Abstract
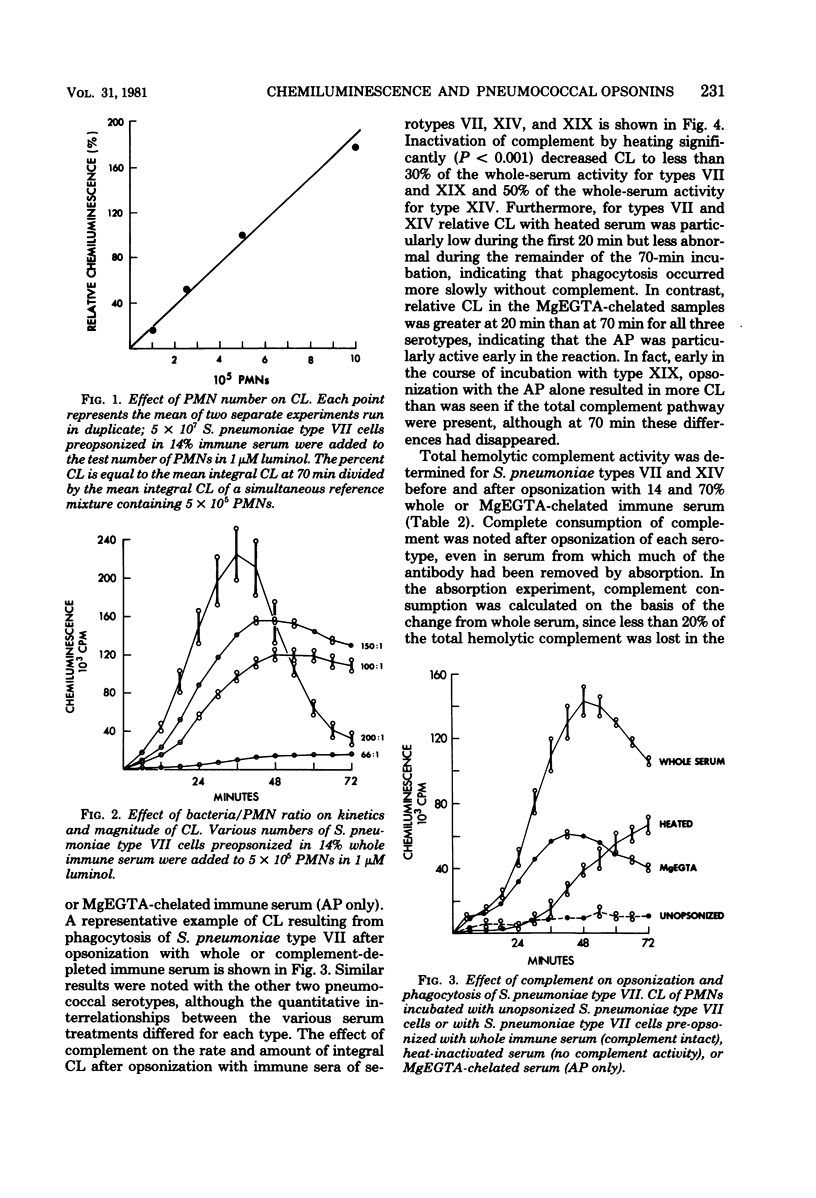
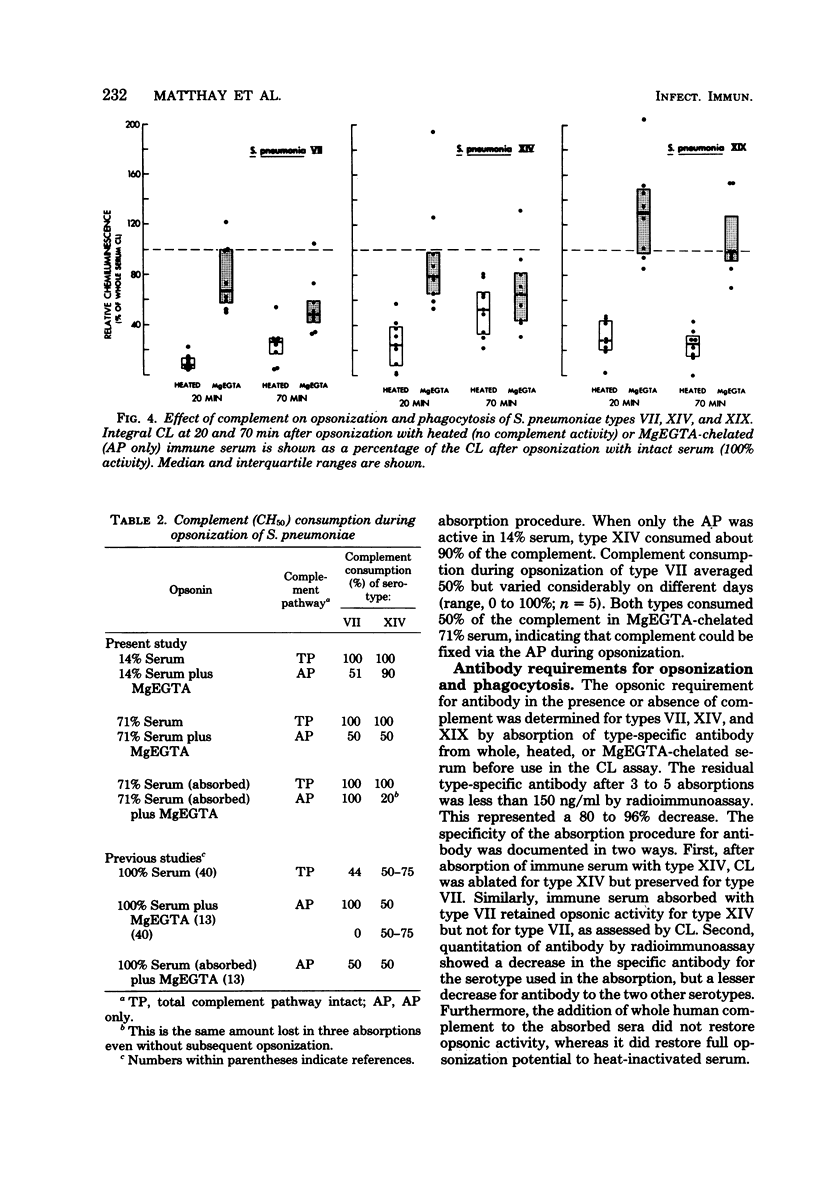
A luminol-enhanced chemiluminescence assay was used to investigate opsonic requirements for phagocytosis of STreptococcus pneumoniae serotypes VII, XIV, and XIX. After opsonization with whole immune sera (with antibody and total complement pathway), heat-inactivated immune sera (with antibody alone), or magnesium dichloride-ethylene glycol tetraacetic acid-chelated immune sera (with antibody and alternative complement pathway), live S. pneumoniae cells were incubated at 37 degrees C with normal polymorphonuclear leukocytes while serial chemiluminescence measurements were recorded. The amount of chemiluminescence observed correlated closely with evidence of phagocytosis as observed by microscopy. Complement was required for efficient opsonization, since all three serotypes showed a slower rise and less integral chemiluminescence after opsonization with heat-inactivated serum as compared with whole serum. The alternative pathway provided opsonic activity equal to that of the total complement pathway for type XIX, but only intermediate activity for types VII and XIV. Type-specific antibody was also required for effective opsonization of all three serotypes since chemiluminescence was markedly reduced when bacteria were opsonized with antibody-depleted serum (serum absorbed with type-specific S. pneumoniae cells at 4 degrees C). Thus, chemiluminescence proved to be an effective means of defining the requirement for both antibody and complement in the opsonization and phagocytosis of S. pneumoniae.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahonkhai V. I., Landesman S. H., Fikrig S. M., Schmalzer E. A., Brown A. K., Cherubin C. E., Schiffman G. Failure of pneumococcal vaccine in children with sickle-cell disease. N Engl J Med. 1979 Jul 5;301(1):26–27. doi: 10.1056/NEJM197907053010106. [DOI] [PubMed] [Google Scholar]
- Allen R. C. Evaluation of serum opsonic capacity by quantitating the initial chemiluminescent response from phagocytizing polymorphonuclear leukocytes. Infect Immun. 1977 Mar;15(3):828–833. doi: 10.1128/iai.15.3.828-833.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann A. J., Addiego J., Wara D. W., Lubin B., Smith W. B., Mentzer W. C. Polyvalent pneumococcal-polysaccharide immunization of patients with sickle-cell anemia and patients with splenectomy. N Engl J Med. 1977 Oct 27;297(17):897–900. doi: 10.1056/NEJM197710272971701. [DOI] [PubMed] [Google Scholar]
- Andersen B. R., Brendzel A. M. Use of a unique chemiluminescence spectrometer in a study of factors influencing granulocyte light emission. J Immunol Methods. 1978;19(2-3):279–287. doi: 10.1016/0022-1759(78)90187-4. [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Edwards M. S., Baker C. J. Luminol-enhanced chemiluminescence for evaluation of type III group B streptococcal opsonins in human sera. J Infect Dis. 1980 Mar;141(3):370–381. doi: 10.1093/infdis/141.3.370. [DOI] [PubMed] [Google Scholar]
- Appelbaum P. C., Shaikh B. S., Widome M. D., Gordon R. A., Austrian R. Fatal pneumococcal bacteremia in a vaccinated splenectomized child. N Engl J Med. 1979 Jan 25;300(4):203–204. doi: 10.1056/NEJM197901253000428. [DOI] [PubMed] [Google Scholar]
- Austrian R., Douglas R. M., Schiffman G., Coetzee A. M., Koornhof H. J., Hayden-Smith S., Reid R. D. Prevention of pneumococcal pneumonia by vaccination. Trans Assoc Am Physicians. 1976;89:184–194. [PubMed] [Google Scholar]
- Bjornson A. B., Gaston M. H., Zellner C. L. Decreased opsonization for Streptococcus pneumoniae in sickle cell disease: studies on selected complement components and immunoglobulins. J Pediatr. 1977 Sep;91(3):371–378. doi: 10.1016/s0022-3476(77)81303-6. [DOI] [PubMed] [Google Scholar]
- Burke J. P., Klein J. O., Gezon H. M., Finland M. Pneumococcal bacteremia. Review of 111 cases, 1957--1969, with special reference to cases with undetermined focus. Am J Dis Child. 1971 Apr;121(4):353–359. [PubMed] [Google Scholar]
- Cadman E., Cohen M. S., Root R. K., Ryan J. L., Minor D. R. Impaired response to pneumococcal vaccine in Hodgkin's disease. N Engl J Med. 1978 Dec 7;299(23):1317–1318. doi: 10.1056/NEJM197812072992322. [DOI] [PubMed] [Google Scholar]
- Cowan M. J., Ammann A. J., Wara D. W., Howie V. M., Schultz L., Doyle N., Kaplan M. Pneumococcal polysaccharide immunization in infants and children. Pediatrics. 1978 Nov;62(5):721–727. [PubMed] [Google Scholar]
- Des Prez R. M., Bryan C. S., Hawiger J., Colley D. G. Function of the classical and alternate pathways of human complement in serum treated with ethylene glycol tetraacetic acid and MgCl2-ethylene glycol tetraacetic acid. Infect Immun. 1975 Jun;11(6):1235–1243. doi: 10.1128/iai.11.6.1235-1243.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Fine D. P. Pneumococcal type-associated variability in alternate complement pathway activation. Infect Immun. 1975 Oct;12(4):772–778. doi: 10.1128/iai.12.4.772-778.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Quie P. G. Influence of the alternate complement pathway in opsonization of several bacterial species. Infect Immun. 1974 Aug;10(2):402–404. doi: 10.1128/iai.10.2.402-404.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebink G. S., Foker J. E., Kim Y., Schiffman G. Serum antibody and opsonic responses to vaccination with pneumococcal capsular polysaccharide in normal and splenectomized children. J Infect Dis. 1980 Mar;141(3):404–412. doi: 10.1093/infdis/141.3.404. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Grebner J. V., Kim Y., Quie P. G. Serum opsonic deficiency produced by Streptococcus pneumoniae and by capsular polysaccharide antigens. Yale J Biol Med. 1978 Sep-Oct;51(5):527–538. [PMC free article] [PubMed] [Google Scholar]
- Giebink G. S., Schiffman G., Krivit W., Quie P. G. Vaccine-type pneumococcal pneumonia. Occurrence after vaccination in an asplenic patient. JAMA. 1979 Jun 22;241(25):2736–2737. doi: 10.1001/jama.241.25.2736. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Verhoef J., Peterson P. K., Quie P. G. Opsonic requirements for phagocytosis of Streptococcus pneumoniae types VI, XVIII, XXIII, and XXV. Infect Immun. 1977 Nov;18(2):291–297. doi: 10.1128/iai.18.2.291-297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray B. M., Converse G. M., 3rd, Dillon H. C., Jr Serotypes of Streptococcus pneumoniae causing disease. J Infect Dis. 1979 Dec;140(6):979–983. doi: 10.1093/infdis/140.6.979. [DOI] [PubMed] [Google Scholar]
- Grebner J. V., Mills E. L., Gray G. H., Quie P. G. Comparison of phagocytic and chemiluminescence response of human polymorphonuclear neutrophils. J Lab Clin Med. 1977 Jan;89(1):153–159. [PubMed] [Google Scholar]
- Harvath L., Andersen B. R. Defective initiation of oxidative metabolism in polymorphonuclear leukocytes. N Engl J Med. 1979 May 17;300(20):1130–1135. doi: 10.1056/NEJM197905173002003. [DOI] [PubMed] [Google Scholar]
- Hatch G. E., Gardner D. E., Menzel D. B. Chemiluminescence of phagocytic cells caused by N-formylmethionyl peptides. J Exp Med. 1978 Jan 1;147(1):182–195. doi: 10.1084/jem.147.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming V. G., Hall R. T., Rhodes P. G., Shigeoka A. O., Hill H. R. Assessment of group B streptococcal opsonins in human and rabbit serum by neutrophil chemiluminescence. J Clin Invest. 1976 Dec;58(6):1379–1387. doi: 10.1172/JCI108593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof D. G., Repine J. E., Peterson P. K., Hoidal J. R. Phagocytosis by human alveolar macrophages and neutrophils: qualitative differences in the opsonic requirements for uptake of Staphylococcus aureus and Streptococcus pneumoniae in vitro. Am Rev Respir Dis. 1980 Jan;121(1):65–71. doi: 10.1164/arrd.1980.121.1.65. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loda F. A., Collier A. M., Glezen W. P., Strangert K., Clyde W. A., Jr, Denny F. W. Occurrence of Diplococcus pneumoniae in the upper respiratory tract of children. J Pediatr. 1975 Dec;87(6 Pt 2):1087–1093. doi: 10.1016/s0022-3476(75)80120-x. [DOI] [PubMed] [Google Scholar]
- Minor D. R., Schiffman G., McIntosh L. S. Response of patients with Hodgkin's disease to pneumococcal vaccine. Ann Intern Med. 1979 Jun;90(6):887–892. doi: 10.7326/0003-4819-90-6-887. [DOI] [PubMed] [Google Scholar]
- Moore D. H., Shackelford P. G., Robson A. M., Rose G. M. Recurrent pneumococcal sepsis and defective opsonization after pneumococcal capsular polysaccharide vaccine in a child with nephrotic syndrome. J Pediatr. 1980 May;96(5):882–885. doi: 10.1016/s0022-3476(80)80568-3. [DOI] [PubMed] [Google Scholar]
- Overturf G. D., Field R., Edmonds R. Death from type 6 pneumococcal septicemia in a vaccinated child with sickle-cell disease. N Engl J Med. 1979 Jan 18;300(3):143–143. doi: 10.1056/NEJM197901183000318. [DOI] [PubMed] [Google Scholar]
- Riley I. D., Tarr P. I., Andrews M., Pfeiffer M., Howard R., Challands P., Jennison G. Immunisation with a polyvalent pneumococcal vaccine. Reduction of adult respiratory mortality in a New Guinea Highlands community. Lancet. 1977 Jun 25;1(8026):1338–1341. doi: 10.1016/s0140-6736(77)92552-1. [DOI] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman G., Douglas R. M., Bonner M. J., Robbins M., Austrian R. A radioimmunoassay for immunologic phenomena in pneumococcal disease and for the antibody response to pneumococcal vaccines. I. Method for the radioimmunoassay of anticapsular antibodies and comparison with other techniques. J Immunol Methods. 1980;33(2):133–144. doi: 10.1016/s0022-1759(80)80004-4. [DOI] [PubMed] [Google Scholar]
- Shigeoka A. O., Santos J. I., Hill H. R. Functional analysis of neutrophil granulocytes from healthy, infected, and stressed neonates. J Pediatr. 1979 Sep;95(3):454–460. doi: 10.1016/s0022-3476(79)80535-1. [DOI] [PubMed] [Google Scholar]
- Siber G. R., Weitzman S. A., Aisenberg A. C., Weinstein H. J., Schiffman G. Impaired antibody response to pneumococcal vaccine after treatment for Hodgkin's disease. N Engl J Med. 1978 Aug 31;299(9):442–448. doi: 10.1056/NEJM197808312990903. [DOI] [PubMed] [Google Scholar]
- Siegel J. D., Poziviak C. S., Michaels R. H. Serotypically defined pneumococcal infections in children. J Pediatr. 1978 Aug;93(2):249–250. doi: 10.1016/s0022-3476(78)80507-1. [DOI] [PubMed] [Google Scholar]
- Smit P., Oberholzer D., Hayden-Smith S., Koornhof H. J., Hilleman M. R. Protective efficacy of pneumococcal polysaccharide vaccines. JAMA. 1977 Dec 12;238(24):2613–2616. [PubMed] [Google Scholar]
- Stephens C. G., Williams R. C., Jr, Reed W. P. Classical and alternative complement pathway activation by pneumococci. Infect Immun. 1977 Aug;17(2):296–302. doi: 10.1128/iai.17.2.296-302.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens P., Young L. S. Quantitative granulocyte chemiluminescence in the rapid detection of impaired opsonization of Escherichia coli. Infect Immun. 1977 Jun;16(3):796–804. doi: 10.1128/iai.16.3.796-804.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjernholm R. L., Allen R. C., Steele R. H., Waring W. W., Harris J. A. Impaired chemiluminescence during phagocytosis of opsonized bacteria. Infect Immun. 1973 Feb;7(2):313–314. doi: 10.1128/iai.7.2.313-314.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. L., Ochs H. D., Schiffman G., Hammerschlag M. R., Miser J., Vichinsky E., Wedgwood R. J. Immune response after splenectomy. Lancet. 1978 Jan 28;1(8057):178–181. doi: 10.1016/s0140-6736(78)90612-8. [DOI] [PubMed] [Google Scholar]
- Tofte R. W., Peterson P. K., Kim Y., Quie P. G. Influence of serum concentration on opsonization by the classical and alternative complement pathways. Infect Immun. 1980 Feb;27(2):693–696. doi: 10.1128/iai.27.2.693-696.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel R. E., Vella P. P., McLean A. A., Woodhour A. F., Davidson W. L., Hilleman M. R. Studies in human subjects of polyvalent pneumococcal vaccines (39894). Proc Soc Exp Biol Med. 1977 Oct;156(1):144–150. doi: 10.3181/00379727-156-39894. [DOI] [PubMed] [Google Scholar]
- Winkelstein J. A., Drachman R. H. Deficiency of pneumococcal serum opsonizing activity in sickle-cell disease. N Engl J Med. 1968 Aug 29;279(9):459–466. doi: 10.1056/NEJM196808292790904. [DOI] [PubMed] [Google Scholar]



