- 10. Mahendroo MS, Porter A, Russell DW, Word RA. 1999. The parturition defect in steriod Salpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol 13:981–992 [DOI] [PubMed] [Google Scholar]
- 11. Vedernikov YP, Mayes M, Moore E, Gei A, Saade GR, Garfield RE. 2000. The effects of pregnancy and smooth muscle contractility on cervical distensibility in the rat. Am J Obstet Gynecol 182:905–908 [DOI] [PubMed] [Google Scholar]
- 12. Havelock JC, Keller P, Muleba N, Mayhew BA, Casey BM, Rainey WE, Word RA. 2005. Human myometrial gene expression before and during parturition. Biol Reprod 72:707–719 [DOI] [PubMed] [Google Scholar]
- 13. Ulmsten U, Wingerup L, Belfrage P, Ekman G, Wiqvist N. 1982. Intracervical application of prostaglandin gel for induction of term labor. Obstet Gynecol 59:336–339 [PubMed] [Google Scholar]
- 14. Woodward DF, Pepperl DJ, Burkey TH, Regan JW. 1995. 6-Isopropoxy-9-oxoxanthene-2-carboxylic acid (AH 6809), a human EP2 receptor antagonist. Biochem Pharmacol 50:1731–1733 [DOI] [PubMed] [Google Scholar]
- 15. Karakurum M, Shreeniwas R, Chen J, Pinsky D, Yan SD, Anderson M, Sunouchi K, Major J, Hamilton T, Kuwabara K. 1994. Hypoxic induction of interleukin-8 gene expression in human endothelial cells. J Clin Invest 93:1564–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu XH, Kirschenbaum A, Lu M, Yao S, Dosoretz A, Holland JF, Levine AC. 2002. Prostaglandin E2 induces hypoxia-inducible factor-1α stabilization and nuclear localization in a human prostate cancer cell line. J Biol Chem 277:50081–50086 [DOI] [PubMed] [Google Scholar]
- 17. Andersson S, Minjarez D, Yost NP, Word RA. 2008. Estrogen and progesterone metabolism in the cervix during pregnancy and parturition. J Clin Endocrinol Metab 93:2366–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hassan SS, Romero R, Haddad R, Hendler I, Khalek N, Tromp G, Diamond MP, Sorokin Y, Malone J., Jr 2006. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol 195:778–786 [DOI] [PubMed] [Google Scholar]
- 19. Hassan SS, Romero R, Pineles B, Tarca AL, Montenegro D, Erez O, Mittal P, Kusanovic JP, Mazaki-Tovi S, Espinoza J, Nhan-Chang CL, Draghici S, Kim CJ. 2010. MicroRNA expression profiling of the human uterine cervix after term labor and delivery. Am J Obstet Gynecol 202:80.e1–80.e8 [DOI] [PubMed] [Google Scholar]
- 20. Pavlides S, Tsirigos A, Migneco G, Whitaker-Menezes D, Chiavarina B, Flomenberg N, Frank PG, Casimiro MC, Wang C, Pestell RG, Martinez-Outschoorn UE, Howell A, Sotgia F, Lisanti MP. 2010. The autophagic tumor stroma model of cancer: role of oxidative stress and ketone production in fueling tumor cell metabolism. Cell Cycle 9:3485–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stiehl DP, Jelkmann W, Wenger RH, Hellwig-Bürgel T. 2002. Normoxic induction of the hypoxia-inducible factor 1α by insulin and interleukin-1β involves the phosphatidylinositol 3-kinase pathway. FEBS Lett 512:157–162 [DOI] [PubMed] [Google Scholar]
- 22. Yoshida K, Kirito K, Yongzhen H, Ozawa K, Kaushansky K, Komatsu N. 2008. Thrombopoietin (TPO) regulates HIF-1α levels through generation of mitochondrial reactive oxygen species. Int J Hematol 88:43–51 [DOI] [PubMed] [Google Scholar]
- 23. Liu SP, Lee SD, Lee HT, Liu DD, Wang HJ, Liu RS, Lin SZ, Su CY, Li H, Shyu WC. 2010. Granulocyte colony-stimulating factor activating HIF-1α acts synergistically with erythropoietin to promote tissue plasticity. PloS One 5:e10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Richard DE, Berra E, Pouyssegur J. 2000. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1α in vascular smooth muscle cells. J Biol Chem 275:26765–26771 [DOI] [PubMed] [Google Scholar]
- 25. Görlach A, Diebold I, Schini-Kerth VB, Berchner-Pfannschmidt U, Roth U, Brandes RP, Kietzmann T, Busse R. 2001. Thrombin activates the hypoxia-inducible factor-1 signaling pathway in vascular smooth muscle cells: role of the p22(phox)-containing NADPH oxidase. Circ Res 89:47–54 [DOI] [PubMed] [Google Scholar]
- 26. Goldenberg RL, Iams JD, Mercer BM, Meis PJ, Moawad A, Das A, Miodovnik M, Vandorsten PJ, Caritis SN, Thurnau G, Dombrowski MP. 2001. The preterm prediction study: toward a multiple-marker test for spontaneous preterm birth. Am J Obstet Gynecol 185:643–651 [DOI] [PubMed] [Google Scholar]
- 27. van Uden P, Kenneth NS, Rocha S. 2008. Regulation of hypoxia-inducible factor-1α by NF-κB. Biochem J 412:477–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Therrien M, Drouin J. 1993. Cell-specific helix-loop-helix factor required for pituitary expression of the pro-opiomelanocortin gene. Mol Cell Biol 13:2342–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Drewes PG, Yanagisawa H, Starcher B, Hornstra I, Csiszar K, Marinis SI, Keller P, Word RA. 2007. Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. Am J Pathol 170:578–589 [DOI] [PMC free article] [PubMed] [Google Scholar]