Abstract
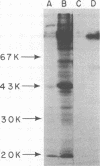
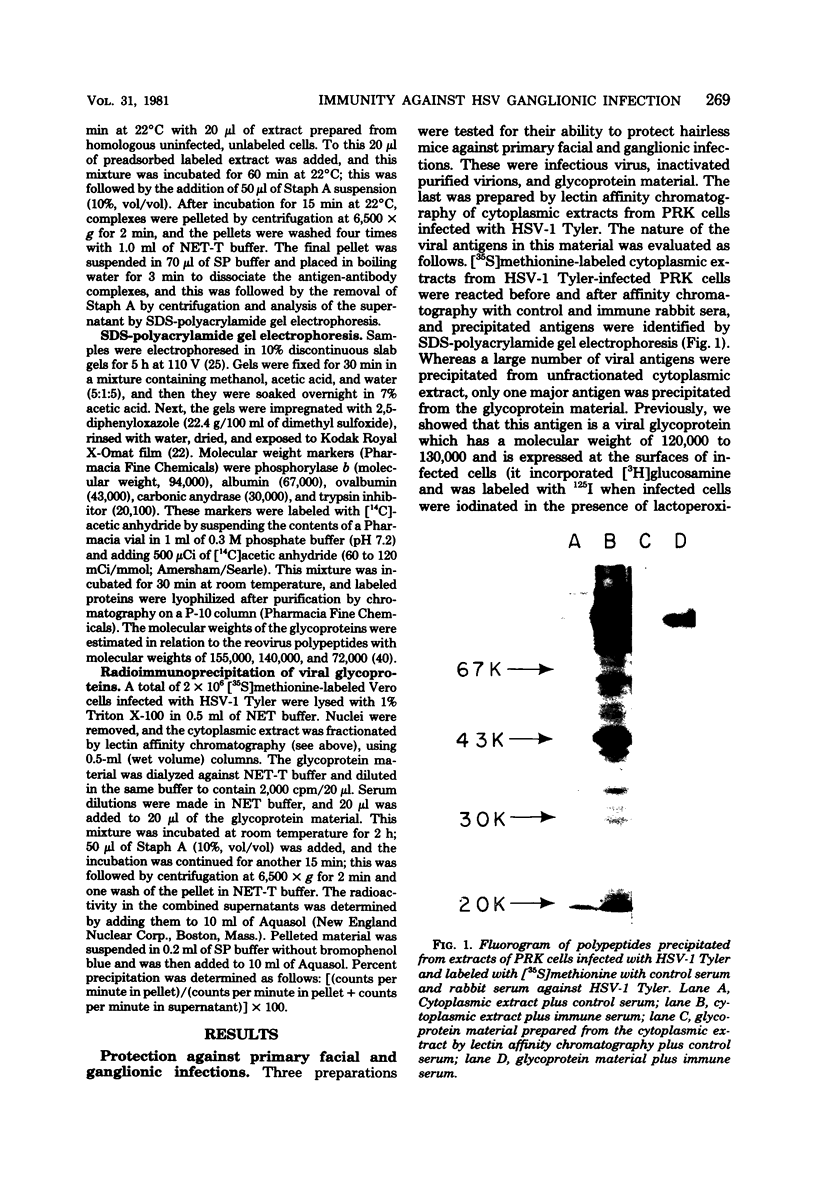
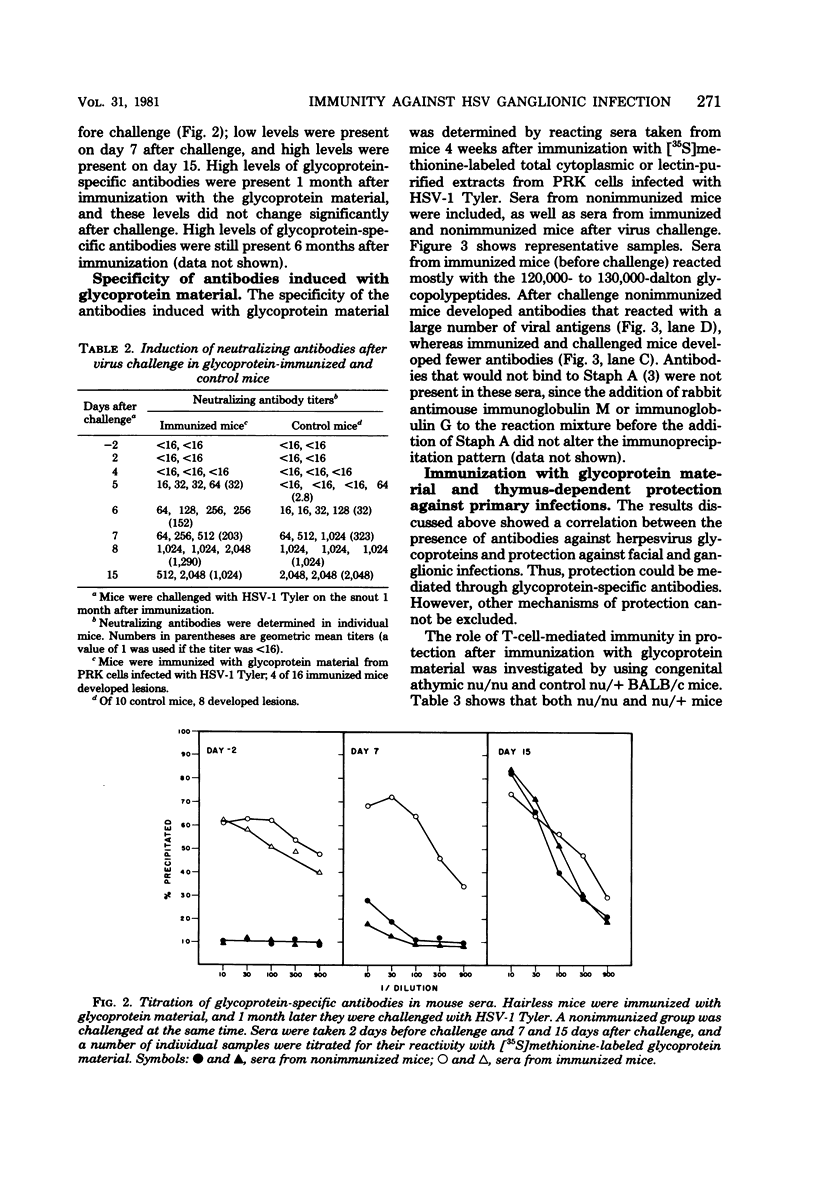
We performed experiments with mice to determine the nature of the immune response(s) that prevents primary infections of the skin and the trigeminal ganglia with herpes simplex virus. Immunization with infectious herpes simplex virus, inactivated virus, or material enriched for viral glycoproteins protected hairless mice against primary facial and ganglionic infections. Live and inactivated viruses induced neutralizing antibodies, whereas glycoprotein material did not. Instead, glycoprotein material induced antibodies that were largely directed against two glycopolypeptides with molecular weights of 120,000 to 130,000. Hairless mice immunized with glycoprotein material responded faster than control mice in the synthesis of neutralizing antibodies after challenge with infectious virus. Congenital athymic BALB/c (nu/nu) mice were protected against primary facial infections after immunization with glycoprotein material, but glycoprotein-specific antibodies were not induced.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asher L. V., Walz M. A., Notkins A. L. Effect of immunization on the development of latent ganglionic infection in mice challenged intravaginally with herpes simplex virus types 1 and 2. Am J Obstet Gynecol. 1978 Aug 1;131(7):788–791. doi: 10.1016/0002-9378(78)90248-x. [DOI] [PubMed] [Google Scholar]
- Brunda M. J., Minden P., Sharpton T. R., McClatchy J. K., Farr R. S. Precipitation of radiolabeled antigen-antibody complexes with protein A-containing Staphylococcus aureus. J Immunol. 1977 Jul;119(1):193–198. [PubMed] [Google Scholar]
- Cappel R. Comparison of the humoral and cellular immune response after immunization with live, UV inactivated herpes simplex virus and a subunit vaccine and efficacy of these immunizations. Arch Virol. 1976;52(1-2):29–35. doi: 10.1007/BF01317862. [DOI] [PubMed] [Google Scholar]
- Ching C. Y., López C. A type-specific antiserum induced bya major herpesvirus type 1 glycoprotein. J Immunol Methods. 1980;32(4):383–391. doi: 10.1016/0022-1759(80)90030-7. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey L., Reeves W. C., Holmes K. K. Cellular immune response in genital herpes simplex virus infection. N Engl J Med. 1978 Nov 2;299(18):986–991. doi: 10.1056/NEJM197811022991805. [DOI] [PubMed] [Google Scholar]
- Douglas R. G., Jr, Couch R. B. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970 Feb;104(2):289–295. [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster cells after exposure to herpes simplex virus type 2. Nat New Biol. 1971 Sep 8;233(36):48–50. doi: 10.1038/newbio233048a0. [DOI] [PubMed] [Google Scholar]
- Glorioso J. C., Wilson L. A., Fenger T. W., Smith J. W. Complement-mediated cytolysis of HSV-1 and HSV-2 infected cells: plasma membrane antigens reactive with type-specific and cross-reactive antibody. J Gen Virol. 1978 Aug;40(2):443–454. doi: 10.1099/0022-1317-40-2-443. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Veldhuisen G., Wilkie N. M. Infectious herpesvirus DNA. Nat New Biol. 1973 Oct 31;245(148):265–266. doi: 10.1038/newbio245265a0. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Djeu J., Kay H. D., Ortaldo J. R., Riccardi C., Bonnard G. D., Holden H. T., Fagnani R., Santoni A., Puccetti P. Natural killer cells: characteristics and regulation of activity. Immunol Rev. 1979;44:43–70. doi: 10.1111/j.1600-065x.1979.tb00267.x. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kirchner H., Darai G., Hirt H. M., Keyssner K., Munk K. In vitro mitogenic stimulation of murine spleen cells by herpes simplex virus. J Immunol. 1978 Feb;120(2):641–645. [PubMed] [Google Scholar]
- Kitces E. N., Morahan P. S., Tew J. G., Murray B. K. Protection from oral herpes simplex virus infection by a nucleic acid-free virus vaccine. Infect Immun. 1977 Jun;16(3):955–960. doi: 10.1128/iai.16.3.955-960.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. J., Friedman-Kien A. E., Brady E. Latent herpes simplex virus in ganglia of mice after primary infection and reinoculation at a distant site. Arch Virol. 1978;57(2):161–166. doi: 10.1007/BF01315677. [DOI] [PubMed] [Google Scholar]
- Klein R. J. Pathogenetic mechansims of recurrent herpes simplex virus infections. Arch Virol. 1976;51(1-2):1–13. doi: 10.1007/BF01317829. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lawrence W. C. Purification of equine herpesvirus type 1. J Gen Virol. 1976 Apr;31(1):81–91. doi: 10.1099/0022-1317-31-1-81. [DOI] [PubMed] [Google Scholar]
- Lopez C., O'Reilly R. J. Cell-mediated immune responses in recurrent herpesvirus infections. I. Lymphocyte proliferation assay. J Immunol. 1977 Mar;118(3):895–902. [PubMed] [Google Scholar]
- McKendall R. R. Efficacy of herpes simplex virus type 1 immunization in protecting against acute and latent infection by herpes simplex virus type 2 in mice. Infect Immun. 1977 May;16(2):717–719. doi: 10.1128/iai.16.2.717-719.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki D., Hedrick S., Watson J., Kingsbury D. T. The interaction of Herpes Simplex Virus with murine lymphocytes. I. Mitogenic properties of herpes simplex virus. J Exp Med. 1977 Dec 1;146(6):1500–1510. doi: 10.1084/jem.146.6.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C., Andersen H. K. Role of activated macrophages in resistance of congenitally athymic nude mice to hepatitis induced by herpes simplex virus type 2. Infect Immun. 1978 Mar;19(3):792–798. doi: 10.1128/iai.19.3.792-798.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C. Role of macrophages in natural resistance to virus infections. Microbiol Rev. 1979 Mar;43(1):1–26. doi: 10.1128/mr.43.1.1-26.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan P. S., Glasgow L. A., Crane J. L., Jr, Kern E. R. Comparison of antiviral and antitumor activity of activated macrophages. Cell Immunol. 1977 Feb;28(2):404–415. doi: 10.1016/0008-8749(77)90122-8. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Roizman B. Infection with herpes-simplex viruses 1 and 2. 1. N Engl J Med. 1973 Sep 27;289(13):667–674. doi: 10.1056/NEJM197309272891305. [DOI] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Nahmias A. J. Herpes simplex virus glycoproteins: participation of individual herpes simplex virus type 1 glycoprotein antigens in immunocytolysis and their correlation with previously identified glycopolypeptides. J Virol. 1979 Dec;32(3):741–748. doi: 10.1128/jvi.32.3.741-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes J. E., Rosemond-Hornbeak H. Antibody-mediated recovery from subcutaneous herpes simplex virus type 2 infection. Infect Immun. 1978 Aug;21(2):489–495. doi: 10.1128/iai.21.2.489-495.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager-Zisman B., Merigan T. C. A useful quantitative semimicromethod for viral plaque assay. Proc Soc Exp Biol Med. 1973 Apr;142(4):1174–1179. doi: 10.3181/00379727-142-37202. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Tompkins W. A., Melnick J. L. The association of herpesvirus type 2 and carcinoma of the uterine cervix. Am J Epidemiol. 1969 May;89(5):547–554. doi: 10.1093/oxfordjournals.aje.a120967. [DOI] [PubMed] [Google Scholar]
- Schlabach A. J., Martinez D., Field A. K., Tytell A. A. Resistance of C58 mice to primary systemic herpes simplex virus infection: macrophage dependence and T-cell independence. Infect Immun. 1979 Nov;26(2):615–620. doi: 10.1128/iai.26.2.615-620.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L., Jordan M. C. Reactivation of latent Herpes simplex virus after pneumococcal pneumonia in mice. Infect Immun. 1975 Apr;11(4):635–639. doi: 10.1128/iai.11.4.635-639.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz M. A., Yamamoto H., Notkins A. L. Immunological response restricts number of cells in sensory ganglia infected with herpes simplex virus. Nature. 1976 Dec 9;264(5586):554–556. doi: 10.1038/264554a0. [DOI] [PubMed] [Google Scholar]
- Wise T. G., Pavan P. R., Ennis F. A. Herpes simplex virus vaccines. J Infect Dis. 1977 Nov;136(5):706–711. doi: 10.1093/infdis/136.5.706. [DOI] [PubMed] [Google Scholar]
- Zawatzky R., Hilfenhaus J., Krichner H. Resistance of nude mice to herpes simplex virus and correlation with in vitro production of interferon. Cell Immunol. 1979 Oct;47(2):424–428. doi: 10.1016/0008-8749(79)90352-6. [DOI] [PubMed] [Google Scholar]