Abstract
Ascorbate peroxidase (AP) is a key enzyme that scavenges potentially harmful H2O2 and thus prevents oxidative damage in plants, especially in N2-fixing legume root nodules. The present study demonstrates that the nodule endodermis of alfalfa (Medicago sativa) root nodules contains elevated levels of AP protein, as well as the corresponding mRNA transcript and substrate (ascorbate). Enhanced AP protein levels were also found in cells immediately peripheral to the infected region of soybean (Glycine max), pea (Pisum sativum), clover (Trifolium pratense), and common bean (Phaseolus vulgaris) nodules. Regeneration of ascorbate was achieved by (homo)glutathione and associated enzymes of the ascorbate-glutathione pathway, which were present at high levels. The presence of high levels of antioxidants suggests that respiratory consumption of O2 in the endodermis or nodule parenchyma may be an essential component of the O2-diffusion barrier that regulates the entry of O2 into the central region of nodules and ensures optimal functioning of nitrogenase.
All N2-fixing organisms face a critical dilemma. Nitrogenase, the enzyme that catalyzes the conversion of N2 into NH3, is quickly and irreversibly inactivated by O2, yet N2 fixation is an extremely energy-demanding process, with at least 16 mol of ATP being consumed per mol of N2 fixed (Ljones and Burris, 1972). Therefore, relying on aerobic respiration to supply this energy puts the organism at risk of inactivating nitrogenase. This “O2 problem” in N2-fixing organisms is handled by a variety of mechanisms, including anaerobic metabolism (Clostridium spp.); consumption of O2 by extremely rapid rates of respiration (Azotobacter spp.); physical compartmentalization of nitrogenase (heterocysts of cyanobacteria and vesicles of Frankia spp., the symbiont in roots of alder and other nonlegumes); and temporal separation of photosynthesis and N2 fixation (nonheterocystous cyanobacteria).
In legume root nodules, the O2 problem is dealt with by three mechanisms (Appleby, 1984; Witty et al., 1986; Hunt et al., 1987; Denison, 1992): an abundant amount of the O2-binding protein leghemoglobin to facilitate the flux of O2 to symbiotic bacteria (Rhizobium or Bradyrhizobium), while maintaining an extremely low, nontoxic concentration of free O2; a high rate of respiratory O2 consumption; and a variable diffusion barrier that controls the entry of O2 into the central, infected regions. The principles by which this barrier operates are not clear, but they are responsible for the physiological control of the size, distribution, and content of intercellular spaces. The diffusion of O2 into the nodule interior can thus be regulated by alterations in relative amounts of air, liquid, or occluding glycoproteins within these intercellular spaces (James et al., 1991; Van Cauwenberghe et al., 1993). The effectiveness of the control of O2 levels in nodules has been unequivocally established with measurements by O2 microelectrodes that show that the O2 concentration rapidly decreases from atmospheric levels in the nodule outer cortex to nearly anaerobic levels in the nodule central region (Tjepkema and Yocum, 1974; Witty et al., 1987).
Activated forms of O2, such as H2O2 and the superoxide radical, constitute another aspect of the O2 problem in legume nodules. Nodules have a high capacity to produce these damaging chemical species because of the high rates of respiration, the strong reducing conditions required to reduce N2, the tendency of leghemoglobin to autoxidize, and the likely ability of nitrogenase to directly reduce O2 (Dalton, 1995). A major defense against activated O2 in nodules is provided by AP (EC 1.11.1.11), a hemoprotein that uses the reducing power of ascorbate to scavenge H2O2. Although AP may be regarded as a nearly universal “housekeeper” in the cytosol and chloroplasts of plant cells, it is especially abundant in the cytosol of N2-fixing root nodules, where it makes up almost 1% of the total soluble protein.
There is considerable variation between species in the structure of the peripheral cell layers in nodules (Dakora and Atkins, 1989; Parsons and Day, 1990; Hirsch, 1992; Brown and Walsh, 1996). This report follows the terminology of Hirsch (1992), in which the organization (from exterior toward the center) is referred to as the nodule cortex, nodule endodermis, nodule parenchyma, and the N2-fixing (infected) zone. Vascular bundles are present in the nodule parenchyma layer. One advantage of this system is that the same terms apply to both determinate and indeterminate nodules, although there are some structural differences, such as the presence of a scleroid layer in the nodule parenchyma of some determinate nodules.
In this report we have used various techniques of microscopy to define the location of antioxidants, especially AP, in the peripheral cell layers of nodules from several legume species. We hypothesize that antioxidants may be involved in a type of “respiratory protection” that limits the entry of O2 into the nodule interior.
MATERIALS AND METHODS
Plant Culture
The following plant species and rhizobial symbionts were used: soybean (Glycine max cv Williams) with Bradyrhizobium japonicum 122DES; alfalfa (Medicago sativa cv Ladak) with Rhizobium meliloti 104A14; clover (Trifolium pratense cv Kenland) with Rhizobium leguminosarum bv trifolii 162P28; pea (Pisum sativum cv Lincoln) with R. leguminosarum bv viceae NLV8; cowpea (Vigna unguiculata cv California blackeye no. 5) with Bradyrhizobium sp. 32H1; and bean (Phaseolus vulgaris cv Contender) with R. leguminosarum bv phaseoli 3622. Plant growth conditions were as described by Dalton et al. (1986).
Northern-Blot Analysis
RNA was isolated by the guanidinium-thiocyanate method using RNAzolB (Tel-Test, Friendswood, TX). Electrophoresis, blotting, and detection were as described by (Sambrook et al. 1989) with a 33P-labeled probe synthesized using the EcoRI-EcoRI full-length cDNA insert of pSOYAP75 (Chatfield and Dalton, 1993) as a template. Each lane was loaded with an equal amount (10 μg/lane) of total RNA based on A265.
Immunodetection
Root nodules of various legume species, including alfalfa, soybean, pea, clover, and common bean, were embedded in London White resin and cut into 1-μm sections. Nodules were between 28 and 40 d old when harvested. At least five nodules from five separate plants were examined for each of the observations reported here, but in most cases the number examined was much higher. Immunodetection was performed with a primary rabbit polyclonal antibody raised against purified soybean AP (Dalton et al., 1993). The primary antibody was used at a dilution of 1:50 for a 2-h incubation at room temperature.
For light-microscopic immunogold-Ag staining, the secondary antibody consisted of affinity-purified goat anti-rabbit antibodies conjugated to colloidal gold (Auroprobe LM, Amersham) and was used according to the manufacturer's recommended procedures. For light-microscopic immunofluorescence staining, the secondary antibody consisted of affinity-purified goat anti-rabbit antibodies conjugated to a Cy3 fluorophore (excitation wavelength, 550 nm; emission wavelength, 570 nm; Jackson ImmunoResearch Laboratories, West Grove, PA) at a dilution of 1:300 for 30 min. Sections were viewed with a microscope (Laborlux S, Leitz, Wetzlar, Germany) equipped with a rhodamine filter. Negative controls for both detection procedures were performed using rabbit normal serum. Additional negative controls were performed by preincubating 150 μL of diluted (1:50) primary antibody with 1 μg of purified recombinant AP (Dalton et al., 1996) for 2 h with gentle shaking at room temperature.
In Situ Hybridization
Digoxigenin-labeled antisense RNA probes for AP transcript were made using pSOYAP75 (Chatfield and Dalton, 1993) digested with BamHI as the template. The synthesis reaction contained T7 polymerase and the components of the RNA-labeling kit (Genius, Boehringer Mannheim). The sense probe (negative control) was constructed using XhoI to linearize the plasmid and T3 polymerase. Details of the hybridization protocol were described by Li et al. (1993). After an initial hybridization period of 15 h at 42°C, samples were incubated with rabbit anti-digoxin, washed, and then incubated with protein-A gold (15 nm, Amersham) at a 1:100 dilution for 90 min. Specimens were photographed with dark-field microscopy, because this accentuates the label and minimizes problems of resolution that arise because the label and underlying tissue are in slightly different planes of focus.
Histochemical Procedures
Localization of ascorbate was performed by incubating thin, hand-cut sections of alfalfa nodules in 4% (w/v) AgNO3 in 100 mm sodium acetate, pH 4.5, for 24 h at room temperature (Chinoy, 1984). For (homo)glutathione localization, fresh cowpea or bean nodules were halved with a sharp razor blade. To one-half, 20 μL of 50 mm K2-PO4, pH 7.0 (control), was immediately added, and the other half received the same buffer containing 4 mm monochlorobimane (Thiolyte MC, Calbiochem; Sanchez-Fernández et al., 1997). This compound conjugates specifically with (homo)- glutathione, yielding a fluorochrome (maximum excitation, 380 nm; maximum emission, 480 nm). After incubation for 1 to 2 min, both halves were washed three times with the same buffer, and the turquoise blue fluorescence was viewed (excitation filter, 355–425 nm; suppression filter, 460 nm) and photographed with a fluorescence microscope (Ortholux II, Leitz). To visualize respiratory activity, intact alfalfa nodules were submerged in 3 mm 2,3,5-triphenyltetrazolium chloride in 10 mm sodium phosphate buffer, pH 7.0, in the dark for 2 h, sectioned with a razor blade, and viewed without further staining.
Enzyme and Metabolite Assays
Nodules of cowpea and bean were dissected into cortex (including nodule parenchyma) and infected tissue under a binocular dissecting microscope. These species were chosen because of the relatively large size of their nodules and, thus, the ease of separating tissue layers. Extracts were prepared and assayed for activities of AP, monodehydroascorbate reductase (EC 1.6.5.4), dehydroascorbate reductase (EC 1.8.5.1), glutathione reductase (EC 1.6.4.2), and catalase (EC 1.11.1.6), as well as for concentrations of ascorbate and (homo)glutathione, using procedures described by Gogorcena et al. (1995). Samples containing similar amounts of whole nodules were run in parallel and used as controls.
RESULTS AND DISCUSSION
Abundance of AP Transcript
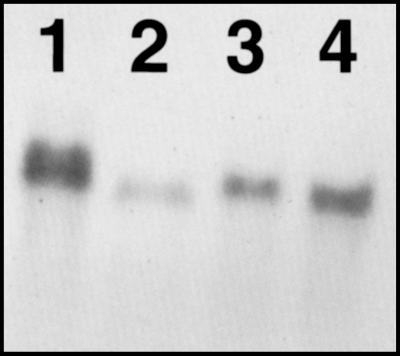
Blots of total RNA from nodules, roots, shoots, and leaves of soybean showed that the transcript level of AP was strongly enhanced in nodules (Fig. 1). The transcript level in nodules was 10-fold higher than in uninfected roots, 2.4-fold higher than in shoots, and 1.5-fold higher than in leaves when quantified with NIH Image software. These observations are consistent with earlier results (Dalton et al., 1987) in which we estimated that the large amount of AP protein in nodules corresponds to about 0.9% of the total soluble protein.
Figure 1.
Northern-blot analysis showing abundance of AP mRNA in soybean nodules (lane 1), roots (lane 2), shoots (lane 3), and leaves (lane 4). Each lane contained 10 μg of total RNA.
The slightly higher position of nodule AP mRNA (Fig. 1, lane 1) as opposed to AP bands from other tissue types may be a gel artifact caused by excess target, but the possibility of a slightly larger-molecular-weight form in nodules cannot be excluded.
Immunolocalization of AP
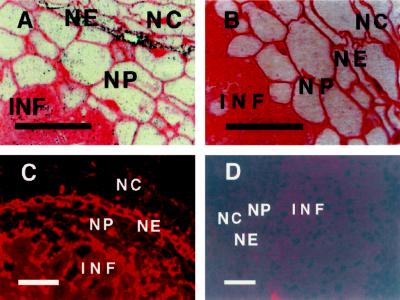
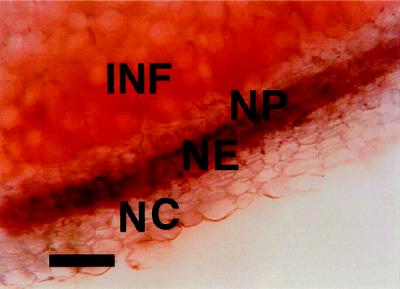
Immunostaining with rabbit polyclonal antibodies raised against soybean AP indicated that the level of this protein was strongly enhanced in the nodule endodermis of alfalfa nodules (Fig. 2). Quantification of AP as Ag-grain deposition per cell area indicated that levels of AP were 3.5- or 2.6-fold more abundant in the endodermis region than in the nodule cortex or in the infected zone, respectively. The antibodies used in these studies recognized a single band in sensitive chemiluminescent western immunoblots of crude extracts of total protein from nodules of soybean, alfalfa, clover, common bean, and red alder (Alnus rubra; not shown). They also recognized recombinant soybean AP expressed in Escherichia coli (Dalton et al., 1996).
Figure 2.
Immunolocalization of AP in alfalfa nodules. A, Typical section showing Ag particles (AP protein) concentrated in the infected region (INF) and in the nodule endodermis (NE) between the nodule cortex (NC) and the nodule parenchyma (NP). B, Negative control indicating the absence of labeling with Ag when normal rabbit serum is used in place of antibody raised against AP. C, Similar to A, except that detection is based on Cy3 fluorescence. D, Negative control with a fluorescent probe in which the primary antibody was blocked by preincubation with purified recombinant AP. Each bar indicates 100 μm.
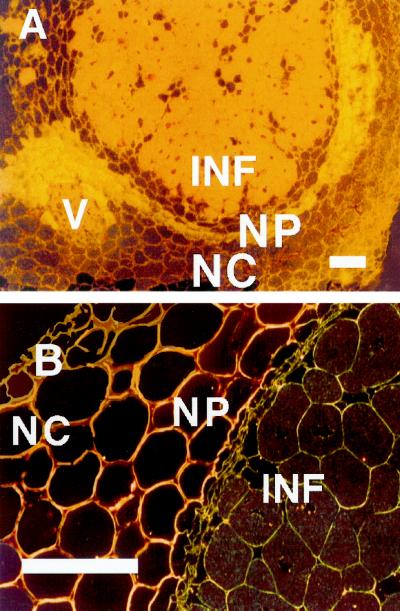
Enhanced immunostaining of AP was consistently observed in the endodermis of indeterminate nodules from several species (alfalfa, Fig. 2, A and C; pea and clover, not shown) with detection procedures based on either Ag precipitation or fluorescence. A different labeling pattern was observed in determinate nodules (bean, Fig. 3A; soybean, not shown). In these species the labeling was most intense at the exterior edge of the nodule parenchyma (just interior to the scleroid layer of soybean) and diminished inwardly before sharply intensifying in the infected region. This region with enhanced AP expression corresponds to the “boundary layer” referred to in other studies (e.g. Parsons and Day, 1990). Enhanced labeling was especially apparent in the parenchyma immediately surrounding vascular bundles (Fig. 3A). As with indeterminate nodules, controls with normal rabbit serum showed essentially no labeling of bean (Fig. 3B) or soybean (not shown) nodules.
Figure 3.
Immunolocalization of AP in bean nodules (A) and negative control (B) using normal rabbit serum. Micrographs were taken using dark-field illumination, which makes Ag grains (AP) appear as bright dots. V, Vascular bundle. Other abbreviations are as in Figure 2. Each bar indicates 100 μm.
Ag immunolabeling of AP could be seen with both bright-field (Fig. 2A) and dark-field microscopy (Fig. 3A); however, this labeling was more clearly seen with a dark field. Although Ag precipitation is a proven and stable technique for localization of both proteins and transcripts, the resultant micrographs suffer from limited resolution because the Ag grains and the target tissue are in different planes of focus. Dark-field microscopy maximizes the visibility of the Ag particles by allowing them to appear as bright spots of light on an in-focus background.
Cell wall artifacts are sometimes observed with immunostaining of plant tissues. Several lines of evidence strongly suggest that our observations were not attributable to such artifacts. Electron-microscopic studies (Dalton et al., 1993; also repeated here but not shown) indicate clearly that the labeling is present almost exclusively in the cytoplasm. Sections of alfalfa nodules treated with normal rabbit serum instead of antiserum to AP showed no labeling in the endodermis and very little in the infected regions, even if the serum was used at an undiluted strength (Fig. 2B). Furthermore, pretreating of primary antibody by the addition of purified, homogeneous recombinant AP (Dalton et al., 1996) resulted in a complete absence of labeling, with amounts of blocking antigen ranging from 1 μg to 1 ng in 150 μL of antibody solution (Fig. 2D). These controls also eliminated the possibility of misleading results due to autofluorescence.
In Situ Hybridization
The use of digoxigenin-labeled RNA antisense probes confirmed that the endodermis region of alfalfa nodules contained high levels of transcript for AP relative to adjacent regions of the cortex (Fig. 4A) and that central, infected cells had high levels relative to adjacent, uninfected cells (Fig. 4B). Controls performed identically with sense RNA probes showed only very light, nonlocalized labeling. The apparent labeling of the epidermis in Figure 4A was an artifact that was also seen in controls.
Figure 4.
In situ localization of mRNA transcript for AP (A and B) and histochemical localization of ascorbate (C and D) in alfalfa nodules. A, In situ hybridization with antisense RNA probe for AP showing enrichment in the nodule endodermis when viewed by dark-field illumination. B, Similar in situ hybridization showing high levels of mRNA labeling in an infected cell (I) and lower levels in an adjacent, uninfected cell (U). The label is evident as small bright dots. Other features include numerous bacteroids (nearly continuous bumps in I) and starch grains in uninfected cells. C, Ag particles precipitated from AgNO3, indicating the presence of ascorbate in high levels in the endodermis region. D, Negative control for C in which the tissue was preincubated in pH 9.0 buffer to oxidize the ascorbate before exposure to AgNO3. Other abbreviations are as in Figure 2. Bar indicates 100 μm in A, C, and D; 10 μm in B.
As was the case with Ag immunolabeling, labeling of AP transcript with Ag could be seen with both bright-field (not shown) and dark-field microscopy, with the latter producing much clearer images.
Histochemistry
Staining with AgNO3 indicated a distinct band of highly elevated ascorbate concentrations in the periphery of alfalfa nodules (Fig. 4C). This band was apparently located at the periphery of the nodule parenchyma, slightly interior to the endodermis, although this distinction is difficult to make in fresh, hand-cut sections. It may be that high utilization of ascorbate by AP locally depletes the ascorbate in the endodermis region. This would account for the absence of Ag deposition in the cell layers immediately exterior to the region of highest ascorbate concentration (Fig. 4C). If this interpretation is correct, then ascorbate could be resupplied to the endodermis through the abundant adjacent supply in the nodule parenchyma. The procedure for ascorbate localization was based on the specific reduction of Ag+ to dark crystals of elemental Ag by ascorbate (Chinoy, 1984). No labeling was present in control sections in which ascorbate was first destroyed by oxidation with either high pH or copper sulfate before exposure to AgNO3 (Fig. 4D). Additional evidence for elevated ascorbate levels was provided by staining of fresh sections with 2,6-dichloro-indophenol, in which case there was a region of clearing (indicating reduction of the dye by ascorbate) in the same peripheral band in which Ag deposition had indicated high ascorbate levels (data not shown).
Histochemical techniques indicated a high concentration of (homo)glutathione in the nodule parenchyma of cowpea (Fig. 5) and bean nodules (not shown). Glutathione (γGlu-Cys-Gly) is a major antioxidant in many organisms. Curiously, legumes partially rely on a homolog, homoglutathione (γGlu-Cys-βAla), with antioxidant properties presumably similar to glutathione found in other plant families (Klapheck, 1988). Because glutathione and homoglutathione are not distinguishable by the techniques used here, this report uses the term (homo)glutathione to indicate both compounds. In plants one of (homo)glutathione's chief roles involves a coupled series of redox reactions by which ascorbate is regenerated for continuing H2O2 scavenging (Dalton, 1995).
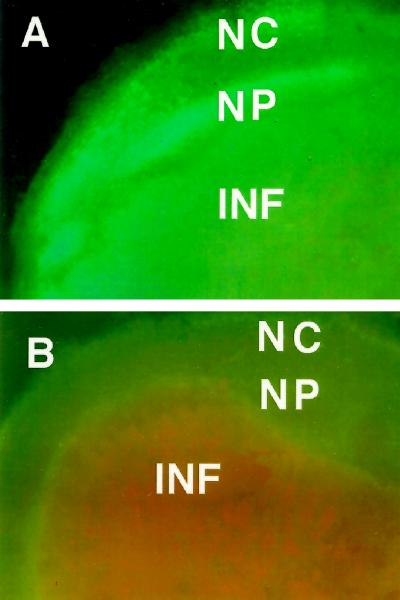
Figure 5.
Histochemical localization of (homo)glutathione in cowpea nodules. A, Turquoise blue fluorescence in the nodule parenchyma emitted by the adduct formed between (homo)glutathione and monochlorobimane. B, Negative control treated similarly except for the omission of monochlorobimane. Abbreviations are as in Figure 2.
Enzyme Activities and Metabolite Concentrations
Additional evidence for the enhanced antioxidant properties of the peripheral regions of nodules was obtained by microdissection of nodules into cortical and infected tissue. The corresponding extracts were analyzed for concentrations of antioxidant metabolites and activities of antioxidant enzymes (Table I). This technique did not allow a fine-enough separation to distinguish between discrete cell layers, such as the endodermis versus other peripheral layers, but it did provide a clear separation of peripheral versus central infected tissue. Ascorbate contents were 44 and 67% higher in the peripheral layers of bean and cowpea nodules, respectively, whereas (homo)glutathione was 44 and 120% higher (Table I). Considering that 85% of the nodules is water, the average concentration of ascorbate in the periphery can be estimated as 1 to 1.5 mm and that of (homo)glutathione as 0.5 mm.
Table I.
Antioxidant metabolites and enzymes in the periphery (cortex plus nodule parenchyma) and infected zone of bean and cowpea nodules
| Antioxidant | Common Bean
|
Cowpea
|
||
|---|---|---|---|---|
| Periphery | Infected zone | Periphery | Infected zone | |
| Ascorbate | 124 ± 9 a | 86 ± 11 b | 50 ± 4 a | 30 ± 4 b |
| (Homo)glutathionea | 37.8 ± 1.0 a | 26.3 ± 2.0 b | 26.4 ± 2.0 a | 12.0 ± 1.2 b |
| AP | 1.08 ± 0.03 a | 1.01 ± 0.05 a | 1.40 ± 0.07 a | 1.38 ± 0.02 a |
| Dehydroascorbate reductase | 51.6 ± 4.9 a | 29.1 ± 2.7 b | 35.1 ± 2.9 a | 40.2 ± 1.7 a |
| Monodehydroascorbate reductase | 384 ± 16 a | 244 ± 5 b | 340 ± 22 a | 217 ± 9 b |
| (Homo)glutathione reductaseb | 41.0 ± 2.9 a | 52.1 ± 1.2 b | 44.3 ± 2.2 a | 41.1 ± 1.4 a |
| Catalase | 125 ± 3 a | 163 ± 5 b | 82 ± 8 a | 150 ± 12 b |
Metabolite contents are expressed as nmol mg−1 protein. Activities are expressed as μmol substrate min−1 mg−1 protein (catalase and AP) or in nmol substrate or product min−1 mg−1 protein (others). Values are means ± se (n = 4–7). For each species, means denoted by the same letter do not differ significantly at P = 0.05 based on Duncan's multiple-range test.
Reduced plus oxidized (homo)glutathione.
Assayed using oxidized glutathione as a substrate.
Enzymes assayed in the peripheral and infected tissue included AP and the related enzymes of H2O2 scavenging: dehydroascorbate reductase, monodehydroascorbate reductase, and glutathione reductase. These enzymes participate in the ascorbate-glutathione pathway by which H2O2 is scavenged in higher plants (Dalton et al., 1986; Dalton, 1995). The specific activity of AP was similar in the peripheral and infected regions of bean and cowpea nodules. However, this does not contradict the immunolocalization data indicating enhancement in the nodule parenchyma, because the peripheral extracts are diluted by regions that immunolocalization studies indicate contain very little of this enzyme, whereas the infected regions contain a uniform, moderately high level.
In terms of specific activity, the peripheral cells of both legume nodules contain 57% more monodehydroascorbate reductase activity than the corresponding infected regions. Dehydroascorbate reductase activity was 77% higher in the peripheral cells of bean nodules compared with levels in the infected zone; however, no such elevation of dehydroascorbate reductase was observed in the periphery of cowpea nodules (Table I). These elevated activities may allow the functioning of the ascorbate-glutathione pathway at high rates in the nodule periphery by continuously regenerating ascorbate from its oxidized forms. However, glutathione reductase activity was similar in the periphery and infected zone of cowpea nodules, and 27% higher in the infected zone of bean nodules. This observation suggests that this enzyme may have additional roles in the infected tissue, unrelated to the ascorbate-glutathione pathway, such as the maintenance of high levels of (homo)glutathione to reduce ferryl leghemoglobin to the ferric form (Puppo et al., 1993).
Catalase activity, another direct scavenger of H2O2 in plants, was markedly higher in the central zone than in the periphery of both legume nodules (Table I), an observation consistent with the primary role of catalase in the elimination of H2O2 generated as a consequence of ureide synthesis in the central zone. Ureides are the primary form by which N is transported out of nodules in these species. Despite its considerable reputation, catalase is not a primary means of antioxidant defenses in the cytoplasm of plant cells because of its very high Km for H2O2 and its restricted location in peroxisomes (Dalton, 1995).
Implications for the O2-Diffusion Barrier
The elevated levels of antioxidants in the endodermis or nodule parenchyma suggest that this region plays a critical role in the O2 relations of nodules. In particular, we suggest that O2 diffusion into the interior of nodules is restricted by respiratory demand at the endodermis region (indeterminate nodules) or boundary layer (determinate nodules). Aerobic respiration invariably produces activated forms of O2, primarily by leakage of electrons from dehydrogenases in the electron transport chain. Elevated levels of AP and ascorbate are thus required to provide adequate antioxidant defenses. Staining with triphenyl tetrazolium dye (an indicator of respiratory activity) indicated that the endodermis region of alfalfa nodules does indeed have high rates of respiration, as opposed to the rates in the nodule cortex (Fig. 6). This procedure also revealed diminished staining in the nodule parenchyma and the central infected regions that may be interpreted as a consequence of limited diffusion of the dye or as an indication of lesser respiratory activity.
Figure 6.
Localization of respiratory activity in the periphery of alfalfa nodules. Dark-red staining in the endodermis/nodule parenchyma region is caused by reduction of tetrazolium by respiratory dehydrogenases. Abbreviations are as in Figure 2. The bar indicates 100 μm.
Because the phloem tissue in nodules lies directly adjacent to the endodermis, there is likely to be an abundant supply of photosynthate to support the high rates of respiration. The respiratory production of H2O2 is centered in mitochondria, whereas AP is primarily cytosolic. However, this separation does not refute the above arguments because H2O2 readily crosses biological membranes.
The high respiratory demand of nodules can be attributed in part to bacteroid (as opposed to host plant cell) activity. AP is not present in bacteroids (Dalton et al., 1986), and this would have no direct role in bacteroid antioxidant defenses. However, nodule respiration occurs in large part in the plant host cell, where AP is essential. Nevertheless, the respiratory consumption of O2 cannot account for much of the well-known variability of the O2-diffusion barrier. Numerous studies have provided evidence that diffusion is regulated by physical changes in the intercellular spaces of the nodule periphery (Dakora and Atkins, 1989; James et al., 1991; Atkins et al., 1993). These physical changes alter the rate at which O2 diffuses into the nodule interior such that the interior concentration of O2 remains unaffected by changes in the external concentration of O2.
In short-term studies (minutes to hours), this compensation does not involve any changes in the observed rates of CO2 evolution, indicating that a variable rate of respiratory O2 consumption does not account for differences in rates of O2 entry into the nodule interior and that there is no respiratory protection of nitrogenase (Sheehy et al., 1983; Hunt et al., 1987; Weisz and Sinclair, 1987; Dakora and Atkins, 1989). However, the exposure of nodules to supra-ambient levels of O2 does result in a 194% increase in the rate of CO2 evolution after 5 d (Dalton et al., 1991). This long-term response also involves a concomitant increase of 81% in the activity of AP and a 53% increase in ascorbate concentration. The observations presented here regarding enhanced antioxidants in the nodule periphery further implicate a role of respiration in controlling O2 entry into nodules, and support the suggestion of Minchin et al. (1992) that the diffusion barrier contains a respiration component that reflects respiratory activity of nodule parenchyma and vascular bundles.
Respiratory O2 consumption at the nodule endodermis (indeterminate nodules) or nodule parenchyma (determinate nodules) could be an important component of the diffusion barrier that operates in conjunction with poorly understood changes in intercellular spaces to provide for maximum regulation of O2 entry into the nodule interior. Although protective respiration does not appear to be involved in short-term variation in O2 diffusion, respiration is almost certainly important as a baseline defense and as a component of long-term variability. Respiratory activity is high at the endodermis and nodule parenchyma, where supplies of O2 and photosynthate are maximal. Enhanced respiration requires enhanced antioxidant defenses, as shown by the elevated levels of ascorbate and AP. Therefore, AP and related antioxidants play a critical, supporting role in maintaining the microaerobic conditions required to prevent the inactivation of nitrogenase in the infected, central region of nodules.
ACKNOWLEDGMENTS
Technical support, assistance, and advice were provided by Dr. Vince Franceschi and Chris Davitt, Electron Microscopy Center, Washington State University, Pullman, and Dr. Maria Herrero, Servicio de Investigación Agraria, Spain. We thank Dr. Frank Minchin for making valuable comments about the manuscript.
Abbreviation:
- AP
ascorbate peroxidase
Footnotes
Work done at Reed College and West Virginia State College was supported by National Science Foundation grant no. IBN-9507491. Additionally, partial support was provided by a grant from the Howard Hughes Medical Institute to the Reed College Biology Department under the 1991 Undergraduate Biological Sciences Initiative. Work done at Consejo Superior de Investigaciones Científicas (Zaragoza) was supported by grant no. PB95-0091 from Dirección General de Enseñanza Superior (Ministry of Education and Culture, Spain).
LITERATURE CITED
- Appleby CA. Leghemoglobin and Rhizobium respiration. Annu Rev Plant Physiol. 1984;35:443–478. [Google Scholar]
- Atkins CA, Hunt S, Layzell DB. Gaseous diffusive properties of soybean nodules cultured with non-ambient pO2. Physiol Plant. 1993;87:89–95. [Google Scholar]
- Brown SM, Walsh KB. Anatomy of the legume nodule cortex: species survey of suberisation and intercellular glycoprotein. Aust J Plant Physiol. 1996;23:211–225. [Google Scholar]
- Chatfield JM, Dalton DA. Ascorbate peroxidase from soybean root nodules. Plant Physiol. 1993;103:661–662. doi: 10.1104/pp.103.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinoy NJ (1984) The Role of Ascorbic Acid in Growth, Differentiation and Metabolism of Plants. Martinus Nijhoff/Dr. Junk, The Hague, The Netherlands, pp 41–50
- Dakora FD, Atkins CA. Diffusion of O2 in relation to structure and function in legume root nodules. Aust J Plant Physiol. 1989;16:131–140. [Google Scholar]
- Dalton DA. Antioxidant defenses of plants and fungi. In: Ahmad S, editor. Oxidative Stress and Antioxidant Defenses in Biology. New York: Chapman & Hall; 1995. pp. 298–355. [Google Scholar]
- Dalton DA, Baird LM, Langeberg L, Taugher CY, Anyan WA, Vance CP, Sarath G. Subcellular localization of oxygen defense enzymes in soybean (Glycine max [L] Merr) root nodules. Plant Physiol. 1993;102:481–489. doi: 10.1104/pp.102.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DA, Diaz del Castillo L, Kahn ML, Joyner SL, Chatfield JM. Heterologous expression and characterization of soybean cytosolic ascorbate peroxidase. Arch Biochem Biophys. 1996;328:1–8. doi: 10.1006/abbi.1996.0135. [DOI] [PubMed] [Google Scholar]
- Dalton DA, Hanus FJ, Russell SA, Evans HJ. Purification, properties and distribution of ascorbate peroxidase in legume root nodules. Plant Physiol. 1987;83:789–794. doi: 10.1104/pp.83.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DA, Post CJ, Langeberg L. Effects of ambient oxygen and of fixed nitrogen on concentrations of glutathione, ascorbate and associated enzymes in soybean root nodules. Plant Physiol. 1991;96:812–818. doi: 10.1104/pp.96.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DA, Russell SA, Hanus FJ, Pascoe GA, Evans HJ. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci USA. 1986;83:3811–3815. doi: 10.1073/pnas.83.11.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison FR. Mathematical modeling of oxygen diffusion and respiration in legume root nodules. Plant Physiol. 1992;98:901–907. doi: 10.1104/pp.98.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogorcena Y, Iturbe-Ormaetxe I, Escuredo PR, Becana M. Antioxidant defenses against activated oxygen in pea nodules subjected to water stress. Plant Physiol. 1995;108:753–759. doi: 10.1104/pp.108.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch AM. Developmental biology of legume nodulation. New Phytol. 1992;122:211–237. doi: 10.1111/j.1469-8137.1992.tb04227.x. [DOI] [PubMed] [Google Scholar]
- Hunt S, King BJ, Canvin DT, Layzell DB. Steady and nonsteady state gas exchange characteristics of soybean nodules in relation to the oxygen diffusion barrier. Plant Physiol. 1987;84:164–172. doi: 10.1104/pp.84.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James EK, Sprent JI, Minchin FR, Brewin NJ. Intercellular location of glycoprotein in soybean nodules: effect of altered rhizosphere oxygen concentration. Plant Cell Environ. 1991;14:467–476. [Google Scholar]
- Klapheck S. Homoglutathione: isolation, quantification and occurrence in legumes. Physiol Plant. 1988;74:727–732. [Google Scholar]
- Li X, Franceschi VR, Okita TW. Segregation of storage protein mRNAs on the rough endoplasmic reticulum membranes of rice endosperm cells. Cell. 1993;72:869–879. doi: 10.1016/0092-8674(93)90576-c. [DOI] [PubMed] [Google Scholar]
- Ljones T, Burris RH. ATP hydrolysis and electron transfer in the nitrogenase reaction with different combinations of the iron protein and the molybdenum-iron protein. Biochim Biophys Acta. 1972;275:93–101. doi: 10.1016/0005-2728(72)90027-8. [DOI] [PubMed] [Google Scholar]
- Minchin FR, Iannetta PPM, Fernandez-Pascual M, De Lorenzo C, Witty JF, Sprent JI. A new procedure for the calculation of oxygen diffusion resistance in legume nodules from flow-through gas analysis data. Ann Bot. 1992;70:283–289. [Google Scholar]
- Parsons R, Day DA. Mechanism of soybean nodule adaptation to different oxygen pressures. Plant Cell Environ. 1990;13:501–512. [Google Scholar]
- Puppo A, Monny C, Davies MJ. Glutathione-dependent conversion of ferryl leghaemoglobin into the ferric form: a potential protective process in soybean (Glycine max) root nodules. Biochem J. 1993;289:435–438. doi: 10.1042/bj2890435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sánchez-Fernández R, Fricker M, Corben LB, White NS, Sheard N, Leaver CJ, Van Montagu M, Inzé D, May MJ. Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proc Natl Acad Sci USA. 1997;94:2745–2750. doi: 10.1073/pnas.94.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy JE, Minchin FR, Witty JF. Biological control of the resistance to oxygen flux in nodules. Ann Bot. 1983;52:565–571. [Google Scholar]
- Tjepkema JD, Yocum CS. Measurement of oxygen partial pressure within soybean nodules by oxygen microelectrodes. Planta. 1974;119:351–360. doi: 10.1007/BF00388335. [DOI] [PubMed] [Google Scholar]
- Van Cauwenberghe OR, Newcomb W, Canny MJ, Layzell DB. Dimensions and distribution of intercellular spaces in cryo-planed soybean nodules. Physiol Plant. 1993;89:252–261. [Google Scholar]
- Weisz PR, Sinclair TR. Regulation of soybean nitrogen fixation in response to rhizosphere oxygen. I. Role of nodule respiration. Plant Physiol. 1987;84:900–905. doi: 10.1104/pp.84.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty JF, Minchin FR, Skøt L, Sheehy JE. Nitrogen fixation and oxygen in legume root nodules. Oxf Surv Plant Mol Cell Biol. 1986;3:275–314. [Google Scholar]
- Witty JF, Skøt L, Revsbech NP. Direct evidence for changes in the resistance of legume root nodules to O2 diffusion. J Exp Bot. 1987;38:1129–1140. [Google Scholar]