Abstract
Idiopathic pulmonary fibrosis (IPF), the most common fibrotic lung disease, is a chronic disease of unknown etiology with a very high mortality. Personalized medicine focuses on the use of the individual’s molecular and ‘omic’ (i.e., genomic, epigenomic and proteomic) information to direct more efficient and cost-effective strategies for prevention, diagnosis, outcome prediction and treatment of diseases. In this review, we describe the use and promise of applying ‘omic’ technologies to the familial and sporadic forms of IPF as a means to personalize diagnosis and outcome prediction in IPF. The validation and implementation of such approaches will be crucial to personalize IPF patient care, prioritize lung transplant and stratify patients for drug studies, as well as, in the future, predict response to therapies as they emerge.
Keywords: idiopathic pulmonary fibrosis, MMP7, MUC5B, PCMI, personal clinic and molecular mortality prediction index, peripheral blood mononuclear cells
Idiopathic pulmonary fibrosis (IPF), the most common idiopathic interstitial pneumonia (IIP) [1], is a chronic, fibrosing interstitial lung disease of unknown etiology, characterized by the complex interaction of environmental [2–4], immunologic [5–9], developmental [10–13], genetic [14–20] and epigenetic factors [21–28], contributing at variable levels in disease development and progression. IPF is a lethal disease with a median survival of 3–3.5 years [29], with no therapy proven to be beneficial for survival with the exception of lung transplantation [30].
Personalized medicine is an innovative and expanding healthcare concept, which focuses on the use of the individual’s genomic and molecular information to direct more efficient and cost-effective strategies for prevention, diagnosis, outcome prediction and treatment of diseases. The use of genomic studies looking at the level of expression of a large number of genes and their targeted protein validation, as well as the analysis of genetic mutations, polymorphisms and epigenetics in lung fibrosis, are increasingly contributing to a better understanding of the pathogenesis of IPF and some of the other IIPs, providing multiple candidate biomarkers for diagnosis and disease monitoring, as well as clues for the development of effective therapies [31].
In this review article we will outline the need for personalized medicine in IPF and review the contributions of genomics and targeted proteomic studies to the identification of person-specific IPF diagnosis, outcome prediction and risk stratification.
The need for personalized medicine in IPF
The areas where personalized medicine could potentially be applied to IPF are the study of disease susceptibility, diagnosis, risk stratification and outcome prediction. We will discuss each one in turn.
Disease susceptibility
The use of genetic testing has been applied to evaluate disease susceptibility in order to offer preventive measures to high-risk populations in other diseases, the perfect example being testing for BRCA1/BRCA2 mutations in patients with significant family history of breast and/or ovarian cancer [32,33]. In IPF, modifiable factors such as cigarette smoking [2] and several occupational factors (i.e., farming, livestock, hairdressing, metal dust, raising birds, stone cutting/polishing and vegetable dust/animal dust) [3] have been repeatedly associated with the development of the disease [34]. Since the majority of patients with similar exposures do not develop IPF, identifying genetic polymorphisms and molecular markers predictive of IPF could help researchers to determine specific relationships between genetic and environmental factors, as well as developing preventive strategies and early-stage pharmacologic interventions.
Diagnosis
According to the most recent American Thoracic Society/European Respiratory Society/ Japanese Respiratory Society/American Lung Association of Texas statement [34], IPF diagnosis is based on the exclusion of known causes of lung fibrosis and the presence of a usual interstitial pneumonia (UIP) pattern on high-resolution computed tomography scan (HRCT) in patients who do not undergo lung biopsy or the specific combinations of typical HRCT and the UIP pattern on surgical lung biopsy [34]. Three major concerns arise from the way we currently diagnose this disease:
IPF is a diagnosis of exclusion that often changes over time as patients present with new signs of their disease, making frequent follow-ups and constant re-evaluations in individuals not subjected to lung biopsy necessary;
Surgical lung biopsies are procedures associated with significant morbidity and mortality [35,36], and they are only helpful when the results are consistent with other non-UIP patterns that are typically more steroid responsive;
Despite the success of HRCT in demonstrating ‘UIP-like’ radiological patterns in IPF with an acceptable interobserver variability [37–39], other interstitial lung diseases such as chronic hypersensitivity pneumonitis [40] and collagen vascular disease associated with interstitial lung disease [41] can also present with an UIP pattern on lung biopsy and HRCT, making the diagnosis of IPF challenging at times.
The identification of genetic or molecular markers specific to IPF will improve the diagnostic yield of the above-mentioned diagnostic modalities and reduce the need for costly and sometimes dangerous interventions, as well as identify patients who may response to specific therapeutic modalities.
Risk stratification & outcome prediction
Another challenge in IPF is outcome prediction; the course of IPF among individual patients is highly variable and extremely difficult to forecast; some patients can remain stable over time, while others can either decline quickly or present with acute exacerbations and die [42]. Disease progression in current clinical practice is monitored mainly by forced vital capacity (FVC), diffusion capacity for carbon monoxide [43,44] and oxygen desaturation studies [45,46], as well as imaging studies, most commonly HRCT [47–49]; even though previous studies have shown associations of serial measures of these clinical variables with disease progression and poor outcomes [4,50–52], serial physiological measures require follow up, are costly, are not predictive at presentation, are usually not mechanistically related to the disease, and in some cases highly variable. The clinical need for outcome prediction in IPF is derived from the need to prioritize patients for transplant. In order to be considered a candidate for lung transplant, patients require a cost-intensive evaluation that, in turn, provides them with a score determined by the lung allocation score system [53]; this score significantly influences the timing of lung transplantation. However, despite the improvement in the new lung allocation score system, the timing to transplant in IPF is not accurate enough and IPF patients still have the highest mortality rate while waiting on the transplant list [54–56]. Identifying reproducible molecular and genomic markers that predict deterioration in clinically similar patients will improve the efficiency of organ utilization and prevent unnecessary evaluations.
The prediction of outcome is also critically important for drug studies. Given the fact that IPF is a relatively uncommon, highly variable and unpredictable disease, researchers need to recruit patients that are likely to progress or die during the study in order to demonstrate an outcome benefit from a drug. They also need to assign patients with the same likelihood of progression or death to the arms of a drug study in order to prevent spurious results. Standard tools for clinical evaluation cannot address those needs. The use of genomic and molecular tools for patient stratification in drug studies will enhance the efficiency of the studies, reduce the cost and risk of failure, and eventually lead to the discovery of an effective therapy in IPF.
Gene mutations, polymorphisms & disease susceptibility in lung fibrosis
Several mutations associated with familial and sporadic forms of IPF have been reported. Thomas and colleagues were the first to identify surfactant protein mutations in familial IPF [15]. They studied a large family of 97 individual patients’ kindred, including six adults affected with UIP, and recognized the presence of a heterozygous exon 5 + 128 T→A transversion, substituting glutamine for leucine at the C terminal region of the pro-surfactant protein C molecule (pro-SFTPC), causing alveolar type II cell atypia with numerous lamellar bodies, dense fibrosis and architectural destruction in the affected individuals. Contrary to what was recognized in familial IPF, SFTPC mutations were infrequently found in sporadic cases of IPF [57]. The study of surfactant protein mutations continued with Wang and colleagues [18]; they analyzed a large family where early pulmonary fibrosis and cancer cosegregated in an autosomal dominant fashion and found a transversion mutation (GGG→GTG) in codon 231 of one of the SFTPA2 alleles, predicting the substitution of a highly conserved glycine residue to valine, suggesting a predisposition to lung cancer and fibrosis in the presence of this mutation.
The presence of genetic mutations in familial and sporadic IPF has not only proven to be relevant for the identification of individuals with increased disease susceptibility, but also provided clues to study the pathways associated with these mutations and their relationship with disease pathogenesis; as an example, Lawson and colleagues studied the possible link between surfactant protein mutations and the predisposition to lung fibrosis and demonstrated, in vitro, the presence of activation of unfolded protein response (UPR) in A549 cells expressing mutant SFTPC. They subsequently identified the increased expression of UPR markers in alveolar epithelial cells (AECs) in the lungs of patients with SFTPC mutation-associated fibrosis, as well as in patients with familial and sporadic IPF [58]. The investigators also examined the herpesvirus effect in AECs, as it can induce endoplasmic reticulum (ER) stress, and identified that herpes-virus protein expression in AECs of IPF patients colocalized with UPR markers, leading to the hypothesis that ER stress and UPR activation could contribute to disease progression in the presence of altered surfactant protein processing or chronic herpesvirus infection. More interestingly, Korfei and colleagues also searched for ER stress mediators in explanted lung tissue only in sporadic IPF cases and demonstrated a severe ER stress response in the AECIIs of these patients, findings indicative that mutation-associated pathways in lung fibrosis appear to be relevant to disease pathogenesis even in the absence of specific mutations [59].
Different studies have suggested that telomerase mutations in telomerase reverse transcriptase confer increased susceptibility for developing adult-onset IPF. One of the first reports describing the occurrence of telomerase mutations associated with short telomeres in familial IPF was published by Armanios and colleagues in six individuals from a registry of 73 probands from the Vanderbilt Pulmonary Fibrosis registry, demonstrating heterozygous mutations in the hTERT or hTR genes [21]. Tsakiri and colleagues reported similar findings confirming the presence of TERT mutations in two large families with cases of IPF and, in one family, a heterozygous mutation of TERC (the RNA component of telomerase required for telomerase integrity) [60]. By sequencing the probands of 44 additional unrelated families and 44 sporadic cases of interstitial lung disease, the investigators discovered another five mutations in TERT. Mushiroda and colleagues also identified polymorphisms within TERT (SNP in intron 2 of the TERT gene – rs2736100) in a genome-wide association study including a derivation cohort of 159 sporadic IPF patients and 934 controls, as well as a replication cohort of 83 sporadic IPF cases and 535 controls, adding more evidence to the previously described findings [22]. With respect to telomere shortening and IPF susceptibility, Cronkhite and colleagues measured telomere lengths of genomic DNA of circulating leukocytes in 201 normal control subjects and 59 probands with familial pulmonary fibrosis and 73 sporadic pulmonary fibrosis cases without TERT or TERC mutations and discovered telomere lengths less than the 10th percentile in familial pulmonary fibrosis (24%) and sporadic case subjects (23%) when compared with control subjects (p = 2.6 × 10−8) [61]. Similarly, Alder and colleagues demonstrated that IIP patients had shorter leukocyte telomeres when compared with age-matched controls (p < 0.0001) [62]. The authors also demonstrated the presence of shorter telomeres in alveolar epithelial cells of IPF patients when compared with age-matched individuals (p < 0.0001). This body of evidence suggests the potential role of telomere shortening as a marker of increased predisposition for adult-onset pulmonary fibrosis.
Other genetic variants have been described in IPF; as an example, Hodgson and colleagues, analyzed six multiplex families with familial IPF and identified a common haplotype comprising ELMOD2 and LOC152586, genes located in chromosome 4, significantly associated with familial IPF when compared with controls [16]. Interestingly, ELMOD2, a molecule that potentially regulates antiviral response [63], was extremely downregulated and nearly absent by in situ hybridization in familial IPF subjects. Polymorphisms in other genes, such as IL-1 [64], CR-1 [65], IL12p40 and IFN-γ [66], NOD2/CARD15 [67], MMP-1 [17], ENA-78, IP-10 and VEGF [68], CD16b [69], IL-8 [70] and HER2 [71] have also been described in IPF, confirming the complex and variable genetic characteristics of this disease, but most of these studies were not replicated.
More recently, Seibold and colleagues published what promises to be one of the most important contributions to personalized medicine in IPF. The investigators performed linkage analysis of 82 multiplex families and a case–control association study in 83 subjects with familial interstitial pneumonia (FIP) [19]. They also analyzed 492 subjects with sporadic IPF and 322 controls. A SNP in the putative promoter of MUC5B (rs35705950) exhibited the strongest association with FIP (minor allele frequency of 34%; p = 1.2 × 10−15) and IPF (minor allele frequency of 38%; p = 2.5 × 10−37); in controls, the minor allele frequency was 9%. The odds ratios were 6.2 (95% CI: 3.7–10.4) for FIP and 8.3 (95% CI: 5.8–11.9) for IPF, and the MUC5B expression was 14.1-times higher in IPF (p < 0.001) when compared with controls. The findings by Seibold and colleagues were simultaneously confirmed by Zhang and colleagues in an independent case–control, collaborative study that included 341 IPF and 801 control subjects [20]. They found strikingly similar results with a minor allele frequency in the combined cohort of 34.3% in patients with IPF, and 11.1% in controls (allelic association; p = 7.6 × 10−40). The odds ratios for IPF in subjects who were heterozygous or homozygous for the minor allele of rs35705950 were 5.9 (95% CI: 4.4–7.8) and 9.7 (95% CI: 4.7–19.9), respectively.
Towards personalized diagnosis of IPF in the context of disease pathogenesis
Several studies [72–77] have looked into the potential of developing IPF-specific gene expression signatures in the context of disease pathogenesis by using either the ‘cherry picking’ approach, focusing mostly on statistically significant genes with possible pathogenic relevance, or the ‘systems approach’, focusing on functional gene group analysis [78]. In this section, irrespective of the approached used for the analysis of expression data we are going to focus on the most relevant genomic, proteomic, miRNA and epigenetic studies for IPF diagnosis.
The first gene expression study in IPF was performed by Zuo and colleagues [72]; the authors identified a signature of 164 genes that differentiated IPF lungs from healthy controls and recognized that upregulated genes in IPF were related to functional groups associated with smooth-muscle proliferation, cellular growth, extracellular matrix formation, degradation and signaling. The investigators also recognized the upregulation of protease-coding genes in IPF, such as MMP-1, -2, -9 and, particularly, -7 (the most informative gene in IPF), and demonstrated that MMP-7-knockout mice were relatively protected from bleomycin-induced pulmonary fibrosis, indicating the potential role of proteases in the pathogenesis of IPF. Selman and colleagues also studied the differences between IPF and hypersensitivity pneumonitis (HP) patients at the gene-expression levels in lungs, and found that overexpression of genes in the HP group was primarily associated with inflammation and immune response, a markedly different functional gene group signature as to that observed in IPF [75]. These authors also attempted to classify nonspecific interstitial pneumonia (NSIP) patients based on the gene-expression signature that differentiated IPF from HP, and discovered a NSIP subgroup that was not classified as either IPF or HP, suggesting the existence of a unique gene-expression signature for idiopathic NSIP; however, despite being different entities at the clinicopathological level, IPF and NSIP express surprisingly similar gene-expression patterns [79]. Interestingly, this phenomenon is also apparent when the gene expression of UIP lungs of IPF and scleroderma pulmonary fibrosis (SSc-PF) patients are compared with each other, as described by Hsu and colleagues [77], although a unique signature of 25 genes seems to differentiate these two etiologies of lung fibrosis.
Rosas and colleagues performed one of the initial and most successful efforts to translate lung gene expression studies to the peripheral blood [80]. Based on the findings of Zuo and colleagues and, in an attempt to evaluate if other peripheral blood proteins previously demonstrated to be relevant to IPF pathogenesis were able to differentiate IPF patients from other chronic lung diseases, the investigators applied a targeted proteomic approach and identified a protein signature including MMP-1, MMP-7, MMP-8, IGFBP-1 and TNFRSA1F, characteristic of IPF [72]. They established that this combinatorial signature was able to distinguish IPF from healthy controls with a sensitivity of 98.6% and specificity of 98.1%. Two members of this signature, MMP-1 and MMP-7 were also able to differentiate IPF from subactue/chronic HP patients with a sensitivity of 96.3% and specificity of 87.2%; finally, this study provided the first clue of MMP-7, as potential marker for early detection, as it was found to be significantly higher in patients with subclinical ILD compared with control individuals (p = 0.019) and significantly lower in full-blown IPF patients (p < 0.0001). The use of a similar approach to potentially validate plasma protein components of the genomic signature differentiating IPF from SSc-PF and IPF from other interstitial lung diseases could be very useful in clinical practice, as distinguishing these conditions can be sometimes challenging in daily clinical practice.
A different method to identified IPF exclusive diagnostic signatures in the context of disease pathogenesis comes from the use of miRNAs; these molecules are a family of small noncoding RNAs (21–25 nucleotides) that bind to the 3′-untranslated region of their target mRNAs. miRNAs regulate gene expression mostly by repressing protein synthesis [81]; so far, 1527 sequences [201] of these short RNA regulators have been identified in humans. Pandit and colleagues discovered that 10% of miRNAs measured were significantly differentially expressed in IPF lungs compared with controls [82]. The investigators focused on let-7d, one of the differentially expressed, downregulated miRNAs in IPF lungs. They demonstrated that TGF-β signaling leads to inhibition of let-7d through binding of SMAD3 to the let-7d promoter. When they inhibited let-7d in vitro in epithelial cells or in vivo in murine lungs they observed increased expression of mesenchymal markers in epithelial cells, as well as changes consistent with early fibrosis in the mouse lung. Liu and colleagues described an opposing phenomenon, first by recognizing the overexpression of miR-21 in IPF and in primary lung fibroblasts stimulated with TGF-β1 and, second, by the attenuation of a bleomycin murine model after miR-21 suppression, confirming the existence of a role of miRNAs in IPF pathogenesis [83]. The recognition of the expression of miRNAs in the peripheral blood in other disease entities [84,85], as well as their potential role in lung fibrosis pathogenesis, is an encouraging factor for the use of these molecules as biomarker tools in IPF diagnosis and outcome prediction.
Different studies have shown the potential role of epigenetically mediated mechanisms in IPF pathogenesis, as an example, Thy-1, an important cell–cell and cell–matrix mediator [86], is not expressed in fibroblastic foci fibroblast of IPF patients. Thy-1(−) fibroblasts demonstrate a more fibrogenic phenotype including increased proliferative capacity, collagen gel contraction and the ability to induce the production of MMP-9 [87]. The reason why these fibroblasts did not express Thy-1 was unknown until Sanders and colleagues discovered that the Thy-1 promoter was hypermethylated in IPF samples [23]. The investigators were able to restore the expression of Thy-1 in these fibroblasts by a DNA methyltransferase inhibitor, supporting the potential role of DNA methylation in regulation of Thy-1 expression in IPF. Coward and colleagues identified changes in histone acetylation that led to diminished expression of cyclooxygenase 2 in fibroblasts from IPF patients [24]. These changes resulted in a defect in the production of the antifibrotic mediator prostaglandin E2. Within the same pathway, Huang and colleagues provided evidence that DNA hypermethylation was responsible for the relative resistance to the antifibrotic effects of prostaglandin E2 by causing a decrease in E prostanoid 2 (EP2) receptor expression [25]. Coward and colleagues also identified other epigenetically regulated targets in IPF by confirming the repression of IP-10, an inhibitor of angiogenesis in chronic fibroproliferative diseases [88], via histone deacetylation and histone H3 hypermethylation, adding more evidence for the role of epigenetic mechanisms in IPF pathogenesis [26]. Finally, Rabinovich and colleagues compared the methylation patterns between IPF, lung cancer samples and normal histology controls from the patients with cancer (as well as the gene expression associated with these changes) [28]. The investigators identified IPF-specific methylation changes, but also recognized that IPF lungs displayed an intermediate methylation profile when compared with lung cancer and controls with 402 differentially methylated CpG islands overlapping between IPF and cancer. Interestingly, IPF lungs did not exhibit hypomethylation of LINE-1 retrotransposon while lung adenocarcinoma samples did, suggesting different origins of methylation changes in IPF and lung cancer.
Outcome prediction & risk stratification in IPF using personalized-based approaches
The recognition that patients with IPF exhibit a disease course that is variable and unpredictable ignited a significant interest in identifying molecular biomarkers indicative of disease activity and outcome prediction. Selman and colleagues studied the microarray gene-expression patterns of IPF patients whose symptoms started 6 months prior to their initial presentation (‘rapid’ progressors) and compared them with IPF patients with more than 24 months of symptoms (‘slower’ progressors), and found a signature of 437 differentially expressed genes between the two groups [89]. The ‘rapid’ progressors had overexpression of genes strongly involved in morphogenesis, oxidative stress, apoptosis, cell migration and proliferation – pathways previously involved in the pathogenesis of IPF – suggesting the potential use of gene-based signatures to differentiate patients with higher likelihood of progression and death. The gene expression differences between ‘progressive’ and ‘relatively stable’ IPF patients were also analyzed by Boon and colleagues. Using serial analysis of gene expression, resulting in 243 differentially expressed transcripts between the studied groups, the investigators found upregulation of the MAPK–EGR1–HSP70 pathway involved in cigarette-smoke-induced inflammation [90], suggesting a role of cigarette effect in disease progression in IPF. These findings also helped to confirm the potential use of gene-based signatures to monitor disease progression in IPF, a discovery that, despite being novel and revolutionary, was clearly limited by the requirement of lung tissue obtained via invasive procedures, a nonideal way to monitor IPF progression in daily clinical practice.
Acute exacerbations of IPF (AE-IPF) are episodes of precipitous decline in respiratory status without an identifiable cause (i.e., infections) [91], leading to a 50% in-hospital morality [92]. Konishi and colleagues compared the gene expression in lungs of patients with AE-IPF lungs versus stable IPF and recognized a signature of 579 differentially expressed genes between these two groups that was, as expected, not indicative of an infectious or inflammatory etiology, as this entity usually does not respond to antibiotics or immunosuppression [93]. The investigators did find evidence of overwhelming apoptosis by terminal deoxynucleotidyl transferase dUTP nick end labeling in the AE-IPF lung as well as the upregulation of cyclinA2, a cell-cycle mediator, and Ki-67, a proliferation marker, both in the alveolar epithelium. The presence of proliferation and cell-cycle regulation markers in the presence of apoptosis suggests an aberrant proliferative response of the alveolar epithelium in response to apoptosis during AE-IPF. α-defensins, a group of innate antimicrobial peptides [94], were also upregulated in the AE-IPF lungs at the mRNA levels as well as at the plasma protein level of AE-IPF patients when compared with controls and stable IPF, suggesting the potential role of these molecules as biomarkers for the diagnosis of AE-IPF.
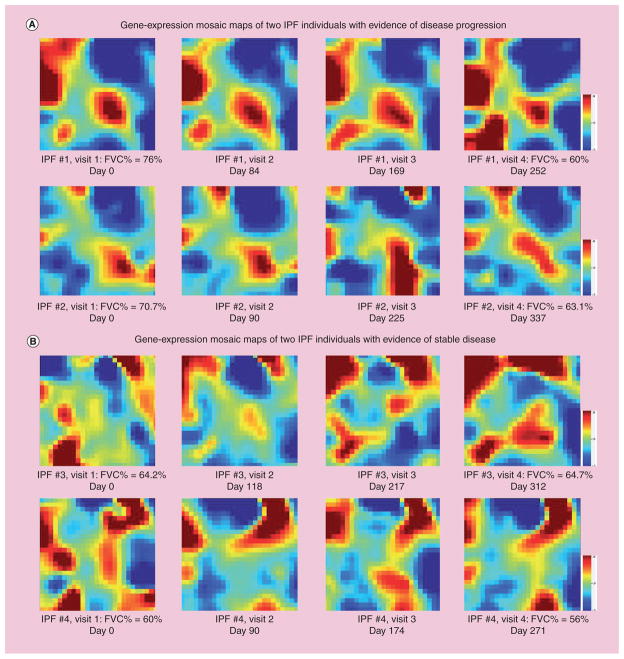
Preliminary work performed by our group illustrates that peripheral blood mononuclear cells are also a potential source of personalized information for disease monitoring and potentially, outcome prediction in IPF [95]. Figure 1 depicts the whole human (29,807 gene probes) gene-expression pattern of four IPF patients evaluated at four different time points (after obtaining signed, informed consent), using gene-expression dynamic inspector [96] mosaic maps. The overall gene-expression pattern of two IPF patients with evidence of disease progression (Figure 1A), measured by a 10% decline in FVC % predicted, is clearly different than the pattern seen in patients with stable disease. In both groups of patients (Figure 1A & B), gene-expression dynamic changes can be observed thorough subsequent visits. These results suggest that studying peripheral blood mononuclear cell gene expression in IPF could help differentiate patients that are likely to progress and die from patients with stable disease [95].
Figure 1. Whole human gene-expression representation (29,807 gene probes) of four idiopathic pulmonary fibrosis patients, at four different time points.
Red denotes gene upregulation, blue denotes downregulation and green indicates no change in gene expression. (A) demonstrate the similarities and dynamic changes across subsequent office visits of two IPF patients with evidence of disease progression measured by a decline of more than 10% of the FVC% predicted between the first and last visit. A different pattern can be observed in two patients with stable disease in (B), suggesting that peripheral blood mononuclear cell gene expression can be used to monitor disease progression and, potentially, outcome prediction in IPF.
FVC: Forced vital capacity; IPF: Idiopathic pulmonary fibrosis.
Recently, it has been suggested that changes in the peripheral blood in 27 patients may be indicative of outcome at presentation. Yokoyama and colleagues showed in 27 patients an association of high KL-6 with decreased survival in IPF [97]. In a prospective cohort of 72 patients, Prasse and colleagues demonstrated that serum CCL18 levels were able to predict outcomes in IPF [98]. In a cohort of 81 patients, Kinder and colleagues established that serum surfactant protein A was a predictor of early mortality in IPF [99]. Moeller and colleagues demonstrated in a cohort of 51 patients that circulating fibrocytes were an indicator of poor prognosis in IPF [100]. Gilani and colleagues determined in a cohort of 89 IPF patients that downregulation of CD28 in circulating CD4 T cells was a marker of poor prognosis [7]. We applied a targeted proteomic approach [101] and screened 95 proteins (some of them previously discovered by genomic studies) in the plasma of 140 IPF subjects in a derivation cohort and validated the results in a replication cohort (101 patients). High plasma concentrations of MMP-7, ICAM-1 and IL-8 were predictive of poor overall survival in both cohorts. We then derived a personal clinic and molecular mortality prediction index (PCMI) in the derivation cohort, using the stepAIC approach [102], a statistical function that performs stepwise model selection to automate the process of variable selection in multivariate regression models approach.
This index was highly predictive of early mortality, with C-statistics exceeding 80%. This study – the first in IPF to integrate clinical and molecular information, as well as to have two cohorts – demonstrates the feasibility of obtaining individualized clinical prediction rules using patient personal clinical and molecular information, and lays the foundation for personalized medicine in IPF.
Future perspective
As outlined, it seems evident that the scientific community has generated enough information to evaluate disease susceptibility, diagnosis, risk stratification and outcome prediction in IPF. Taking this wealth of information into consider ation, are we routinely testing family members of IPF patients or high-risk individuals for known genetic variations associated with the disease? Are we using peripheral blood-based tests to diagnose IPF? Are we using biomarkers to monitor disease progression for earlier referral to transplant or randomization in drug studies? The answer is simply ‘no’. So, what do we need in order to translate the information we have generated so far from genomic studies and proteomic validations in order to finally influence patient care? The answer is in the generation of sufficiently powered, strictly designed, multicenter studies for the final validation of previously identified, easily accessible biomarkers using personalized-based approaches.
In order to achieve this, we have to continue the collaborative efforts between major lung fibrosis centers given the low prevalence of IPF. The concept of collaborative genomic studies has already been applied by the NIH and most recently the National Heart, Lung, and Blood Institute in the Lung Tissue Research Consortium [202] and the Lung Genomic Research Consortium [203]. However, much larger studies and industry involvement in multicenter consortia that assess biomarkers in the context of clinical research as well as common practice are required, potentially through the agreement to share the data obtained from the placebo arms of drug studies.
One could argue that the cost of ‘omic’ studies for biomarker discovery in IPF could be very high, especially in times when research resources are limited. However, the validation and use of reliable peripheral blood biomarkers in IPF could counterbalance this argument, with a substantial potential for reduction in healthcare cost by decreasing the number of lung biopsies for diagnosis (as well as the cost related to the morbidity and mortality associated with these procedures) and CT scans for disease progression monitoring. In addition, there may be a potential decrease in intensive care unit admissions and hospital stay if we can use these biomarkers for acute exacerbation prediction in an attempt to triage these patients earlier for lung transplantation. In the case of drug studies, randomized clinical trials could be stopped earlier (and hence result in a reduction in research cost) if, along with a clinical response, there is an easily measureable molecular response. These reasons make a strong case for the continuation of biomarker studies in IPF. Finally, another future direction should be the study of the different IPF and ILD disease subphenotypes (concomitant chronic obstructive pulmonary disease, lung cancer, pulmonary arterial hypertension, drug responsiveness and so on) and the identification of biomarkers associated with quality-of-life measures (i.e., cough, dyspnea, oxygen use and so on) in order to develop monitoring strategies and potentially drug targets to these specific patient subpopulations.
To address many of the challenges, the field of pulmonary fibrosis we have to integrate discoveries from genomic and other ‘omics’ disciplines in our design of clinical studies, prioritize lung transplantation and eventually guide therapeutic interventions. This integration will lead to implementation of personalized medicine approaches in lung fibrosis, and will dramatically transform the care of these patients.
Executive summary.
The need for personalized medicine in idiopathic pulmonary fibrosis
Personalized medicine in idiopathic pulmonary fibrosis (IPF) should be applied for the evaluation of disease susceptibility, diagnosis, risk stratification and outcome prediction.
Familial & sporadic IPF mutations, polymorphisms & disease susceptibility
Gene mutations have been identified to be associated with familial and sporadic forms of IPF. Some of them are: MUC5B, SFTPC, SFPTA2, telomerase, ELMOD2, LOC152586, IL-1, CR-1, IL12p40, IFN-γ, NOD2/CARD15, MMP-1, ENA-78, IP-10, VEGF, CD16b, IL-88 and, most recently, HER2.
Towards personalized diagnosis of IPF in the context of disease pathogenesis
Gene-expression patterns in lungs differentiate IPF from controls, hypersensitivity pneumonitis and scleroderma pulmonary fibrosis patients.
Peripheral blood proteins, identified based on genomic studies in IPF, can be used in combinatorial signatures to diagnose IPF and to differentiate IPF from subacute/chronic hypersensitivity pneumonitis patients.
miRNA expression patterns in lungs differentiate IPF patients from controls.
IPF lungs do not appear to exhibit hypomethylation of LINE-1 retrotransposon, while lung cancer samples do.
Outcome prediction & risk stratification in IPF using personalized-based approaches
Gene expression in lungs of IPF differentiates patients with distinct patterns of disease progression.
Gene-expression patterns in lungs of acute exacerbations of IPF (AE-IPF) are different from stable IPF patients and controls.
α-defensins, overexpressed genes in the AE-IPF lungs, are also elevated in the plasma of AE-IPF patients.
Peripheral blood mononuclear cell gene-expression patterns in peripheral blood seem to differentiate IPF patients with different rates of disease progression.
Peripheral blood proteins and cellular subpopulations (KL-6, surfactant protein A, CCL18, MMP-7, ICAM, IL-8, fibrocytes and CD4+CD28null cells) predict poor outcomes in IPF.
The personal clinic and molecular mortality prediction index using gender, forced vital capacity percentage predicted, diffusion capacity of lung carbon monoxide percentage predicted and MMP-7 protein levels predicts mortality in IPF with high accuracy.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
N Kaminski’s work is supported by The Dorothy P and Richard P Simmons Endowed Chair for Pulmonary Research, as well as the NIH grants: RC2HL101715, RO1HL095397, R01LM009657, U01HL108642. N Kaminski and JD Herazo-Maya are both inventors in a patent application regarding the use of peripheral blood markers in IPF. N Kaminski is an inventor in a patent application regarding the use of miRNAs to treat IPF. N Kaminski is a consultant to Stromedix and Sanofi-Aventis and is a recipient of investigator-initiated grants from Gilead and Celgene. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155(1):242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner KB, Samet JM, Coultas DB, et al. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: a multicenter case–control study. Collaborating Centers. Am J Epidemiol. 2000;152(4):307–315. doi: 10.1093/aje/152.4.307. [DOI] [PubMed] [Google Scholar]
- 4.King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164(7):1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 5.Marchal-Somme J, Uzunhan Y, Marchand-Adam S, et al. Cutting edge: nonproliferating mature immune cells form a novel type of organized lymphoid structure in idiopathic pulmonary fibrosis. J Immunol. 2006;176(10):5735–5739. doi: 10.4049/jimmunol.176.10.5735. [DOI] [PubMed] [Google Scholar]
- 6.Feghali-Bostwick CA, Tsai CG, Valentine VG, et al. Cellular and humoral autoreactivity in idiopathic pulmonary fibrosis. J Immunol. 2007;179(4):2592–2599. doi: 10.4049/jimmunol.179.4.2592. [DOI] [PubMed] [Google Scholar]
- 7.Gilani SR, Vuga LJ, Lindell KO, et al. CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLoS One. 2010;5(1):e8959. doi: 10.1371/journal.pone.0008959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taille C, Grootenboer-Mignot S, Boursier C, et al. Identification of periplakin as a new target for autoreactivity in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(6):759–766. doi: 10.1164/rccm.201001-0076OC. [DOI] [PubMed] [Google Scholar]
- 9.Xue J, Gochuico BR, Alawad AS, et al. The HLA class II allele DRB1*1501 is over-represented in patients with idiopathic pulmonary fibrosis. PLoS One. 2011;6(2):e14715. doi: 10.1371/journal.pone.0014715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med. 2008;5(3):e62. doi: 10.1371/journal.pmed.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konigshoff M, Balsara N, Pfaff EM, et al. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One. 2008;3(5):e2142. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vuga LJ, Ben-Yehudah A, Kovkarova-Naumovski E, et al. WNT5A is a regulator of fibroblast proliferation and resistance to apoptosis. Am J Respir Cell Mol Biol. 2009;41(5):583–589. doi: 10.1165/rcmb.2008-0201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konigshoff M, Kramer M, Balsara N, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119(4):772–787. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪.Nogee LM, Dunbar AE, 3rd , Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Eng J Med. 2001;344(8):573–579. doi: 10.1056/NEJM200102223440805. The first report of surfactant protein C mutations in a patient diagnosed with desquamative interstitial pneumonitis and her newborn daughter diagnosed with nonspecific interstitial pneumonitis. [DOI] [PubMed] [Google Scholar]
- 15.Thomas AQ, Lane K, Phillips J, 3rd, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165(9):1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 16.Hodgson U, Pulkkinen V, Dixon M, et al. ELMOD2 is a candidate gene for familial idiopathic pulmonary fibrosis. Am J Hum Genet. 2006;79(1):149–154. doi: 10.1086/504639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Checa M, Ruiz V, Montano M, Velazquez-Cruz R, Selman M, Pardo A. MMP-1 polymorphisms and the risk of idiopathic pulmonary fibrosis. Hum Genet. 2008;124(5):465–472. doi: 10.1007/s00439-008-0571-z. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Kuan PJ, Xing C, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84(1):52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪▪.Seibold MA, Wise AL, Speer MC, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Eng J Med. 2011;364(16):1503–1512. doi: 10.1056/NEJMoa1013660. Relatively large study and the first to demonstrate the strong association between the MUC5B single-nucleotide promoter polymorphism in familial interstitial pneumonia and idiopathic pulmonary fibrosis (IPF) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪▪.Zhang Y, Noth I, Garcia JG, Kaminski N. A variant in the promoter of MUC5B and idiopathic pulmonary fibrosis. N Eng J Med. 2011;364(16):1576–1577. doi: 10.1056/NEJMc1013504. This independent case–control study confirmed the MUC5B promoter polymorphism in a cohort of IPF patients evaluated at the University of Pittsburgh and at the University of Chicago. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪.Armanios MY, Chen JJ, Cogan JD, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Eng J Med. 2007;356(13):1317–1326. doi: 10.1056/NEJMoa066157. A key report describing telomerase mutations resulting in short telomeres associated with cases of familial pulmonary fibrosis. [DOI] [PubMed] [Google Scholar]
- 22.Mushiroda T, Wattanapokayakit S, Takahashi A, et al. A genome-wide association study identifies an association of a common variant in TERT with susceptibility to idiopathic pulmonary fibrosis. J Hum Genet. 2008;45(10):654–656. doi: 10.1136/jmg.2008.057356. [DOI] [PubMed] [Google Scholar]
- 23.Sanders YY, Pardo A, Selman M, et al. Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39(5):610–618. doi: 10.1165/rcmb.2007-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coward WR, Watts K, Feghali-Bostwick CA, Knox A, Pang L. Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. Mol Cell Biol. 2009;29(15):4325–4339. doi: 10.1128/MCB.01776-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang SK, Fisher AS, Scruggs AM, et al. Hypermethylation of PTGER2 confers prostaglandin E2 resistance in fibrotic fibroblasts from humans and mice. Am J Pathol. 2010;177(5):2245–2255. doi: 10.2353/ajpath.2010.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coward WR, Watts K, Feghali-Bostwick CA, Jenkins G, Pang L. Repression of IP-10 by interactions between histone deacetylation and hypermethylation in idiopathic pulmonary fibrosis. Mol Cell Biol. 2010;30(12):2874–2886. doi: 10.1128/MCB.01527-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz De Leon A, Cronkhite JT, Katzenstein AL, et al. Telomere lengths, pulmonary fibrosis and telomerase (TERT ) mutations. PLoS One. 2010;5(5):e10680. doi: 10.1371/journal.pone.0010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabinovich EI, Kapetanaki MG, Steinfeld I, et al. Global methylation patterns in idiopathic pulmonary fibrosis. PLoS One. 2012;7(4):e33770. doi: 10.1371/journal.pone.0033770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez Perez ER, Daniels CE, Schroeder DR, et al. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest. 2010;137(1):129–137. doi: 10.1378/chest.09-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thabut G, Mal H, Castier Y, et al. Survival benefit of lung transplantation for patients with idiopathic pulmonary fibrosis. J Thorac Cardiovasc Surg. 2003;126(2):469–475. doi: 10.1016/s0022-5223(03)00600-7. [DOI] [PubMed] [Google Scholar]
- 31.Kass DJ, Kaminski N. Evolving genomic approaches to idiopathic pulmonary fibrosis: moving beyond genes. Clin Transl Sci. 2011;4(5):372–379. doi: 10.1111/j.1752-8062.2011.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burke W, Daly M, Garber J, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II BRCA1 and BRCA2 Cancer Genetics Studies Consortium. JAMA. 1997;277(12):997–1003. [PubMed] [Google Scholar]
- 33.Nelson HD, Huffman LH, Fu R, Harris EL Force USPST. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;143(5):362–379. doi: 10.7326/0003-4819-143-5-200509060-00012. [DOI] [PubMed] [Google Scholar]
- 34.Raghu G, Collard HR, Egan JJ, et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Utz JP, Ryu JH, Douglas WW, et al. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J. 2001;17(2):175–179. doi: 10.1183/09031936.01.17201750. [DOI] [PubMed] [Google Scholar]
- 36.Lettieri CJ, Veerappan GR, Helman DL, Mulligan CR, Shorr AF. Outcomes and safety of surgical lung biopsy for interstitial lung disease. Chest. 2005;127(5):1600–1605. doi: 10.1378/chest.127.5.1600. [DOI] [PubMed] [Google Scholar]
- 37.Swensen SJ, Aughenbaugh GL, Myers JL. Diffuse lung disease: diagnostic accuracy of CT in patients undergoing surgical biopsy of the lung. Radiology. 1997;205(1):229–234. doi: 10.1148/radiology.205.1.9314990. [DOI] [PubMed] [Google Scholar]
- 38.Flaherty KR, Thwaite EL, Kazerooni EA, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax. 2003;58(2):143–148. doi: 10.1136/thorax.58.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aziz ZA, Wells AU, Hansell DM, et al. HRCT diagnosis of diffuse parenchymal lung disease: inter-observer variation. Thorax. 2004;59(6):506–511. doi: 10.1136/thx.2003.020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Churg A, Muller NL, Flint J, Wright JL. Chronic hypersensitivity pneumonitis. Am J Surg Pathol. 2006;30(2):201–208. doi: 10.1097/01.pas.0000184806.38037.3c. [DOI] [PubMed] [Google Scholar]
- 41.Kim EA, Lee KS, Johkoh T, et al. Interstitial lung diseases associated with collagen vascular diseases: radiologic and histopathologic findings. Radiographics. 2002;22(Spec No):S151–165. doi: 10.1148/radiographics.22.suppl_1.g02oc04s151. [DOI] [PubMed] [Google Scholar]
- 42.Martinez FJ, Safrin S, Weycker D, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med. 2005;142(12 Pt 1):963–967. doi: 10.7326/0003-4819-142-12_part_1-200506210-00005. [DOI] [PubMed] [Google Scholar]
- 43.Martinez FJ, Flaherty K. Pulmonary function testing in idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3(4):315–321. doi: 10.1513/pats.200602-022TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erbes R, Schaberg T, Loddenkemper R. Lung function tests in patients with idiopathic pulmonary fibrosis. Are they helpful for predicting outcome? Chest. 1997;111(1):51–57. doi: 10.1378/chest.111.1.51. [DOI] [PubMed] [Google Scholar]
- 45.Stephan S, De Castro Pereira CA, Coletta EM, Ferreira RG, Otta JS, Nery LE. Oxygen desaturation during a 4-minute step test: predicting survival in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2007;24(1):70–76. [PubMed] [Google Scholar]
- 46.Shitrit D, Rusanov V, Peled N, Amital A, Fuks L, Kramer MR. The 15-step oximetry test: a reliable tool to identify candidates for lung transplantation among patients with idiopathic pulmonary fibrosis. J Heart Lung Transplant. 2009;28(4):328–333. doi: 10.1016/j.healun.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 47.Lynch DA, Godwin JD, Safrin S, et al. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med. 2005;172(4):488–493. doi: 10.1164/rccm.200412-1756OC. [DOI] [PubMed] [Google Scholar]
- 48.Battista G, Zompatori M, Fasano L, Pacilli A, Basile B. Progressive worsening of idiopathic pulmonary fibrosis. High resolution computed tomography (HRCT) study with functional correlations. Radiol Med. 2003;105(1–2):2–11. [PubMed] [Google Scholar]
- 49.Flaherty KR, Mumford JA, Murray S, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168(5):543–548. doi: 10.1164/rccm.200209-1112OC. [DOI] [PubMed] [Google Scholar]
- 50.Zappala CJ, Latsi PI, Nicholson AG, et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35(4):830–836. doi: 10.1183/09031936.00155108. [DOI] [PubMed] [Google Scholar]
- 51.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 52.Du Bois RM, Weycker D, Albera C, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med. 2011;184(12):1382–1389. doi: 10.1164/rccm.201105-0840OC. [DOI] [PubMed] [Google Scholar]
- 53.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6(5 Pt 2):1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 54.Shorr AF, Davies DB, Nathan SD. Outcomes for patients with sarcoidosis awaiting lung transplantation. Chest. 2002;122(1):233–238. doi: 10.1378/chest.122.1.233. [DOI] [PubMed] [Google Scholar]
- 55.Gries CJ, Mulligan MS, Edelman JD, Raghu G, Curtis JR, Goss CH. Lung allocation score for lung transplantation: impact on disease severity and survival. Chest. 2007;132(6):1954–1961. doi: 10.1378/chest.07-1160. [DOI] [PubMed] [Google Scholar]
- 56.Neurohr C, Huppmann P, Thum D, et al. Potential functional and survival benefit of double over single lung transplantation for selected patients with idiopathic pulmonary fibrosis. Transpl Int. 2010;23(9):887–896. doi: 10.1111/j.1432-2277.2010.01071.x. [DOI] [PubMed] [Google Scholar]
- 57.Lawson WE, Grant SW, Ambrosini V, et al. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax. 2004;59(11):977–980. doi: 10.1136/thx.2004.026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawson WE, Crossno PF, Polosukhin VV, et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol. 2008;294(6):L1119–L1126. doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- 59.Korfei M, Ruppert C, Mahavadi P, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178(8):838–846. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsakiri KD, Cronkhite JT, Kuan PJ, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA. 2007;104(18):7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cronkhite JT, Xing C, Raghu G, et al. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178(7):729–737. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alder JK, Chen JJ, Lancaster L, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 2008;105(35):13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pulkkinen V, Bruce S, Rintahaka J, et al. ELMOD2, a candidate gene for idiopathic pulmonary fibrosis, regulates antiviral responses. FASEB J. 2010;24(4):1167–1177. doi: 10.1096/fj.09-138545. [DOI] [PubMed] [Google Scholar]
- 64.Hutyrova B, Pantelidis P, Drabek J, et al. Interleukin-1 gene cluster polymorphisms in sarcoidosis and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002;165(2):148–151. doi: 10.1164/ajrccm.165.2.2106004. [DOI] [PubMed] [Google Scholar]
- 65.Zorzetto M, Ferrarotti I, Trisolini R, et al. Complement receptor 1 gene polymorphisms are associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168(3):330–334. doi: 10.1164/rccm.200302-221OC. [DOI] [PubMed] [Google Scholar]
- 66.Latsi P, Pantelidis P, Vassilakis D, Sato H, Welsh KI, Du Bois RM. Analysis of IL-12 p40 subunit gene and IFN-gamma G5644A polymorphisms in idiopathic pulmonary fibrosis. Respir Res. 2003;4:6. doi: 10.1186/1465-9921-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zorzetto M, Ferrarotti I, Campo I, et al. NOD2/CARD15 gene polymorphisms in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22(3):180–185. [PubMed] [Google Scholar]
- 68.Liu L, Dai HP, Xiao B, Zhang S, Ban CJ, Xin P. [Association of ENA-78, IP-10 and VEGF gene polymorphism with idiopathic pulmonary fibrosis] Zhonghua Yi Xue Za Zhi. 2009;89(38):2690–2694. [PubMed] [Google Scholar]
- 69.Bournazos S, Bournazou I, Murchison JT, et al. Fcgamma receptor IIIb (CD16b) polymorphisms are associated with susceptibility to idiopathic pulmonary fibrosis. Lung. 2010;188(6):475–481. doi: 10.1007/s00408-010-9262-3. [DOI] [PubMed] [Google Scholar]
- 70.Ahn MH, Park BL, Lee SH, et al. A promoter SNP rs4073T>A in the common allele of the interleukin 8 gene is associated with the development of idiopathic pulmonary fibrosis via the IL-8 protein enhancing mode. Respir Res. 2011;12:73. doi: 10.1186/1465-9921-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martinelli M, Pacilli AM, Rivetti S, et al. A role for epidermal growth factor receptor in idiopathic pulmonary fibrosis onset. Mol Biol Rep. 2011;38(7):4613–4617. doi: 10.1007/s11033-010-0594-0. [DOI] [PubMed] [Google Scholar]
- 72▪▪.Zuo F, Kaminski N, Eugui E, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA. 2002;99(9):6292–6297. doi: 10.1073/pnas.092134099. This study, the first gene-expression analysis of IPF lungs, demonstrated the upregulation of MMP-7 in fibrotic human lungs and the protection of MMP-7-knockout mice against bleomycin-induced pulmonary fibrosis, showing the importance of this molecule in IPF pathogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cosgrove GP, Brown KK, Schiemann WP, et al. Pigment epithelium-derived factor in idiopathic pulmonary fibrosis: a role in aberrant angiogenesis. Am J Respir Crit Care Med. 2004;170(3):242–251. doi: 10.1164/rccm.200308-1151OC. [DOI] [PubMed] [Google Scholar]
- 74.Pardo A, Gibson K, Cisneros J, et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005;2(9):e251. doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Selman M, Pardo A, Barrera L, et al. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2006;173(2):188–198. doi: 10.1164/rccm.200504-644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bridges RS, Kass D, Loh K, Glackin C, Borczuk AC, Greenberg S. Gene expression profiling of pulmonary fibrosis identifies Twist1 as an antiapoptotic molecular “rectifier” of growth factor signaling. Am J Pathol. 2009;175(6):2351–2361. doi: 10.2353/ajpath.2009.080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsu E, Shi H, Jordan RM, Lyons-Weiler J, Pilewski JM, Feghali-Bostwick CA. Lung tissues in patients with systemic sclerosis have gene expression patterns unique to pulmonary fibrosis and pulmonary hypertension. Arthritis Rheum. 2011;63(3):783–794. doi: 10.1002/art.30159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaminski N, Rosas IO. Gene expression profiling as a window into idiopathic pulmonary fibrosis pathogenesis: can we identify the right target genes? Proc Am Thorac Soc. 2006;3(4):339–344. doi: 10.1513/pats.200601-011TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosas IO, Kaminski N. When it comes to genes – IPF or NSIP, familial or sporadic – they’re all the same. Am J Respir Crit Care Med. 2007;175(1):5–6. doi: 10.1164/rccm.200610-1415ED. [DOI] [PubMed] [Google Scholar]
- 80▪▪.Rosas IO, Richards TJ, Konishi K, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008;5(4):e93. doi: 10.1371/journal.pmed.0050093. This study was the first to establish a combined, genomic targeted, proteomic signature including MMP-1 and MMP-7 to differentiate IPF from controls and other chronic lung diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bazzini AA, Lee MT, Giraldez AJ. Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science. 2012;336(6078):233–237. doi: 10.1126/science.1215704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82▪▪.Pandit KV, Corcoran D, Yousef H, et al. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;182(2):220–229. doi: 10.1164/rccm.200911-1698OC. The investigators discovered the first differentially expressed miRNA signature in IPF lungs and identified a potential mechanism of lung fibrosis pathogenesis by the inhibition of let-7d, one of the downregulated miRNAs in IPF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu G, Friggeri A, Yang Y, et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207(8):1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dai Y, Huang YS, Tang M, et al. Microarray analysis of microRNA expression in peripheral blood cells of systemic lupus erythematosus patients. Lupus. 2007;16(12):939–946. doi: 10.1177/0961203307084158. [DOI] [PubMed] [Google Scholar]
- 85.Hausler SF, Keller A, Chandran PA, et al. Whole blood-derived miRNA profiles as potential new tools for ovarian cancer screening. Br J Cancer. 2010;103(5):693–700. doi: 10.1038/sj.bjc.6605833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rege TA, Hagood JS. Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival, and cytokine/growth factor responses. Biochim Biophys Acta. 2006;1763(10):991–999. doi: 10.1016/j.bbamcr.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramirez G, Hagood JS, Sanders Y, et al. Absence of Thy-1 results in TGF-beta induced MMP-9 expression and confers a profibrotic phenotype to human lung fibroblasts. Lab Invest. 2011;91(8):1206–1218. doi: 10.1038/labinvest.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strieter RM, Belperio JA, Keane MP. CXC chemokines in angiogenesis related to pulmonary fibrosis. Chest. 2002;122(6 Suppl):298S–301S. doi: 10.1378/chest.122.6_suppl.298s. [DOI] [PubMed] [Google Scholar]
- 89.Selman M, Carrillo G, Estrada A, et al. Accelerated variant of idiopathic pulmonary fibrosis: clinical behavior and gene expression pattern. PLoS One. 2007;2(5):e482. doi: 10.1371/journal.pone.0000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boon K, Bailey NW, Yang J, et al. Molecular phenotypes distinguish patients with relatively stable from progressive idiopathic pulmonary fibrosis (IPF) PLoS One. 2009;4(4):e5134. doi: 10.1371/journal.pone.0005134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176(7):636–643. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37(2):356–363. doi: 10.1183/09031936.00159709. [DOI] [PubMed] [Google Scholar]
- 93.Konishi K, Gibson KF, Lindell KO, et al. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;180(2):167–175. doi: 10.1164/rccm.200810-1596OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 95.Herazo JD, Gibson K, Juan-Guardela B, et al. Peripheral blood monuclear cells gene expression patterns predict mortality in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(A5306) [Google Scholar]
- 96.Eichler GS, Huang S, Ingber DE. Gene Expression Dynamics Inspector (GEDI): for integrative analysis of expression profiles. Bioinformatics. 2003;19(17):2321–2322. doi: 10.1093/bioinformatics/btg307. [DOI] [PubMed] [Google Scholar]
- 97.Yokoyama A, Kondo K, Nakajima M, et al. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology. 2006;11(2):164–168. doi: 10.1111/j.1440-1843.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 98.Prasse A, Probst C, Bargagli E, et al. Serum CC-chemokine ligand 18 concentration predicts outcome in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179(8):717–723. doi: 10.1164/rccm.200808-1201OC. [DOI] [PubMed] [Google Scholar]
- 99.Kinder BW, Brown KK, Mccormack FX, et al. Serum surfactant protein-A is a strong predictor of early mortality in idiopathic pulmonary fibrosis. Chest. 2009;135(6):1557–1563. doi: 10.1378/chest.08-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moeller A, Gilpin SE, Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179(7):588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 101▪▪.Richards TJ, Kaminski N, Baribaud F, et al. Peripheral blood proteins predict mortality in idiopathicpulmonary fibrosis. Am J Respir Crit Care Med. 2012;185(1):67–76. doi: 10.1164/rccm.201101-0058OC. By developing a personal clinic and molecular mortality prediction index, which combines clinical and demographic parameters with MMP-7 protein levels in the peripheral blood, this study set the grounds for the use of personalized based approaches for outcome prediction in IPF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Venables WN, Ripley BD. Modern Applied Statistics with S. 4. Springer; NY, USA: 2002. [Google Scholar]
Websites
- 201.miRBase. www.mirbase.org/cgi-bin/mirna_summary.pl?org=hsa.
- 202.Lung Tissue Research Consortium. www.ltrcpublic.com/
- 203.Lung Genomics Research Consortium. www.lung-genomics.org.