Abstract
Background
Studies report mixed findings regarding antidepressant agents and suicide risks, and few examine suicide deaths. Studies using observational data can accrue the large sample sizes needed to examine suicide death but selection biases must be addressed. We assessed associations between suicide death and treatment with the seven most commonly used antidepressants in a national sample of VA patients in depression treatment. Multiple analytic strategies were used to address potential selection biases.
Methods
We identified VA patients with depression diagnoses and new antidepressant starts between April 1, 1999 and September 30, 2004 (N=502,179). Conventional Cox regression models, Cox models with inverse probability of treatment weighting, propensity stratified Cox models, marginal structural models (MSM), and instrumental variable (IV) analyses were used to examine relationships between suicide and exposure to: bupropion, citalopram, fluoxetine, mirtazapine, paroxetine, sertraline, and venlafaxine.
Results
Crude suicide rates varied from 88 to 247/100,000 person-years across antidepressant agents. In multiple Cox and MSM models, sertraline and fluoxetine had lower risks for suicide death than paroxetine. Bupropion had lower risks than several antidepressants in Cox but not MSM models. IV analyses did not find significant differences across antidepressants.
Discussion
Most antidepressants did not differ in their risk for suicide death. However, across several analytic approaches, although not IV analyses, fluoxetine and sertraline had lower risks of suicide death than paroxetine. These findings are congruent with the FDA meta-analysis of RCTs reporting lower risks for “suicidality” for sertraline and a trend towards lower risks with fluoxetine than for other antidepressants. Nevertheless, divergence in findings by analytic approach suggests caution when interpreting results.
INTRODUCTION
Patients with depressive disorders are at high risk for suicide.1,2 Although antidepressant medications are effective in reducing depressive symptoms among adults, concerns have re-emerged that antidepressant medications may increase rather than decrease suicide risks, particularly when first initiated or when the dosage is changed. Beginning in 2003, the FDA warned of increased suicide risks with specific antidepressant agents when used by youth (i.e., paroxetine in June, 2003; 10 antidepressants in early 2004).3 However, based on meta-analyses of randomized controlled trials (RCTs), the most recent FDA “black box” warning included all antidepressant agents when prescribed for children, adolescents, and young adults. 4,5
However, even though the FDA ‘s most recent warning included all antidepressants, there are reasons to believe that antidepressant agents may differ in their associated suicide risks. Antidepressants have different pathophysiolgical effects and differ in their impact on concomitant anxiety, pain, sleep onset, and sleep architecture. They also differ in the likelihood of adverse discontinuation effects. 6 All of these factors, in turn, have been linked to increased suicide risks. 7-9
Despite these theoretical possibilities, studies that have compared suicide risks across antidepressant agents have reported mixed results. 10-15 Studies have found limited differences in suicide risk by antidepressant class, 10 although a few have reported signals for differential risks by specific antidepressant agent. The 2004 FDA meta-analysis of RCTs that assessed risks among youth reported a clearer signal for suicide risks with venlafaxine than for other antidepressants when compared to placebo. There was also a possible indication of increased risks with paroxetine.4 A secondary analysis indicated that antidepressants with longer half-lives were associated with lower risks, and that fluoxetine had the lowest risk ratio.16 The 2006 FDA meta-analysis ofRCTs among adults reported an indication for lower risks with sertraline and a trend for lower risks with fluoxetine than for other antidepressants.5,17
Studies using large observational datasets to examine risks associated with specific antidepressants have also reported mixed results with a few studies reporting increased risks with venlafaxine and decreased risks with sertraline or fluoxetine, but others finding no differences. 12,15,18-20
Such mixed results might be expected, given the considerable challenges faced by studies examining the rare outcome of suicide, the variety of methodologies employed, the different outcomes assessed, and the different populations studied. Studies using data from RCTs face challenges due to limited sample sizes and often use proxy outcomes (ideation or attempts) rather than suicide death. Studies using observational data face challenges due to confounding of the relationship between antidepressant choice and suicide, as prescribers may preferentially use a particular agent for patients with certain characteristics (e.g., sleep issues or pain) and these characteristics may also be associated suicide risks. Studies that assess suicide risk by antidepressant class rather than by specific antidepressant agent face challenges if agents within a class have similar effects on neurotransmitters yet vary in their impact on sleep, energy, and pain.
In this study, we use data from the Department of Veterans Affairs (VA) Health System, the largest organized healthcare system in the US, to examine differential risks of suicide death in a large cohort of patients (N= 502,179) with depression diagnoses who received any of the seven most commonly prescribed antidepressant medications in the VA between 1999 and 2004. We employed multiple analytic approaches to address potential treatment selection biases, assessing consistency across approaches.
METHODS
This study was reviewed and approved by the Human Subjects Committee at the Veterans Affairs Ann Arbor Health System and the University of Michigan.
Data Source
Data were obtained from the Department of Veterans Affairs (VA) Health System National Depression Registry (NARDEP) which includes data on pharmacy fills, diagnoses, visits, and hospitalizations for VA patients who have had at least one diagnosis of a depressive disorder. Data were also obtained from the National Death Index (NDI), which is compiled from state-mandated death certificates and considered the “gold standard” in US mortality databases.21
Patient Cohort
Employing a new user design, 22 the study cohort consisted of 502,179 patients who received both a depression diagnosis and a new fill of one of the seven most commonly used antidepressant medications between 4/1/1999 and 9/30/2004 in the VA health system (fluoxetine, sertraline, paroxetine, citalopram, venlafaxine, bupropion, and mirtazapine). Depression diagnoses were identified using the ICD-9 codes: 296.2x, 296.3x, 296.90, 296.99, 298.0, 300.4, 311, 293.83, 301.12, 309.0, or 309.1. A new antidepressant start was defined as a fill of any of the seven study antidepressant agents, after at least 6 months without any observed antidepressant fills. Patients who did not have a new start of one of these antidepressant agents were not included in the study. Patients were also excluded if they received a diagnosis of bipolar I, schizophrenia, or schizoaffective disorder during the 12 months prior to the index date or during the study period as these diagnoses make it less likely that patients have a depressive disorder (study inclusion criterion) and may substantially change associations between antidepressant exposure and suicide risks.
Outcome Measure
The primary outcome, death due to suicide, was assessed using data from the National Death Index. A death was categorized as a suicide based on International Classification of Diseases, tenth revision, codes X60-X84 and Y87.0.
Antidepressant Exposure
The primary predictor variable was exposure to the specific antidepressant agent used for the new antidepressant start (fluoxetine, sertraline, paroxetine, citalopram, venlafaxine, bupropion, and mirtazapine). These seven antidepressant agents accounted for approximately 90% of all VA antidepressant fills during the study period. Because mirtazapine is frequently used as a hypnotic in low doses, mirtazapine was considered to be a new antidepressant start only if prescribed in doses of ≥ 15 mg per day.
Days of exposure to the antidepressant agent started on the date of the first new fill and continued until the days’ supply of the last fill were exhausted. In the main study analyses, patients were considered to have been exposed to an additional 10% of the days’ supplies for each fill, to account for some continued exposure from later ingestion of missed doses and/or continued blood levels during short gaps in coverage.
In the Cox models, exposure time to the new antidepressant was censored at the time the days’ supply of the last fill were exhausted or at the time of a switch to a second antidepressant medication. In marginal structural models (MSM), exposure time after antidepressant discontinuation was counted as time “unexposed” to the first agent, with censoring at 6 months or 12 months following treatment initiation.
Patient Characteristics
Patient age was assessed at the time of the first new antidepressant fill and categorized as <40, 40-49, 50-64, and ≥65 years. Patient race was categorized as Black, White, Other, or Unknown, and a dichotomous indicator was constructed for Hispanic ethnicity.
Indicators were constructed for diagnoses of alcohol abuse/dependence, drug abuse/dependence, post-traumatic stress disorder (PTSD), personality disorder, other anxiety disorder, major depression versus “other depression” diagnosis, and tobacco use disorder. Medical burden was assessed using the Charlson comorbidity index, with the cormorbidity score categorized as 0, 1, 2, or ≥ 3. 23,24Study models also included the numbers of in-patient psychiatric stays (0, 1, ≥2), total outpatient visits (0-3, 4-12, or ≥13), outpatient mental health visits (0, 1-2, ≥3), number of psychotropic medications filled, receipt of any psychotherapy CPT codes, and whether Medicare reimbursed services were used. We also constructed indicators for whether patients were service connected for disabilities occurring or exacerbated by military service, as this is associated with higher levels of VA services access. We included indicators for whether a suicide attempt was recorded in the prior 3 years and the time since the patient’s first depression diagnosis, with truncation at 730 days.
Most of the covariates for Cox models (with and without inverse probability of treatment weighting (IPTW) or propensity stratification) were based on data for the 12 months prior to the first new antidepressant start. In MSM models, several covariates were time varying (antidepressant exposures, other psychotropic medication exposures, utilization variables and comorbid conditions), and these time-varying covariates were updated monthly, based on the prior 12 months of data.
Two facility-level variables were included in study models: the geographic region (Northeast, West, Midwest, South) of the VA facility most used by the patient in the year of their index antidepressant start (or VA facility used most often in the past 12 months for MSM models), and VA location in an urban versus a rural area based on Metropolitan Statistical Area designation.
Finally, although all seven study drugs were available throughout the study period,25 we included fiscal year of new start to control for secular practice trends that might result from the recency of an antidepressant’s market introduction or its inclusion in VA formulary (i.e., likelihood of “channeling” of newly introduced medications to more severely ill patients).
Data Analyses
We first examined the distributions of all continuous variables and their functional relationships with suicide risks, and we categorized continuous variables when there was observed non-linearity.
Crude suicide rates were calculated for patients exposed to each of the seven antidepressant medications over the entire exposure period using Poisson regression. Exact methods were used to calculate 95% confidence intervals. We also generated plots of log hazards to explore suicide risk ratios for the antidepressant agents during the year following treatment initiation.
To compare suicide risks associated with different antidepressant agents, we used multiple approaches to address potential treatment selection biases, including conventional Cox models, Cox models with inverse probability of treatment weighting (IPTW), propensity stratified Cox models, marginal structural models, a linear probability model with IPTW, and instrumental variable (IV) analyses. For all approaches, except propensity stratified model estimates, citalopram was used as the reference antidepressant.
Cox models with IPTW method and propensity-stratified Cox models attempt to control for “treatment by indication” by adjusting for the likelihood that a given patient will receive a specific agent.26 For the IPTW Cox model, propensities were estimated using a multinomial logit model as the predicted probability of receiving the patient’s antidepressant agent, given all baseline covariate values and relevant interactions. The Cox model was then weighted by the inverse of the estimated propensities. Additional pair-wise contrasts of antidepressant agents were obtained based on post-hoc linear contrasts.
As the IPTW method can provide extreme weights to the very few people in the tails of the propensity distribution,27 potentially multiplying any residual bias, the distributions were examined for extreme weights and suicide risks were examined by antidepressant agents across the propensity distribution to assess for notable non-uniform relative risks.
The propensity stratified Cox models compared 21 pairs of antidepressant agents separately and thus the reference antidepressant changed depending upon the specific pairs being compared. Relative risk estimates were obtained using Cox models stratified by balanced blocks of propensity scores estimated using binary logistic regression models. Propensity blocks were obtained using stratification matching where the number of blocks for each pair-wise comparison ranged from 20 to 32.28 For each stratified analysis, propensity blocks in the extreme propensity distribution with less than 3 observations in one treatment group were trimmed as an additional step to further remove residual confounding. We also completed a more traditional propensity analysis stratified by propensity quintiles, after truncating the cohort to those in the area of overlapping propensities. In both propensity-weighted and propensity-stratified methods, models used to obtain propensity scores were fit without consideration for parsimony and included all baseline covariates. Most continuous variables were included without categorization and with quadratic terms. The model also included relevant two-way interactions. In addition to using propensities, the Cox models included a limited set of baseline covariates to adjust for their effects on suicide risk.
Marginal structural models (MSM) were used to adjust for time-varying exposure as well as potential time-varying confounders.29 Because more than 90% of the patients filled the newly initiated antidepressant for less than 12 months, MSM models were completed using 6 and 12 months of follow-up data.
All time-varying exposures and covariates were updated monthly, and a pooled logistic regression model was used to approximate the Cox’s model where an individual was assigned monthly weights until suicide, death, or the end of follow-up time.29 The contribution of each patient to the calculation at each follow-up month was weighted by the product of the inverse probability-of-treatment weight and the inverse probability-of-censoring weight with each weight estimated from the observed data. Probability of receiving the patient’s antidepressant treatment was estimated for each month, given his/her own treatment history up to the current time, given past time-varying treatment, covariate and utilization history, using multinomial logistic regression. Time varying treatment history included exposures to other antidepressants, in addition to exposures to other psychotropics. Other antidepressants were grouped as selective serotonin reuptake inhibitors, older non-selective or dual action reuptake inhibitors, newer mixed or dual-action agents, or other. Censoring weights were estimated monthly using logistic regression and a similar set of covariates as predictors, plus the treatment exposure types in the prior month.
Lastly, we used linear probability models for the dichotomous outcome of suicide using both IPTW approach and an instrumental variable (IV) approach to address treatment selection biases. The IV approach has the potential to address unobserved confounding variables, if the instruments are valid.30,31 IV analyses involve the estimation of two different regression models. The first model predicts the treatment choice (in this case antidepressant agent) and the second model predicts the outcome of interest (suicide death) as a function of the predicted treatment. IV analyses require observable instruments that are predictive of antidepressant choice and are not correlated with suicide death except via antidepressant choice. To examine the relationship between 7 commonly used antidepressants and suicide death, we included 6 instrumental variables. The IVs were the percentages of all antidepressant fills at patient’s facility of most use that were fills for each of the specific agents (all except citalopram which was the reference antidepressant).
The theoretical basis for using IVs of fractional fills for each antidepressant at the patient’s facility is that they are expected to influence the choice of antidepressant agent, and yet patients do not generally choose facilities based on these prescribing patterns. Thus these IVs are not expected to be directly related to patient characteristics or outcomes. 32 F-statistics and partial R2 wesre obtained to assess if IVs were predictive of antidepressant choice at the patient level in the first-stage linear regressions.33 All analyses were adjusted for potential clustering at the facility level using robust sandwich estimation.
Because suicide deaths are rare, statistical significance was set at 5% and we did not adjust for multiple comparisons. When evaluating results, the consistency of results across analytic approaches and the size of the estimated relative risks were emphasized.
RESULTS
Study population
The study population has been previously described. 34 In keeping with the VA population, the majority of patients were male (92%), white (75%) and aged 50 or older (73%). In the 12 months prior to the first antidepressant start, 12% had had a diagnosis of alcohol use disorder and 13% had had a diagnosis of PTSD. Forty-seven percent had a mental health outpatient visit in the prior 12 months.
Sertraline was prescribed for 27.0% of patients with a new antidepressant starts, citalopram for 26.1%, fluoxetine for 14.0% and paroxetine for 13.3%. Of the non-SSRI’s, bupropion was prescribed most often (10.9%), followed by mirtazapine (4.2%) and venlafaxine (4.4%).
Crude Suicide Rates for Each Antidepressant
Table 1 presents the crude suicide rates per 100,000 person-years of exposure to each of the seven most commonly prescribed antidepressant agents. These crude rates ranged from 88/100,000 person-years to 247/100,000 person-years, with unadjusted suicide rates being highest among patients with new starts of mirtazapine. Venlafaxine had the second highest crude suicide rate, followed by paroxetine, citalopram, sertraline, fluoxetine, and bupropion.
Table 1.
Crude suicide rates during exposure for users of commonly prescribed antidepressants
| Antidepressant | Suicides | Exposure | Suicide Rate* | Suicide Rate* by Time Periods Following Initiation |
||
|---|---|---|---|---|---|---|
| Agent | N | Days | [0-90 days) | [91-180 days) | [181-365 days) | |
|
|
|
|||||
| Citalopram | 77 | 17,086,258 | 165 (130, 206) | 633 (475, 826) | 805 (440, 1350) | 60 (20, 141) |
| Bupropion | 12 | 4,963,443 | 88 (46, 154) | 189 (82, 372) | 341 (41, 1232) | 117 (14, 422) |
| Fluoxetine | 26 | 9,279,959 | 102 (67, 150) | 451 (276, 697) | 204 (25, 737) | 91 (25, 232) |
| Venlafaxine | 15 | 2,815,656 | 195 (109, 321) | 698 (335, 1284) | 703 (85, 2539) | 145 (18, 522) |
| Sertraline | 57 | 18,921,772 | 110 (83, 143) | 515 (374, 691) | 285 (92, 664) | 22 (2.6, 78) |
| Paroxetine | 45 | 9,141,532 | 180 (131, 241) | 799 (553, 1116) | 0 | 134 (49, 293) |
| Mirtazapine | 15 | 2,221,194 | 247 (138, 407) | 456 (183, 940) | 1173 (242, 3427) | 319 (66, 933) |
Note: Values in brackets are 95% confidence intervals.
Per 100,000 person years
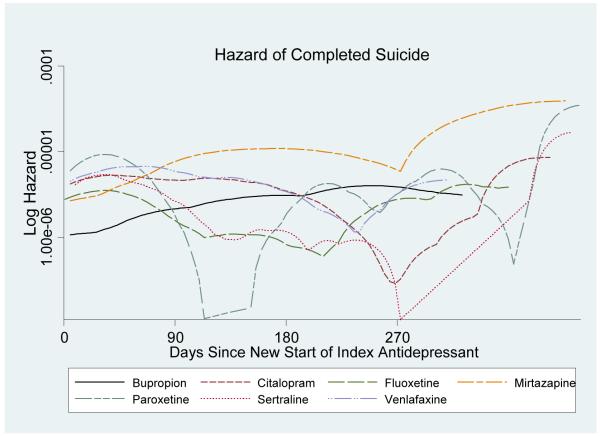
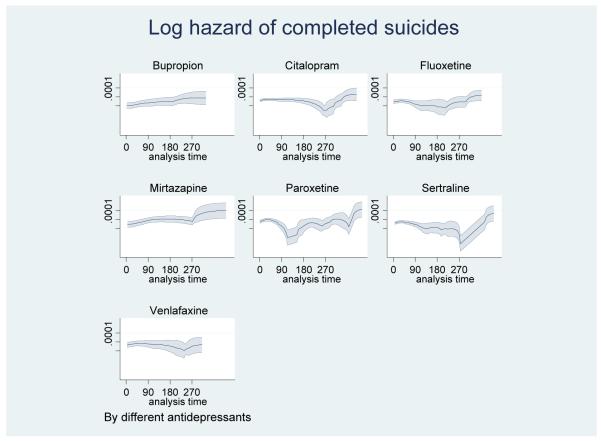
Table 1, Figure 1, and Figure 2 show the hazards of suicide with the individual agents over time. In the figures of smoothed log hazards, most antidepressants showed constant or somewhat decreasing hazards over time; however, mirtazapine showed higher suicide risks in more distal periods. Figure 2 includes 95% point-wise confidence bands.
Figure 1.
Log hazard of completed suicide over the exposure days since new start of index antidepressant
Figure 2.
Log hazard of completed suicide (95% confidence bands) by exposure days since new antidepressant start
Multivariate Analysis
In conventional Cox regression, IPTW, and propensity stratified Cox models (Table 2 and 3), patients with new starts of bupropion had lower suicide risks than those with new starts of citalopram, mirtazapine, and paroxetine. Patients with new fluoxetineor sertraline starts had lower suicide risks than paroxetine. The conventional Cox model, IPTW, and propensity stratified Cox model gave similar relative risk estimates. Although not shown, results from the analysis stratified by propensity quintiles also gave similar results to those using propensity stratification matching.
Table 2.
Adjusted* hazard ratios for suicide death during antidepressant exposure using traditional Cox regression model (N=502,179)
| Antidepressant Agent | Hazard Ratio | |
|---|---|---|
| Citalopram | 1.0 | |
| Bupropion | 0.45 (0.26, 0.81)† | |
| Fluoxetine | 0.63 (0.38, 1.06) | |
| Sertraline | 0.70 (0.48, 1.02) | |
| Venlafaxine | 0.89 (0.51, 1.56) | |
| Paroxetine | 1.06 (0.75, 1.51) | |
| Mirtazapine | 1.11 (0.56, 2.19) | |
| Age at time of first new start (reference = < 40 years) | 40-49 yrs | 0.79 (0.47, 1.32) |
| 50-64 yrs | 0.70 (0.47, 1.05) | |
| 65+ yrs | 0.99 (0.61, 1.62) | |
| Sex | Male | 2.57 (1.27, 5.19)† |
| Race (reference = White) | Black | 0.18 (0.08, 0.44)‡ |
| Other | 0.42 (0.10, 1.76) | |
| Unknown | 2.33 (1.53, 3.54)‡ | |
| Hispanic ethnicity | 0.26 (0.11, 0.63)‡ | |
| Alcohol use | 1.48 (0.90, 2.41) | |
| Post traumatic stress disorder | 0.60 (0.36, 0.99)† | |
| Major depression | 1.97 (1.44, 2.69)‡ | |
| Other anxiety disorder | 1.37 (0.99, 1.89) | |
| Suicide attempt | 2.96 (1.30, 6.73)† | |
| No. of Charlson comorbidities (reference = 0) | 1 | 0.92 (0.67, 1.25) |
| 2 | 0.73 (0.46, 1.17) | |
| 3+ | 0.65 (0.34, 1.27) | |
| No. of in-patient psychiatric stays (reference = 0) | 1 | 4.28 (2.55, 6.20)‡ |
| >1 | 2.93 (1.33, 6.46)† | |
| No. of outpatient mental health visits (reference = 0) | 1-2 | 0.87 (0.64, 1.17) |
| >2 | 0.68 (0.41, 1.13) | |
| No. of psychiatric medications (reference = 0) | 1 | 1.26 (0.89, 1.78) |
| >1 | 2.21 (1.48, 3.29)‡ | |
| Patient is service connected | 0.85 (0.63, 1.14) | |
| Days♣ since first depression diagnosis to the new start | 0.95 (0.90, 0.99)† | |
| Region of facility of most use (reference = West) | Midwest | 0.71 (0.52, 0.97)† |
| Northeast | 0.77 (0.52, 1.14) | |
| South | 0.85 (0.61, 1.18) | |
| Fiscal year of first new start (reference = 1999) | 2000 | 1.07 (0.61, 1.89) |
| 2001 | 1.42 (0.75, 2.68) | |
| 2002 | 0.81 (0.42, 1.57) | |
| 2003 | 0.84 (0.43, 1.62) | |
| 2004 | 0.75 (0.39, 1.41) |
Note: Values in brackets are 95% confidence intervals. All diagnosis and service use variables are based on one year prior to the new start of the antidepressant agents, except for suicide attempt and whether the patient is service connected or not, which are based on three years prior to the new start of the antidepressant
In addition to the variables included in the table, adjusted also for any drug use disorder, tobacco use disorder and personality disorder diagnosis, Medicare use in prior year, number of outpatient visits and urban location facility
In units of 100 days and truncated at 2 yrs
p < 0.05
p < 0.005
Table 3.
Adjusted hazard ratios for suicide death associated with each antidepressant agent vs. citalopram and other pair-wise contrasts based on traditional Cox regression, inverse probability of treatment weighted (IPTW) model, propensity stratified (PS) model and marginal structural model (MSM) (N = 502,179).
| Antidepressant Agent | Traditional Cox | IPTW | PS | MSM♣ | MSM¶ |
|---|---|---|---|---|---|
| Citalopram | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Bupropion | 0.45 (0.26, 0.81)† | 0.50 (0.27, 0.92)† | 0.48 (0.26,0.91)† | 0.55 (0.20, 1.53) | 0.46 (0.16, 1.34) |
| Fluoxetine | 0.63 (0.38, 1.06) | 0.61 (0.36, 1.03) | 0.64 (0.36, 1.13) | 0.45 (0.28, 0.72)‡ | 0.52 (0.32, 0.85)† |
| Sertraline | 0.70 (0.48, 1.02) | 0.66 (0.45, 0.98)† | 0.71 (0.47, 1.06) | 0.49 (0.33, 0.72)‡ | 0.60 (0.40, 0.90)† |
| Venlafaxine | 0.89 (0.51, 1.56) | 0.94 (0.51, 1.73) | 0.84 (0.48, 1.49) | 0.86 (0.48, 1.56) | 0.91 (0.49, 1.70) |
| Paroxetine | 1.06 (0.75, 1.51) | 1.06 (0.74, 1.53) | 1.10 (0.75, 1.63) | 0.85 (0.56, 1.31) | 0.95 (0.60, 1.50) |
| Mirtazapine | 1.11 (0.56, 2.19) | 0.97 (0.47, 1.98) | 1.13 (0.53, 2.39) | 2.12 (0.92, 4.86) | 0.88 (0.43, 1.76) |
|
| |||||
| Other Pair-wise Contrasts* | |||||
|
| |||||
| Bupropion vs. Fluoxetine | 0.72 (0.36, 1.44) | 0.82 (0.39, 1.73) | 0.86 (0.40, 1.85) | 1.23 (0.43, 3.51) | 0.89 (0.30, 2.63) |
| Bupropion vs. Sertraline | 0.65 (0.36, 1.18) | 0.76 (0.39, 1.47) | 0.68 (0.37, 1.25) | 1.13 (0.41, 3.11) | 0.77 (0.27, 2.20) |
| Bupropion vs. Venlafaxine | 0.50 (0.24, 1.04) | 0.53 (0.23, 1.25) | 0.59 (0.25, 1.41) | 0.64 (0.21, 1.89) | 0.51 (0.16, 1.59) |
| Bupropion vs. Paroxetine | 0.43 (0.23, 0.78)† | 0.47 (0.25, 0.91)† | 0.34 (0.17, 0.67)‡ | 0.64 (0.23, 1.79) | 0.49 (0.17, 1.41) |
| Bupropion vs. Mirtazapine | 0.41 (0.18, 0.94)† | 0.52 (0.22, 1.20) | 0.45 (0.20, 1.01) | 0.26 (0.08, 0.89)† | 0.53 (0.16, 1.72) |
| Fluoxetine vs. Sertraline | 0.91 (0.59, 1.40) | 0.92 (0.60, 1.43) | 0.87 (0.54, 1.39) | 0.92 (0.58, 1.45) | 0.86 (0.54, 1.38) |
| Fluoxetine vs. Venlafaxine | 0.69 (0.35, 1.38) | 0.65 (0.32, 1.32) | 0.59 (0.30, 1.20) | 0.52 (0.27, 0.97)† | 0.57 (0.29, 1.11) |
| Fluoxetine vs. Paroxetine | 0.60 (0.39, 0.90)† | 0.58 (0.37, 0.90)† | 0.53 (0.34, 0.81)† | 0.52 (0.32, 0.85)† | 0.54 (0.33, 0.90)† |
| Fluoxetine vs. Mirtazapine | 0.57 (0.26, 1.25) | 0.63 (0.28, 1.41) | 0.49 (0.22, 1.06) | 0.21 (0.09, 0.51)‡ | 0.59 (0.28, 1.24) |
| Sertraline vs. Venlafaxine | 0.76 (0.42, 1.38) | 0.71 (0.36, 1.37) | 0.70 (0.37, 1.33) | 0.56 (0.32, 1.00) | 0.66 (0.36, 1.21) |
| Sertraline vs. Paroxetine | 0.62 (0.47, 0.93)† | 0.62 (0.44, 0.88)† | 0.64 (0.44, 0.92)† | 0.57 (0.38, 0.86)† | 0.63 (0.41, 0.96)† |
| Sertraline vs. Mirtazapine | 0.63 (0.31, 1.28) | 0.68 (0.33, 1.45) | 0.53 (0.26, 1.09) | 0.23 (0.10, 0.53)‡ | 0.69 (0.35, 1.35) |
| Venlafaxine vs. Paroxetine | 0.86 (0.46, 1.61) | 0.88 (0.44, 1.78) | 0.75 (0.38, 1.48) | 1.01 (0.56, 1.84) | 0.96 (0.51, 1.81) |
| Venlafaxine vs. Mirtazapine | 0.83 (0.35, 1.96) | 0.97 (0.37, 2.52) | 0.65 (0.26, 1.63) | 0.41 (0.16, 1.04) | 1.04 (0.45, 2.39) |
| Paroxetine vs. Mirtazapine | 0.96 (0.45, 2.04) | 1.10 (0.50, 2.42) | 0.98 (0.44, 2.19) | 0.40 (0.17, 0.95)† | 1.09 (0.54, 2.21) |
Note: Results are from first episode of treatment only and adjusted for within facility clustering. Values in brackets are 95% confidence intervals.
p < 0.05
p < 0.005
For traditional Cox model, IPTW model and marginal structural model based estimates, other pair wise contrasts are done using post-hoc linear combination estimation based on a model fit using all new starts of the seven antidepressants and using citalopram as the reference group. For propensity stratified model estimates, each of the 21 pair-wise comparisons are done based on new starts of specific pairs of antidepressants. All estimates, including the MSM model estimates, were based on additional adjustment for a limited set of baseline covariates.
Based on 12 months follow-up data
Based on 6 months follow-up data.
When increasing or decreasing risk ratios in time by medication were modeled using time by medication interaction in the Cox model, mirtazapine showed increasing risks in time (p = 0.04), and sertraline showed decreasing risks over time (p = 0.05).
In the MSM models, lower suicide risks were again seen for fluoxetine and sertraline compared to paroxetine. However, MSM models did not show reduced suicide risks for patients on bupropion compared to citalopram or paroxetine. Only the MSM model censored at 6 months showed reduced risks for bupropion compared to mirtazapine.
In IV analyses, the F-statistics were larger than 30, and the majority of partial R2 values ranged from 0.012 to 0.0283, although R2 values were lower for venlafaxine (0.003) and bupropion (0.005). Thus, our six IV instruments consisting of fractional fills for each antidepressant at the patient’s facility of most use were predictive of antidepressant choice. In the IV model that included these 6 instruments, no antidepressant agent showed significantly different risks of suicide death than citalopram in the 12 months following treatment initiation (Table 4). Subsequent pair-wise antidepressant agent contrasts also did not show different risks of suicide death between antidepressant agents. Results based on an IV model optimized using generalized method of moments35 gave similar results to those based on 2-stage least squares.
Table 4.
Adjusted difference* in risk of suicide estimated by inverse probability of treatment weighted (IPTW) model and two-stage instrumental variable (IV) method
| Variables | IPTW estimates | IV estimates |
|---|---|---|
| Bupropion vs. Citalopram | −35.58 (−58.50, −12.65)† | −78.31 (−360.53, 203.91) |
| Fluoxetine vs. Citalopram | −18.98 (−40.56, 2.59) | −42.53 (−138.73, 53.67) |
| Sertraline vs. Citalopram | −19.89 (−37.87, −1.91)† | −8.04 (−109.53, 93.45) |
| Venlafaxine vs. Citalopram | −1.02 (−38.42, 36.38) | −45.36 (−556.58, 465.86) |
| Paroxetine vs. Citalopram | 1.11 (−23.00, 25.21) | −60.95 (−193.77, 71.87) |
| Mirtazapine vs. Citalopram | −9.99 (−48.66, 28.67) | −149.17 (−406.23, 107.89) |
Note: Based on linear probability model of completed suicide within one year of newly starting an antidepressant; includes only first episode of new start of antidepressant treatment per patient. Values in brackets are 95% confidence intervals.
Risk difference expressed as per 100,000, and adjusted for age, gender, race, ethnicity, any alcohol use disorder, drug use disorder, tobacco use disorder, post-traumatic stress disorder diagnosis, major depression, other anxiety disorder, personality disorder diagnosis, Medicare use in prior year, receipt of psychotherapy in prior year, suicide attempt, numbers of Charlson comorbidities, inpatient psychiatric days, outpatient visits, outpatient mental health visits and psychiatric medications, service connectedness, number of days since first depression diagnosis to new start, urban location facility, region of facility, fiscal year of first new start
p < 0.05
The IPTW linear probability model (Table 4) gave results that were congruent with relative risk estimates based on various non-linear models (Table 3).
DISCUSSION
This is the largest study, to date, to examine the relationship between specific antidepressant agents and suicide death. Comprehensive longitudinal data for a large sample of high-risk patients allowed us to employ multiple analytic strategies to address potential treatment biases, 36,34 the major methodological issue for studies using observational data. Analytic approaches included conventional Cox models with covariate adjustment, Cox models with IPTW, propensity stratified Cox models, marginal structural models, and an instrumental variable approach.
The results from these different approaches converged in most instances and indicated that most antidepressant agents did not differ in their risks for suicide death.
However, conventional Cox models and the IPTW and propensity stratified Cox models all suggested lower risks with sertraline and fluoxetine than with paroxetine and suggested lower risks with bupropion than for several other antidepressants. MSM models confirmed the findings for sertraline and fluoxetine but not for bupropion.
IV models did not indicate any differences in suicide risks with specific antidepressant agents. Given appropriate instruments, the IV approach theoretically can better account for unmeasured confounders. However, the IV approach typically involves a loss of statistical power, because it is based on variation in treatment due to IVs rather than the full variation in treatment. This loss in power may limit the application of this approach where the event of interest (e.g., suicide) has a very low base rate and power is a pivotal issue. Nevertheless, the finding in IV analyses of no differences in risk across antidepressants is consistent with findings of Schneeweiss et. al, who used a high-dimensional propensity adjusted analytic approach.19
We note that the study findings of lower risks of suicide with fluoxetine and sertraline than paroxetine across several analytic approaches is consistent with a few observational studies that reported lower risks with fluoxetine than for other antidepressants among adults discharged from hospitals in Finland18 or a trend towards lower risks with sertraline among adult Medicaid patients discharged from the hospital.15
However, it may be more informative to compare the current study’s results to those of studies using data from RCTs, as RCT findings may be limited by sample size and use of proxy outcomes but are not subject to treatment selection biases. Of interest, the FDA meta-analyses of RCTs in adults which examined the relationship between a composite measure of “suicidality” and antidepressant use, also reported lower risks for sertraline than for other SSRI and non-SSRI agents when compared to placebo. In this meta-analysis, there was also a trend (p=.11) for decreased risk ratio for fluoxetine. However, the FDA analysis reported no particular signal for increased risks with paroxetine.
A re-analyses of FDA data from the meta-analysis for antidepressants among youth also reported a relationship between antidepressant half-life and suicide risks, with fluoxetine use being associated with the lowest risk (RR, 0.52).16
However, despite some degree of congruency of this study’s findings with the FDA meta-analysis of RCTs, study results may still be affected by residual confounding. The FDA meta-analysis did not find indications of lower risks with bupropion, and we suspect the present study’s limited findings regarding bupropion were due to remaining confounding. Bupropion is often used for smoking cessation, and despite the requirement of a depression diagnosis and adjustment for comorbid tobacco use disorder, patients with milder depression may still have been more likely to receive bupropion for smoking cessation and have also been at less risk of suicide due to milder symptoms. Schneeweiss et al. excluded patients receiving bupropion because of this alternative treatment indication.19
Limitations
The VA study population consists predominantly of men and older individuals, and study findings may not generalize to other treatment populations. Overall suicide risks associated with antidepressant use differ by patient age, 5,17 and it is possible that risks associated with specific antidepressant agents may also vary with age.
We relied on antidepressant prescription fills to determine antidepressant exposure. Some patients may have used mental health services outside of the VA health system and have had antidepressant starts that were not recorded in our dataset. However, during the study period, patients eligible for VA coverage often exclusively used VA pharmacies because of the VA’s generous drug benefit.37
There are limited data on patient symptoms in administrative data and inclusion of more detailed patient data might have allowed improved adjustment for potential confounders.
We excluded patients who developed bipolar disorder or schizophrenia during the study period as we believed such patients may have been misdiagnosed as having a depressive disorder. However, this may have resulted in some misspecification of risk for specific antidepressants if patients with less certain depression diagnoses were given an agent preferentially and were also less likely to live long enough to receive a second, corrected diagnosis of bipolar disorder or schizophrenia.38
We also assessed most covariates for the 12 months prior to the index date of the new antidepressant but required only a 6-month clean period to define a new antidepressant start. This might have differentially affected some covariate values (e.g., number of outpatient visits) if specific antidepressant agents were more likely to be preceded by antidepressant exposure during the 12 to 6 month period before the index date.
Finally, we completed multiple comparisons for antidepressant agents, using several different analytic approaches to address selection biases. Given the low base rate of suicide, we did not correct for multiple comparisons, and instead emphasized results that were consistent across analytic approaches and that had substantial effect sizes.
Summary
Head-to-head comparisons of suicide risks of different antidepressant agents are difficult to conduct given the enormous sample sizes required. We completed the largest study to date, examining this relationship in over 500,000 high-risk VA patients. Across a variety of models, fluoxetine and sertraline appeared to have lower risks than paroxetine, findings that are reasonably congruent with those of the FDA meta-analysis of RCTs, which found indications that these agents might have lower risks than other antidepressants. However, we note that IV analyses did not provide additional support for these findings. The divergence observed in findings by analytic approach and the possibility of residual confounding suggests continued caution when using observational data to assess suicide risks associated with antidepressant agents.
Acknowledgements
The funding sources for this work were the Department of Veterans Affairs, Health Services Research and Development Service, IIR 04-211-1, MRP 03-320, CD2 07-206-1,CDA 10-036-1, and the National Institute of Mental Health, R01-MH078698-01. Resources were also contributed by SMITREC, Ann Arbor, MI. We wish to acknowledge the contributions of Drs. Robert Gibbon and Hendricks C. Brown who graciously read this manuscript and provided comments.
Footnotes
All authors will complete the Unified Competing Interest form. No authors have had organizational support that might appear to have influenced the submitted work.
References
- 1.Harris EC, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205–28. doi: 10.1192/bjp.170.3.205. [DOI] [PubMed] [Google Scholar]
- 2.Ilgen MA, Bohnert AS, Ignacio RV, et al. Psychiatric diagnoses and risk of suicide in veterans. Arch Gen Psychiatry. 2010;67:1152–8. doi: 10.1001/archgenpsychiatry.2010.129. [DOI] [PubMed] [Google Scholar]
- 3.Reports of Suicidality in Pediatric Patients Being Treated with Antidepressant Medications for Major Depressive Disorder (MDD) US Food and Drug Administration; [Accessed December 30, 2010]. 2003. 2010, at http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm16 8828.htm. [Google Scholar]
- 4.Office of Drug Safety Cover Memorandum Follow-up Consult of August 16, 2004 by Andrew Mosholder on Suicidality in pediatric clinical trials with paroxetine and other antidepressant drugs. 2004 Accessed at http://www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4065b1-11-TAB09a-Mosholder-review.pdf.
- 5. [Accessed December 13, 2006];Overview for December 13 Meeting of Psychpharmacologic Drugs Advisory Committee. at http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4272b1-01-FDA.pdf.
- 6.Racagni G, Popoli M. The pharmacological properties of antidepressants. Int Clin Psychopharmacol. 2010;25:117–31. doi: 10.1097/YIC.0b013e3283311acd. [DOI] [PubMed] [Google Scholar]
- 7.Pfeiffer PN, Ganoczy D, Ilgen M, et al. Comorbid anxiety as a suicide risk factor among depressed veterans. Depress Anxiety. 2009;26:752–7. doi: 10.1002/da.20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilgen MA, Zivin K, McCammon RJ, Valenstein M. Pain and suicidal thoughts, plans and attempts in the United States. Gen Hosp Psychiatry. 2008;30:521–7. doi: 10.1016/j.genhosppsych.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wojnar M, Ilgen MA, Wojnar J, et al. Sleep problems and suicidality in the National Comorbidity Survey Replication. J Psychiatr Res. 2009;43:526–31. doi: 10.1016/j.jpsychires.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fergusson D, Doucette S, Glass KC, et al. Association between suicide attempts and selective serotonin reuptake inhibitors: systematic review of randomised controlled trials. BMJ. 2005;330:396. doi: 10.1136/bmj.330.7488.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez C, Rietbrock S, Wise L, et al. Antidepressant treatment and the risk of fatal and non-fatal self harm in first episode depression: nested case-control study. BMJ. 2005;330:389. doi: 10.1136/bmj.330.7488.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubino A, Roskell N, Tennis P, et al. Risk of suicide during treatment with venlafaxine, citalopram, fluoxetine, and dothiepin: retrospective cohort study. BMJ. 2007;334:242. doi: 10.1136/bmj.39041.445104.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292:338–43. doi: 10.1001/jama.292.3.338. [DOI] [PubMed] [Google Scholar]
- 14.Juurlink DN, Mamdani MM, Kopp A, et al. The risk of suicide with selective serotonin reuptake inhibitors in the elderly. Am J Psychiatry. 2006;163:813–21. doi: 10.1176/ajp.2006.163.5.813. [DOI] [PubMed] [Google Scholar]
- 15.Olfson M, Marcus SC, Shaffer D. Antidepressant drug therapy and suicide in severely depressed children and adults: A case-control study. Arch Gen Psychiatry. 2006;63:865–72. doi: 10.1001/archpsyc.63.8.865. [DOI] [PubMed] [Google Scholar]
- 16.Smith EG. Association between antidepressant half-life and the risk of suicidal ideation or behavior among children and adolescents: confirmatory analysis and research implications. J Affect Disord. 2009;114:143–8. doi: 10.1016/j.jad.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Stone M, Laughren T, Jones ML, et al. Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug Administration. BMJ. 2009;339:b2880. doi: 10.1136/bmj.b2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiihonen J, Lonnqvist J, Wahlbeck K, Klaukka T, et al. Antidepressants and the risk of suicide, attempted suicide, and overall mortality in a nationwide cohort. Arch Gen Psychiatry. 2006;63:1358–67. doi: 10.1001/archpsyc.63.12.1358. [DOI] [PubMed] [Google Scholar]
- 19.Schneeweiss S, Patrick AR, Solomon DH, et al. Variation in the risk of suicide attempts and completed suicides by antidepressant agent in adults: a propensity score-adjusted analysis of 9 years’ data. Arch Gen Psychiatry. 2010;67:497–506. doi: 10.1001/archgenpsychiatry.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneeweiss S, Patrick AR, Solomon DH, et al. Comparative safety of antidepressant agents for children and adolescents regarding suicidal acts. Pediatrics. 2010;125:876–88. doi: 10.1542/peds.2009-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowper DC, Kubal JD, Maynard C, et al. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12:462–8. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 22.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Disease. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Schneeweiss S, Maclure M. Use of comorbidity scores for control of confounding in studies using administrative databases. Int J Epidemiol. 2000;29:891–8. doi: 10.1093/ije/29.5.891. [DOI] [PubMed] [Google Scholar]
- 25.Kim HM, Zivin K, Ganoczy D, et al. Predictors of alternative antidepressant agent initiation among U. S. veterans diagnosed with depression. Pharmacoepidemiol Drug Saf. 2010;19:1049–56. doi: 10.1002/pds.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Kurth T, Walker AM, Glynn RJ, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163:262–70. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 28.Becker SO, Ichino A. Estimation of average treatment effects based on propensity scores. The Stata Journal. 2002;2:358–77. [Google Scholar]
- 29.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–70. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Newhouse JP, McClellan M. Econometrics in outcomes research: the use of instrumental variables. Annu Rev Public Health. 1998;19:17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 31.Angrist JD, Imbens G, Rubin DB. Identifcation of causal effects using instrumental variables. Journal of the American Statistical Association. 1996;99:444–55. [Google Scholar]
- 32.Brookhart MA, Rassen JA, Schneeweiss S. Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf. 2010;19:537–54. doi: 10.1002/pds.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rassen JA, Brookhart MA, Glynn RJ, et al. Instrumental variables I: instrumental variables exploit natural variation in nonexperimental data to estimate causal relationships. J Clin Epidemiol. 2009;62:1226–32. doi: 10.1016/j.jclinepi.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HM, Zivin K, Ganoczy D, Pfeiffer P, Hoggatt K, McCarthy JF, Downing K, Valenstein M. Predictors of alternative antidepressant agent initiation among U. S. veterans diagnosed with depression. Pharmacoepidemiol Drug Saf. 2010;19(10):1049–56. doi: 10.1002/pds.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.IVREG2: Stata module for extended instrumental variables/2SLS, GMM and AC/HAC, LIML, and k-class regression. Boston College Department of Economics, Statistical Software Components S425401; [Accessed December, 2010, 2010]. 2007. http://ideas.repec.org/c/boc/bocode/s425401.html. [Google Scholar]
- 36.Zivin K, Kim HM, McCarthy JF, et al. Suicide mortality among individuals receiving treatment for depression in the Veterans Affairs health system: associations with patient and treatment setting characteristics. Am J Public Health. 2007;97:2193–8. doi: 10.2105/AJPH.2007.115477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piette JD, Heisler M. Problems due to medication costs among VA and non-VA patients with chronic illnesses. Am J Manag Care. 2004;10:861–8. [PubMed] [Google Scholar]
- 38.Schneeweiss S. A basic study design for expedited safety signal evaluation based on electronic healthcare data. Pharmacoepidemiol Drug Saf. 2010;19:858–68. doi: 10.1002/pds.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]