Abstract
T-box 3 (Tbx3) is a member of the T-box family of genes. Mutations that result in the haploinsufficiency of TBX3 cause Ulnar Mammary Syndrome (UMS) in humans characterized by mammary gland hypoplasia as well as other congenital defects. In mice, homozygous mutations are embryonic lethal, suggesting that Tbx3 is essential for embryo development. Studies in mice have shown that Tbx3 is essential in the maintenance of mouse embryonic stem cell (ESC) self-renewal and in their differentiation into extra-embryonic endoderm (ExEn). The role TBX3 plays in regulating human ESCs has not been explored. Since mouse and human ESCs are known to represent distinct pluripotent states, it is important to address the role of TBX3 in human ESC self-renewal and differentiation. Using over-expression and knockdown strategies, we found that TBX3 over-expression promotes human ESC proliferation possibly by repressing the expression of both NFκBIB and p14ARF, known cell cycle regulators. During differentiation, TBX3 knockdown resulted in decreased neural rosette formation and in decreased expression of neuroepithelial and neuroectoderm markers (PAX6, LHX2, FOXG1, RAX). Taken together, our data suggests a role for TBX3 in human ESC proliferation and reveals an unrecognized novel role of TBX3 in promoting neuroepithelial differentiation. Our results suggest that TBX3 plays distinct roles in regulating self-renewal and differentiation in both human and mouse ESCs.
Keywords: TBX3, human embryonic stem cells, self-renewal, differentiation, neuroepithelial
INTRODUCTION
Tbx3 is a member of the T-box gene family. The T-box family of transcription factors plays an important role in regulating early developmental processes and in regulating gene expression networks involved in specifying cell lineage [1–3]. Homozygous mutations of Tbx3 are embryonic lethal in mice suggesting that Tbx3 is important during the earliest phases of embryo development [4]. In humans, heterozygous mutations of TBX3 are sufficient to cause Ulnar Mammary syndrome characterized by defects within the limbs, apocrine and mammary gland, similar to those in mouse models [4–11].
In addition to its role in regulating early embryo and mammary gland development, Tbx3 has been identified as a key regulator of mouse ESC pluripotency and differentiation. In mouse ESCs undergoing retinoic acid (RA) induced differentiation, Tbx3 was significantly down-regulated along with known pluripotency regulators Oct4 and Nanog [12]. Knockdown of Tbx3 resulted in the loss of pluripotency and differentiation of mouse ESCs, suggesting that Tbx3 is necessary to maintain self-renewal [12]. While over-expression of Tbx3 was found to be sufficient to maintain mouse ESCs in their undifferentiated state in the absence of LIF [13], Tbx3 over-expression also induced differentiation into extra-embryonic endoderm through direct regulation of Gata6 [14]. A more recent study has shown that Tbx3 not only maintains mouse ESC self-renewal, but also plays an essential role in their differentiation [14]. Knockdown of Tbx3, during mouse embryoid body formation, prevented extra-embryonic endoderm differentiation, while enhancing ectoderm and trophectoderm differentiation [14]. In addition, expression of Tbx3 during somatic cell reprogramming has been shown to improve the overall quality of induced pluripotent stem (iPS) cells [15]. These studies confirm that Tbx3 is not only necessary to maintain self-renewal but also plays a role in regulating differentiation of mouse ESCs into extraembryonic endoderm.
Although accumulating evidence suggests Tbx3 play roles in regulating the self-renewal and differentiation of mouse ESCs, the function of TBX3 in regulating human ESC self-renewal and differentiation remains largely unexplored. Molecular analyses suggest that human and mouse ESCs represent distinct pluripotent states, in which mouse ESCs are considered to be at a more naïve pluripotent state, and as a result have very different biological properties [16,17]. This is reflected by the fact that the same transcriptional and signaling pathways regulate mouse and human ESC pluripotency differently. Addition of LIF or BMPs to mouse ESCs is necessary to maintain pluripotency while these same factors cause differentiation in human ESCs [18–22]. Due to the differences between their pluripotent states, TBX3 may function very differently in human ESCs than in mouse ESCs. Unraveling human ESC transcriptional circuitry is fundamental to understanding ESC differentiation and specification in early human development. It is also a key step for the development of improved methods to derive, culture, and differentiate human ESCs into cells of a specific developmental pathway for potential therapeutic use.
In this study we generated TBX3 over-expressing and TBX3 knockdown human ESC lines to investigate the role of TBX3 in regulating human ESC self-renewal and differentiation. In contrast to mouse ESC studies, we found that neither over-expression nor knockdown of TBX3 was able to attenuate self-renewal ability to induce differentiation in undifferentiated human ESCs. Instead, over-expression of TBX3 promoted stem cell proliferation possibly by repressing the expression of cell cycle regulators, NFκBIB and p14ARF. Also, knockdown of TBX3 during differentiation reduced neural rosette formation as well as the expression of neuroepithelial and neuroectoderm markers (FOXG1, PAX6, LHX2, and RAX). Our study suggests TBX3 stimulates human ESC proliferation and promotes neuroepithelial differentiation. Our data demonstrates that TBX3 plays distinct roles in human and mouse ESCs in regulating self-renewal and differentiation.
MATERIALS AND METHODS
Maintenance of human ESCs
Human ESCs (H9, H1) were obtained from WiCell (Madison, Wisconsin). Cells were maintained on mitomycin C inactivated mouse embryonic fibroblast (MEF) feeder layer and fed daily with human ESC medium (knockout-Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% Knockout Serum replacement, 1% Glutamax, 1% nonessential amino acids, 6 ng/ml basic fibroblast growth factor (bFGF) (all from Invitrogen), and 0.1 mM β-mercaptoethanol (Sigma-Aldrich). Cells were passaged enzymatically every 4–6 days with 1 mg/ml collagenase type IV (Invitrogen) and seeded onto MEF feeder layers. When appropriate, human ESCs were also cultured on matrigel (BD Biosciences) and fed daily with conditioned medium (CM) supplemented with 6 ng/ml bFGF.
Human ESC differentiation
To induce differentiation of human ESCs as embryoid bodies (EBs), colonies of undifferentiated human ESCs were treated with 1 mg/ml collagenase type IV for 10 minutes and dissociated cells into clumps. Clumps of undifferentiated human ESCs were cultured in suspension and fed ESC medium without bFGF (differentiation medium) every other day for 5 or 7 days. On the 5th or 7th day, EBs were collected and plated onto 0.2% gelatin coated plates. Plated EBs were fed every other day with differentiation medium or differentiation medium supplemented with 10% Fetal Bovine Serum (FBS), 100 ng/ml Bone Morphogenetic Protein-4 (BMP-4), or 1 μM Retinoic Acid (RA) for an additional 11 or 14 days.
Directed Neuroepithelial Differentiation
Human ESCs were induced to differentiate towards neuroepithelia as previously described by Shi et al [23]. Briefly, human ESCs (H9) were passaged using 1 mg/ml collagenase and plated on Matrigel-coated 12-well plates in MEF conditioned medium supplemented with 10 ng/ml bFGF2. Once the cells reached 90% confluency, neural induction was initiated by changing the culture medium to one that supports neural induction; a 1:1 mixture of N2- and B27-containing media referred to as 3N medium. N2 medium consisted of DMEM/F12, N2 (Gibco), 5 μg/ml insulin, 1 mM l-glutamine, 100 μM non-essential amino acids, 100 μM 2-mercaptoethanol, 50 U/ml penicillin and 50 mg/ml streptomycin. B27 medium consisted of Neurobasal (Invitrogen), B27 (Gibco), 200 mM glutamine, 50 U/ml penicillin and 50 mg/ml streptomycin. 3N medium was supplemented with 1 μM retinoic acid, 1 μM Dorsomorphin (Sigma), and 10 μM SB431542 (Sigma) [23]. Cells were fed every other day for 14 days. The efficiency of neural induction was monitored by the appearance of cells with characteristic neuroepithelial cell morphology (neural rosette formation) and expression of established neuroepithelial markers, PAX6, NESTIN, and the apical re-distribution of ZO-1.
Plasmids
For over-expression constructs, we digested the FUGW-PGK-eGFP-Puromycin plasmid with restriction enzymes, BamHI and BsrGI, to excise eGFP. Digested plasmid was run on a 1% agarose gel and purified using Qiagen Gel Extraction Kit (Qiagen) according to manufacturer’s protocol. Next, the full length cDNA of TBX3, along with a myc tag, was PCR amplified out of pcDNA-myc-TBX3, digested with restriction enzymes (BamHI and BsrGI), and ligated into the FUGW-PGK-Puromycin plasmid. Positive clones were verified by restriction enzyme digestion and sequencing. The control plasmid was constructed in a similar fashion except only the myc tag was ligated into the FUGW-PGK-Puromycin plasmid. For TBX3 knock-down constructs, the plko.1-control and various plko.1-TBX3shRNA plasmids were a generous gift from Dr. Anand Ganesan.
Verification of plko.1-TBX3 shRNA lentiviral vectors
Nearly confluent MCF7 cells, over-expressing TBX3-eGFP fusion protein, were transfected with 6 μg of control or TBX3 shRNA lentiviral vectors using Lipofectamine 2000 (Invitrogen) transfection reagent, according to manufacturer’s protocol. Seventy-two hours after transfection, cell lysates were harvested and used to perform western blot to verify knockdown.
Western Blotting
Cell lysates were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on a NuPAGE 4–12% gradient Bis-Tris gel (Invitrogen) and electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Invitrogen). Membranes were incubated in 5% blocking buffer (Non-fat dry milk in PBS containing 0.1% Tween) for 3 three hours at room temperature. Membranes were probed with mouse anti-myc (Clonetech, 1:2000) overnight at 4°C. Membranes were then washed three times with PBS-T and incubated with goat anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibody (1:2000; Pierce) for 1 ½ hours at 4°C. HRP was detected using ECL Western Blotting Detection Reagents (Amersham Biosciences).
Lentivirus production
Lentivirus was produced by transient co-transfection of three plasmids into 293T cells. Briefly, 293T cells were plated onto poly-D-lysine coated plates at approximately 80–90% confluency. 293T cells were transfected using Lipofectamine 2000 reagent (Invitrogen), according to the manufacturer’s protocol. A total of 16 μg of plasmid DNA per 100 mm dish was used: 2 μg of the VSV-G envelope plasmid, pMD.G, 8 μg of the packaging plasmid, pCMVR8.91, and 6 μg of the Lentiviral transducing vector. The medium was replaced 24 hours after transfection with virus-collecting medium (DMEM supplemented with 1% FBS). The viral supernatant was collected at 48 and 72 hours after transfection and filtered through 0.45 μm filters. Virus was concentrated using the Amicon Ultra-15 Centrifugal filter device (Millipore) as recommended by the manufacturer and immediately added to cells.
Transduction of human ESCs to create TBX3 over-expressing and knockdown cell lines
Twenty four hours prior to transduction, human ESCs were mechanical passaged from MEFs onto matrigel to eliminate MEFs during transduction. The following day, concentrated lentivirus was added to fresh CM supplemented with 6 μg/ml polybrene and added to the cells. Human ESCs were transduced twice, once at the 48 hour viral harvest and once at the 72 hour viral harvest. Forty-eight hours after the second transduction, puromycin (1 μg/ml) was used to select for transduced ESCs. Single clones were picked, expanded and verified for either TBX3 over-expression or knockdown.
Generation of H9-hOCT4-eGFP cell line
The hOCT4-eGFP plasmid was a generous gift from Dr. Wei Cui laboratory. Human ESCs were transfected as previously described [24]. Briefly, 24 hours prior to transfection, human ESCs were mechanically passaged onto matrigel. The following day, 10 μg of ApaLI linearized hOCT4-eGFP plasmid was transfected into human ESCs using Fugene 6 (Roche), following manufacturer’s instructions. The DNA: Fugene ratio of 1:1.5 was used. Forty-eight hours later, transfected cells were selected for using G418 at 200 μg/ml. Single clones were picked and expanded.
Transient TBX3 over-expression experiments
Prior to transduction, OCT4-eGFP human ESCs were mechanically passaged onto matrigel and then transduced at 24 and 48 hours after plating, with myc or myc-TBX3 lentivirus. Six days after initial transduction, cells were fixed with 4% paraformaldehyde.
Quantitative Real Time-Polymerase Chain Reaction analysis (QRT-PCR)
RNA was isolated and purified using the RNeasy Plus Mini Kit (Qiagen), according to the manufacturer’s recommendations. SYBR Green quantitative real-time polymerase chain reactions (qPCRs) were performed in triplicate for each primer set using the ABI 7900HT Sequence Detection System (Applied Biosystems). Primers were designed to span introns or at exon junctions. To ensure specificity of PCR, melt curve analyses were performed at the end of all PCRs. Gene expression levels were normalized to GAPDH and then analyzed using the 2ΔΔCt method.
Immunocytochemistry
For immunocytochemistry analyses, cells were fixed with 4% paraformaldehyde in PBS for 20 minutes. Fixed cells were permeabilized with 0.1% Triton X-100 and 10% goat serum in PBS (10% Blocking buffer) for 1 hour at room temperature. The cells were incubated with primary antibodies in 10% Blocking buffer overnight at 4°C, followed by incubation with appropriate Alexa Fluor 488 or 594-conjugated secondary antibodies (1:400; Molecular Probes) in 10% Blocking buffer for 1 ½ hours at 4°C. Cell nuclei were visualized by 4′-6-diamidino-2-phenylindole (DAPI, 1:400) staining. Immunofluorescence images were captured on a Nikon Eclipse Ti Inverted Microscope. Antibodies against the following proteins were used: myc (1:200; Clonetech), TBX3 (1:50; Zymed), OCT4 (1:200; Abcam), NANOG (1:100; Cell Signaling), PAX6 (1:300; Covance), NESTIN (1:200; Invitrogen); SOX1 (1:300; BD Pharmingen), and ZO-1 (1:300; Invitrogen).
Mitotic index
Human ESCs were fixed with 4% Paraformaldehyde in PBS for 20 minutes. Fixed cells were permeabilized with 0.1% Triton X-100 and incubated with DAPI (1:400) for 1 hour at room temperature. DAPI images were captured on a Nikon Eclipse Ti Inverted Microscope. The Image J software was used to count mitotic nuclei and total number of nuclei present. To calculate the mitotic index, the number of mitotic nuclei was divided by the total number of nuclei. Approximately 2,000–5,000 cells were scored.
BrdU incorporation assay
Control and TBX3 over-expressing human ESC proliferation was assessed by 5-bromo-2-deoxyuridine (BrdU) incorporation. Cells were seeded onto 12-well matrigel coated plates in MEF-conditioned medium supplemented with 10 ng/ml bFGF and allowed to grow for 4 days. On the fourth day, BrdU was diluted into fresh MEF-conditioned medium to a final concentration of 10 μM and incubated at 37°C for 4 hours. Cells were washed with PBS and fixed with 4% paraformaldehyde for 15 minutes. Fixed cells were washed, treated with 1.5 M HCl for 30 minutes at room temperature and washed once more. BrdU that had been incorporated into newly synthesized DNA was detected by immunocytochemistry as described above (Immunocytochemistry). Mouse anti-BrdU (1:1000, Millipore) and Goat anti-Mouse Alexa Fluor 594-conjugated secondary antibodies (1:400; Molecular Probes) were used to visualize incorporated BrdU. Cell nuclei were visualized by 4′-6-diamidino-2-phenylindole (DAPI). Approximately 3,000 cells from control and TBX3 over-expressing human ESC lines were quantified and normalized to the total cell number in each field using the Image J software.
Quantification of neural rosettes
Differentiated human ESCs were fixed with 4% paraformaldehyde in PBS for 20 min, washed, and subsequently incubated with 10% Blocking serum (10% Goat serum and 0.1% Triton X-100 in PBS) containing DAPI (1:400) for 1 hour. DAPI images were taken of all neural rosette structures for control and TBX3 shRNA cell lines using a Nikon Eclipse Ti Inverted Microscope. Image J software was used to count total number of neural rosette structures. The total number of neural rosettes was then normalized to the number of EBs plated per cell line or the total number of cells at time of fixation. This experiment was performed in triplicate for control and TBX3 shRNA cell lines.
RESULTS
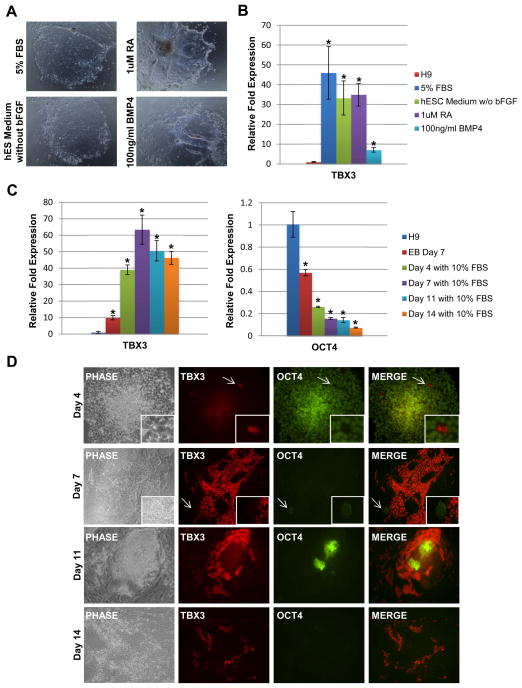
TBX3 is up-regulated in differentiating human ESCs
Although it has been shown that Tbx3 is necessary to maintain mouse ESC self-renewal and pluripotency, it is important to verify whether TBX3 exhibits a similar function in human ESCs. In order to gain insight of TBX3’s function in human ESC pluripotency or differentiation, we first determined the expression pattern of TBX3 in undifferentiated and differentiated human ESCs. Human ESCs were induced to differentiate through an EB intermediate for 5 days. On the fifth day, EBs were collected, plated and then treated with various differentiating agents such as FBS (5%), BMP-4 (100 ng/ml), and RA (1 μM) for an additional 11 days. Human ESCs began to differentiate, characterized by the loss of tight colony morphology (Figure 1A). By day 11 of treatment with FBS, RA, BMP-4 or withdrawal of bFGF, TBX3 expression was significantly up-regulated (Figure 1B). To determine the time course of TBX3 up-regulation during differentiation, we induced formation of EBs for 7 days and induced differentiation for an additional 14 days with 10% FBS. We selected 10% FBS as our differentiating agent to maximize EB adherence. Quantitative RT-PCR analysis was performed at day 7 of EB formation and at days 4, 7, 11 and 14 of differentiation with 10% FBS. As shown in Figure 1C, qRT-PCR analysis revealed that TBX3 expression is up-regulated during EB formation and as early as day 4 of FBS-induced differentiation. OCT4 expression level decreased with increasing time of differentiation, confirming that these cells are differentiating cells (Figure 1C). At each time point of differentiation with 10% FBS, immunocytochemistry of TBX3 and OCT4 was performed and results were consistent with our qRT-PCR data. As shown in Figure 1D, TBX3 expression began to increase in differentiated cells as early as day 4 and continued increasing to day 11 (Figure 1D). More importantly, cells that highly expressed TBX3 did not express OCT4. This was demonstrated at every time point during differentiation (Figure 1D, insets). In contrast to mouse ESC studies in which Tbx3 expression is down-regulated upon EB formation and differentiation with RA [12,25–27], our results show that TBX3 is highly up-regulated upon differentiation of human ESCs. The reciprocal expression patterns of TBX3 and OCT4 imply that TBX3 may promote differentiation of human ESCs by inhibiting OCT4 expression.
Figure 1.
TBX3 is up-regulated upon differentiation of human ESCs. (A) Cell morphologies of adhered EBs at day 11 of differentiation. Images were taken at 4X magnification. (B) Quantitative RT-PCR analysis of relative TBX3 expression levels in differentiated human ESCs at day 11 (undifferentiated H9=1.0). *, P < 0.05. TBX3 is up-regulated approximately 45-fold, 30-fold, 35-fold and 8-fold in H9 cells treated with FBS, withdrawal of bFGF, RA, or BMP-4, respectively. (C) Quantitative RT-PCR analysis of relative TBX3 and OCT4 expression levels in EBs and 10% FBS induced differentiated human ESCs at day 4, 7, 11, and 14 (undifferentiated H9=1.0). TBX3 is up-regulated in EBs at day 7 and in 10% FBS induced differentiated human ESCs at day 4, 7, 11, and 14, respectively. OCT4 expression levels decreased as time of differentiation increased. *, P < 0.05 (D) Immunocytochemistry of TBX3 and OCT4 expression in 10% FBS induced differentiated human ESCs at day 4, 7, 11, and 14. Double staining for TBX3 (red) and OCT4 (green). TBX3 and OCT4 expression do not overlap at any time point (white arrow and insets). Images were captured at 10X magnification.
TBX3 does not regulate OCT4 promoter activity
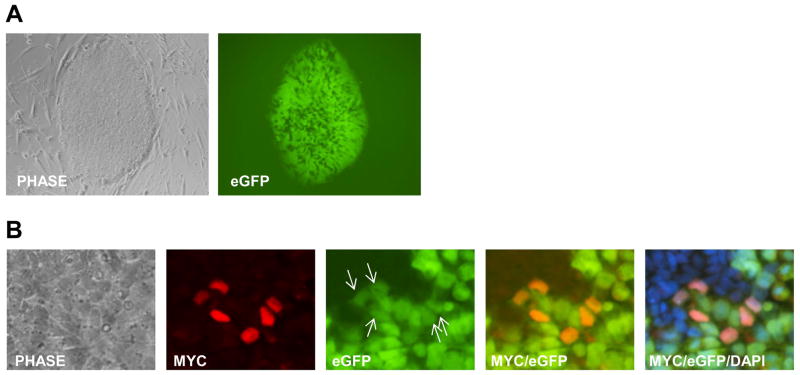
As shown in Figure 1D, TBX3 expression did not overlap with OCT4 expression at any time during differentiation, suggesting that TBX3 may function to inhibit OCT4 expression and promote differentiation in human ESCs. To test this, a hOCT4-eGFP human ESC reporter line was created by transfection with the reporter construct, phOCT4-eGFP. To determine whether TBX3 inhibits OCT4 expression, these cells were transduced with lentivirus to over-express TBX3. This construct has been used to generate stable human ESC lines and exhibited similar regulation of OCT4p-eGFP expression to that of endogenous OCT4 in parental human ESCs [24]. Figure 2A shows the human ESC line expressing the enhanced green fluorescence protein (eGFP) reporter gene under the control of the OCT4 promoter. As shown in Figure 2B (arrows), TBX3 over-expression does not inhibit the expression of eGFP, suggesting that TBX3 does not directly regulate the OCT4 promoter. This data also suggests that the converse expression of TBX3 and OCT4 seen in differentiating human ESCs, in Figure 1D, may represent two different stages of differentiation and that the absence of OCT4 in TBX3 positive cells is independent of the function of TBX3.
Figure 2.
TBX3 does not directly inhibit the human OCT4 promoter activity. (A) Human OCT4p-eGFP ESC line. (B) Over-expression of TBX3 co-localizes with eGFP expression and does not inhibit the OCT4 promoter to decrease eGFP expression. Images were taken at 20X magnification.
Over-expression of TBX3 in undifferentiated human ESCs promotes stem cell proliferation, but is not sufficient to induce differentiation
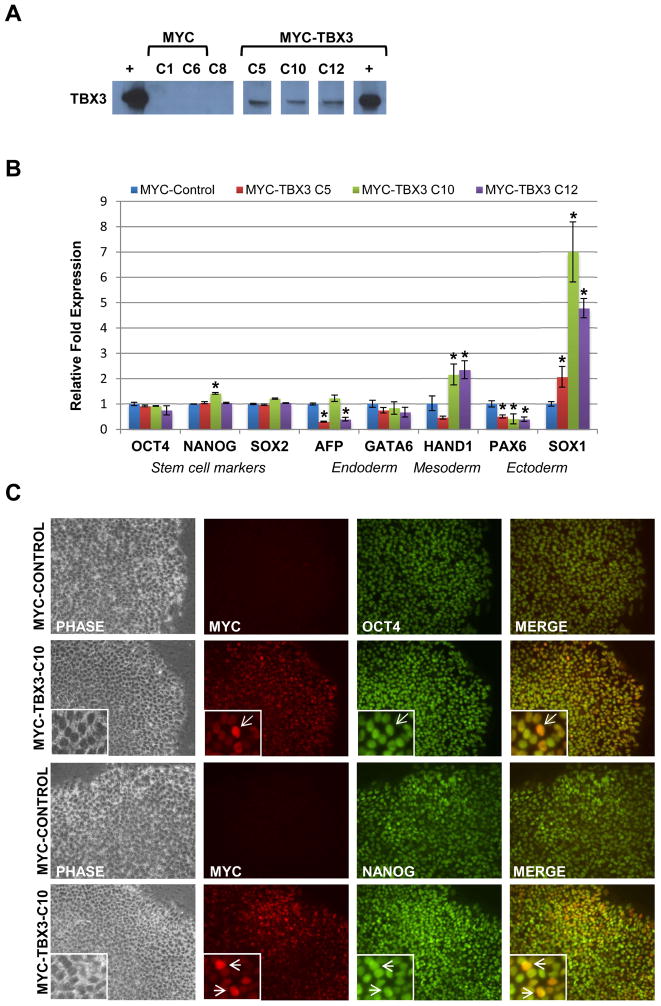
Since TBX3 is highly expressed during differentiation of human ESCs, it is important to determine if over-expression of TBX3, alone, is sufficient to initiate differentiation. In this experiment, we used lentivirus to generated stable human ESCs lines over-expressing TBX3 tagged with Myc. Over-expression of TBX3 was verified by western blot (Figure 3A) and qRT-PCR (data not shown). For immunocytochemistry and western blot analyses, anti-myc antibody was used to detect ectopically expressed TBX3 and to differentiate endogenous TBX3 (Figure 3A and C). Three independent ESC lines over-expressing TBX3 (C5, C10, and C12) were used in subsequent experiments. Results of qRT-PCR analysis revealed no significant changes in the expression of pluripotency (OCT4, NANOG, SOX2), endoderm (AFP) or mesoderm (HAND1) markers in all TBX3 over-expressing clones, except for PAX6 (ectoderm) which was significantly down-regulated, and SOX1 (ectoderm) which was significantly up-regulated (Figure 3B). Confirming our qRT-PCR data, immunocytochemistry analysis of OCT4 and NANOG revealed no change in the expression of these pluripotent markers upon over-expression of TBX3 (Figure 3C). A recent study suggested that over-expression of Tbx3 in mouse ESCs induced differentiation into extra-embryonic endoderm by direct up-regulation of Gata6 expression [14]. To test if TBX3 plays the same role in human ESCs, we examined GATA6 expression with qRT-PCR. Our result showed no change in GATA6 expression, suggesting over-expression alone is not sufficient to drive undifferentiated human ESCs to a specific lineage. This data shows that the over-expression of TBX3 alone is not sufficient to induce differentiation of undifferentiated human ESCs, further supporting that TBX3 functions differently in human than in mouse ESCs.
Figure 3.
Over-expression of TBX3 in undifferentiated human ESCs does not affect pluripotency. (A) Verification of ectopically expressed myc-TBX3 in human ESCs. TBX3 over-expression was verified by western blot. (B) Quantitative real-time PCR (QRT-PCR) analysis of pluripotency and differentiation markers in TBX3 over-expressing clones. Expression levels of pluripotency (OCT, NANOG, and SOX2) and differentiation-related genes (AFP, HAND1, PAX6 and SOX1) were analyzed by qRT-PCR in TBX3 over-expressing clones. (Control H9 cells =1.0) *, P<0.05. (C) Immunocytochemistry of TBX3, OCT4 and NANOG expression in control and TBX3 over-expressing human ESC lines. TBX3 over-expressing cells maintained normal OCT4 (2nd row) and NANOG (4th row) expression when compared to control cells (1st and 3rd rows). Images were taken at 20X magnification.
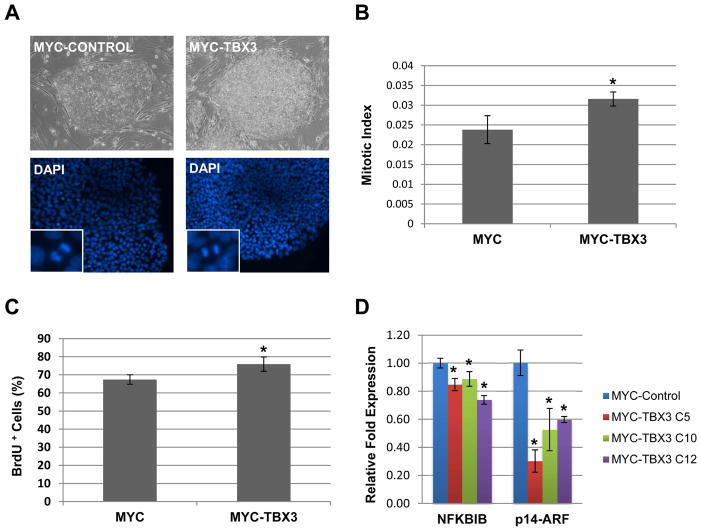
Although TBX3 over-expression didn’t induce differentiation, the colonies over-expressing TBX3 appeared more compact and needed to be passaged more often than control ESCs (Figure 4A). As shown in Figure 4A, approximately 72 hours after passage, while control ESC colonies contained only a single layer of cells, TBX3 over-expressing colonies had multiple cell layers. To determine whether this difference was due to increased cell proliferation, the mitotic index was calculated. Using DAPI staining, total and mitotic cells were identified and counted (Figure 4A, insets). TBX3 over-expressing cells had a significantly higher mitotic index suggesting that these cells were undergoing increased cell proliferation (Figure 4B). We also performed a BrdU incorporation assay to further verify our mitotic index results. As shown in Figure 4C, human ESCs over-expressing TBX3 had a significantly higher percentage of cells that incorporated BrdU, suggesting that TBX3 over-expression promotes human ESC proliferation in the undifferentiated state.
Figure 4.
Over-expression of TBX3 increases stem cell proliferation possibly by repressing the expression of NFκBIB and p14ARF. (A) Phase images of control (left) and TBX3 over-expressing (right) human ESC colonies cultured on MEFs. Examples of DAPI images used to calculate mitotic index. Mitotic cells are shown in insets. (B) TBX3 over-expressing ESCs have a significantly higher mitotic index than control ESCs. *, p<0.05. (C) TBX3 over-expression increases the percentage of BrdU positive ESCs cells. *, p<0.05 (D) TBX3 over-expression in human ESCs results in a significant decrease in the expression of cell cycle regulators, NFκBIB and p14ARF. *, p<0.05.
TBX3 has been shown to inhibit p14ARF expression to promote proliferation and immortalization of mouse embryo fibroblasts [7,28–32]. We have recently shown that TBX3 directly binds to and inhibits the promoter activity of NFκBIB, an inhibitor of NFκB, to promote mammary epithelial cell proliferation [50]. Since the NFκB pathway is known to regulate cell cycle progression and proliferation [33,34], we decided to examine NFκBIB and p14ARF expression levels. As shown in Figure 4D, both NFκBIB and p14ARF expression were significantly decreased upon over-expression of TBX3, suggesting that TBX3 over-expression may increase human ESC proliferation by repressing NFκBIB and p14ARF expression.
Knockdown of TBX3 impairs neuroepithelial differentiation in differentiating human ESCs
To further investigate the role of TBX3 during human ESC differentiation processes, we established human ESC lines that were stably integrated with shRNA targeting TBX3. Since undifferentiated human ESCs express low levels of endogenous TBX3, we used a readily available MCF7-TBX3-eGFP cell line, expressing a high level of the TBX3-eGFP fusion protein, to test the efficiency of the TBX3 shRNA lentiviral constructs. Western blot analysis of MCF7-TBX3-eGFP cells transfected with lentiviral constructs, harboring shRNA targeted at various exons of TBX3, efficiently knocked-down TBX3 expression (Figure 5A). TBX3 level was not affected in cells transfected with the control vector or the 3′UTR shRNA vector, as expected (Figure 5A). To create stable TBX3 shRNA human ESC lines, a lentiviral vector harboring shRNA targeted at exon 2 of TBX3 was used to produce lentivirus and transduce human ESCs. Two clones of control (Control-1 and -2) and TBX3 shRNA (TBX3shRNA-1 and -2) ESC lines were used for subsequent experiments.
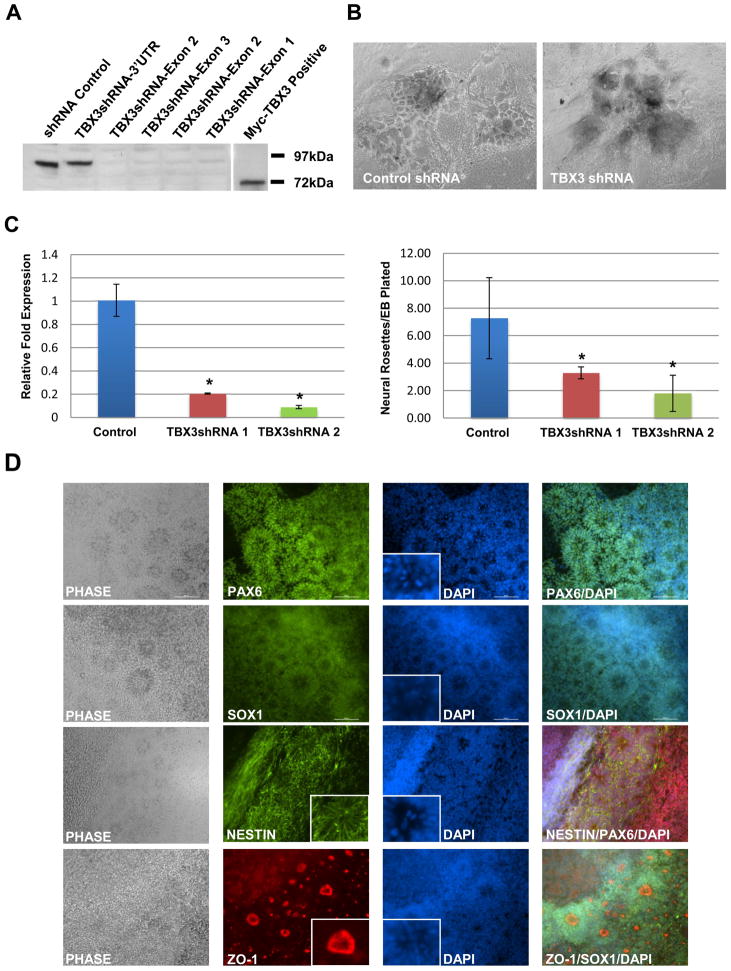
Figure 5.
TBX3 knockdown inhibits neural rosette formation in differentiated human ESCs. (A) Verification of TBX3 knockdown. MCF7-TBX3-eGFP cell line was transfected with various TBX3 shRNA lentiviral vectors. Cell lysates were harvested and a western blot was performed, using a rabbit anti-TBX3 antibody, to check the efficiency of TBX3 knockdown. TBX3-eGFP fusion protein runs at 97kDa while myc-TBX3 protein runs at 72kDa. (B) Phase images of cell morphologies at day 8 of differentiation with 10% FBS suggest a decrease in the number of neural rosette structures. (C) Quantification of neural rosettes in control and TBX3 knockdown cell lines. The loss of TBX3 expression coincided with a significant loss in neural rosette formation.*, p<0.05. (D) Characterization of neural rosettes. Immunocytochemistry was performed with antibodies for PAX6, SOX1, NESTIN and ZO-1. Radial formation of NESTIN, apical localization of ZO-1, localization of mitotic cells within the apical lumen can be seen within the insets. Images were taken at 10X magnification.
Previous studies in mouse ESCs have shown that knockdown of Tbx3 compromises self-renewal and results in differentiation, suggesting that Tbx3 is important for maintaining self-renewal [12–14]. However, our human TBX3shRNA ESC lines showed no obvious changes to colony morphology (Supporting Information Figure S1A). Furthermore, qRT-PCR analysis of pluripotency markers, OCT4 and NANOG, showed no significant change in expression when compared to control cells (Supporting Information Figure S1B). To examine if the knockdown of TBX3 in undifferentiated human ESCs affected stem cell proliferation, the mitotic index was calculated as previously described. Knockdown of TBX3 did not affect cell proliferation in undifferentiated human ESCs (Supporting Information Figure S1C). Since TBX3 expression in undifferentiated human ESCs is extremely low, possibly due to TBX3 being epigenetically repressed by Sirtuin-1(NAD+-dependent class III histone deacetylase) [35], further knockdown of TBX3 at this stage was not expected to affect pluripotency or cell proliferation.
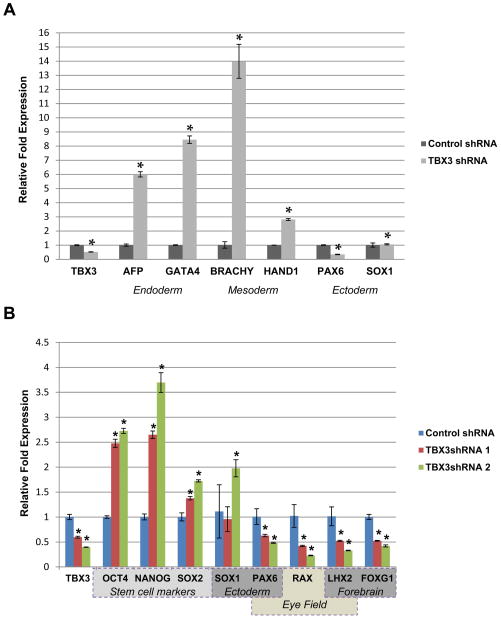
To determine whether TBX3 plays a role in differentiating human ESCs, TBX3 shRNA ESC lines were induced to form EBs and cultured in suspension for seven days without bFGF. On the seventh day, EBs were plated and fed with human ESC medium supplemented with 10% FBS for 8 additional days. Colony morphology was examined. As shown in Figure 5B, there was a decrease in the number of neural rosette-like structures in the TBX3 knockdown ESC line. To verify that these structures were indeed neural rosettes, we performed immunocytochemistry of PAX6, SOX1, NESTIN, and ZO-1 (Figure 5D). PAX6 and SOX1 are early neural transcription factors and commonly used as markers of neural rosettes [36–41]. Besides the typical radial cell morphology seen by DAPI staining, the expression pattern of PAX6 and SOX1 is consistent with neural rosettes (Figure 5D). NESTIN is a well-known neural cell marker that is expressed within rosettes [42,43]. NESTIN expression with radial appearance is consistent with morphology of neural rosettes (Figure 5D, inset). Formation of neural rosettes is initiated by acquiring cell polarity that is illustrated by the redistribution of ZO-1, a tight junction protein [44]. As shown in Figure 5D, ZO-1 expression was localized to the apical lumen of our structures as observed in neural rosettes [44]. Neural rosettes are also characterized by the presence of mitotic cells within the apical lumen [40]. Mitotic cells were identified in the apical lumen of these structures (Figure 5D, DAPI insets). Together, expression of these markers confirms that the identified structures are neural rosettes. After characterization, neural rosettes were quantified and normalized to the number of EBs plated. The number of neural rosettes/EB plated significantly decreased upon knockdown of TBX3 in differentiating cells (Figure 5C). Quantitative RT-PCR analysis revealed that knockdown of TBX3 up-regulated mesoderm (BRACHYURY and HAND1) and endoderm markers (AFP and GATA4), suggesting that TBX3 may function to inhibit differentiation into mesoderm and endoderm lineages (Figure 6A). Interestingly, the ectoderm marker, PAX6, was down-regulated while SOX1 expression remained unchanged, suggesting that knockdown of TBX3 impairs differentiation into neuroepithelia (Figure 6A and B). This result is consistent with the decrease in neural rosette formation. Expression of OCT4, NANOG and SOX2 are up-regulated in TBX3 knockdown cell lines (Figure 6B), suggesting that TBX3 may function to enhance repression of these markers during differentiation.
Figure 6.
TBX3 knockdown inhibits neuroepithelial, neuroectoderm and eye field marker expression. Expression levels of pluripotency and differentiation related markers in TBX3 knockdown cell lines at day 8 of differentiation with 10% FBS were analyzed. (A) Quantitative RT-PCR was performed on the expression of TBX3, endoderm markers (AFP and GATA4), mesoderm markers (BRACHYURY and HAND1) and neuroepithelial markers (PAX6 and SOX1). Control cells= 1.0. *, p<0.05. (B) Quantitative RT-PCR was performed on the expression of TBX3, pluripotency markers (OCT4, NANOG, and SOX2), neuroepithelial markers (PAX6 and SOX1), forebrain markers (FOXG1 and LHX2) and eye field markers (PAX6, LHX2, RAX). *, p<0.05.
To confirm that TBX3 knockdown specifically affects neuroepithelial differentiation, we differentiated both control and TBX3 shRNA human ESC lines using a well-defined neuroepithelial differentiation protocol previously described by Shi et al [23]. Briefly, control and TBX3 shRNA ESC lines were plated onto matrigel and once cells reached 90% confluency, neural induction was initiated by changing the culture medium to one that supports neural induction, referred to as 3N medium [23], for an additional 14 days. The efficiency of neural induction between control and TBX3 shRNA cell lines was monitored by the appearance of cells with characteristic neuroepithelial cell morphology (neural rosette formation). After characterization, neural rosettes were quantified and normalized to the number of cells present at day 14. Using this defined neuroepithelial differentiation protocol, we have found that knockdown of TBX3 results in a significant decrease in neural rosette formation, as compared to control cells (Supporting Information Figure S1D). This result is consistent with our differentiation protocol and suggests that TBX3 plays an important role in early neuroepithelial differentiation.
Tbx3’s role in Xenopus eye field formation has been well characterized [45]. Eye field transcription factors or EFTFs (pax6, rx1, tbx3/ET, six3, lhx2 and six6) are considered important for eye field formation [45]. Over-expression of these factors in the developing Xenopus embryos can induce eye-like structures [46]. Moreover, BMP-4 regulates Tbx3 expression in the developing optic cup of mice eyes [47,48]. TBX3 was found to be expressed in the ciliary epithelium of the adult mammalian eye [49]. To test if knockdown of TBX3 affected eye field specification, we performed qRT-PCR analysis on the expression of eye field markers (PAX6, LHX2, RAX). Since commitment towards a retinal lineage begins with the establishment of the eye field within the anterior neuroepithelium [50], we also performed qRT-PCR on anterior/forebrain markers (LHX2 and FOXG1). Interestingly, the expression of both anterior/forebrain and eye field markers were significantly reduced in TBX3 knockdown differentiated cells (Figure 6B). These results suggest that TBX3 may not only function to promote neuroepithelial differentiation but may also promote eye field specification.
DISCUSSION
In this study, we have defined new roles for TBX3 in human ESCs. We found that TBX3 over-expression, in the undifferentiated state, promotes increased stem cell proliferation possibly through the repression of NFκBIB and p14ARF. Knockdown experiments, during differentiation, revealed an unrecognized role of TBX3 in neuroepithelial differentiation. To gain insight into whether TBX3 plays a role in promoting human ESCs self-renewal or differentiation, we examined TBX3 expression during differentiation of human ESCs. To our surprise, we found TBX3 expression to be significantly up-regulated as early as EB formation and further up-regulated during differentiation with BMP4, FBS and RA, suggesting a possible role of TBX3 in promoting human ESCs differentiation. This result is completely opposite of what has been shown in mouse ESCs. In mouse ESCs, Tbx3 is highly expressed in the undifferentiated state and is down-regulated upon EB formation and differentiation with RA or PI3K inhibitor [12,14]. ChIP-sequencing has shown that Tbx3 binds to the Oct4 promoter in mouse ESCs [15] to promote self-renewal and pluripotency. Since human and mouse ESCs represent different stages of pluripotency, our study suggests that they may also have distinct responses to the manipulation of TBX3. Examples in which human and mouse ESCs respond differently to similar transcriptional and signaling pathways have been well documented. Addition of LIF or BMPs to mouse ESCs is necessary to maintain pluripotency while these same factors cause differentiation in human ESCs [18–22].
Although human ESC lines over-expressing TBX3 had no effect on the expression of pluripotency markers (OCT4, NANOG and SOX2), we found that ectoderm markers PAX6 was significantly down-regulated while SOX1 was significantly up-regulated. Currently there are no studies suggesting that TBX3 directly regulates PAX6 or SOX1 in human ESCs. However, a study by Pankratz et al showed PAX6 to be down-regulated and SOX1 to be up-regulated by day 6 of EB formation when compared to undifferentiated human ESCs [41]. The similar PAX6 and SOX1 expression pattern between these two studies invites further investigation into the relationship between PAX6, SOX1 and TBX3 in human ESCs. Over-expression of Tbx3 was found to promote mouse ESC differentiation into extra-embryonic endoderm by direct up-regulation of Gata6 expression. In our study, TBX3 over-expression did not affect GATA6 expression to induce differentiation, confirming that TBX3 plays different roles in human and mouse ESCs.
TBX3 over-expressing clones had a significantly higher mitotic index and BrdU incorporation than control cells and both p14ARF and NFκBIB were significantly repressed. These results are consistent with TBX3 function, identified by our and other groups, to promote cell proliferation possibly by repressing p14ARF [29,30,51,52]. ARF transcript levels are repressed in iPS and ESCs compared to MEFs [53]. In hematopoietic and neural stem cells, Bmi-1 was found to support self-renewal by suppressing the expression of p19/p14ARF [54–56], suggesting that p14/p19ARF inhibits proliferation for various types of stem cells. Our study suggests that by inhibiting p14ARF, TBX3 promotes human ESC proliferation. NFκBIB, an inhibitor of NFκB, is also repressed by TBX3. NF-κB associated pathways also play an important role in cell proliferation, differentiation and apoptosis [57]. Aberrant activation of NFκB results in excessive cellular proliferation [58]. Our results are consistent with our recent study which showed that TBX3 over-expression directly inhibits NFκBIB expression to promote mammary epithelial proliferation [51]. However, NFκB signaling in human ESCs is controversial [59,60]. In this study, we showed that TBX3 inhibited NFκBIB expression, suggesting additional pathways that promote human ESC proliferation. Further studies assessing the expression of targets of the p14ARF and NFκB signaling pathway will further elucidate the t mechanism by which TBX3 expression regulates human ESC proliferation.
Our study showed that knockdown of TBX3 in the undifferentiated state of human ESCs did not attenuate self-renewal or pluripotency to induce differentiation. This is consistent with previous findings in which TBX3 expression is expected to be extremely low in the undifferentiated state [35] and thus further reducing the level of TBX3, at this stage, would not affect self-renewal or pluripotency. However, TBX3 knockdown during differentiation of human ESCs resulted in an up-regulation of OCT4, NANOG, and SOX2. Since our over-expression studies showed that TBX3 does not directly regulate the expression of OCT4 and NANOG, TBX3 may influence differentiation through another mechanism. BMP signaling promotes human ESC differentiation. TBX3 has been identified both upstream and downstream of BMP signaling [61,62]. Additional studies to determine whether TBX3 regulates the BMP signaling pathway to enhance human ESC differentiation will facilitate our understanding of these mechanisms. Knockdown of TBX3 resulted in a significant decrease in neural rosette formation, suggesting a role for TBX3 in promoting neuroepithelial differentiation. This was confirmed by a decrease in PAX6 expression. However, the expression of another neuroepithelial marker, SOX1, did not change. Studies have shown that during neural differentiation of human ESCs, initial neuroepithelial cells express PAX6 much earlier than SOX1 [38,41]. PAX6 expression was detected as early as day 6 of differentiation while SOX1 expression was detected at day 14. Thus, change of SOX1 expression in our TBX3 knockdown cells may not have been initiated yet. If we extended our time point to allow further differentiation, we may be able to better assess if knockdown of TBX3 also affects SOX1 expression. In these TBX3 shRNA differentiation experiments, we used a non-defined hESC culture system with MEF and FBS. It is important to note that this differentiation method had certain limitations, especially since non-defined factors from MEF and FBS could influence differentiation towards one specific lineage over another. Thus, the direct effect of TBX3 knockdown on neuroepithelial differentiation could be skewed by non-specific effects based on our initial differentiation method. To eliminate this possible effect, and examine the effect of TBX3 knockdown on neuroepithelial differentiation, we repeated our TBX3 shRNA differentiation experiment using a previously published and well defined neuroepithelial differentiation protocol, as described by Shi et al. Using this protocol, we have recapitulated our previous results in which TBX3 knockdown results in a decrease in neural rosette formation, further suggesting that the lack of TBX3 specifically affects differentiation towards neuroepithelial cells.
It is very interesting that knockdown of TBX3 impaired neural rosette formation and decreased the expression of anterior/forebrain (FOXG1, LHX2) and eye field markers (LHX2, PAX6, and RAX), further suggesting a role for TBX3 in regulating neuroepithelial differentiation. This result is consistent with findings in Xenopus in which Tbx3 regulates eye field specification [45,46]. To confirm TBX3’s role in regulating differentiation towards neuroepithelia is not due to the aberrant differentiation of other germ layers during EB formation, additional experiments with lineage-specific differentiation protocols will clarify the function of TBX3 in eye field and neuroepithelial development.
This study demonstrates that TBX3 functions differently in human ESCs. In mouse ESCs, Tbx3 expression is necessary for maintaining self-renewal, while over-expression promotes differentiation into extra-embryonic endoderm [12–14]. In undifferentiated human ESCs, TBX3 promotes ESC proliferation possibly through the repression of NFκBIB and p14ARF. During differentiation, TBX3 may promote neuroepithelial and eye field specification. Human and mouse ESCs represent distinct pluripotent states which may explain why TBX3 functions differently in human ESCs than it does in mouse ESCS. Human ESCs are more similar to mouse-derived epiblast stem cells (mEpiSCs) than mouse ESCs [17,63,64], suggesting that human ESCs are at a later developmental stage that is more primed for differentiation. Due to their different pluripotent and developmental states, human and mouse ESCs may respond to TBX3 manipulation differently.
CONCLUSION
Our results suggest TBX3 promotes human ESC proliferation in the undifferentiated state and plays a role to promote neuroepithelial differentiation. These results provide new insight into the roles of TBX3 in regulating self-renewal and differentiation of human ESCs. Further investigation of the molecular mechanisms by which TBX3 regulates neuroepithelial differentiation will contribute to our understanding of ESC differentiation and specification in early human development.
Supplementary Material
Figure S1. Knockdown of TBX3 does not affect human ESC pluripotency or cell proliferation. (A) Knockdown of TBX3 in human ESCs did not affect colony morphology. (B) Quantitative RT-PCR analysis of pluripotency markers, OCT4 and NANOG. (Control H9 cells =1.0) TBX3 knockdown did not significantly affect OCT4 or NANOG expression. (C) Knockdown of TBX3 did not affect cell proliferation. Mitotic index was calculated as described previously for the over-expression studies. (D) Quantification of neural rosettes in control and TBX3 knockdown cell lines using a defined neuroepithelial differentiation protocol. The loss of TBX3 expression coincided with a significant loss in neural rosette formation.*, p<0.05.
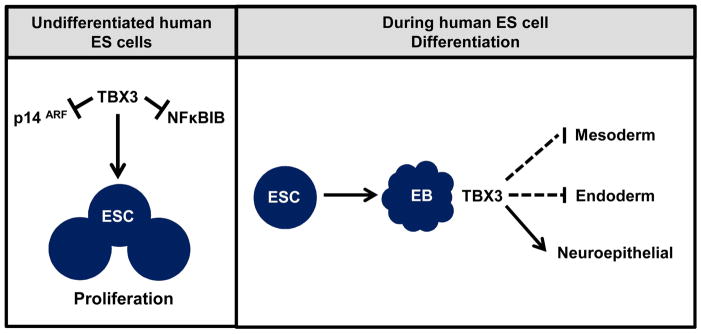
Figure 7.
Model for TBX3 regulation of human ESC proliferation and differentiation. In undifferentiated human ESCs, TBX3 may promote cell proliferation by repressing cell cycle regulators, NFκBIB and p14ARF. During differentiation, TBX3 may function to promote neuroepithelial differentiation while inhibiting differentiation into mesoderm and endoderm.
Acknowledgments
We thank Chengkang Zhang for cloning vectors used in this study. We would also like to thank the UCI Stem Cell Core Facility for providing training and cell culture space. The authors are grateful to Milad Riazifar and the Huang Lab for critically reading this manuscript. This work was supported by the National Cancer Institute R01CA121876 grant to TH and R01CA121876 minority supplemental to TE.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTERESTS
The authors indicate no potential conflicts of interest.
Author Contributions:
T.E.: Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; T.H.: Conception and design, financial support, provision of study material, data analysis and interpretation, manuscript writing, final approval of manuscript
References
- 1.Wilson V, Conlon FL. The T-box family. GENOME BIOL. 2002;3:REVIEWS3008. doi: 10.1186/gb-2002-3-6-reviews3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papaioannou VE, Silver LM. The T-box gene family. BIOESSAYS. 1998;20:9–19. doi: 10.1002/(SICI)1521-1878(199801)20:1<9::AID-BIES4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 3.Packham EA, Brook JD. T-box genes in human disorders. HUM MOL GENET. 2003;12(Spec No 1):R37–44. doi: 10.1093/hmg/ddg077. [DOI] [PubMed] [Google Scholar]
- 4.Davenport TG, Jerome-Majewska LA, Papaioannou VE. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. DEVELOPMENT. 2003;130:2263–2273. doi: 10.1242/dev.00431. [DOI] [PubMed] [Google Scholar]
- 5.Brummelkamp TR, Kortlever RM, Lingbeek M, et al. TBX-3, the gene mutated in Ulnar-Mammary Syndrome, is a negative regulator of p19ARF and inhibits senescence. J BIOL CHEM. 2002;277:6567–6572. doi: 10.1074/jbc.M110492200. [DOI] [PubMed] [Google Scholar]
- 6.Carlson H, Ota S, Campbell CE, et al. A dominant repression domain in Tbx3 mediates transcriptional repression and cell immortalization: relevance to mutations in Tbx3 that cause ulnar-mammary syndrome. HUM MOL GENET. 2001;10:2403–2413. doi: 10.1093/hmg/10.21.2403. [DOI] [PubMed] [Google Scholar]
- 7.Lingbeek ME, Jacobs JJ, van Lohuizen M. The T-box repressors TBX2 and TBX3 specifically regulate the tumor suppressor gene p14ARF via a variant T-site in the initiator. J BIOL CHEM. 2002;277:26120–26127. doi: 10.1074/jbc.M200403200. [DOI] [PubMed] [Google Scholar]
- 8.Bamshad M, Lin RC, Law DJ, et al. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. NAT GENET. 1997;16:311–315. doi: 10.1038/ng0797-311. [DOI] [PubMed] [Google Scholar]
- 9.Bamshad M, Le T, Watkins WS, et al. The spectrum of mutations in TBX3: Genotype/Phenotype relationship in ulnar-mammary syndrome. AM J HUM GENET. 1999;64:1550–1562. doi: 10.1086/302417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klopocki E, Neumann LM, Tonnies H, et al. Ulnar-mammary syndrome with dysmorphic facies and mental retardation caused by a novel 1.28 Mb deletion encompassing the TBX3 gene. EUR J HUM GENET. 2006 doi: 10.1038/sj.ejhg.5201696. [DOI] [PubMed] [Google Scholar]
- 11.He M, Wen L, Campbell CE, et al. Transcription repression by Xenopus ET and its human ortholog TBX3, a gene involved in ulnar-mammary syndrome. PROC NATL ACAD SCI U S A. 1999;96:10212–10217. doi: 10.1073/pnas.96.18.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanova N, Dobrin R, Lu R, et al. Dissecting self-renewal in stem cells with RNA interference. NATURE. 2006;442:533–538. doi: 10.1038/nature04915. [DOI] [PubMed] [Google Scholar]
- 13.Niwa H, Ogawa K, Shimosato D, et al. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. NATURE. 2009;460:118–122. doi: 10.1038/nature08113. [DOI] [PubMed] [Google Scholar]
- 14.Lu R, Yang A, Jin Y. Dual functions of T-box 3 (Tbx3) in the control of self-renewal and extraembryonic endoderm differentiation in mouse embryonic stem cells. J BIOL CHEM. 2011;286:8425–8436. doi: 10.1074/jbc.M110.202150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han J, Yuan P, Yang H, et al. Tbx3 improves the germ-line competency of induced pluripotent stem cells. NATURE. 2010;463:1096–1100. doi: 10.1038/nature08735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. SCIENCE. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 17.Tesar PJ, Chenoweth JG, Brook FA, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. NATURE. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 18.Williams RL, Hilton DJ, Pease S, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. NATURE. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 19.Daheron L, Opitz SL, Zaehres H, et al. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. STEM CELLS. 2004;22:770–778. doi: 10.1634/stemcells.22-5-770. [DOI] [PubMed] [Google Scholar]
- 20.Ying QL, Nichols J, Chambers I, et al. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. CELL. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang P, Li J, Tan Z, et al. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. BLOOD. 2008;111:1933–1941. doi: 10.1182/blood-2007-02-074120. [DOI] [PubMed] [Google Scholar]
- 22.Xu RH, Chen X, Li DS, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. NAT BIOTECHNOL. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y, Kirwan P, Smith J, et al. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. NAT NEUROSCI. 2012;15:477–86. S1. doi: 10.1038/nn.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerrard L, Zhao D, Clark AJ, et al. Stably transfected human embryonic stem cell clones express OCT4-specific green fluorescent protein and maintain self-renewal and pluripotency. STEM CELLS. 2005;23:124–133. doi: 10.1634/stemcells.2004-0102. [DOI] [PubMed] [Google Scholar]
- 25.Storm MP, Kumpfmueller B, Thompson B, et al. Characterization of the phosphoinositide 3-kinase-dependent transcriptome in murine embryonic stem cells: identification of novel regulators of pluripotency. STEM CELLS. 2009;27:764–775. doi: 10.1002/stem.3. [DOI] [PubMed] [Google Scholar]
- 26.Galan-Caridad JM, Harel S, Arenzana TL, et al. Zfx controls the self-renewal of embryonic and hematopoietic stem cells. CELL. 2007;129:345–357. doi: 10.1016/j.cell.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu R, Yang A, Jin Y. Dual functions of T-box 3 (Tbx3) in the control of self-renewal and extraembryonic endoderm differentiation in mouse embryonic stem cells. J BIOL CHEM. 2011;286:8425–8436. doi: 10.1074/jbc.M110.202150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoogaars WM, Barnett P, Rodriguez M, et al. TBX3 and its splice variant TBX3 + exon 2a are functionally similar. PIGMENT CELL MELANOMA RES. 2008;21:379–387. doi: 10.1111/j.1755-148X.2008.00461.x. [DOI] [PubMed] [Google Scholar]
- 29.Yarosh W, Barrientos T, Esmailpour T, et al. TBX3 is overexpressed in breast cancer and represses p14 ARF by interacting with histone deacetylases. CANCER RES. 2008;68:693–699. doi: 10.1158/0008-5472.CAN-07-5012. [DOI] [PubMed] [Google Scholar]
- 30.Platonova N, Scotti M, Babich P, et al. TBX3, the gene mutated in ulnar-mammary syndrome, promotes growth of mammary epithelial cells via repression of p19ARF, independently of p53. CELL TISSUE RES. 2007;328:301–316. doi: 10.1007/s00441-006-0364-4. [DOI] [PubMed] [Google Scholar]
- 31.Rowley M, Grothey E, Couch FJ. The role of Tbx2 and Tbx3 in mammary development and tumorigenesis. J MAMMARY GLAND BIOL NEOPLASIA. 2004;9:109–118. doi: 10.1023/B:JOMG.0000037156.64331.3f. [DOI] [PubMed] [Google Scholar]
- 32.Silva J, Dominguez G, Silva JM, et al. Analysis of genetic and epigenetic processes that influence p14ARF expression in breast cancer. ONCOGENE. 2001;20:4586–4590. doi: 10.1038/sj.onc.1204617. [DOI] [PubMed] [Google Scholar]
- 33.Van Waes C. Nuclear factor-kappaB in development, prevention, and therapy of cancer. CLIN CANCER RES. 2007;13:1076–1082. doi: 10.1158/1078-0432.CCR-06-2221. [DOI] [PubMed] [Google Scholar]
- 34.Karin M. Nuclear factor-kappaB in cancer development and progression. NATURE. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 35.Calvanese V, Lara E, Suarez-Alvarez B, et al. Sirtuin 1 regulation of developmental genes during differentiation of stem cells. PROC NATL ACAD SCI U S A. 2010;107:13736–13741. doi: 10.1073/pnas.1001399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhara SK, Stice SL. Neural differentiation of human embryonic stem cells. J CELL BIOCHEM. 2008;105:633–640. doi: 10.1002/jcb.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhara SK, Hasneen K, Machacek DW, et al. Human neural progenitor cells derived from embryonic stem cells in feeder-free cultures. DIFFERENTIATION. 2008;76:454–464. doi: 10.1111/j.1432-0436.2007.00256.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Huang CT, Chen J, et al. Pax6 is a human neuroectoderm cell fate determinant. CELL STEM CELL. 2010;7:90–100. doi: 10.1016/j.stem.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elkabetz Y, Studer L. Human ESC-derived neural rosettes and neural stem cell progression. COLD SPRING HARB SYMP QUANT BIOL. 2008;73:377–387. doi: 10.1101/sqb.2008.73.052. [DOI] [PubMed] [Google Scholar]
- 40.Elkabetz Y, Panagiotakos G, Al Shamy G, et al. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. GENES DEV. 2008;22:152–165. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pankratz MT, Li XJ, Lavaute TM, et al. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. STEM CELLS. 2007;25:1511–1520. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaneko Y, Sakakibara S, Imai T, et al. Musashi1: an evolutionally conserved marker for CNS progenitor cells including neural stem cells. DEV NEUROSCI. 2000;22:139–153. doi: 10.1159/000017435. [DOI] [PubMed] [Google Scholar]
- 43.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. CELL. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 44.Itoh M, Nagafuchi A, Yonemura S, et al. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J CELL BIOL. 1993;121:491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuber ME, Gestri G, Viczian AS, et al. Specification of the vertebrate eye by a network of eye field transcription factors. DEVELOPMENT. 2003;130:5155–5167. doi: 10.1242/dev.00723. [DOI] [PubMed] [Google Scholar]
- 46.Viczian AS, Solessio EC, Lyou Y, et al. Generation of functional eyes from pluripotent cells. PLoS BIOL. 2009;7:e1000174. doi: 10.1371/journal.pbio.1000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Behesti H, Papaioannou VE, Sowden JC. Loss of Tbx2 delays optic vesicle invagination leading to small optic cups. DEV BIOL. 2009;333:360–372. doi: 10.1016/j.ydbio.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Behesti H, Holt JK, Sowden JC. The level of BMP4 signaling is critical for the regulation of distinct T-box gene expression domains and growth along the dorso-ventral axis of the optic cup. BMC DEV BIOL. 2006;6:62. doi: 10.1186/1471-213X-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gualdoni S, Baron M, Lakowski J, et al. Adult ciliary epithelial cells, previously identified as retinal stem cells with potential for retinal repair, fail to differentiate into new rod photoreceptors. STEM CELLS. 2010;28:1048–1059. doi: 10.1002/stem.423. [DOI] [PubMed] [Google Scholar]
- 50.Meyer JS, Shearer RL, Capowski EE, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. PROC NATL ACAD SCI U S A. 2009;106:16698–16703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J, Esmailpour T, Shang X, et al. TBX3 over-expression causes mammary gland hyperplasia and increases mammary stem-like cells in an inducible transgenic mouse model. BMC DEV BIOL. 2011;11:65. doi: 10.1186/1471-213X-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan W, Huang X, Chen C, et al. TBX3 and its isoform TBX3+2a are functionally distinctive in inhibition of senescence and are overexpressed in a subset of breast cancer cell lines. CANCER RES. 2004;64:5132–5139. doi: 10.1158/0008-5472.CAN-04-0615. [DOI] [PubMed] [Google Scholar]
- 53.Li H, Collado M, Villasante A, et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. NATURE. 2009;460:1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Molofsky AV, Pardal R, Iwashita T, et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. NATURE. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park IK, Qian D, Kiel M, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. NATURE. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 56.Rizo A, Olthof S, Han L, et al. Repression of BMI1 in normal and leukemic human CD34(+) cells impairs self-renewal and induces apoptosis. BLOOD. 2009;114:1498–1505. doi: 10.1182/blood-2009-03-209734. [DOI] [PubMed] [Google Scholar]
- 57.Karin M. Nuclear factor-kappaB in cancer development and progression. NATURE. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 58.Wu JT, Kral JG. The NF-kappaB/IkappaB signaling system: a molecular target in breast cancer therapy. J SURG RES. 2005;123:158–169. doi: 10.1016/j.jss.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Armstrong L, Hughes O, Yung S, et al. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. HUM MOL GENET. 2006;15:1894–1913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]
- 60.Kang HB, Kim YE, Kwon HJ, et al. Enhancement of NF-kappaB expression and activity upon differentiation of human embryonic stem cell line SNUhES3. STEM CELLS DEV. 2007;16:615–623. doi: 10.1089/scd.2007.0014. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki T, Takeuchi J, Koshiba-Takeuchi K, et al. Tbx Genes Specify Posterior Digit Identity through Shh and BMP Signaling. DEV CELL. 2004;6:43–53. doi: 10.1016/s1534-5807(03)00401-5. [DOI] [PubMed] [Google Scholar]
- 62.Tumpel S, Sanz-Ezquerro JJ, Isaac A, et al. Regulation of Tbx3 expression by anteroposterior signalling in vertebrate limb development. DEV BIOL. 2002;250:251–262. [PubMed] [Google Scholar]
- 63.Vallier L, Touboul T, Chng Z, et al. Early cell fate decisions of human embryonic stem cells and mouse epiblast stem cells are controlled by the same signalling pathways. PLoS ONE. 2009;4:e6082. doi: 10.1371/journal.pone.0006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hanna J, Cheng AW, Saha K, et al. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. PROC NATL ACAD SCI U S A. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Knockdown of TBX3 does not affect human ESC pluripotency or cell proliferation. (A) Knockdown of TBX3 in human ESCs did not affect colony morphology. (B) Quantitative RT-PCR analysis of pluripotency markers, OCT4 and NANOG. (Control H9 cells =1.0) TBX3 knockdown did not significantly affect OCT4 or NANOG expression. (C) Knockdown of TBX3 did not affect cell proliferation. Mitotic index was calculated as described previously for the over-expression studies. (D) Quantification of neural rosettes in control and TBX3 knockdown cell lines using a defined neuroepithelial differentiation protocol. The loss of TBX3 expression coincided with a significant loss in neural rosette formation.*, p<0.05.