Abstract
Aim
To identify human hair cell progenitors from adult inner ear sensory epithelium.
Materials & methods
We collected discarded utricles from translabyrinthine surgery and isolated human utricular sensory epithelial cells (HUCs) to explore whether they can proliferate and obtain features of stem/progenitor cells in vitro using reverse transcription PCR and immunofluorescence.
Results
When cultured in vitro, HUCs expressed genes and proteins that are usually present in prosensory cells and stem cells. Additionally, dissociated HUCs expanded on the substrates and presented properties of mesenchymal cells via epithelial-to-mesenchymal transition.
Conclusion
The results reveal that sensory epithelial cells from the adult human inner ear can re-enter the cell cycle and adopt a stem/progenitor cell fate. The outcomes of this study may open avenues for human hair cell progenitor generation, which could potentially provide a novel stem cell-based replacement for hearing loss and other inner ear disorders.
Keywords: cell replacement, epithelial-to-mesenchymal transition, epithelium, hair cell, hearing loss, proliferation, prosensory cell, regeneration, stem cell
Mammalian sensory hair cells are vulnerable to numerous insults, such as overstimulation, aging, genetic disorders and ototoxic drugs. Damage to a mammalian sensory hair cell is usually irreversible; therefore, degeneration of hair cells generally causes permanent hearing loss, tinnitus, motion sickness and other inner ear disorders. Generation of hair cell progenitors with the capability of proliferation and differentiation has been suggested as one of the critical steps in hair cell regeneration [1,2]. It has been documented that mouse inner ear sensory epithelia possess stem/ progenitor cells, which show the ability to proliferate and differentiate in vitro [3,4]. However, it remains undetermined how adult human utricular cells respond when dissociated and cultured in vitro and whether stem/progenitor cells exist in adult human inner ear sensory epithelia.
It is a challenge to obtain inner ear sensory epithelial cells from adult human beings. The human inner ear lies within the temporal bone, which is not as easy to access as other tissues, such as skin. Owing to ethical considerations, it is virtually impossible to obtain inner ear tissues from normal adults. Fortunately, an alternative exists in the discarded tissues from surgery. Vestibular schwannomas (also called acoustic neuromas) are benign tumors arising from the schwann cells of cranial nerve VIII. In cases of schwannomas with indications for a trans-labyrinthine surgical approach [5], the vestibular system, including the utricle and semicircular canals, are removed and discarded as part of the surgical approach to the tumor. It is therefore feasible to collect these discarded tissues from surgery and use them as a human model to investigate whether adult human utricle sensory epithelial cells (HUCs) are able to proliferate when cultured in vitro. During embryonic development, prosensory cells in the developing inner ear (otocyst) give rise to either vestibular or cochlear sensory patch, which, in turn, differentiates into its final fate as hair cells and supporting cells [6]. One of the major aims of this study is to determine whether adult human inner ear sensory epithelial cells can adopt a pro-sensory cell fate in vitro, which is the first step for an effective regeneration strategy. In future studies, we will use these stem/progenitor cells to produce large numbers of new sensory hair cells as replacement cell resources for in vitro and in vivo research.
Materials & methods
Isolation of sensory epithelial cells from human utricles & generation of cloned HUC cell lines
All human sample collection procedures have been approved by the local Human Investigation Committee. Owing to the fact that discarded surgical specimens were collected in such a manner that participants cannot be identified, this study qualifies for informed consent exemption under US federal regulations. Pure sensory epithelial sheets were harvested from utricles discarded during two vestibular schwannoma surgeries that used a translabyrinthine approach. The utricles were treated with 0.5 mg/ml thermolysin (Sigma) at 37°C for 30 min and the sensory epithelium was lifted from the stroma using the tip of a 27 ga needle [1,7]. All the edges of the sensory epithelial sheet were trimmed away so that only the central part of the sheet was collected. The pure sensory epithelial sheets were cut into 1–2 mm2 pieces, which were rinsed with 0.1 M phosphate-buffered saline, then transferred into a new 15-ml centrifuge tube [7]. Following dissociation with 1 ml papain mixture (Sigma) at 37°C for 1 h [8], the sensory epithelial pieces were treated with 9 ml of DMEM/F12 with 10% fetal bovine serum (FBS; all from Invitrogen) to end dissociation. The cell suspension was centrifuged at 200 g for 5 min. The supernatant was removed and the cells were resuspended in 1 ml DMEM/F12 supplemented with 10% FBS. The cell suspension was gently triturated 10–15 times and plated into a 24-well plate precoated with 0.1% gelatin (Millipore) and containing prewarmed primary culture medium (DMEM/ F12, 15% FBS, 1% insulin transferrin selenium [Invitrogen], 0.1% 2-mercaptoethanol [Invitrogen], 0.1% ampicillin [Fishersci], 20 ng/ml FGF2 [Invitrogen] and 20 ng/ml EGF [Invitrogen]). The cells were then cultured in humidified 5% CO2 and 95% air at 37°C. Half of the culture medium was replaced every 2–3 days. The primary culture was replicated twice using discarded utricles from schwannoma surgery. When the cells in the primary culture reached 70–80% confluence in the culture wells, TrypLE™ (Invitrogen) was used to dissociate cells, followed by serum-containing medium to stop dissociation. The cell suspension was centrifuged at 200 g for 3 min, and the cells were resuspended into 1 ml of culture medium. A hemocytometer was used to evaluate the cell number and the cells were plated into a T25 culture flask (Nunc) at a density of approximately 2000 cells/cm2. These passage 1 cells were cultured in the expansion culture medium (DMEM/F12, 10% FBS, 1% insulin transferrin selenium, 0.1% 2-mercaptoethanol and 0.1% ampicillin). Samples of passage 1 cells were cultured on a glass cover slip and maintained for 4–6 days, and then fixed for immunofluorescence. When passage 1 cells reached 70–80% confluence, the cells were harvested, centrifuged and suspended using the aforementioned methods. Cell suspensions were diluted and added into 96-well plates at the ratio of 0–2 cells/well. Solitary cells wereidentified and their growth followed for 5–6 passages to obtain sufficient number of cells for the study. To maintain cell lines, some of the cells were frozen in culture medium supplemented with 5% dimethyl sulfoxide. Samples of cloned HUCs (cHUCs) after passage 6–7 were used for experimental purposes.
Proliferation assay
Two methods were applied to characterize cell proliferation. Firstly, cHUCs (passage 8) were cultured for 24 h in the culture media containing 3 μg/ml 5-bromo-2-deoxyuridine (BrdU; Sigma) at culture days 1, 3, 5 and 7 [7]. At the end of culture periods, cHUCs were fixed in 4% paraformaldehyde followed by DNA denaturization in 1 M HCl at room temperature for 30 min. The samples were blocked in 10% donkey serum and 0.2% Triton™ X-100 (Sigma Aldrich) at room temperature for 30 min followed by mouse anti-BrdU antibodies overnight at 4°C. Dylight® 549-conjugated secondary antibodies (Jackson ImmunoResearch) were applied to the samples to visualize cells using a Leica epifluorescence microscope with appropriate filters. DAPI (4′,6-diamidino-2-phenylindole) was used for the detection of all nuclei in the samples. Three microscope fields of each sample were randomly selected, and the images were captured using a digital monochrome cooled CCD camera (Q Imaging). The total cell number and the number of cells labeled by both BrdU and DAPI were counted in each field and the percentage of cells incorporating BrdU was calculated at each culture period.
Secondly, a f luorescence-detection-based CyQuant® NF cell proliferation assay (Invitrogen) was used to determine cell proliferation. This method can directly observe cell growth without the need for nuclear incorporation. Passage 10 cHUCs were used to set up a standard curve with a microplate reader (Molecular devices) using the manufacturer’s instruction in order to evaluate the correlation between cell number and fluorescence intensity. To evaluate cell proliferation, approximately 1000 cHUCs (passage 10) were seeded into each well of a 96-well plate and incubated for at least 4 h for cell attachment. The fluorescence intensity of the cells was evaluated at 4 h and 1, 2, 4, 5, 6, 7 and 8 days. The fluorescence intensity of the cells was detected when the culture medium was removed and CyQuant NF dye (100 μl) was applied for 40 min. Cell number was evaluated using the aforementioned standard curve. Six replicates were used for each time point.
RNA preparation & reverse transcription PCR
An RNeasy® mini extract kit (Qiagen) was used to extract total RNA from samples of pure utricular sensory epithelial sheets and passage 8–25 cHUCs. A reverse transcription kit (Qiagen) was used to synthesize cDNA. All procedures were performed according to the manufacturers’ instructions. Primers and conditions for reverse transcription PCR (RT-PCR) are listed in Table 1.
Table 1.
Primers and conditions for reverse transcription PCR.
| Gene name | Forward | Reverse | Product length (bp) | Annealing temperature (°C) |
|---|---|---|---|---|
| KRT18 | 5′-ATCTCAGGACCTCGCCAAGA-3′ | 5′-TGTTCTCCAAGCTGGCCTTC-3′ | 245 | 55 |
| KRT18 | 5′-GCATCGTTCTGCAGATTGACA-3′ | 5′-GGATGTCTGCCATGATCTTGG-3′ | 312 | 55 |
| CDH1 | 5′-TGGATAGAGAACGCATTGCCA-3′ | 5′-GTGTTCACATCATCGTCCGC-3′ | 226 | 55 |
| CDH1 | 5′-GGCTGGAGATTAATCCGGACA-3′ | 5′-AATGGTCCAGTTGGCACTCG-3′ | 326 | 55 |
| CDH1 | 5′-CTTGTCATTGAGCCTGGCAA-3′ | 5′-CTGTGCACACCTGGAATTGG-3′ | 229 | 55 |
| ZEB1 | 5′-ACCCTTGAAAGTGATCCAGCC-3′ | 5′-GCTGTCACGTTCTTCCGCTT-3′ | 417 | 55 |
| CDH2 | 5′-TGCTCAGGACCCAGATCGATA-3′ | 5′-TGGAGTTTCGCAAGTCTCTGC-3′ | 289 | 55 |
| FN1 | 5′-AACGACACATTCCACAAGCGT-3′ | 5′-TGAGATGGCTGTGGTGCATTC-3′ | 329 | 55 |
| SNAI2 | 5′-GTCAAGAAGCATTTCAACGCC-3′ | 5′-TTGGAGGAGGTGTCAGATGGA-3′ | 272 | 55 |
| VIM | 5′-GCTTCGCCAACTACATCGACA-3′ | 5′-TCAAGGTCAAGACGTGCCAG-3′ | 325 | 55 |
| BMP4 | 5′-ATTCCGTAGTGCCATCCCGA-3′ | 5′-GCCCAAACATCTGCAGAAGTG-3′ | 325 | 55 |
| EYA1 | 5′-GCGCTGTGCAAACATCTCA-3′ | 5′-TTTCACTGCTGCTCATTGGC-3′ | 239 | 55 |
| SIX1 | 5′-TGCCGTCGTTTGGCTTTAC-3′ | 5′-TGGTTGTGAGGCGAGAACTG-3′ | 228 | 55 |
| DLX5 | 5′-CAACTTTGCCCGAGTCTTCAG-3′ | 5′-TTGCCATTCACCATTCTCACC-3′ | 294 | 55 |
| HES1 | 5′-ACAGTGAAGCACCTCCGGAA-3′ | 5′-TTGATCTGGGTCATGCAGTTG-3′ | 202 | 55 |
| JAG1 | 5′-AGTCGTGCATGCTCCAATCG-3′ | 5′-TCCAACTCGAACTGACCCGA-3′ | 254 | 55 |
| CDKN1B | 5′-GACCCGGGAGAAAGATGTCAA-3′ | 5′-CGAAATTCCACTTGCGCTG-3′ | 201 | 55 |
| LFNG | 5′-CATGAATACGGCTGAGCGGAT-3′ | 5′-TACAGGTGGCAGTGGATGGA-3′ | 266 | 55 |
| NOTCH1 | 5′-TGCACSOCCATGGTACCAATCA-3′ | 5′-GCTGGAGCATCTTCTTCGGA-3′ | 293 | 55 |
| POU5F1 | 5′-GTACTCCTCGGTCCCTTTCC-3′ | 5′-CAAAAACCCTGGCACAAACT-3′ | 344 | 55 |
| NANOG | 5′-AGCCCTGATTCTTCCACCAGT-3′ | 5′-CTTCTGCGTCACACCATTGC-3′ | 236 | 55 |
| SOX2 | 5′-GAACACCAATCCCATCCACAC-3′ | 5′-TCCTGCAAAGCTCCTACCGT-3′ | 281 | 55 |
| GAPDH | 5′-TGAAGGTCGGAGTCAACGGA-3′ | 5′-GATGGCATGGACTGTGGTCA-3′ | 533 | 55 |
Immunofluorescence
Samples from utricles, passage 1 HUCs and passages 8–25 cHUCs were fixed with 4% paraformaldehyde for 15–30 min at room temperature followed by immunofluorescence. Primary antibodies used in this study included: antibodies specific for epithelial cells, such as anti-E-cadherin (Santa Cruz), anti-ZO1 (Zymed), anti-pan-cytokeratin (cytokeratins 4, 5, 6, 8, 10, 13 and 18; Abcam); antibodies specific for mesenchymal cells, such as anti-Snail2 (slug; Developmental Studies Hybridoma Bank [DSHB]), vimentin (DSHB), N-cadherin (DSHB), fibronectin (Millipore); antibodies specific for prosensory cells, such as BMP4 (Millipore) and Islet1 (DSHB); and antibodies specific for pluripotent or multipotent stem cells/ neural stem cells, such as Oct4 (R&D systems), SSEA-4 (DSHB), Nestin (Millipore) and GFAP (Covance). Cell samples were incubated in primary antibodies overnight at 4°C. DyLight488, DyLight549 or DyLight649 conjugated secondary antibodies (Jackson Immunoresearch) and appropriate filters were used to observe the samples using Leica SPE, Olympus FV1000 confocal microscopy or Leica epifluorescence microscopy.
Results
Generation of HUCs from human inner ear utricles
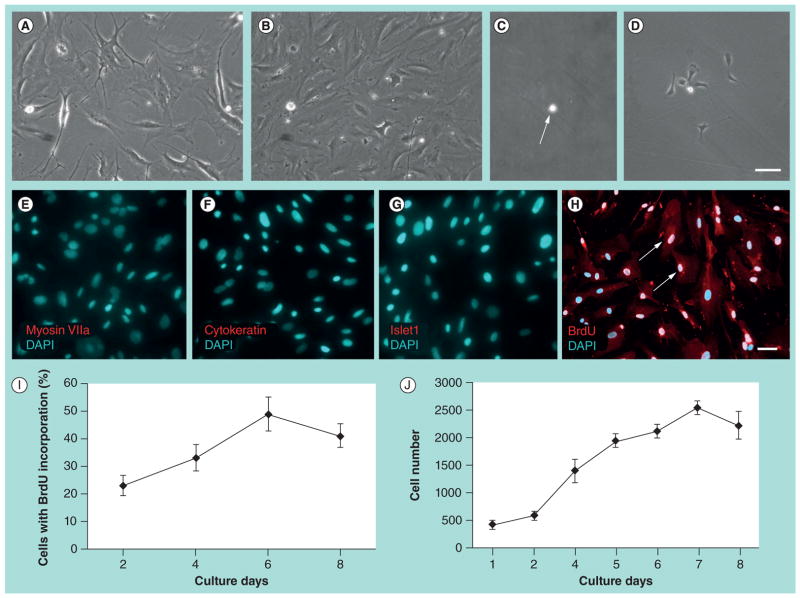
When dissociated primary culture of HUCs was plated into a 24-well plate containing culture medium supplemented with EGF and FGF2, the cells doubled their number in 3–4 days (Figure 1B). When cells were cultured for 5–7 days and reached approximately 70–80% confluence in the culture wells, they were dissociated and transferred to culture flasks. Samples of passage 1 cells were fixed and immunofluorescence showed that they did not express the hair cell marker myosin VIIa, the supporting cell marker cytokeratin or the prosensory marker Islet1 (Figure 1E–G).
Figure 1. Cloned human utricular sensory epithelial cells proliferate when cultured in vitro.
At primary culture, dissociated human utricular sensory epithelial cells (HUCs) expanded on 2D substrates in 24 h (A) and increased the cell number in 3–4 days in the presence of EGF and FGF2 (B). At passage 1, a solitary cloned HUC (cHUC; arrow in C) grew to a small cell island in 3–4 days (D). Samples of passage 1 cells were fixed and immunofluorescence showed that they did not express the hair cell marker myosin VIIa (E), the supporting cell marker cytokeratin (F) and the prosensory markers Islet1 (G). DAPI was used to label the nuclei of cells. BrdU was added to the culture media of passage 8 cHUCs at culture day 1, 3, 5 and 7 for 24 h. Immunofluorescence results reveal that many cells incorporated BrdU (red, arrows in H), indicating that these cells had entered S-phase and possessed the potential to divide. The percentage of cells incorporating BrdU increased from culture days 1–6, then decreased at days 6–8 (I). CyQuant® NF cell proliferation assay showed that cHUCs grew rapidly during culture days 1–7, while cell growth rate decreased mildly during culture days 7–8 (J). The slow cell growth rate after culture days 6–7 may have been related to the space limitation in the culture dish.
Scale bars: (A–D) 50 μm, shown in (D); (E–H) 50 μm, shown in (H).
BrdU: 5-bromo-2-deoxyuridine; DAPI: 4′,6-diamidino-2-phenylindole.
At passage 2, the cells were plated into 96-well plates and the solitary cells were identified in 31 wells, five of which grew to cell clones. The cloned cells were expanded in subsequent cultures for up to 25 passages and termed cHUCs in this study. Expansion of the cell clone was repeated in five clones.
Proliferation of HUCs
We found that cHUCs proliferated for up to 25 passages over 4 months. We used two methods to characterize the time-course of cell growth of cHUCs. First, we added BrdU to the culture medium of passage 8 cHUCs at culture day 1, 3, 5 and 7 for 24 h. Immunofluorescence results revealed that many cells incorporated BrdU (Figure 1H). The percentage of cells incorporating BrdU increased from culture day 1 to day 6, then decreased slightly during days 7–8 (Figure 1I). Additionally, CyQuant NF cell proliferation assay, a fluorescence-detection-based method that can directly observe cell growth, was performed in this study. We found that the cell population expanded rapidly during culture days 1–7, while cell growth rate mildly decreased during culture days 7–8 (Figure 1J).
cHUCs acquired properties of stem/progenitors when cultured in vitro
In this study, in order to obtain a sufficient number of cells for analyses, cHUCs were gradually passaged from small multiwell plates to large tissue culture flasks during passage 2–7. Samples of cHUCs from passages 8–25 were harvested for the detection of candidate markers for inner ear prosensory cells [6,9,10]. It is reported that some of the prosensory genes such as SOX2, CDKN1B (P27KIP1), HES1, JAG1 and NOTCH1, are detectable in mouse inner ear sensory epithelia [4,11,12]. Our RT-PCR results indicated that both cHUCs and adult human utricular sensory epithelial sheets expressed SOX2, CDKN1B (P27KIP1), HES1, JAG1 and NOTCH1 (Figure 2A). However, some of the prosensory genes, such as BMP4, EYA1, SIX1, DLX5 and LFNG, were detected in passage 8–25 cHUCs, but not in adult human utricular sensory epithelial sheets (Figure 2A). Moreover, immunofluorescence of passage 8 cHUCs revealed the expression of prosensory transcriptional factor Islet1 in the nuclei (Figure 2C) and the secreting protein BMP4 located in the cytoplasm (Figure 2E), which were not observed in adult human utricular sensory epithelia (Figure 2B–D). In addition, cHUCs have been passaged 25 times, indicating their capacity to behave as stem cells. These data indicate that cHUCs express genes (BMP4, EYA1, SIX1, DLX5 and LFNG) and proteins (Islet1 and BMP4) usually shown in inner ear prosen-sory cells [6], suggesting that cHUCs may have acquired the characteristics of prosensory cells and, therefore, the potential to differentiate into sensory epithelial cells.
Figure 2. Human utricular sensory epithelial cells acquire properties of stem/progenitor cell in vitro.
Reverse transcription PCR (RT-PCR) indicates that passage 8–25 cloned HUCs (cHUCs) expressed a number of genes usually seen in prosensory cells, such as BMP4, EYA1, SIX1, DLX5 and LFNG (A). It is notable that some prosensory genes were detectable in both utricular sensory epithelial sheet and cHUCs, including SOX2, CDKN1B, HES1, JAG1 and NOTCH1. Expression of the epithelial markers such as KRT18 (cytokeratin 18) and CDH1 (E-cadherin) were observed at the mRNA level in both the utricular sensory epithelial sheet and cHUCs using RT-PCR. cHUCs expressed ZEB1, CDH2 (N-cadherin), FN1 (fibronectin), SNAI2 and VIM (vimentin), which were not detectable in the utricular sensory epithelial sheet (A). Immunofluorescence of passage 8 cHUCs revealed the expression of transcriptional factor Islet1 in the nuclei (arrow in C) and the secreting protein BMP4 located in the cytoplasm (E). The mature utricular sensory epithelia did not express Islet1 and BMP4 (B & D). These data indicate that cHUCs may have acquired the characteristics of prosensory cells. Additionally, cHUCs expressed POU5F1 (Oct4) and NANOG in RT-PCR (A). Protein expression of Oct4 (arrow in G, double-labeled nuclei shown in pink), SSEA-4, GFAP and Nestin was identified in cHUCs but not in utricular sensory epithelium using immunofluorescence (F–M), suggesting that cHUCs may have obtained some of the general features of stem/progenitor cells.
Scale bars: (B, D, F, H, J & L) 20 μm; (C, E, G, I, K & M) 50 μm.
HUC: Human utricular sensory epithelial cell.
To further investigate whether cHUCs shared general features of stem/progenitor cells, passage 8–25 cHUCs were investigated with markers widely present in human stem cells, such as Oct4, Nanog, SSEA-4, GFAP and Nestin [13–15]. We found that cHUCs expressed POU5F1 (OCT4) and NANOG using RT-PCR (Figure 2A). Protein expression of Oct4, SSEA-4, GFAP and Nestin was identified in passage 8–25 cHUCs, but not in adult human utricular sensory epithelium using immunofluorescence (Figure 2F–M), indicating that cHUCs may have obtained some of the general features of stem/progenitor cells.
HUCs aquired features of mesenchymal cells when cultured on 2D substrates
It is known that sensory epithelial cells from the mouse utricle are capable of becoming mesenchymal-like cells that express features of stem/progenitor cells via EMT [7]. However, it is unclear whether human sensory epithelial cells have the same ability. In this study, when cultured in vitro, HUCs started to dedifferentiate and undergo the following morphological, genetic and protein changes.
Morphological changes
When cultured on 2D substrates, primary culture of HUCs started to expand on the substrate and take on a more pleomorphic phenotype (Figur E 1A & B). Phalloidin-labeled F-actin was observed to assemble into irregular ‘stress fiber’ (Figur E 3D & H) that is usually found in mesenchymal cells [16].
Figure 3. Human utricular sensory epithelial cells acquire mesenchymal properties in vitro.
When cultured in vitro, passage 1 HUCs did not express the epithelial markers E-cad, cytokeratin and ZO-1 (E–G), which are usually observed in mature utricular sensory epithelia (A–C). Phalloidin-labeled F-actin was changed into irregular ‘stress fibers’ that are usually seen in mesenchymal cells (D & H). Protein expression of mesenchymal markers, including slug (red, double-labeled nuclei shown in pink, arrows in M), fibronectin (N), vimentin (O) and N-cadherin (P) was found using immunofluorescence. These findings suggest that cloned HUCs are able to adopt a mesenchymal trait.
Scale bars: (A–L) 20 μm; (M–P) 50 μm.
E-cad: E-cadherin; HUC: Human utricular sensory epithelial cell.
Changes of epithelial junction markers
E-cadherin, encoded by the CDH1 gene, is located at the lateral membrane in adherens junction and serves as one of the hallmarks of epithelial cells (Figure 3A). RT-PCR showed that CDH1 gene expression was detected in passages 8–25 cHUCs when three groups of primers were used (Figure 2A & Table 1). However, immunofluorescence revealed that E-cadherin protein was not detectable in HUCs as early as passage 1 (Figure 3E). Cytokeratin subunits, such as subunit 18, are usually expressed in adult human inner ear sensory epithelia (Figur E 3B) [17]. We found that KRT18, a gene encoding cytokeratin subunit 18, was expressed in passages 8–25 cHUCs using RT-PCR, whereas cytokeratin was not detectable when anti-pan-cytokeratin (cytokeratins 4, 5, 6, 8, 10, 13 and 18) antibodies were used (Figure 3F). Additionally, ZO1, a tight junction protein among utricular epithelial cells (Figure 3C) [11], was not detected in HUCs as early as passage 1 using immunofluorescence (Figure 3G).
Expression of mesenchymal markers
RT-PCR indicates that passage 8–25 cHUCs expressed genes that are usually found in mesenchymal cells, such as ZEB1, CDH2 (N-cadherin), FN1 (fibronectin), SNAI2 (snail2) and VIM (vimentin) (Figure 2A). Immunof luorescence showed that cHUCs (as early as passage 1) expressed proteins of mesenchymal cell types, such as Snail2 (slug), fibronectin, vimentin, and N-cadherin (Figure 3M–P), which were not observed in utricular sensory epithelia (Figure 3I–L).
Discussion & conclusion
In birds, fish and amphibians, supporting cells in the inner ear usually respond to sensory hair cell loss by generating new supporting cells and replacement hair cells via dedifferentiation of supporting cells or direct cell type conversion [18–20]. Mammalian supporting cells, however, do not have such robust abilities as those of non-mammalian vertebrates [21,22]. Insults to the human inner ear usually cause permanent inner ear disorders, such as hearing loss, tinnitus and balance disability that affect the daily lives of over 10% of the population. Generation of human hair cell progenitors is one of the major challenges in sensory hair cell regeneration research. Although it has been shown that rodent hair cells can be regenerated in vitro or via modulation gene expression during development [3,4,23–25], generation of human hair cells or hair cell progenitors is rarely reported. One of the major reasons is that it is difficult to obtain inner ear samples from normal humans for use in identifying and culturing hair cell progenitors. It is reported that stem cells can be identified from human fetal inner ear [26]. However, the clinical application of these fetal-derived cells is severely limited owing to ethical considerations. In the translabyrinthine surgical approach for removal of vestibular schwannomas, the peripheral vestibular system, including utricles, is not usually affected by the tumor, but must be sacrificed in order to access the tumor for the purpose of radical removal. In this study, these discarded vestibular tissues were collected followed by isolation of sensory epithelial sheets from the utricles. The sensory epithelial cells were dissociated and cultured in vitro. Obtaining human samples from discarded tissues of inner ear surgery for in vitro study represents a novel approach to identifying progenitor cells for stem cell-based regenerative strategies.
In this study, we found that sensory epithelial cells derived from human utricles obtained the ability to proliferate for over 25 passages, as evidenced by cell passage, BrdU incorporation and fluorescence-detection-based Cyquant dye study. In addition, we found that the proliferation ability of cHUCs is associated with the acquisition of mesenchymal properties (Figures 2A & 3). Adult mammalian sensory epithelial cells usually lose the ability to proliferate, while mesenchymal cells generally retain the capability of proliferation. Epithelial-to-mesenchymal transition (EMT) plays a critical role in the formation of the body plan and generation of tissues and organs in normal development [27]. In adults, EMT is important in tissue repair, organ fibrosis, and carcinoma progression [28]. Recently, EMT was found to contribute to the in vitro expansion of epithelial cells. When epithelial cells from pancreatic islets are cultured on 2D substrates in vitro, they dedifferentiate into mesenchymal-like cells, which can be expanded to a large number for use in regeneration studies [29,30]. In our previous studies of inner ear regeneration, both chick and mouse inner ear sensory epithelial cells are found to undergo EMT to acquire the capability of proliferation [1,7]. Our data in this study suggest that cHUCs may have a similar ability to undergo EMT.
We recently used a mouse model to determine that mouse utricular cells (MUCs) can acquire features of mesenchymal cells via EMT [7]. When MUCs undergo EMT, RT-PCR and immunofluorescence show that MUCs lose the expression of epithelial markers, such as Cdh1 (E-cadherin) and ZO-1 [7]. In conjunction with downregulation of epithelial markers, MUCs express mesenchymal markers, such as Vim (vimentin), Fn1 (fibronectin) and Cdh2 (N-cadherin). In addition, F-actin of MUCs is arranged in a ‘stress fiber’ pattern, which is usually seen in mesenchymal cells. In this study, F-actin of cHUCs also showed a ‘stress fiber’ pattern and cHUCs consistently expressed mesenchymal markers, such as SNAI2 (snail2), ZEB1, VIM (vimentin), FN1 (fibronectin), and CDH2 (N-cadherin) when they were cultured in vitro. Immunofluorescence indicates that cHUCs (passage 1 and 8–25) lack expression of E-cadherin, ZO-1 and cytokeratins, which is in accordance with the observation in MUCs. However, expression of CDH1 and KRT18 is consistently detected in cHUCs using RT-PCR, suggesting that cHUCs may retain expression of epithelial markers such as CDH1 and KRT18 at the mRNA level, while protein expression of these epithelial markers is not detectable using immunofluorescence. These results indicate that cHUCs cultured in vitro acquire mesenchymal cell features, although they may retain mRNA expression of epithelial markers. The reason for the difference in epithelial marker expression between MUCs and cHUCs is unclear. We speculate that it may relate to species diversity. It is also possible that cHUCs may adjust their molecular profiles under challenging conditions in cultures.
Mesenchymal status is crucial for the acquisition and maintenance of the multi-/pluripotency of stem/progenitor cells. When human embryonic stem cells are grown on matrigels, these cells undergo EMT to express mesenchymal markers, while retaining the expression of pluripotent markers such as Oct4 and Nanog [31]. It has been revealed that epithelial cells from pancreatic islets are able to dedifferentiate into mesenchymal-like multipotent stem/progenitor cells via EMT [29,30]. A direct link between the EMT and the gain of epithelial stem cell properties has also been documented [32]. In our previous study using birds as a model, we found that chick utricular cells are able to undergo EMT to proliferate and produce hair cells via mesenchymal-to-epithelial transition [1]. We recently used a mouse model to determine that MUCs can acquire features of prosensory cells and stem/progenitor cells via EMT [7]. In order to study whether sensory epithelial cells derived from adult humans have similar capabilities, in this study we cultured cHUCs and found that cHUCs did express genes and proteins usually expressed in prosensory cells and stem/progenitor cells [6,9,10], such as BMP4, EYA1, SIX1, DLX5, HES1, JAG1, CDKN1B (P27kip1), LFNG, NOTCH1, POU5F1 (Oct4), NANOG and SOX2 using RT-PCR, and BMP4, Islet1, Oct4, SSEA-4, GFAP and Nestin using immunofluorescence. These data suggest that cHUCs have a similar ability to MUCs to acquire properties of prosensory cells and stem/ progenitor cells, and therefore the potential to differentiate into sensory epithelial cells, including hair cells and supporting cells. In future studies, we will induce these cHUCs to differentiate into new hair cells, and characterize them with tests of hair cell markers, hair bundle markers, ultrastructural study of hair bundles using electron microscopy and functional evaluation using electrophysiology.
Conclusion
In conclusion, discarded human inner ear tissues were collected from surgery in this study. HUCs obtain the ability to proliferate and they can be cultured in vitro for over 25 passages. Remarkably, HUCs acquire features of prosensory cells and stem/progenitor cells, indicating a novel resource for in vitro human hair cell generation. Isolated HUCs are able to change their cell fate, and express features of mesenchymal cells via EMT when they are cultured in vitro. Future study will be directed to the induction of HUCs to differentiate into human sensory hair cells, followed by detailed characterization of hair cells using morphological, genetic and protein expression methods at critical stages throughout the process. Once human hair cells can be differentiated from cHUCs, generation of hair cell progenitor cells via EMT may open avenues for hair cell regeneration research, which will benefit millions of patients with inner ear disorders, such as hearing loss and tinnitus.
Future perspective
Generation and characterization of hair cell progenitors will be one of the major challenges in the field. The cell resources for hair cell progenitors include discarded human inner ear tissues/surgical specimens, human embryonic stem cells and human induced pluripotent stem cells. Hair cell progenitors isolated from inner ear tissues will be a good model to study the signals controlling hair cell regeneration. Although there is still a learning curve for induced pluripotent stem cell technology, the possibility exists to utilize personalized stem cells in future clinical tests, thus avoiding ethical and immune-rejection issues. The genetics, morphological and electrophysiological properties of nascent hair cells will be investigated to ascertain their potentials for replacing degenerated hair cells in hearing loss, tinnitus, and other inner ear disorders.
Executive summary.
Fully differentiated human utricular sensory epithelial cells are able to acquire the ability to proliferate in vitro for over 25 passages.
Isolated adult human utricular sensory epithelial cells can be cultured in vitro and acquire features of prosensory cells and stem/progenitor cells.
Isolated human utricular sensory epithelial cells are able to undergo epithelial-to-mesenchymal transition to adopt mesenchymal features.
Human prosensory-like progenitor cells can be generated via dedifferentiation from an epithelial fate to a mesenchymal fate.
Obtaining human samples from discarded tissues of inner ear surgery for in vitro study represents a novel approach to identifying progenitor cells for stem cell-based regenerative strategies.
Acknowledgments
The authors thank B Zhang, C Li, Y Wu and S Wang for their technical support, and antibodies from the Developmental Studies Hybridoma Bank.
Footnotes
Financial & competing interests disclosure
This study is supported by the Deafness Research Foundation, the American Hearing Research Foundation, the American Academy of Audiology Foundation and NIDCD/NIH (R03DC011597). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
For reprint orders, please contact: reprints@futuremedicine.com
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been exempted by local Human Investigation Committee according to US federal regulations because anonymized samples were collected in this study.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1▪.Hu Z, Corwin JT. Inner ear hair cells produced in vitro by a mesenchymal-to-epithelial transition. Proc Natl Acad Sci USA. 2007;104(42):16675–16680. doi: 10.1073/pnas.0704576104. First study to reveal that chick inner ear sensory epithelial cells are able to undergo epithelial-to-mesenchymal transition (EMT) to proliferate and produce hair cells via mesenchymal-to-epithelial transition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okano T, Kelley MW. Stem cell therapy for the inner ear: recent advances and future directions. Trends Amplif. 2012;16(1):4–18. doi: 10.1177/1084713812440336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3▪.Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9(10):1293–1299. doi: 10.1038/nm925. First study to isolate pluripotent stem cells from the adult mouse utricle. [DOI] [PubMed] [Google Scholar]
- 4▪.White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441(7096):984–987. doi: 10.1038/nature04849. First study to show that mammalian supporting cells can proliferate and differentiate into hair-like cells. [DOI] [PubMed] [Google Scholar]
- 5.Baguley DM, Jones S, Wilkins I, Axon PR, Moffat DA. The inhibitory effect of intravenous lidocaine infusion on tinnitus after translabyrinthine removal of vestibular schwannoma: a double-blind, placebo-controlled, crossover study. Otol Neurotol. 2005;26(2):169–176. doi: 10.1097/00129492-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 6▪.Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7(11):837–849. doi: 10.1038/nrn1987. Reviews the major features of prosensory cells, which will eventually give rise to hair cells and supporting cells. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Hu Z. Sensory epithelial cells acquire features of prosensory cells via epithelial to mesenchymal transition. Stem Cells Dev. 2012;21(10):1812–1821. doi: 10.1089/scd.2011.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Jiang H, Hu Z. Concentration-dependent effect of nerve growth factor on cell fate determination of neural progenitors. Stem Cells Dev. 2011;20(10):1723–1731. doi: 10.1089/scd.2010.0370. [DOI] [PubMed] [Google Scholar]
- 9.Hartman BH, Reh TA, Bermingham-McDonogh O. Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proc Natl Acad Sci USA. 2010;107(36):15792–15797. doi: 10.1073/pnas.1002827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neves J, Parada C, Chamizo M, Giraldez F. Jagged 1 regulates the restriction of Sox2 expression in the developing chicken inner ear: a mechanism for sensory organ specification. Development. 2011;138(4):735–744. doi: 10.1242/dev.060657. [DOI] [PubMed] [Google Scholar]
- 11.Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol. 2008;9(1):65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batts SA, Shoemaker CR, Raphael Y. Notch signaling and Hes labeling in the normal and drug-damaged organ of Corti. Hear Res. 2009;249(1–2):15–22. doi: 10.1016/j.heares.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramirez JM, Gerbal-Chaloin S, Milhavet O, et al. Brief report: benchmarking human pluripotent stem cell markers during differentiation into the three germ layers unveils a striking heterogeneity: all markers are not equal. Stem Cells. 2011;29(9):1469–1474. doi: 10.1002/stem.681. [DOI] [PubMed] [Google Scholar]
- 14.Moore RN, Cherry JF, Mathur V, Cohen R, Grumet M, Moghe PV. E-cadherin-expressing feeder cells promote neural lineage restriction of human embryonic stem cells. Stem Cells Dev. 2011;21(1):30–41. doi: 10.1089/scd.2010.0434. [DOI] [PubMed] [Google Scholar]
- 15.Ahmadi N, Razavi S, Kazemi M, Oryan S. Stability of neural differentiation in human adipose derived stem cells by two induction protocols. Tissue Cell. 2012;44(2):87–94. doi: 10.1016/j.tice.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Pollack V, Scheiber K, Pfaller W, Schramek H. Loss of cytokeratin expression and formation of actin stress fibers in dedifferentiated MDCK-C7 cell lines. Biochem Biophys Res Commun. 1997;241(2):541–547. doi: 10.1006/bbrc.1997.7837. [DOI] [PubMed] [Google Scholar]
- 17.Bauwens LJ, Degroot JC, Ramaekers FC, Veldman JE, Huizing EH. Cytokeratin expression in the epithelia of the adult human cochlea. Eur Arch Otorhinolaryngol. 1991;248(5):293–297. doi: 10.1007/BF00176758. [DOI] [PubMed] [Google Scholar]
- 18.Brignull HR, Raible DW, Stone JS. Feathers and fins: non-mammalian models for hair cell regeneration. Brain Res. 2009;1277:12–23. doi: 10.1016/j.brainres.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci Lett. 1996;205(1):17–20. doi: 10.1016/0304-3940(96)12367-3. [DOI] [PubMed] [Google Scholar]
- 20.Stone JS, Cotanche DA. Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol. 2007;51(6–7):633–647. doi: 10.1387/ijdb.072408js. [DOI] [PubMed] [Google Scholar]
- 21.Groves AK. The challenge of hair cell regeneration. Exp Biol Med (Maywood) 2010;235(4):434–446. doi: 10.1258/ebm.2009.009281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwan T, White PM, Segil N. Development and regeneration of the inner ear. Ann NY Acad Sci. 2009;1170:28–33. doi: 10.1111/j.1749-6632.2009.04484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell. 2010;141(4):704–716. doi: 10.1016/j.cell.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126(8):1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- 25.Sage C, Huang M, Karimi K, et al. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005;307(5712):1114–1118. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- 26.Chen W, Johnson SL, Marcotti W, Andrews PW, Moore HD, Rivolta MN. Human fetal auditory stem cells can be expanded in vitro and differentiate into functional auditory neurons and hair cell-like cells. Stem Cells. 2009;27(5):1196–1204. doi: 10.1002/stem.62. [DOI] [PubMed] [Google Scholar]
- 27.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial–mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119(6):1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29▪▪.Gershengorn MC, Hardikar AA, Wei C, Geras-Raaka E, Marcus-Samuels B, Raaka BM. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science. 2004;306(5705):2261–2264. doi: 10.1126/science.1101968. First study to reveal that epithelial cells from pancreatic islets are able to undergo EMT to dedifferentiate into mesenchymal-like multipotent stem/progenitor cells that can proliferate and differentiate. [DOI] [PubMed] [Google Scholar]
- 30.Gallo R, Gambelli F, Gava B, et al. Generation and expansion of multipotent mesenchymal progenitor cells from cultured human pancreatic islets. Cell Death Differ. 2007;14(11):1860–1871. doi: 10.1038/sj.cdd.4402199. [DOI] [PubMed] [Google Scholar]
- 31▪.Eastham AM, Spencer H, Soncin F, et al. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007;67(23):11254–11262. doi: 10.1158/0008-5472.CAN-07-2253. Shows that human embryonic stem cells are able to undergo EMT to express mesenchymal markers, while retaining the expression of pluripotent markers. [DOI] [PubMed] [Google Scholar]
- 32▪▪.Mani SA, Guo W, Liao MJ, et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. Illustrates a direct link between the EMT and the gain of epithelial stem cell properties. [DOI] [PMC free article] [PubMed] [Google Scholar]