Summary
Background
The optimum treatment for high-risk soft-tissue sarcoma (STS) in adults is unclear. Regional hyperthermia concentrates the action of chemotherapy within the heated tumour region. Phase 2 studies have shown that chemotherapy with regional hyperthermia improves local control compared with chemotherapy alone. We designed a parallel-group randomised controlled trial to assess the safety and efficacy of regional hyperthermia with chemotherapy.
Methods
Patients were recruited to the trial between July 21, 1997, and November 30, 2006, at nine centres in Europe and North America. Patients with localised high-risk STS (≥5 cm, Fédération Nationale des Centres de Lutte Contre le Cancer [FNCLCC] grade 2 or 3, deep to the fascia) were randomly assigned to receive either neo-adjuvant chemotherapy consisting of etoposide, ifosfamide, and doxorubicin (EIA) alone, or combined with regional hyperthermia (EIA plus regional hyperthermia) in addition to local therapy. Local progression-free survival (LPFS) was the primary endpoint. Efficacy analyses were done by intention to treat. This trial is registered with ClinicalTrials.gov, number NCT 00003052.
Findings
341 patients were enrolled, with 169 randomly assigned to EIA plus regional hyperthermia and 172 to EIA alone. All patients were included in the analysis of the primary endpoint, and 332 patients who received at least one cycle of chemotherapy were included in the safety analysis. After a median follow-up of 34 months (IQR 20–67), 132 patients had local progression (56 EIA plus regional hyperthermia vs 76 EIA). Patients were more likely to experience local progression or death in the EIA-alone group compared with the EIA plus regional hyperthermia group (relative hazard [RH] 0.58, 95% CI 0.41–0.83; p=0.003), with an absolute difference in LPFS at 2 years of 15% (95% CI 6–26; 76% EIA plus regional hyperthermia vs 61% EIA). For disease-free survival the relative hazard was 0.70 (95% CI 0.54–0.92, p=0.011) for EIA plus regional hyperthermia compared with EIA alone. The treatment response rate in the group that received regional hyperthermia was 28.8%, compared with 12.7% in the group who received chemotherapy alone (p=0.002). In a pre-specified per-protocol analysis of patients who completed EIA plus regional hyperthermia induction therapy compared with those who completed EIA alone, overall survival was better in the combined therapy group (HR 0.66, 95% CI 0.45–0.98, p=0.038). Leucopenia (grade 3 or 4) was more frequent in the EIA plus regional hyperthermia group compared with the EIA-alone group (128 of 165 vs 106 of 167, p=0.005). Hyperthermia-related adverse events were pain, bolus pressure, and skin burn, which were mild to moderate in 66 (40.5%), 43 (26.4%), and 29 patients (17.8%), and severe in seven (4.3%), eight (4.9%), and one patient (0.6%), respectively. Two deaths were attributable to treatment in the combined treatment group, and one death was attributable to treatment in the EIA-alone group.
Interpretation
To our knowledge, this is the first randomised phase 3 trial to show that regional hyperthermia increases the benefit of chemotherapy. Adding regional hyperthermia to chemotherapy is a new effective treatment strategy for patients with high-risk STS, including STS with an abdominal or retroperitoneal location.
Funding
Deutsche Krebshilfe, Helmholtz Association (HGF), European Organisation of Research and Treatment of Cancer (EORTC), European Society for Hyperthermic Oncology (ESHO), and US National Institute of Health (NIH).
Introduction
Adult soft-tissue sarcomas (STS) are an uncommon, but problematic malignancy, with an estimated 10 390 new cases each year in the USA. Half of all patients eventually die as a result of the disease.1,2 The goals of therapy include increasing local control for both extremity lesions and other locations while preserving function and reducing distant metastases. Adjuvant radiotherapy and conservative surgery have significantly improved local control and functional outcome in patients with extremity STS.3,4,5 However, the risk of disease progression is highly dependent on tumour size, histological grade, and depth.6 Attempts to improve outcomes using upfront anthracycline-based and ifosfamide-based chemotherapy or chemoradiotherapy have been confounded either by non-randomised study designs or severe toxicity, and the single randomised phase 2 study of neo-adjuvant chemotherapy done to date failed to show any benefit.7,8,9 Regional hyperthermia has been shown to act synergistically with radiotherapy or chemotherapy.10 In randomised trials combining regional hyperthermia with radiotherapy, loco-regional control and disease-free survival was improved in patients with melanoma, recurrent breast cancer, and cervical cancer.11,12,13
Our previous prospective phase 2 trials showed that neo-adjuvant chemotherapy combined with regional hyperthermia shrank the tumour and prevented early progression, and potentially improves survival in patients with primary, recurrent, or inadequately resected high-risk STS.14,15 We designed the European Society for Hyperthermic Oncology (ESHO) and European Organisation for Research and Treatment of Cancer (EORTC)–Soft Tissue and Bone Sarcoma Group (STBSG) 62961 multicentre, randomised phase 3 study to assess the efficacy and safety of adjuvant chemotherapy with regional hyperthermia in patients with localised high-risk STS.
Methods
Patients
Between July 21, 1997, and November 30, 2006, eligible patients were recruited from nine centres in four countries (six centres in Germany, one in Norway, one in Austria, one in the USA).
Eligible patients were 18 to 70 years of age and had adult-type STS with the following risk criteria: Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grade 2 or 3, tumour diameter 5 cm or more, deep to the fascia, and no evidence of distant metastases. External pathological review was done by SD on behalf of the EORTC. Patients were enrolled with primary or recurrent disease. In patients who had undergone previous surgery (tumour-free margins ≤1 cm or margins contaminated), random allocation to treatment was allowed within 8 weeks of surgery.
Randomisation and masking
Patients were randomly assigned to either chemotherapy consisting of etoposide, ifosfamide, and doxorubicin (EIA) alone, or EIA combined with regional hyperthermia by block randomisation. Block randomisation was done centrally at the EORTC data centre, with stratification according to site (extremity vs non-extremity), presentation of tumour (primary vs recurrent vs prior surgery), and centre. The randomisation was based on a computer-generated list. After informed consent by a suitable patient, the study centre reported relevant information to the EORTC data centre, and treatment allocation was communicated by fax to the study centre. Blinding was not possible due to the nature of the allocated treatment.
Procedures
The protocol was approved by the EORTC and done according to the intergroup procedures of the EORTC in place at that time. Local ethics approval was obtained at all participating institutions, and written and informed consent was obtained for all patients. Blinded interim reports were given during the recruitment period at the annual EORTC-STBSG meetings, and no interim analyses with respect to efficacy were done. The Independent Data Monitoring Committee (IDMC) of the EORTC reviewed the data in June 2004, and advised the trial be continued.
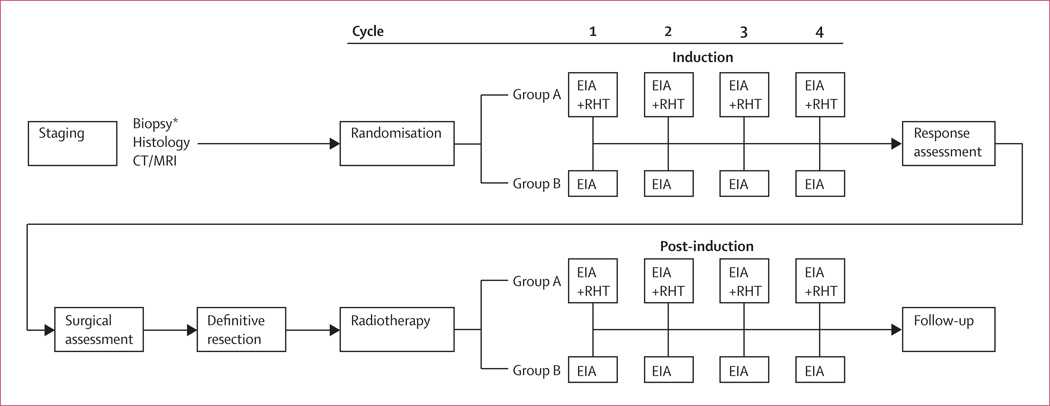
Patients were randomly assigned to either four cycles of chemotherapy (EIA) alone, or to EIA with regional hyperthermia as induction therapy followed by assessment of tumour response by imaging. After the best possible local therapy (surgery and/or radiotherapy), another four cycles of their allocated treatment was given for post-induction therapy. Patients with previous surgery were also assigned to receive the complete induction and post-induction therapy after randomisation (figure 1).
Figure 1. Study design.
*Patients with previous surgery were also assigned to receive the complete induction and post-induction therapy after randomisation. EIA=etoposide+ifosfamide+doxorubicin. RHT=regional hyperthermia.
Cycles of the EIA regimen (etoposide 125 mg/m2 on day 1 and 4, ifosfamide 1500 mg/m2 on days 1–4, and doxorubicin 50 mg/m2 on day 1) were given every 3 weeks unless progressive disease, unacceptable toxic effects, or withdrawal from the study occurred. Dose reductions based on the grade of adverse effects were done according to protocol guidelines.
Regional hyperthermia aiming for tumour temperatures of 42°C for 60 minutes were given on day 1 and 4 of each EIA cycle during both induction and post-induction therapy. Regional hyperthermia and thermal mapping were done according to the ESHO guidelines for quality and safety assurance.16 The BSD-2000 hyperthermia system (BSD Medical Corporation, Salt Lake City, UT, USA) was used. Treatment was stopped or omitted if severe adverse events occurred.
Surgeons were encouraged to definitively resect tumours in all patients within 4–6 weeks of induction therapy, including repeat resection if indicated. In case of early progression, patients were considered for immediate tumour resection (including amputation).
For radiotherapy, if indicated, the dose was defined as 50–60 Gy, with daily fractions of 1.8–2.0 Gy, and a boost up to 66 Gy delivered 4–6 weeks after surgery. The recommended doses were adjusted depending on the individual situation and the neighbouring radiosensitive tissue.
After randomisation, follow-up forms documenting toxicity and recurrence status were required every 3 months during the first year, every 4 months up to 3 years, every 6 months up to 5 years, and yearly thereafter.
The primary outcome was local progression-free survival (LPFS), defined as the time from randomisation to confirmed local progression, relapse, or death, whichever occurred first and irrespective of any occurrence of distant metastases. Patients without confirmed progression, relapse, or death were censored at the time of the last valid assessment. Secondary endpoints were disease-free survival (DFS), overall survival (OS), tumour response after induction therapy, treatment toxicity, and long-term complications.
DFS was defined as the time from randomisation to confirmed local failure, distant metastases, or death due to disease or treatment, whichever occurred first. Overall survival was defined as the time from randomisation to death of any cause, with survivors being censored at the time of last follow-up. Tumour response was assessed after induction therapy using WHO criteria for patients who had measurable disease at baseline. For all responding patients, a blinded independent review was done by board members of the EORTC-STBSG.
Time-averaged tumour temperatures of all regional hyperthermia treatments were used to calculate the maximum (Tmax) temperature and the temperatures achieved in 20%, 50%, and 90% (T20, T50, and T90, respectively), of all measured sites. The method used for tumour temperature measurements during regional hyper thermia treatments has been described in detail previously.17
Adverse events related to chemotherapy were graded according to the Common Toxicity Criteria of the National Cancer Institute (1994). Toxicity related to regional hyperthermia was scored according to protocol guidelines. Based on a sample size of 167 patients per group the study had a power of 80% to detect a hazard ratio (HR) of 0.7, assuming a median LPFS of 43 months in the EIA plus regional hyperthermia group and 30 months in the EIA alone group. A follow-up of 36 months and a total of 209 events were required. An accrual period of 6 years and a follow-up time of 9 years were set.
Statistical analysis
Proportions were compared by χ2 tests, and continuous outcomes by Mann-Whitney-U tests. Time-to-event data were analysed by the Kaplan-Meier method. Differences in survival were assessed by stratified log-rank test. Cumulative incidence functions for the competing risks of local progression, distant failure, and death were calculated, and the effect of treatment regimens was assessed by Gray’s test.18 A two-sided level of significance of 0.05 was applied to all tests.
The confirmative analysis used the proportional-hazards model, stratified by site and presentation of tumours. Sensitivity analyses used a random effect model to adjust for centre heterogeneity.19 Endpoints were also analysed in the patients recruited in the three largest recruiting centres. We assessed treatment differences in selected subgroups by stratified Cox regression20.
To validate former phase 2 results15, a proportional-hazards model stratified by tumour site was calculated in a pre-specified per-protocol analysis of patients with confirmed histology who completed their assigned induction therapy. Patients who discontinued because of early progression were not excluded.
No adjustment for multiple testing was made for secondary endpoints. The safety population consisted of all patients who started induction therapy. All analyses were done using statistical software R (version 2.9.0).21
This trial is registered with ClinicalTrials.gov, number NCT 00003052.
Role of the funding source
The funding source had no influence on the collection, analysis, or interpretation of the data. The corresponding author had full access to all data and the final responsibility to submit the manuscript for publication.
Results
341 patients were randomly assigned to either EIA plus regional hyperthermia (n=169) or EIA alone (n=172) in nine centres, with 310 patients (90.9%) enrolled at three core centres with staff experienced in the use of the technology involved. Median follow-up was 34 months (IQR 20–67). Table 1 shows the baseline demographic and clinical characteristics of patients.
Table 1.
Baseline characteristics of all patients assigned to treatment
| EIA plus RHT (N=169) | EIA (N=172) | |
|---|---|---|
| Median age | ||
| Years (range) | 51.0 (18.0–70.0) | 52.0 (19.0–70.0) |
| Sex | ||
| Male | 95 (56.2) | 94 (54.7) |
| Female | 74 (43.8) | 78 (45.3) |
| WHO performance status | ||
| 0 | 110 (65.1) | 115 (66.9) |
| 1 | 51 (30.2) | 50 (29.1) |
| 2 | 8 (4.7) | 7 (4.1) |
| Site of tumour | ||
| Non-Extremity* | 96 (56.8) | 96 (55.8) |
| Extremity | 73 (43.2) | 76 (44.2) |
| Presentation of tumour | ||
| Primary | 78 (46.2) | 84 (48.8) |
| Recurrent | 19 (11.2) | 18 (10.5) |
| Prior surgery† | 72 (42.6) | 70 (40.7) |
| Size of tumour | ||
| 5.0–7.9 cm | 46 (27.2) | 50 (29.1) |
| 8.0–12.0 cm | 51 (30.2) | 58 (33.7) |
| >12.0 cm | 72 (42.6) | 64 (37.2) |
| Size of tumour (cm) | ||
| Median | 11.0 | 11.3 |
| Range | 5.0–36.0 | 5.0–40.0 |
| Grading | ||
| 2 | 84 (50.0) | 77 (44.8) |
| 3 | 84 (50.0) | 94 (54.7) |
| Pathology | ||
| Liposarcoma‡ | 31 (18.3) | 30 (17.4) |
| Leiomyosacoma | 27 (16.0) | 27 (15.7) |
| Synovial sarcoma | 25 (14.8) | 20 (11.6) |
| Sarcoma NOS | 35 (20.7) | 38 (22.1) |
| Other sarcoma§ | 38 (22.5) | 39 (22.7) |
| Not soft-tissue sarcoma¶ | 2 (1.2) | 5 (2.9) |
| Unreviewed sarcoma | 11 (6.5) | 13 (7.6) |
All data are n (%) unless otherwise specified. EIA=etoposide+ifosfamide+doxorubicin. RHT=regional hyperthermia. NOS=not otherwise specified.
Non-extremity includes tumours localised in the pelvis or abdomen (81%), trunk (18%), and head and neck (1%).
Prior surgery of primary or recurrent tumour with tumour-free margins ≤1 cm or margins contaminated.
All liposarcoma belong to the non-extremity group (G2 16 vs 16; G3 15 vs 14).
Angiosarcoma, rhabdomyosarcoma, fibrosarcoma, myxofibrosarcoma, nerve sheath tumours, gastrointestinal stromal tumours, epitheloid sarcoma, alveolar soft-part sarcoma, extraskeletal myxoid chondrosarcoma, myogenic NOS, haemangiopericytoma or malignant solitary fibrous tumour, Ewing’s sarcoma, myofibrosarcoma.
Anaplastic large-cell lymphoma, solid pseudopapillary neoplasm of the pancreas, pleomorphic T-cell lymphoma, neuroendocrine carcinoma, atypical Burkitt’s lymphoma, giant-cell tumour of tendon sheath, chondrosarcoma (not mesenchymal).
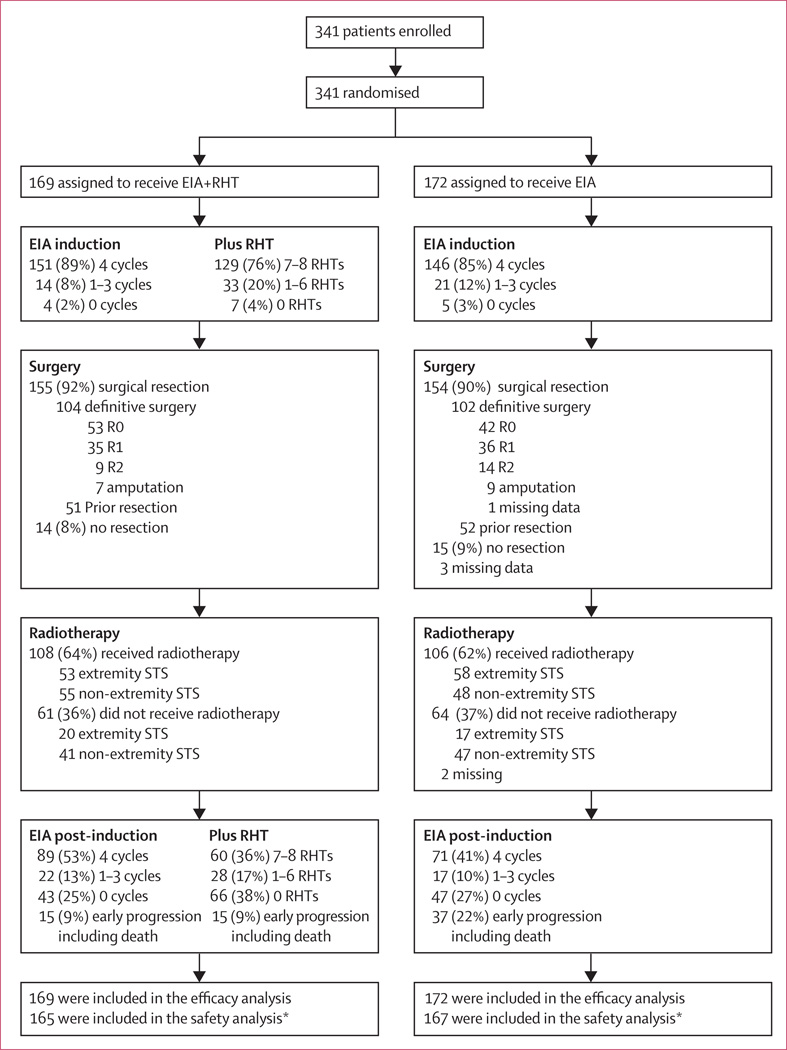
151 patients (89.3%) in the EIA plus regional hyperthermia group and 146 patients (84.9%) in the EIA-alone group completed induction chemotherapy (figure 2). 129 patients (76.3%) in the combined-treatment group received 7–8 regional hyper thermia treatments, 33 patients (19.5%) 1–6 regional hyperthermia treatments, and seven patients (4.1%) none.
Figure 2. Trial profile.
EIA=etoposide+ifosfamide+doxorubicin. RHT=regional hyperthermia. STS=soft-tissue sarcoma. *Four patients in the combined therapy group and five patients in the EIA-alone group did not recevive EIA.
Nearly all patients (90.6%) underwent surgery (155 EIA plus regional hyperthermia vs 154 EIA alone). Around two-thirds of these patients had definitive tumour resection after induction therapy (104 EIA plus regional hyperthermia vs 102 EIA alone), either as first tumour resection (83 vs 86) or after previous inadequate surgery (21 vs 16). The proportion of patients who had R0 resection was 51.0% (53/104) in the combined treatment group versus 41.6% (42/101) in the EIA-alone group, 33.7% (35/104) in the combined treatment group versus 35.6% (36/101) in the EIA-alone group for R1 resections, and 8.7% (9/104) in the combined treatment group versus 13.9% (14/101) in the EIA-alone group for R2 (figure 2). The proportion of patients who underwent amputation was 6.7% (7/104) in the combined treatment group versus 8.9% (9/101) in the EIA-alone group. Patients with extremity STS in the combined treatment group had better surgical outcomes than those in the EIA-alone group, largely because a greater proportion of them underwent R0 (67.9% vs 50.0%; 36/53 vs 29/58) resection including amputation and a lower proportion underwent R1 and R2 resections (26.4% vs 43.1%; 14/53 vs 25/58). Three patients in the combined treatment group and 4 patients in EIA-alone group had no resection (5.7% vs 6.9%; 3/53 vs 4/58). In patients with non-extremity STS, the proportion of patients who underwent R0 (combined treatment 36.9% vs 37.9% in the EIA-alone group; 24/65 vs 22/58) resection including amputation and R1 or R2 resections (combined treatment 46.2% vs 43.1% in the EIA-alone group; 30/65 vs 25/58) were similar in both treatment groups. Eleven patients in the combined treatment group and 11 patients in EIA-alone group had no resection (16.9% vs 19.0%; 11/65 vs 11/58). 51 patients in the combined treatment group and 52 in the EIA-alone group had previous inadequate surgery where a re-resection was not possible. 14 patients in the combined treatment group and 15 in EIA-alone group had no resection, primarily because of non-resectability. The median interval between induction therapy and surgery was 5.9 weeks (range 4.9–7.7 weeks) in the combined treatment group and 5.7 weeks in the EIA-alone group (range 4.3–7.9 weeks).
108 patients in the combined treatment group and 106 in the EIA-alone group received radiotherapy, with a mean dose of 53.2 Gy (SD 8.9) and 52.7 Gy (SD 9.6), respectively. The median interval between surgery and radiotherapy was 6.3 weeks (range 4.7–8.9 weeks) in the combined treatment group and 5.9 weeks (range 4.1–8.9 weeks) in the EIA-alone group. 61 patients in the combined treatment group and 64 in the EIA-alone group did not receive radiotherapy. The main reason for not receiving radiotherapy was an abdominal or retroperitoneal tumour location.
More patients in the combined treatment group completed full post-induction chemotherapy compared with the EIA-alone group (89 [52.7%] vs 71 [41.3%]; p=0.02). Similar numbers of patients did not receive post-induction therapy (43 in the combined treatment group vs 47 in the EIA-alone group) due to non-compliance, whereas the number of patients showing early progression including death before the start of post-induction therapy was significantly lower in the combined treatment group than in the EIA-alone group (15 vs 37; p=0.003). In the combined treatment group, only 60 patients (35.5%) received 7–8 regional hyperthermia treatments, 28 patients (16.6%) 1–6 regional hyperthermia treatments, and 66 patients (38.3%) received none (figure 2). Reasons for not receiving regional hyperthermia were related to side-effects (eg, delayed wound healing, infection, acute reactions due to radiation therapy) or intolerance to further heat treatment (eg, pain due to bolus pressure or heat) after local therapy (surgery or radiation).
The overall duration of study treatment was 32.4 weeks (range 24.9–40.1 weeks) for the combined treatment group versus 29.1 weeks (range 20.2–38.4 weeks) in the EIA-alone group. The median number of cycles of chemotherapy was 8.0 in the combined treatment group versus 5.0 in the EIA-alone group (IQR 4–8 cycles for both treatment groups). Dose reductions (37% vs 21%; 61/165 vs 35/167) and cycles delayed more than 7 days (36.9% vs 22.8%; 59/160 vs 37/162) were mainly due to haematological toxicity. Within the combined treatment group, tumour temperature measurements were done in 71.6% (116/162) of patients. For Tmax the median intratumoural temperature was 41.8°C (IQR 41.1–43.2). The median time-averaged temperatures were 40.8°C for the T20 (IQR 40.1–42.3), 40.3°C for the T50 (IQR 39.5–41.0°C), and 39.2°C for the T90 (IQR 38.5–39.8°C).
56 patients in the combined treatment group had local progression compared with 76 in the EIA-alone group. Response to treatment and survival is shown in table 2.
Table 2.
Response to treatment and survival in all patients assigned to treatment
| EIA plus RHT (N=169) |
EIA (N=172) |
Hazard ratio (95% CI) |
p value* | |
|---|---|---|---|---|
| Median follow-up time (months [IQR]) | ||||
| Median (IQR) | 36 (23–68) | 32 (18–66) | .. | 0.28 |
| Local progression-free survival | ||||
| Median duration (months) | 75 (43 to >120) | 75 | 0.58 (0.41–0.83) | 0.003 |
| Proportion (%) at 2 years | 76 (70–83) | 61 (53–69) | .. | .. |
| Proportion (%) at 4 years | 66 (58–74) | 55 (48–64) | .. | .. |
| Disease-free survival | ||||
| Median duration (months) | 32 (24–49) | 18 (14–26) | 0.70 (0.54–0.92) | 0.011 |
| Proportion (%) at 2 years | 58 (51–66) | 44 (37–52) | .. | .. |
| Proportion (%) at 4 years | 42 (35–51) | 35 (28–43) | .. | .. |
| Overall survival | ||||
| Number of deaths | 74 | 79 | 0.88 (0.64–1.21) | 0.43 |
| Median duration (months) | 79 (54 to >120) | 73 (45 to >120) | .. | .. |
| Proportion (%) at 2 years | 78 (72–84) | 72 (65–79) | .. | .. |
| Proportion (%) at 4 years | 59 (51–67) | 57 (49–65) | .. | .. |
| Response to induction therapy† | 0.002 | |||
| No measurable disease | 51 (30.2) | 46 (26.7) | .. | .. |
| Measurable disease | 118 (69.8) | 126 (73.3) | .. | .. |
| Complete response (n [%]) | 3 (2.5) | 1 (0.8) | .. | .. |
| Partial response (n [%]) | 31 (26.3) | 15 (11.9) | .. | .. |
| Stable disease (n [%]) | 66 (55.9) | 73 (57.9) | .. | .. |
| Progressive disease (n [%]) | 8 (6.8) | 26 (20.6) | .. | .. |
| Could not be evaluated (n [%]) | 10 (8.5) | 11 (8.7) | .. | .. |
| Overall response (%) | 34 (28.8) | 16 (12.7) | .. | .. |
EIA=etoposide+ifosfamide+doxorubicin. RHT=regional hyperthermia.
Calculated by log-rank test.
Response according to WHO criteria was assessed in patients with measurable disease at the time of randomisation. Responses (complete response and partial response) have been confirmed by external review (independent review committee) in 48 of 50 patients.
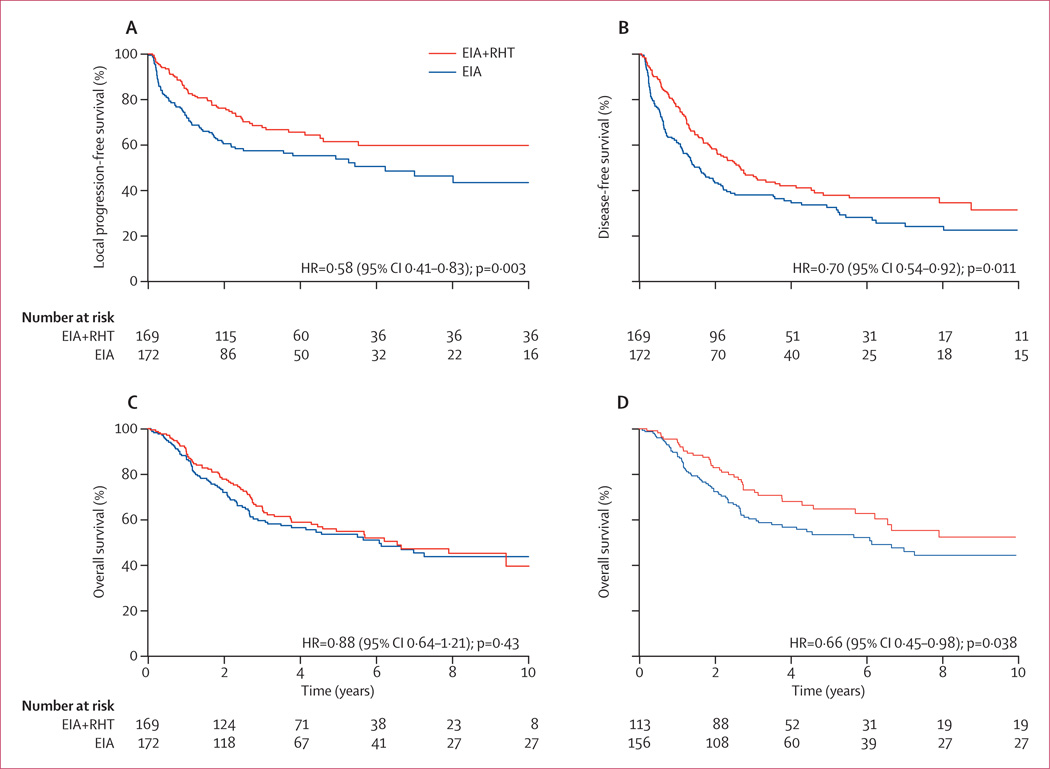
The relative hazard for local progression or death between patients receiving combined therapy or EIA alone was 0.58 (95% CI 0.41–0.84; p=0.003), with a median duration of greater than 120 months (ie, the median is not yet reached) versus 75 months and an absolute difference at 2 years of 15% (95% CI 6–26%; 76% vs 61%; figure 3A). For 149 patients with extremity STS, the local progression-free survival after 2 years was 92% (95%CI 85–98) vs 80% (71–89; HR 0.63, 95%CI 0.32–1.28, p=0.20; table 3). For the predominant group of non-extremity tumours with 192 patients, the benefit of regional hyperthermia was more pronounced and significant with a local progression-free survival after 2 years of 64% (95% CI 55–75) vs 45% (36–57; HR 0.60, 95%CI 0.40–0.90; p=0.012; table 4).
Figure 3. Kaplan-Meier estimates of local progression-free survival (A), disease-free survival (B), and overall survival in all patients randomly allocated treatment (C), and overall survival in the per-protocol-induction population (D).
EIA=etoposide+ifosfamide+doxorubicin. RHT=regional hyperthermia.
Table 3.
Anti-tumour efficacy in the extremity subgroup (N=149; all patients randomly allocated treatment)
| EIA plus RHT (N=76) | EIA (N=73) | Hazard ratio (95% CI) | p value* | |
|---|---|---|---|---|
| Local progression-free survival | ||||
| Exremity (months) | Not reached | Not reached | 0.63 (0.32–1.28) | 0.20 |
| Proportion (%) at 2 years | 92 (85–98) | 80 (71–89) | .. | .. |
| Proportion (%) at 4 years | 82 (73–93) | 76 (66–87) | .. | .. |
| Disease-free survival | ||||
| Median duration (months) | 43 (30 to >120) | 43 (19 to >120) | 0.79 (0.51–1.23) | 0.29 |
| Proportion (%) at 2 years | 70 (60–81) | 57 (47–69) | .. | .. |
| Proportion (%) at 4 years | 49 (38–63) | 49 (39–62) | .. | .. |
| Overall survival | ||||
| Number of deaths | 24 | 26 | 0.93 (0.53–1.62) | 0.80 |
| Median duration (months) | Not reached | Not reached | .. | .. |
| Proportion (%) at 2 years | 89 (82–96) | 81 (72–90) | .. | .. |
| Proportion (%) at 4 years | 67 (56–80) | 69 (59–81) | .. | .. |
EIA=etoposide+ifosfamide+doxorubicin. RHT=regional hyperthermia.
Calculated by log-rank test.
Table 4.
Anti-tumour efficacy in the non-extremity subgroup (N=192; all patients randomly allocated treatment)
| EIA plus RHT (N=96) | EIA (N=96) | Hazard ratio (95% CI) | p value* | |
|---|---|---|---|---|
| Local progression-free survival | ||||
| Median duration (months) | 54 (32 to >120) | 21 (14–59) | 0.60 (0.40–0.90) | 0.012 |
| Proportion (%) at 2 years | 64 (55–75) | 45 (36–57) | .. | .. |
| Proportion (%) at 4 years | 53 (43–65) | 39 (30–52) | .. | .. |
| Disease-free survival | ||||
| Median duration (months) | 22 (17–44) | 13 (9–20) | 0.64 (0.46–0.90) | 0.011 |
| Proportion (%) at 2 years | 50 (41–61) | 33 (24–44) | .. | .. |
| Proportion (%) at 4 years | 37 (29–49) | 23 (15–34) | .. | .. |
| Overall survival | ||||
| Number of deaths | 50 | 53 | 0.87 (0.59–1.28) | 0.49 |
| Median duration (months) | 54 (33 to >120) | 33 (27–87) | .. | .. |
| Proportion (%) at 2 years | 70 (61–79) | 65 (56–75) | .. | .. |
| Proportion (%) at 4 years | 53 (44–65) | 47 (37–58) | .. | .. |
EIA=etoposide+ifosfamide+doxorubicin. RHT=regional hyperthermia.
Calculated by log-rank test.
For the combined treatment group versus the EIA-alone group the relative hazard for disease-free survival was 0.70 (95% CI 0.54–0.92; p=0.011), with a median duration of 32 versus 18 months and an absolute difference at 2 years of 14% (95% CI 4–26; 58% vs 44%; figure 3B).
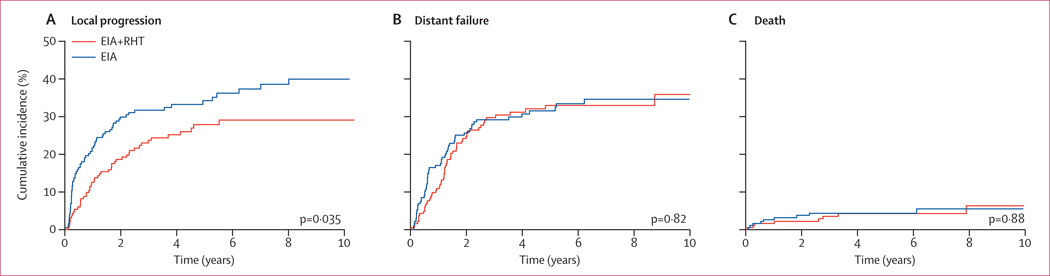
Local progression at 2 years was 19% (95% CI 13%–24%) in the combined treatment group compared with 30% in the EIA-alone group (23–37; p=0.035). The incidence rate of distant failures was not significantly different (24% vs 26%; p=0.82; figure 4B).
Figure 4. Estimated cumulative incidence for competing risks of local progression (A), distant failure (B), and death (C) in all patients randomly allocated to treatment.
153 patients died during the whole follow up period of 128 months (74 in the combined treatment group vs 79 in the EIA-alone group). Overall survival did not differ between the groups (HR 0.88, 95% CI 0.64–1.21; p=0.43; figure 3C). Overall response rate after induction therapy was more than twice as high (28.8%) in the combined treatment group compared with the EIA-alone group (12.7%), and the number of patients with progressive disease was significantly lower in the combined treatment group compared with the EIA-alone group (eight vs 26, p=0.002; table 2). In an analysis of 269 patients (78.9% of the study population) with confirmed histology of STS who either received the complete induction therapy or had early progression (per-protocol-induction population), regional hyperthermia improved overall survival in the combined therapy group compared with the EIA-alone group (HR 0.66, 95% CI 0.45–0.98; p=0.038; figure 3D).
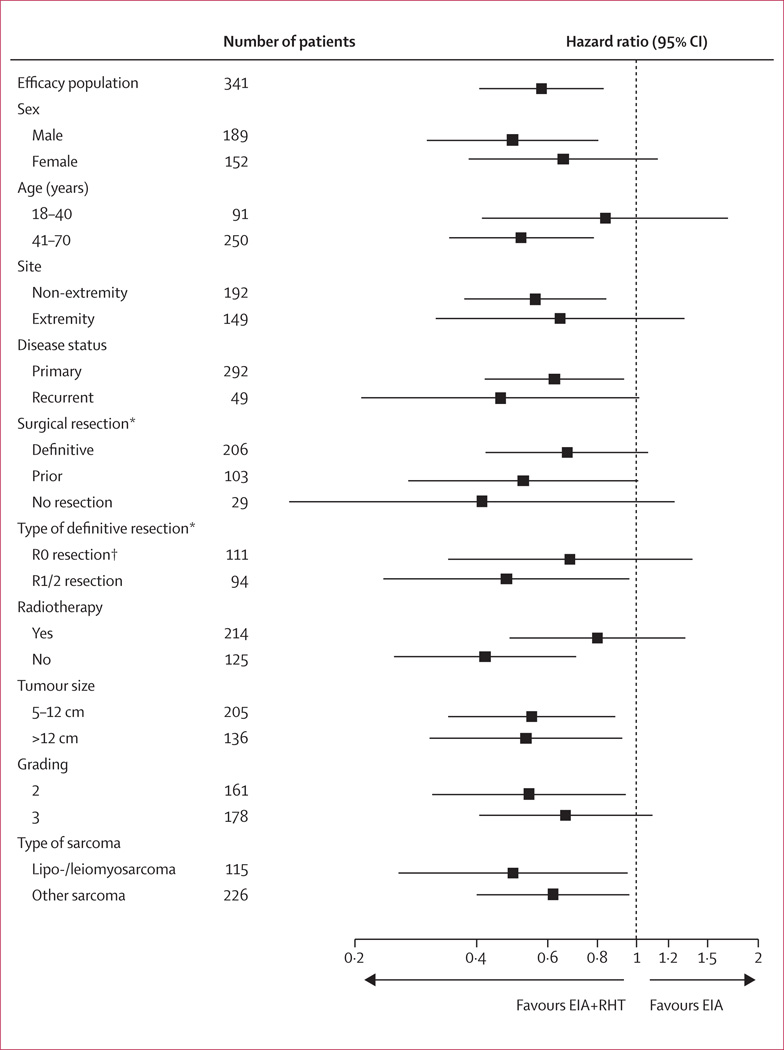
Compared with EIA alone, combined therapy results in better LPFS in all patient subgroups (figure 5). For all 310 patients treated in the three major centres, the results for the relevant endpoints are similar to the overall result (LPFS: HR 0.54, 95% CI 0.37–0.77; DFS: HR 0.67, 0.51–0.89; overall survival: HR 0.87, 0.63–1.21).
Figure 5. Local progression-free survival in the EIA plus regional hyperthermia group compared with the EIA group for selected subgroups of patients allocated to treatment.
EIA=etoposide+ifosfamide+doxorubicin. RHT=regional hyperthermia. *There were missing data for surgical resection for three patients; and for type of definitive resection for one patient. †R0 resection includes amputation.
Among 332 patients included in the safety analysis, 234 patients (70.5%) developed grade 3 or 4 leucopenia, with 128 patients in the combined therapy group compared with 106 patients in the EIA-alone group (77.6% vs 63.5%; p=0.005; table 5). The most frequent non-haematological adverse events were grade 3 or 4 nausea (23 in the combined therapy group vs 26 in the EIA-alone group), vomiting (15 vs 9 patients), and neurotoxicity (15 vs 8 patients). Hyperthermia-related adverse events were pain, bolus pressure, and skin burn, which were mild to moderate in 66 (40.5%), 43 (26.4%), and 29 patients (17.8%), and severe in seven (4.3%), eight (4.9%), and one patient (0.6%), respectively.
Table 5.
Adverse events according to treatment (safety population)
| EIA plus RHT | EIA | p value | |
|---|---|---|---|
| Adverse events related to chemotherapy | |||
| Number of patients | 165 | 167 | .. |
| Number of deaths attributable to treatment* | 2 (1.2%) | 1 (0.6%) | .. |
| Haematological | |||
| Acute Leukaemia† | 3 (1.8%) | 2 (1.2%) | .. |
| Leucopenia grade 3 or 4 | 128 (77.6%) | 106 (63.5%) | 0.005 |
| Thrombocytopenia grade 3 or 4 | 28 (17.0%) | 23 (13.8%) | 0.42 |
| Non-haematological grade 3 or 4 | |||
| Nausea | 23 (13.9%) | 26 (15.6%) | 0.68 |
| Vomiting | 15 (9.1%) | 9 (5.4%) | 0.19 |
| Nephrotoxicity | 2 (1.2%) | 2 (1.2%) | 0.99 |
| Cardiotoxicity | 3 (1.8%) | 4 (2.4%) | 0.71 |
| Neurotoxicity | 15 (9.1%) | 8 (4.8%) | 0.12 |
| Fever of unknown origin | 1 (0.6%) | 5 (3.0%) | 0.10 |
| Adverse events related to hyperthermia | |||
| Number of patients | 163 | .. | .. |
| Pain (power-related) | |||
| Mild to moderate | 66 (40.5%) | .. | .. |
| Severe | 7 (4.3%) | .. | .. |
| Bolus pressure | |||
| Mild to moderate | 43 (26.4%) | .. | .. |
| Severe | 8 (4.9%) | .. | .. |
| Skin burn | |||
| Mild to moderate | 29 (17.8%) | .. | .. |
| Severe | 1 (0.6%) | .. | .. |
| Tissue necrosis | |||
| Mild to moderate | 7 (4.3%) | .. | .. |
| Severe | 4 (2.5%) | .. | .. |
| Localised Infection | |||
| Mild to moderate | 5 (3.1%) | .. | .. |
| Severe | 2 (1.2%) | .. | .. |
| Others‡ | |||
| Mild to moderate | 23 (14.1%) | .. | .. |
| Severe | 14 (8.6%) | .. | .. |
All data are n (%) unless otherwise stated. EIA=etoposide+ifosfamide+ doxorubicin. RHT=regional hyperthermia.
All patients died from neutropenic sepsis.
Four patients developed acute myeloid leukemia, and one patient developed acute lymphoblastic leukemia.
Other reasons: claustrophobia, not power-related pain, wound healing disorder, nausea.
Two deaths were attributable to treatment in the combined treatment group, and one death was attributable to treatment in the EIA-alone group. During follow-up, secondary haematological malignancies were noted in three patients in the combined treatment group and two patients in the EIA-alone group.
Discussion
To our knowledge, this is the first and largest randomised phase 3 trial of neo-adjuvant chemotherapy in patients with high-risk STS. Our results indicate that regional hyperthermia combined with the three-drug-regimen EIA can be given safely with moderate toxicity. However, the higher proportion of patients experiencing thrombocytopenia and leucopenia compared with patients treated with EIA alone (17.0% vs 13.8%, p=0.42; and 77.6% vs 63.5%, p=0.005, respectively) might be related to the heating field involving part of the bone marrow, especially in patients with large abdominal or pelvic tumours (81% of the non-extremity tumours). Thermosensitisation due to the concurrent EIA chemotherapy might also contribute to the increased haematological toxicity in the combined therapy group compared with the EIA-alone group. The water-bolus pressure on the patient inside the regional heating applicator might be responsible for the increased, but non-significant, incidence of grade 3 and 4 vomiting and grade 3 and 4 local neurotoxicity in the combined therapy group compared with the EIA-alone group (9.1% vs 5.4%, p=0.19; and 9.1% vs 4.8%; p=0.12, respectively), especially in patients after surgery and radiation therapy.
Adding regional hyperthermia to EIA results in significantly better LPFS (HR 0.58, p=0.003) and DFS (HR 0.70, p=0.011) compared with EIA alone. An analysis of competing risks shows that the improvement is predominantly attributable to the regional hyperthermia-related prevention of local progression, since numbers of deaths were similar in both treatment groups (figure 4A and 4C). The effect was consistent among all pre-specified risk factors and stratification criteria. The major local adjuvant treatment for STS is radiation therapy, which often cannot be administered to large retroperitoneal lesions, especially after surgery. Our analysis shows that the benefit to LPFS from regional hyperthermia is independent of radiation therapy. The significant benefit of regional hyperthermia experienced by patients with non-extremity (abdominal or retroperitoneal) tumours reflects the marked effect of local control on final outcome for this subgroup, because these patients usually die from local progressive disease with or without distant metastasis.22 The absence of effective second-line therapy partly supports the view that progression-free survival for these patients is an acceptable surrogate for overall survival, as has been discussed recently.23
We defined the study population on the basis of the most relevant risk factors for sarcoma-specific death,24 thus providing a homogeneous cohort of patients. By our stratification to site and type of tumour presentation, the proportional numbers of patients in these subgroups were well-balanced.
The rationale for using regional hyperthermia is that heat kills cells by direct thermal toxicity, increases drug efficacy, and induces tumoricidal immune responses.25 The EIA regimen combines the most active chemotherapeutic agents (doxorubicin and ifosfamide) for STS, with the addition of etoposide based on our earlier experience with anthracycline-refractory STS.17 However, the role of etoposide is now questionable, and might be omitted in future protocols because of its low activity in STS and its leukaemogenic potential. Ifosfamide is most effective at temperatures between 40.5°C and 43.0°C, as shown in human sarcoma xenografts.26 This fits with the tumour-temperature profile achieved in this study (T50 40.3°C, IQR 39.5°C–41.0°C; Tmax 41.8°C IQR 41.1°C–43.2°C).
Evidence suggests that regional hyperthermia synergises with EIA induction chemotherapy, as shown by its effect on overall response and early progression. Adding regional hyperthermia to the EIA regimen resulted in overall response 2.3 times greater than in patients treated with EIA alone (28.8% vs 12.7%, p=0.002). This increased shrinking of the tumour attributable to regional hyperthermia was confirmed by blinded independent review to avoid any overestimation of response rate, as has been noted in other trials. Other studies of neo-adjuvant therapy—mostly in extremity STS—have used other drug combinations9,27,28 or chemoradiotherapy.8 However, the proportion of patients responding to these regimens was similar to the response rate noted for EIA with regional hyperthermia. Importantly, adding regional hyperthermia prevented a considerable proportion of patients from developing progressive disease, compared with EIA alone (6.8% vs 20.6%). This is in contrast with the high rate of early progressive disease in the trials mentioned above.8,9,27,28 In the only randomised trial comparing doxorubicin–ifosfamide chemotherapy as neo-adjuvant treatment, the rate of progression was 18%.9 In two independent reports from the MD Anderson Cancer Center, TX, USA, the rates of progression were 30% and 26%, respectively.27,28 The Radiation Therapy Oncology Group8 using the MAID regimen (mesna, doxorubicin, ifosfamide, and dacarbazine) combined with preoperative split-course radiotherapy reported a progression rate of 14%, but this intensified regimen was associated with high toxicity.29 These previous results are similar to the results in our EIA-alone group, and emphasise the effectiveness of regional hyperthermia for the prevention of early progression. Early progression, rather than response rate, has been shown to be most important for identifying new effective compounds in advanced STS.30
Moreover, the improved surgical outcome in 12% of patients due to more effective tumour mass reduction was not counterbalanced by early disease progression during the preoperative phase of treatment with EIA plus regional hyperthermia. In general, despite high tumour response rates after neo-adjuvant chemotherapy in high-risk patients (T2b, high grade), the risk of early disease progression is of major concern.31
In all patients who were assigned to treatment, there is no evidence of a difference in overall survival between the EIA-alone group and the combined treatment group, although this could be due to the low power to detect any difference because of the relatively few deaths so far (153 patients). However, among the 269 patients who completed induction therapy (4 cycles EIA plus 8 regional hyperthermias vs 4 cycles EIA alone), including the patients with early progression, we noted a significant improvement in overall survival in the combined therapy group compared with the EIA-alone group (HR 0.66, p=0.038). As shown previously in our retrospective analysis of high-risk STS with retroperitoneal or visceral location, response related to EIA plus regional hyperthermia induction therapy was predictive for long-term survival.15 This suggests that the addition of regional hyperthermia leading to higher response rates and prevention of early progression is the main cause of the overall survival benefit in this subgroup.
At present the benefits of this new intervention is restricted to patients with high-risk STS. Whether a similar benefit will be seen in lower risk patients, and whether the safety profile will be the same, and hence the trade off between benefit and harm worthwhile, remains to be established. Current routine clinical practice consists of preoperative and postoperative first-line chemotherapy (eg, doxorubicin and ifosfamide) combined with regional hyperthermia—in addition to preoperative radiotherapy—if possible.
This randomised trial provides the first evidence that regional hyperthermia added to preoperative and postoperative chemotherapy is clinically more effective than chemotherapy alone in a specific population of patients with high-risk STS. This therapeutic strategy offers a new treatment option, and can be integrated in the multimodal treatment approach for these patients.
Acknowledgments
This study was funded by the Deutsche Krebshilfe (M99/94/Wi II, 70-I702-Wi II), Helmholtz Association (VH-VI-140), EORTC 62961, ESHO RHT-95, and NIH P01 CA42745. In addition the following investigators supported the EORTC-ESHO multi-centre trial: Austria: R Windhager; Germany: H J Sauer, J Gellermann, R Wilkowski, M Kuhlencordt, B Weber, Th Licht, F Hörterer, F Berger; Norway: O Dahl, O Mella; USA: M Dewhirst. We thank C Klose and R P Laubender and M Schmidt for data management and statistical analysis. We thank the many investigators in medical oncology, surgical oncology, and radiation oncology who worked with the principal investigator at each participating institution as part of the combined-modality treatment team for the patients. We are indebted to all the patients who took part in the study and to their families.
Footnotes
Contributors
RDI and JV designed the study in collaboration with the European Society for Hyperthermic Oncology (ESHO) and European Organisation for Research and Treatment of Cancer (EORTC). UM supervised data management and statistical analysis. All authors participated in the study implementation, data analysis, and interpretation of results. This report was principally drafted by RDI, LHL, JV, UM, and PH, and was critically reviewed and subsequently approved by each co-author.
Conflicts of interest
RDI, LHL and SA-R have received consulting fees from Medtherm. All other authors declared no conflicts of interest.
Contributor Information
Rolf D Issels, Klinikum der Universität München—Campus Grosshadern, München, Germany; Helmholtz Zentrum München—German Research Center for Environmental Health, München, Germany.
Lars H Lindner, Klinikum der Universität München—Campus Grosshadern, München, Germany; Helmholtz Zentrum München—German Research Center for Environmental Health, München, Germany.
Jaap Verweij, Erasmus University Medical Center Rotterdam, Rotterdam, the Netherlands.
Peter Wust, Charité—Universitätsmedizin Berlin, Berlin, Germany.
Peter Reichardt, Charité—Universitätsmedizin Berlin, Berlin, Germany.
Baard-Christian Schem, Haukeland University Hospital, Bergen, Norway.
Sultan Abdel-Rahman, Klinikum der Universität München—Campus Grosshadern, München, Germany.
Soeren Daugaard, Department of Pathology, Rigshospitalet, Copenhagen, Denmark.
Christoph Salat, Klinikum der Universität München—Campus Grosshadern, München, Germany.
Clemens-Martin Wendtner, Klinikum der Universität München—Campus Grosshadern, München, Germany; Uniklinik Köln, Köln, Germany.
Zeljko Vujaskovic, Duke University Medical Center, Durham, NC, USA.
Rüdiger Wessalowski, Universitätsklinikum Düsseldorf, Düsseldorf, Germany.
Karl-Walter Jauch, Klinikum der Universität München—Campus Grosshadern, München, Germany.
Hans Roland Dürr, Klinikum der Universität München—Campus Grosshadern, München, Germany.
Ferdinand Ploner, Universitätsklinik Graz, Graz, Austria.
Andrea Baur-Melnyk, Klinikum der Universität München—Campus Grosshadern, München, Germany.
Ulrich Mansmann, Klinikum der Universität München—Campus Grosshadern, München, Germany.
Wolfgang Hiddemann, Klinikum der Universität München—Campus Grosshadern, München, Germany.
Jean-Yves Blay, Centre Leon Berard, Lyon, France.
Peter Hohenberger, Charité—Universitätsmedizin Berlin, Berlin, Germany.
References
- 1.Jemal A, Tiari RC, Murray T. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Brennan M, Singer S, Maki RG. Soft tissue sarcoma. In: De Vita VT, Hellmann S, Rosenberg S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Pennsylvania: Lippincott Williams and Wilkins; 2005. pp. 1581–1637. [Google Scholar]
- 3.Pisters PW, Harrison LB, Leung DH, Woodruff JM, Casper ES, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 4.Yang JC, Chang AE, Baker AR. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 5.O’Sullivan B, Davis AM, Turcotte R. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 6.Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353:701–711. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 7.Casali PG, Picci P. Adjuvant chemotherapy for soft tissue sarcoma. Curr Opinion Oncol. 2005;17:361–365. doi: 10.1097/01.cco.0000166652.15546.4f. [DOI] [PubMed] [Google Scholar]
- 8.Kraybill WG, Harris J, Spiro IJ. Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. J Clin Oncol. 2006;24:619–625. doi: 10.1200/JCO.2005.02.5577. [DOI] [PubMed] [Google Scholar]
- 9.Gortzak E, Azzarelli A, Buesa J. A randomized phase II study on neo-adjuvant chemotherapy in high risk adult soft tissue sarcoma. Eur J Cancer. 2001;37:1096–1103. doi: 10.1016/s0959-8049(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 10.Wust P, Hildebrandt B, Sreenivasa G. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3:487–497. doi: 10.1016/s1470-2045(02)00818-5. [DOI] [PubMed] [Google Scholar]
- 11.Overgaard J, Gonzalez Gonzalez D, Hulshof MC. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet. 1995;345:540–543. doi: 10.1016/s0140-6736(95)90463-8. [DOI] [PubMed] [Google Scholar]
- 12.Jones EL, Olseson JR, Prosnitz JR. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23:3079–3085. doi: 10.1200/JCO.2005.05.520. [DOI] [PubMed] [Google Scholar]
- 13.van der Zee J, Gonzalez Gonzalez D, van Rhoon G. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective randomized, multicenter trial. Lancet. 2000;355:1119–1125. doi: 10.1016/s0140-6736(00)02059-6. [DOI] [PubMed] [Google Scholar]
- 14.Issels RD, Abdel-Rahman S, Wendtner CM. Neoadjuvant chemotherapy combined with regional hyperthermia (RHT) for locally advanced primary or recurrent high-risk soft tissue sarcomas (HR-STS) of adults: Long-term results of a phase II study. Eur J Cancer. 2001;37:1599–1608. doi: 10.1016/s0959-8049(01)00183-6. [DOI] [PubMed] [Google Scholar]
- 15.Wendtner CM, Abdel-Rahman S, Krych M. Response to neoadjuvant chemotherapy combined with regional hyperthermia predicts long-term survival for adult patients with retroperitoneal and visceral high-risk soft tissue sarcoma. J Clin Oncol. 2002;20:3156–3164. doi: 10.1200/JCO.2002.07.146. [DOI] [PubMed] [Google Scholar]
- 16.Lagendijk JJ, Van Rhoon GC, Hornsleth SN. ESHO quality assurance guidelines for regional hyperthermia. Int J Hyperthermia. 1998;14:125–133. doi: 10.3109/02656739809018219. [DOI] [PubMed] [Google Scholar]
- 17.Issels RD, Prenninger SW, Nagele A. Ifosfamide plus etoposide combined with regional hyperthermia in patients with locally advanced sarcomas: A phase II study. J Clin Oncol. 1990;8:1818–1829. doi: 10.1200/JCO.1990.8.11.1818. [DOI] [PubMed] [Google Scholar]
- 18.Pintilie M. A practical perspective. Statistics in practice. 1st Edition. New York: John Wiley & Sons; 2006. Competing risks. [Google Scholar]
- 19.Hosmer DW, Lemeshow S. Applied survival analysis: Regression modelling of time to event data. 1st Edition. New York: John Wiley & Sons; 1999. [Google Scholar]
- 20.Cox DR, Oakes D. Analysis of survival data. New York: Chapman and Hall; 1990. [Google Scholar]
- 21.R Development Core Team. Vienna, Austria: R Foundation for Statistical Computing; R: A language and environment for statistical computing. http://www.R-project.org. [Google Scholar]
- 22.Katz MHB, Choi EA, Pollock RE. Current concepts in multimodality therapy for retroperitoneal sarcoma. Exp Rev Anticancer Ther. 2007;7:159–168. doi: 10.1586/14737140.7.2.159. [DOI] [PubMed] [Google Scholar]
- 23.Saad ED, Katz A, Hoff PM, Buyse M. Progression-free survival as surrogate and as true end point: insights from the breast and colorectal cancer literature. Ann Oncol. 2010;21:7–12. doi: 10.1093/annonc/mdp523. [DOI] [PubMed] [Google Scholar]
- 24.Kattan MW, Leung DHY, Brennan MY. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20:791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 25.Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer. 2008;44:2546–2554. doi: 10.1016/j.ejca.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 26.Wiedemann G, Roszinski S, Biersack A, Weiss C, Wagner T. Local hyperthermia enhances cyclophosphamide, ifosfamide and cis-diamminedichloroplatinum cytotoxicity on human-derived breast carcinoma and sarcoma xenografts in nude mice. J Cancer Res Clin Oncol. 1992;118:129–135. doi: 10.1007/BF01187501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pisters PWT, Patel SR, Varma DGK. Preoperative chemotherapy for stage IIIB extremity soft tissue sarcoma: Long-term results from a single institution. J Clin Oncol. 1997;15:3481–3487. doi: 10.1200/JCO.1997.15.12.3481. [DOI] [PubMed] [Google Scholar]
- 28.Meric F, Hess KR, Varma DGK. Radiographic response to neoadjuvant chemotherapy is a predictor of local control and survival in soft tissue sarcomas. Cancer. 2002;95:1120–1126. doi: 10.1002/cncr.10794. [DOI] [PubMed] [Google Scholar]
- 29.Pisters PWT. Preoperative chemotherapy and split-course radiation therapy for patients with localized soft tissue sarcomas: Home run, base hit or strike out? J Clin Oncol. 2006;24:549–551. doi: 10.1200/JCO.2005.04.3026. [DOI] [PubMed] [Google Scholar]
- 30.van Glabbeke M, Verweij J, Judson I, Nielson OS. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer. 2002;38:543–549. doi: 10.1016/s0959-8049(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 31.Pisters PWT, O’Sullivan B, Maki RG. Evidence-based recommendations for local therapy for soft tissue sarcomas. J Clin Oncol. 2007;25:1003–1008. doi: 10.1200/JCO.2006.09.8525. [DOI] [PubMed] [Google Scholar]