Abstract
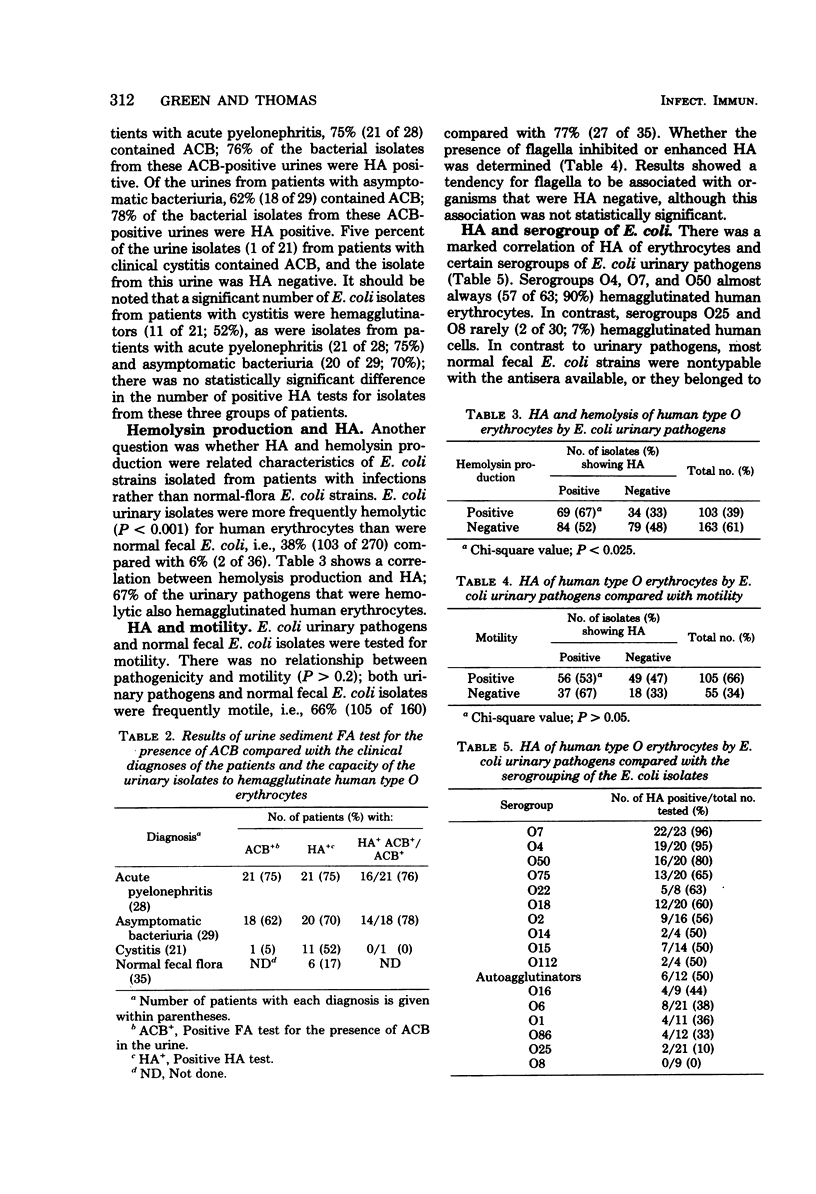
The purpose of this investigation was to study potential virulence factors associated with Escherichia coli urinary pathogens isolated from patients with urinary tract infection. These factors were compared with characteristics of normal-flora E. coli isolated from stool specimens of healthy individuals without a history of urinary tract infection. The potential virulence factor focused on in this study was hemagglutination (HA) of human type O erythrocytes by E. coli urinary pathogens. A total of 265 strains of E. coli isolated from patients with urinary tract infections were tested for their ability to hemagglutinate human type O erythrocytes; of these, 148 (56%) were HA positive. Only 6 of 36 fecal E. coli strains (17%) isolated from healthy controls were HA positive. This significant association of the presence of hemagglutinin on E. coli that causes urinary tract infections indicates the likelihood that HA is a marker of virulence. Only 12% (5 of 43) of Proteus mirabilis and 3% (3 of 104) of Klebsiella pneumoniae urinary isolates were HA positive. There was a trend for HA-positive E. coli to be isolated from patients with kidney infections and positive tests for antibody-coated bacteria rather than bladder infections and negative tests for antibody-coated bacteria, although the difference was not statistically significant. There was a significant correlation (P < 0.025) between hemolysin production and HA; 67% (69 of 103) of the isolates that produced hemolysin also hemagglutinated human type O erythrocytes. There was no significant correlation between HA and motility, although there was a trend for flagellated organisms to be non-hemagglutinators. There was a marked correlation between the presence of hemagglutinin and the serogroup of the E. coli isolate; serogroups O4, O7, and O50 were almost always HA positive (57 of 63; 90%). In contrast, serogroups O8 and O25 were rarely HA positive (2 of 30; 7%).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooke E. M., Ewins S. P. Properties of strains of Escherichia coli isolated from a variety of sources. J Med Microbiol. 1975 Feb;8(1):107–111. doi: 10.1099/00222615-8-1-107. [DOI] [PubMed] [Google Scholar]
- Cooke E. M. Properties of strains of Escherichia coli isolated from the faeces of patients with ulcerative colitis, patients with acute diarrhoea and normal persons. J Pathol Bacteriol. 1968 Jan;95(1):101–113. doi: 10.1002/path.1700950112. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Eriksson B., Hanson L. A., Jodal U., Kaijser B., Janson G. L., Lindberg U., Olling S. Adhesion to normal human uroepithelial cells of Escherichia coli from children with various forms of urinary tract infection. J Pediatr. 1978 Sep;93(3):398–403. doi: 10.1016/s0022-3476(78)81145-7. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Hanson L. A., Jodal U., Lindberg U., Akerlund A. S. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet. 1976 Sep 4;1(7984):490–492. [PubMed] [Google Scholar]
- Edén C. S., Hansson H. A. Escherichia coli pili as possible mediators of attachment to human urinary tract epithelial cells. Infect Immun. 1978 Jul;21(1):229–237. doi: 10.1128/iai.21.1.229-237.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977 Nov;18(2):330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., DuPont H. L. Hemagglutination patterns of enterotoxigenic and enteropathogenic Escherichia coli determined with human, bovine, chicken, and guinea pig erythrocytes in the presence and absence of mannose. Infect Immun. 1979 Feb;23(2):336–346. doi: 10.1128/iai.23.2.336-346.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A. A., Brumfitt W., Howard C. J. K antigens of Escherichia coli and renal involvement in urinary-tract infections. Lancet. 1971 Mar 13;1(7698):514–516. [PubMed] [Google Scholar]
- Kaijser B., Hanson L. A., Jodal U., Lidin-Janson G., Robbins J. B. Frequency of E. coli K antigens in urinary-tract infections in children. Lancet. 1977 Mar 26;1(8013):663–666. doi: 10.1016/s0140-6736(77)92111-0. [DOI] [PubMed] [Google Scholar]
- Kaijser B. Immunology of Escherichia coli: K antigen and its relation to urinary-tract infection. J Infect Dis. 1973 Jun;127(6):670–677. doi: 10.1093/infdis/127.6.670. [DOI] [PubMed] [Google Scholar]
- Ljungh A., Faris A., Wadström T. Hemagglutination by Escherichia coli in septicemia and urinary tract infections. J Clin Microbiol. 1979 Oct;10(4):477–481. doi: 10.1128/jcm.10.4.477-481.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew B. H., Jorgensen J., Counts G. W., Falkow S. Association of hemolysin production, hemagglutination of human erythrocytes, and virulence for chicken embryos of extraintestinal Escherichia coli isolates. Infect Immun. 1978 Apr;20(1):50–54. doi: 10.1128/iai.20.1.50-54.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C. Inhibition of the interaction between fimbrial haemagglutinins and erythrocytes by D-mannose and other carbohydrates. J Gen Microbiol. 1972 Jun;71(1):149–157. doi: 10.1099/00221287-71-1-149. [DOI] [PubMed] [Google Scholar]
- Ottow J. C. Ecology, physiology, and genetics of fimbriae and pili. Annu Rev Microbiol. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- Powell C. J., Jr, Finkelstein R. A. Virulence of Escherichia coli strains for chick embryos. J Bacteriol. 1966 Apr;91(4):1410–1417. doi: 10.1128/jb.91.4.1410-1417.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH H. W. The haemolysins of Escherichia coli. J Pathol Bacteriol. 1963 Jan;85:197–211. doi: 10.1002/path.1700850119. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Halls S. The transmissible nature of the genetic factor in Escherichia coli that controls haemolysin production. J Gen Microbiol. 1967 Apr;47(1):153–161. doi: 10.1099/00221287-47-1-153. [DOI] [PubMed] [Google Scholar]
- Stirm S., Orskov F., Orskov I., Birch-Andersen A. Episome-carried surface antigen K88 of Escherichia coli. 3. Morphology. J Bacteriol. 1967 Feb;93(2):740–748. doi: 10.1128/jb.93.2.740-748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas V., Shelokov A., Forland M. Antibody-coated bacteria in the urine and the site of urinary-tract infection. N Engl J Med. 1974 Mar 14;290(11):588–590. doi: 10.1056/NEJM197403142901102. [DOI] [PubMed] [Google Scholar]
- Vosti K. L. Relationship of hemagglutination to other biological properties of serologically classified isolates of Escherichia coli. Infect Immun. 1979 Aug;25(2):507–512. doi: 10.1128/iai.25.2.507-512.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



