Abstract
Background
In settings with high tuberculosis prevalence, 15–30% of HIV-infected individuals initiating antiretroviral therapy (ART) have undiagnosed tuberculosis. Such patients are usually screened by symptoms and sputum smear, which have poor sensitivity.
Objective
To project the clinical and economic outcomes of using Xpert MTB/RIF(Xpert), a rapid tuberculosis/rifampicin-resistance diagnostic, to screen individuals initiating ART.
Design
We used a microsimulation model to evaluate the clinical impact and cost-effectiveness of alternative TB screening modalities -in all patients or only symptomatic patients - for hypothetical cohorts of individuals initiating ART in South Africa (mean CD4 171/μL; tuberculosis prevalence 22%). We simulated no active screening and four diagnostic strategies: 1) smear microscopy (sensitivity 23%); 2) smear and culture (sensitivity, 100%); 3) one Xpert sample (sensitivity in smear-negative tuberculosis: 43%); 4) two Xpert samples (sensitivity in smear-negative tuberculosis: 62%). Outcomes included projected life expectancy, lifetime costs (2010 USD), and incremental cost-effectiveness ratios (ICERs). Strategies with ICERs <$7,100 (South African gross domestic product per capita) were considered very cost-effective.
Results
Compared with no screening, life expectancy in tuberculosis-infected patients increased by 1.6 months using smear in symptomatic patients and by 6.6 months with 2 Xpert samples in all patients. At 22% tuberculosis prevalence, the ICER of smear for all patients was $2,800/year of life saved (YLS), and of Xpert (2 samples) for all patients was $5,100/YLS. Strategies involving one Xpert sample or symptom screening were less efficient.
Conclusions
Model-based analysis suggests that screening all individuals initiating ART in South Africa with two Xpert samples is very cost-effective.
Keywords: tuberculosis, HIV, cost-effectiveness, diagnostics, antiretroviral therapy
INTRODUCTION
An estimated 350,000 HIV-infected individuals die annually from tuberculosis (TB), an infection for which treatment is widely available throughout the world [1]. While antiretroviral therapy (ART) markedly reduces the risk of developing TB disease, many individuals in high incidence settings develop active TB prior to ART initiation. In high TB-burden countries, up to 30% of individuals referred for ART have unrecognized active TB, which might readily be diagnosed through routine, systematic investigation [2,3]. Symptom screening alone fails to identify 10–20% of sputum culture-positive cases in this population [4]. Sputum smear microscopy has been the mainstay of TB screening, but performs poorly in these immunocompromised patients, because of lower bacillary load in sputa [2,3].
Recently, Xpert MTB/RIF (hereinafter Xpert), a novel PCR-based diagnostic, demonstrated good sensitivity and specificity for diagnosis of TB in international, multi-center validation studies [5,6]. However, Xpert has lower sensitivity among individuals with negative sputum smears, and between 70% and 90% of individuals initiating ART with undiagnosed, culture-positive TB are smear-negative [2,3]. As a result, the sensitivity of Xpert as a routine screening tool in these patients is lower than when investigating overt TB ‘suspects’ [7]. Additionally, costs of Xpert are thought to present a substantial barrier – and represent a logistical concern – to its widespread use in resource-limited settings [8].We modeled the clinical outcomes and cost-effectiveness of Xpert compared with alternative TB screening approaches in patients – with and without TB-associated symptoms—initiating ART in peri-urban South Africa.
METHODS
Overview and Analytic Framework
We modified the previously published Cost-Effectiveness of Preventing AIDS Complications (CEPAC) International model of HIV infection and treatment [9,10] to incorporate the natural history, diagnosis, and treatment of TB in HIV-infected individuals. In a simulated cohort of ART-naïve HIV-infected individuals initiating treatment, we compared no TB screening along with 8 diagnostic strategies: sputum smear microscopy (2 concurrent samples), smear and culture (2 concurrent samples), and either one or two concurrent samples of sputum tested by Xpert MTB/RIF where the strategies involving two samples were deemed positive if either test was positive; each diagnostic strategy was evaluated for use in TB-symptomatic patients only or in all patients irrespective of TB-related symptoms.
We projected the life expectancy and direct costs of care for a cohort of individuals initiating ART using these alternative TB-screening strategies. Future costs and benefits were discounted at 3% per year. We used incremental cost-effectiveness ratios (ICERs) in 2010 U.S. dollars per year of life saved ($/YLS), defined as the additional cost, divided by the additional benefit, of a diagnostic strategy compared with the next less expensive strategy. Strategies with a higher cost and lower life expectancy, or strategies with a lower cost but higher cost-effectiveness ratio, were considered ‘dominated strategies’ and were eliminated from further comparisons [11]. We considered strategies to be very cost-effective if their ICERs were below the yearly per capita Gross Domestic Product (GDP) of South Africa ($7,100 in 2010) and cost-effective if they were below three times the per capita GDP ($21,300) [12].
We denote strategies below according to the diagnostic followed by the population; for example, performing 2 smears among symptomatic patients is denoted ‘Smear-2-Symptoms’. The number following ‘Xpert’ refers to how many samples are performed in the strategy (e.g. ‘Xpert-2-All’ denotes 2 Xpert samples performed in all patients, regardless of symptoms).
Model and Assumptions
The CEPAC International model is a first-order, Monte Carlo microsimulation model of HIV and TB natural history and treatment in resource-limited settings. In brief, a simulated cohort of individuals with HIV enters the model at the time of initial ART evaluation and progresses through health states, according to predefined probabilities determined by CD4 count, HIV RNA level, and history of opportunistic infection. Health states reflect use of ART, level of immunosuppression, presence and history of opportunistic infections, treatment of these infections, drug toxicities, and costs of care. Decisions about ART initiation and ART switches are pre-specified according to rules that depend on CD4 count and history of opportunistic infections, consistent with South African national policies [13]. Simulated individuals accrue monthly costs of care that include clinic visits, hospitalizations, laboratory monitoring and pharmaceutical costs. Additional model details are in the online Appendix.
With respect to TB, individuals may have no infection, latent infection or active disease; infections may be with drug susceptible or multidrug-resistant (MDR) strains. Upon TB diagnosis, patients may receive first-line therapy, a standardized retreatment regimen, or a second-line regimen. The probability of treatment success is determined by the drug resistance of the patient’s isolate and the regimen used [14]. In the model, untreated or unsuccessful regimens put patients at increased risk of mortality attributable to TB. ART reduces mortality from TB and other opportunistic infections and is applied in the model as a reduced risk to the monthly TB mortality probability (Table 1) [15].
Table 1.
Cohort description and selected natural history, treatment and diagnostic model parameters for a model of TB screening strategies in South Africa.
| Parameter | Base Case Value | References |
|---|---|---|
| Baseline cohort characteristics | ||
| Mean age (SD), years | 34 (7) | [7] |
| Women, % | 65.4 | [7] |
| Mean CD4 count (SD), cells/μl | 171 (60) | [7] |
| History of TB treatment, % | 26.5 | [7] |
| Current active TB, % | 22.0 | [3,7] |
| MDR prevalence among previously untreated, % | 3.3 | [19] |
| MDR prevalence among previously treated, % | 7.7 | [19] |
| Natural history and treatment | ||
| Monthly mortality probability with untreated TB | 0.086 | see Appendix |
| Relative risk of TB mortality on ART | 0.44 | [15],see Appendix |
| Monthly probability of relapse after cure | 0.004 | [32] |
| Probability of cure, DS TB (FLD) | 0.8 | [33] |
| Probability of cure, MDR TB (FLD) | 0 | [14] |
| Probability of cure, MDR TB (SLD), no treatment history | 0.68 | [34] |
| Probability of cure, MDR TB (SLD), after treatment failure | 0.55 | [34–36] |
| HIV RNA suppression on ART | 75% at 24 weeks | [37] |
| Diagnostic parameters | ||
| Sensitivity, WHO symptom screen | 84% | [7] |
| Specificity, WHO symptom screen | 33% | [7] |
| Sensitivity, smear | 23% | [3,7] |
| Specificity, smear | 100% | [7] |
| Sensitivity, Xpert for TB (smear positive patients) | 99% | [7] |
| Sensitivity, Xpert for TB (smear negative patients) | 43% | [7] |
| Sensitivity, Xpert for TB (smear negative patients, 2 specimens) | 62% | [7] |
| Specificity, Xpert for TB | 99% | [7] |
| Sensitivity, Xpert for rifampin resistance | 98% | [5–7] |
| Specificity, Xpert for rifampin resistance | 94% | [7] |
| Sensitivity, 2 cultures for TB | 100% | Assumption |
| Specificity, 2 culture for TB | 99% | Assumption |
| Months delay for culture result | 1 | [7] |
| Months delay for diagnosis in smear-negative patients* | 2 | [6,17] |
| Costs of diagnostics (2010 US dollars) | ||
| Smear microscopy | $4.60 | [23] |
| Liquid culture | $14.90 | [23] |
| First-line drug susceptibility testing | $73.20 | [23] |
| Xpert MTB/RIF | $21.60 | [23,24] |
| Costs of Drugs (2010 US dollars/month) | ||
| First-line ARV drugs† | $16.70 | [38] |
| Second-line ARV drugs† | $45.80 | [38] |
| First-line TB therapy | $6.60 | see Appendix |
| Second-line TB therapy | $140.00 | see Appendix |
Assuming no other diagnostic performed (see Appendix for further details).
Excludes costs of clinic visits and laboratory monitoring
SD: standard deviation; MDR: multidrug-resistant; DS: drug-susceptible; FLD: first-line drugs; SLD: second-line drugs; TB: tuberculosis; ART: antiretroviral therapy
Individuals diagnosed by smear microscopy are initiated on first-line drugs if they have no TB history or a standard retreatment regimen if they have a TB history and have been previously treated [16]. Patients diagnosed by Xpert or culture and drug susceptibility testing are started on second-line drugs if they are identified as having drug-resistant TB, otherwise they are treated with first-line or retreatment regimens. False positive and false negative TB tests are included in all strategies. Additionally, false positive and false negative rifampin resistance tests are simulated in the Xpert strategy. Individuals diagnosed as not having rifampicin resistance are initiated on first-line drugs or standardized re-treatment, according to prior TB history. Given reports of false-positive rifampicin resistance results [7], individuals diagnosed as having rifampin resistance by Xpert have confirmatory culture and drug susceptibility testing (DST); those found to have rifampin susceptibility by culture/DST are switched from second-line drugs back to first-line drugs after a one-month delay.
We assume that two sputum specimens are obtained for the smear microscopy and culture strategies. Smear and Xpert results are available on the same day, while culture/DST results are available after one month. We assume treatment is initiated immediately upon diagnosis, but that individuals may default from treatment under any strategy. Sixteen percent of smear-negative individuals awaiting culture are lost prior to treatment, accounting for their incomplete return (Appendix). In individuals with tuberculosis who are smear or Xpert negative, some would be treated empirically, while diagnosis may be substantially delayed in others. To account for these extremes, we assume on average a two-month delay in diagnosis due to false-negative results of smear-negative or Xpert-negative TB, if no other tests are performed, based on results of two studies [6,17]. In strategies where symptomatic patients only were screened, asymptomatic patients are subject to this two-month delay in diagnosis. We assume individuals initiated ART one month after initial evaluation.
Input Data
The model is populated with HIV natural history, treatment, and cost data from the Cape Town AIDS Cohort [9,18]. For cohort characteristics and parameters related to TB diagnosis, we use primary data from a prospective TB screening study among individuals initiating ART in a peri-urban township near Cape Town [7]. We simulate an ART-naïve cohort of patients with mean age of 34 years (standard deviation, 7 years) and mean CD4 count of 171/μl (standard deviation, 60/μl); 26.5% of individuals have a history of TB treatment (Table 1). The undiagnosed TB prevalence is 22.0%, consistent with the aforementioned study and an additional screening study among individuals initiating ART in the same setting [3,7]. MDR prevalence among individuals with active TB is 3.3% among previously untreated and 7.7% among previously treated individuals [19].
In this cohort, 84% of individuals with TB and 67% of individuals without TB have a positive WHO symptom screen, defined as one or more of: current cough, fever, night sweats or weight loss [7,20]. We use sputum culture as the gold standard for TB diagnosis. Compared with culture, the sensitivity of smear is 23%, based on pooled analysis of two screening studies in this immunocompromised population [3,7]. The sensitivity of Xpert ranges from 43% (smear-negative, one sample), to 62% (smear-negative, two samples), to 99% (smear-positive, one or two samples) [7]. A detailed description of data sources for TB and HIV-specific model parameters is available in the Appendix.
Costs
We use a micro-costing approach to estimate costs of care for both HIV and TB. Healthcare utilization data for HIV were from the Cape Town AIDS Cohort [9,18]. Unit costs for inpatient hospitalization, outpatient visits, laboratory monitoring and pharmaceutical costs are from previous studies in South Africa [21]. To simulate TB treatment costs, we use antimycobacterial costs from a provincial TB hospital and resource utilization costs from previous studies [22]. Costs of ART and TB drug toxicities are from prior studies (see Appendix). TB diagnostic costs are derived from the South African National Health Laboratory Service and include labor and materials costs [23]. Cost of a single smear is $4.60, culture is $14.90, and first-line drug susceptibility testing is $73.20. Xpert cost is $21.60 per test. Antiretroviral therapy costs are in Table 1.
Sensitivity Analysis
We performed sensitivity analysis on key parameters by varying each parameter over broad ranges of plausible values, supported by the literature where possible, and assessing the impact on the results (Appendix). To evaluate the spectrum of decreasing test costs over time and increases in costs that might be applied in alternative settings, we varied costs over a broad range. We performed two-way sensitivity analysis by varying two parameters at a time and assessing which strategy conferred the greatest life expectancy using a willingness-to-pay threshold of $7,100/YLS for each parameter pair. Additionally, we examined the potential cost-effectiveness of an additional hypothetical diagnostic with increased sensitivity and associated increased cost, compared with the most effective strategy in the model. We assumed that such a diagnostic, like Xpert, provided results on the same day.
RESULTS
Clinical Impact and Cost-effectiveness
With no TB screening at ART initiation, projected undiscounted life expectancy among individuals with TB was 116.2 months (discounted, 90.6 months). Compared with no screening in a cohort with 22% undiagnosed TB prevalence, all strategies increased life expectancy (Table 2). For those with active TB, Smear-2-Symptoms conferred the smallest gain in life expectancy (undiscounted, 2.2 life months; discounted, 1.6 months;) compared with no screening, while the most effective strategy (Xpert-2-All) conferred an average gain of 8.9 life months (discounted, 6.6 months). On a population basis, Xpert-2-All increased projected undiscounted life expectancy from 151.7 to 153.7 months, a gain of 2.0 life months (discounted, 1.5 months).
Table 2.
Clinical impact, costs, and incremental cost-effectiveness of TB screening strategies among individuals initiating ART in South Africa.
| Strategy | TB-infected LE (months) | Population LE (months) | Lifetime Cost (2010 USD) | ICER ($/YLS) | ||
|---|---|---|---|---|---|---|
| undiscounted | discounted | undiscounted | discounted | |||
| No Screening | 116.2 | 90.6 | 151.7 | 117.0 | 31,240 | -- |
|
| ||||||
| Smear-2-Symptoms* | 118.4 | 92.3 | 152.2 | 117.4 | 31,320 | 2,600 |
|
| ||||||
| Smear-2-All | 118.8 | 92.6 | 152.3 | 117.5 | 31,340 | 2,800 |
|
| ||||||
| Culture-2-Symptoms | 121.4 | 94.5 | 152.9 | 117.9 | 31,520 | dominated† |
|
| ||||||
| Culture-2-All | 122.4 | 95.3 | 153.1 | 118.1 | 31,580 | 5,100‡ |
|
| ||||||
| Xpert-1-Symptoms | 122.3 | 95.2 | 153.1 | 118.0 | 31,610 | dominated† |
|
| ||||||
| Xpert-1-All | 123.4 | 96.0 | 153.3 | 118.2 | 31,670 | dominated† |
|
| ||||||
| Xpert-2-Symptoms | 123.7 | 96.2 | 153.4 | 118.3 | 31,690 | dominated† |
|
| ||||||
| Xpert-2-All | 125.1 | 97.3 | 153.7 | 118.5 | 31,770 | 5,100‡ |
Costs in 2010 U.S. dollars and are discounted. LE: life expectancy. ICER: incremental cost-effectiveness ratio. YLS: year of life saved. Culture strategies included smear microscopy and drug susceptibility testing on cultures.
Numbers in the table have been rounded; small deviations in individual calculations may be due to rounding.
Format of strategies is test-number-population, such that Smear-2-Symptoms refers to two smears in symptomatic individuals, etc.
‘dominated’: strategies with lower life expectancy and higher cost, or a higher ICER than a more effective strategy.
ICER for Culture-2-All ($5,060/YLS) is less than ICER for Xpert-2-All ($5,140/YLS), but appears same due to rounding.
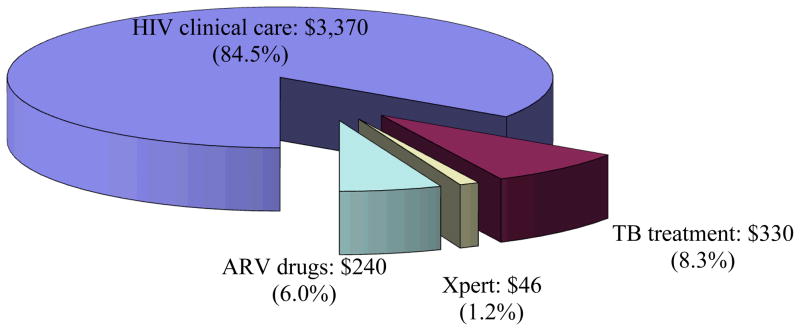
The discounted lifetime costs of care ranged from $31,240 with no screening to $31,770 under the most costly strategy (Xpert-2-All). Average, per person direct healthcare costs in the first year on ART were $3,990 under the Xpert-2-All strategy. With all strategies, HIV care accounted for the majority of costs, followed by TB treatment costs. For example, for the Xpert-2-All strategy, 84.5% of first-year costs (84.6% of total costs) were for HIV clinical care, 6% of first-year costs were for ART drugs (12.5% of total costs), 8.3% of first-year costs (2.7% of total costs) were for TB treatment, and 1.2% of first-year costs (0.2% of total costs) were for Xpert (Figure 1).
Figure 1. Component costs of care for the first year after screening.
Breakdown of the first year of health care costs for an individual initiating ART in South Africa in the Xpert-2-All strategy, a time frame which total costs may be compared for some budgetary purposes. Total per person costs were $3,990. TB: tuberculosis. ARV: antiretroviral.
Compared with no screen, Smear-2-Symptoms and Smear-2-All were very cost-effective, with incremental cost-effectiveness ratios of $2,600/YLS and $2,800/YLS. Compared with Smear-2-All, Culture-2-All had an incremental cost-effectiveness ratio of $5,100/YLS. Xpert-2-All had an incremental cost-effectiveness ratio of $5,100/YLS compared with Culture-2-All and was very cost-effective. Xpert-2-All had greater effectiveness with a lower incremental cost-effectiveness ratio than Culture-2-Symptoms, Xpert-1-All or Xpert-2-Symptoms, which were therefore dominated.
Sensitivity Analyses
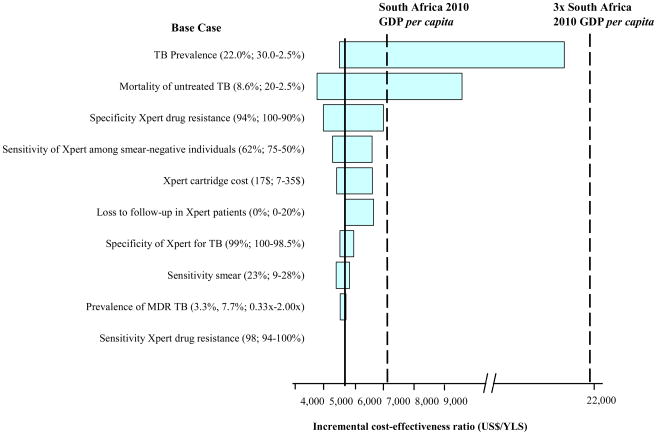
In one-way sensitivity analyses, the most influential parameter on the results was undiagnosed TB prevalence (Figure 2). Xpert-2-All was very cost-effective unless the prevalence of TB was below 7.5% and was cost-effective (<3x GDP) unless the prevalence was below 1%. The cost-effectiveness of Xpert-2-All was moderately sensitive to the mortality from untreated TB and loss-to-follow-up in patients providing samples for Xpert. Xpert-2-All was very cost-effective across all ranges examined for the prevalence of MDR TB and the sensitivity of Xpert in smear-negative individuals.
Figure 2. One-way sensitivity analysis of model parameters.
One-way sensitivity analysis comparing the impact of key model parameters on the incremental cost-effectiveness ratio (ICER) of the Xpert-2-all strategies, compared to the next best, non-dominated strategy. The x-axis is the ICER. Each horizontal bar represents a parameter varied over the range indicated; wider bars indicate larger differences in the ICER seen by varying the parameter. ‘x’ following a number denotes a multiplicative effect on the baseline value of parameter.
MDR: Multidrug-resistant; DS: Drug-susceptible; FLD: First-line drugs; SLD: Second-line drugs; YLS: year of life saved.
In sensitivity analysis on the time to diagnosis among individuals who were smear or Xpert negative, at a time to diagnosis of 1 month, both culture strategies were dominated by Xpert. At a time to diagnosis of 3 months, both smear strategies were dominated by culture, and both culture strategies were cost-effective (Culture-2-Symptoms, ICER: $4200/YLS; Culture-2-All, ICER: $4800/YLS). Xpert-2-All remained very cost-effective, though the ICER was increased ($6700/YLS).
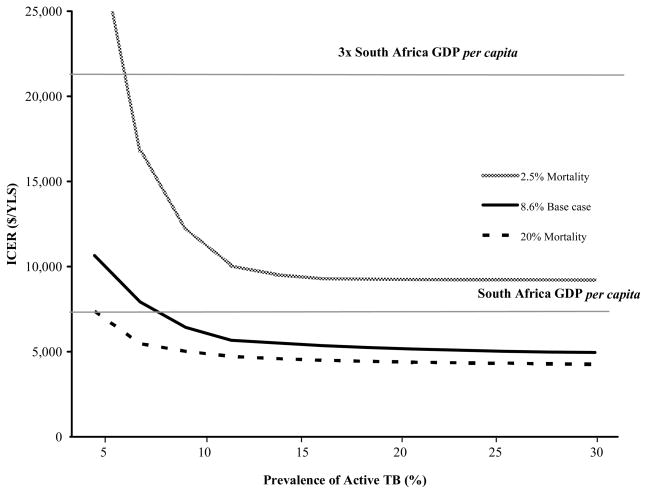
In two-way sensitivity analysis, we examined the impact of varying the mortality of untreated tuberculosis and the prevalence of TB (Figure 3). For the base case mortality estimate of 8.6% per month, Xpert was very cost-effective at a TB prevalence > 8%. For TB mortality of 20%, Xpert was very cost-effective at a TB prevalence > 5%. Xpert was cost effective (but not very-cost effective) at monthly TB mortality > 2.5%, as long as TB prevalence was >7%.
Figure 3. Two-way sensitivity analysis of TB prevalence and mortality of untreated TB on the incremental cost-effectiveness ratio (ICER) for Xpert.
The lower horizontal line indicates the 2010 per capita GDP of South Africa ($7,100), which the WHO defines as ‘very cost-effective’ (see Methods). The upper horizontal line indicates three times the per capita GDP of South Africa ($21,300), which is considered a ‘cost-effective’ intervention.
When a hypothetical diagnostic with higher sensitivity and increased cost was included in the analysis (e.g. adding a 3rd Xpert or another novel test), it was cost-effective at a willingness-to-pay threshold of $7,100/YLS, even at substantially increased costs for moderately increased sensitivity (Appendix, Figure A6). For example, at a sensitivity of 75% (compared to Xpert-2-All sensitivity of 62%), a new diagnostic would be very cost-effective even if it cost up to $96 per test.
DISCUSSION
HIV-infected individuals initiating ART in South Africa have extraordinarily high rates of TB, much of which is undiagnosed by the currently recommended symptom screen and smear microscopy [2,3,7]. Routine screening of these patients with Xpert MTB/RIF can identify a substantial proportion of these cases, potentially averting both deaths and TB transmission. Despite concerns regarding the cost of this new diagnostic test, using a model of HIV/TB co-infection, we found routine screening of individuals initiating ART in South Africa with Xpert to be very cost-effective.
Among the major questions surrounding TB screening for individuals initiating ART is whether symptom screening should be used first to identify individuals who would benefit most from the diagnostic. Although symptom screening in this population has had variable reported sensitivity and specificity for TB [2], the WHO continues to advocate symptom screening. Our results demonstrate that the Xpert strategies were cost-effective when screening all individuals compared with screening symptomatic individuals only. In the absence of a better screening tool, these results suggest that Xpert should be used to screen all patients starting ART in South Africa, regardless of symptoms. Similarly, obtaining two samples for Xpert conferred more benefit than one sample and was highly cost-effective, showing that the added sensitivity of a second test is worth the cost.
The results supporting screening all patients, regardless of symptoms, as well as using two tests, were robust to assumptions about the diagnostic performance, undiagnosed TB prevalence, and TB mortality across a broad range of published values. Moreover, the costs of Xpert, which have received considerable emphasis in the discussion about feasibility of its widespread use [24], had little impact on cost-effectiveness. These findings have been previously described with other diagnostic tests that carry one-time costs [25]. Costs of Xpert here accounted for only 1.2% of the costs of the care in the first year for these patients; the increasing costs associated with use of Xpert were predominantly the costs of treating TB and HIV disease, in individuals whose life expectancy was increased by the better diagnostic. A consequence of this finding is that more costly one-time diagnostics would remain cost-effective across plausible increases in sensitivity. We found that increasing the test sensitivity among smear-negative individuals to 75% would be very-cost effective even if the diagnostic cost were $96. Whether such increased sensitivity could be achieved through repeated specimens of Xpert, using Xpert on other body fluids or tissues, or a different novel diagnostic, is an important area for further research. Given the high prevalence and mortality associated with TB in individuals initiating ART, diagnostics that perform well in this population will likely be highly cost-effective even at substantial cost.
These results suggest that, wherever possible, symptom screening and smear microscopy should be replaced by culture or Xpert, and efforts should be undertaken to expand access to these diagnostics for use in all patients initiating ART. While culture and Xpert provided similar value for money, the pace at which access to culture and drug-susceptibility testing may be expanded is severely limited by substantial laboratory infrastructure requirements. In contrast, Xpert requires few additional laboratory or human resources compared with smear and appears to pose lower infectious risk than smear preparation [26]. The South African National Health Laboratory System plans to roll out over a hundred Xpert devices to its laboratories, considerably expanding access to this diagnostic. Deployment of Xpert to ART clinics would further minimize delays in TB diagnosis, immediately address a highly vulnerable population, and maximize the benefits of its rapid results.
With the increasing burden of MDR TB in South Africa, expanded screening for rifampin resistance by Xpert may result in improved case detection of drug resistant strains. This early diagnosis may reduce mortality, though costs of MDR TB treatment, compared to drug-susceptible TB, remain considerably higher. In sensitivity analysis, we found that increasing prevalence of MDR TB resulted in a higher ICER for Xpert, suggesting that increased diagnosis, MDR TB treatment, and life expectancy were associated with substantial costs.
The results of this study should be interpreted within the limitations of model parameters and assumptions. We assumed that individuals diagnosed with TB by smear or Xpert initiated treatment immediately; pretreatment delays or loss to follow-up may be considerable and would diminish the benefit of rapid diagnostics. We assumed that sputum culture was the gold standard for TB diagnosis, though use of sputum cultures may fail to diagnose cases of extrapulmonary TB. In addition to model-specified mortality due to opportunistic infections, we included substantial unspecified mortality due to AIDS obtained from natural history cohort data; some of this may have been from undiagnosed extrapulmonary TB; this would make our Xpert even more cost-effective. There are few published data on mortality from untreated TB in individuals with HIV. We utilized primary data from a prospective screening study, but deaths were few. However, the findings were robust to estimates of untreated TB mortality. Moreover, the mortality value we utilized was lower than in other observational cohorts [27,28], suggesting our projections of the benefits of early TB diagnosis may also be conservative.
We did not include in this analysis the benefits of earlier diagnosis on reducing further TB transmission. Since the benefits of Xpert are predominantly accrued among smear-negative patients [29], the reduction in transmission may be modest. For MDR TB, the impact of early diagnosis may be more substantial. However, any additional benefits with respect to reduction of transmission would make Xpert even more cost-effective.
While this analysis utilized epidemiologic and resource utilization data from South Africa, results may be generalizable to countries with lower GDPs per capita, since the largest proportion of cost was the cost of HIV care, which is lower in other settings than in South Africa. The cost-effectiveness of the Xpert strategies was comparable to that of ART in South Africa, suggesting that if ART is considered cost-effective, using Xpert to screen individuals, in settings where the prevalence of TB among individuals initiating ART is high, would likewise be cost-effective.
A recent cost-effectiveness analysis of Xpert for tuberculosis diagnosis similarly found it to be cost-effective in comparison with smear; however, ART and other HIV-related costs were excluded [30]. We found HIV-related costs to be critically important. Additionally, our analysis focused on tuberculosis screening of all patients initiating ART, rather than diagnosis in only symptomatic patients presenting to clinics.
The World Health Organization has endorsed the use of Xpert to complement smear microscopy and culture and provided guidance on the infrastructure and resources required to incorporate Xpert into clinical laboratories [31]. While our analysis suggests that Xpert will be cost-effective for screening individuals initiating ART, further studies will be needed to evaluate costs and operational challenges toward implementation of this diagnostic.
Symptom screening and sputum microscopy perform poorly in individuals starting ART in high TB-prevalence settings, but limited laboratory capacity for culture and a dearth of sensitive, rapid TB diagnostics has left clinicians with few alternatives. With the advent of Xpert MTB/RIF, microbiologic screening of individuals initiating ART is now available. In South Africa and other settings with high prevalence of TB in those with HIV, our findings suggest that screening all individuals initiating ART with Xpert MTB/RIF is very cost-effective and should become the standard of care.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by the National Institute of General Medical Sciences (U54 GM088558), the National Institute of Allergy and Infectious Diseases (R01 AI058736 and K01 AI074495), the AIDS Clinical Trials Group (U01 AI068636), the Wellcome Trust (SDL; #088590/Z/09) and the Doris Duke Charitable Foundation (Clinical Scientist Development and ORACTA Awards). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, which played no role in the study design, methods, interpretation of results, the content of this manuscript, or the decision to submit it for publication.
References
- 1.World Health Organization. Global Tuberculosis Control 2011. Geneva: 2011. [Accessed 9 January 2012]. Available: http://www.who.int/tb/publications/global_report/en/index.html. [Google Scholar]
- 2.Bassett IV, Wang B, Chetty S, Giddy J, Losina E, et al. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clinical Infectious Diseases. 2010;51:823–829. doi: 10.1086/656282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawn SD, Kranzer K, Edwards DJ, McNally M, Bekker L-G, et al. Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS. 2010;24:1323–1328. doi: 10.1097/QAD.0b013e3283390dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Getahun H, Kittikraisak W, Heilig CM, Corbett EL, Ayles H, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8:e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawn SD, Brooks SV, Kranzer K, Nicol M, Whitelaw A, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Medicine. 2011;8:e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawn SD, Nicol MP. XpertR MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011;6:1067–1082. doi: 10.2217/fmb.11.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walensky RP, Wood R, Ciaranello AL, Paltiel AD, Lorenzana SB, et al. Scaling up the 2010 World Health Organization HIV Treatment Guidelines in resource-limited settings: a model-based analysis. PLoS Med. 2010;7:e1000382. doi: 10.1371/journal.pmed.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walensky RP, Wood R, Weinstein MC, Martinson NA, Losina E, et al. Scaling up antiretroviral therapy in South Africa: the impact of speed on survival. J Infect Dis. 2008;197:1324–1332. doi: 10.1086/587184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunink M, Glasziou P, Siegel J, Weeks J, Pliskin J, et al. Decision making in health and medicine: integrating evidence and values. Cambridge: University Press; 2001. [Google Scholar]
- 12.World Health Organization. Report of the Commission on Macroeconomics and Health. Geneva, Switzerland: 2001. Macroeconomics and Health: Investing in Health for Economic Development. [Google Scholar]
- 13.Department of Health. The South African antiretroviral treatment guidelines. 2010. [Google Scholar]
- 14.Jones-López EC, Ayakaka I, Levin J, Reilly N, Mumbowa F, et al. Effectiveness of the standard WHO recommended retreatment regimen (category II) for tuberculosis in Kampala, Uganda: A prospective cohort study. PLoS Med. 2011;8:e1000427. doi: 10.1371/journal.pmed.1000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawn SD, Kranzer K, Wood R. Antiretroviral therapy for control of the HIV-associated tuberculosis epidemic in resource-limited settings. Clin Chest Med. 2009;30:685–699. viii. doi: 10.1016/j.ccm.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Department of Health. [Accessed 27 September 2011];The South African Tuberculosis Control Programme Practical Guidelines. 2000 Available: http://www.kznhealth.gov.za/chrp/documents/Guidelines/Guidelines%20National/Tuberculosis/SA%20TB%20Guidelines%202004.pdf.
- 17.Dimairo M, MacPherson P, Bandason T, Zezai A, Munyati SS, et al. The risk and timing of tuberculosis diagnosed in smear-negative TB suspects: a 12 month cohort study in Harare, Zimbabwe. PLoS ONE. 2010;5:e11849. doi: 10.1371/journal.pone.0011849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes CB, Wood R, Badri M, Zilber S, Wang B, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42:464–469. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 19.Cox HS, McDermid C, Azevedo V, Muller O, Coetzee D, et al. Epidemic levels of drug resistant tuberculosis (MDR and XDR-TB) in a high HIV prevalence setting in Khayelitsha, South Africa. PLoS One. 2010;5:e13901. doi: 10.1371/journal.pone.0013901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva: World Health Organization; 2010. [Accessed 27 September 2011]. p. Available: http://www.who.int/hiv/pub/tb/9789241500708/en/index.html. [Google Scholar]
- 21.Badri M, Cleary S, Maartens G, Pitt J, Bekker L-G, et al. When to initiate highly active antiretroviral therapy in sub-Saharan Africa? A South African cost-effectiveness study. Antivir Ther (Lond) 2006;11:63–72. [PubMed] [Google Scholar]
- 22.Sinanovic E, Kumaranayake L. Financing and cost-effectiveness analysis of public-private partnerships: provision of tuberculosis treatment in South Africa. Cost Eff Resour Alloc. 2006;4:11. doi: 10.1186/1478-7547-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Health Laboratory Service. Cost Quotation. 2009. [Google Scholar]
- 24.World Health Organization. Roadmap for rolling out Xpert MTB/RIF for rapid diagnosis of TB and MDR-TB. Geneva: World Health Organization; 2010. [Accessed 27 September 2011]. p. Available: www.who.int/tb/laboratory/roadmap_xpert_mtb-rif.pdf. [Google Scholar]
- 25.Sax PE, Islam R, Walensky RP, Losina E, Weinstein MC, et al. Should resistance testing be performed for treatment-naive HIV-infected patients? A cost-effectiveness analysis. Clin Infect Dis. 2005;41:1316–1323. doi: 10.1086/496984. [DOI] [PubMed] [Google Scholar]
- 26.Banada PP, Sivasubramani SK, Blakemore R, Boehme C, Perkins MD, et al. Containment of bioaerosol infection risk by the Xpert MTB/RIF assay and its applicability to point-of-care settings. J Clin Microbiol. 2010;48:3551–3557. doi: 10.1128/JCM.01053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pablos-Méndez A, Sterling TR, Frieden TR. The relationship between delayed or incomplete treatment and all-cause mortality in patients with tuberculosis. JAMA. 1996;276:1223–1228. doi: 10.1001/jama.1996.03540150025026. [DOI] [PubMed] [Google Scholar]
- 28.Kramer F, Modilevsky T, Waliany AR, Leedom JM, Barnes PF. Delayed diagnosis of tuberculosis in patients with human immunodeficiency virus infection. Am J Med. 1990;89:451–456. doi: 10.1016/0002-9343(90)90375-n. [DOI] [PubMed] [Google Scholar]
- 29.Tostmann A, Kik SV, Kalisvaart NA, Sebek MM, Verver S, et al. Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in the Netherlands. Clin Infect Dis. 2008;47:1135–1142. doi: 10.1086/591974. [DOI] [PubMed] [Google Scholar]
- 30.Vassall A, van Kampen S, Sohn H, Michael JS, John KR, et al. Rapid Diagnosis of Tuberculosis with the Xpert MTB/RIF Assay in High Burden Countries: A Cost-Effectiveness Analysis. PLoS Med. 2011;8:e1001120. doi: 10.1371/journal.pmed.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Rapid implementation of the Xpert MTB/RIF diagnostic test. Geneva: 2011. [Accessed 12 January 2012]. Available: whqlibdoc.who.int/publications/2011/9789241501569_eng.pdf. [Google Scholar]
- 32.Golub JE, Durovni B, King BS, Cavalacante SC, Pacheco AG, et al. Recurrent tuberculosis in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2008;22:2527–2533. doi: 10.1097/QAD.0b013e328311ac4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. Global Tuberculosis Control 2010. Geneva, Switzerland: 2010. [Google Scholar]
- 34.Seung KJ, Omatayo DB, Keshavjee S, Furin JJ, Farmer PE, et al. Early outcomes of MDR-TB treatment in a high HIV-prevalence setting in Southern Africa. PLoS ONE. 2009;4:e7186. doi: 10.1371/journal.pone.0007186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brust JCM, Lygizos M, Chaiyachati K, Scott M, van der Merwe TL, et al. Culture conversion among HIV co-infected multidrug-resistant tuberculosis patients in Tugela Ferry, South Africa. PLoS ONE. 2011;6:e15841. doi: 10.1371/journal.pone.0015841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heller T, Lessells RJ, Wallrauch CG, Bärnighausen T, Cooke GS, et al. Community-based treatment for multidrug-resistant tuberculosis in rural KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis. 2010;14:420–426. [PubMed] [Google Scholar]
- 37.Hammond R, Harry TC. Efficacy of antiretroviral therapy in Africa: effect on immunological and virological outcome measures -- a meta-analysis. Int J STD AIDS. 2008;19:291–296. doi: 10.1258/ijsa.2007.007248. [DOI] [PubMed] [Google Scholar]
- 38.Clinton Health Access Initiative. [Accessed 27 September 2011];Antiretroviral (ARV) Price List. 2010 Available: http://www.clintonfoundation.org/files/chai_arv_priceList_april2010_english.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.