Abstract
Purpose:
To measure patients' assessment of chronic illness care and its variation across primary healthcare (PHC) models.
Methods:
We recruited 776 patients with diabetes, heart failure, arthritis or chronic obstructive pulmonary disease from 33 PHC clinics. Face-to-face interviews, followed by a telephone interview at 12 months, were conducted using the Patient Assessment of Chronic Illness Care (PACIC). Multilevel regression was used in the analysis.
Results:
The mean PACIC score was low at 2.5 on a scale of 1 to 5. PACIC scores were highest among patients affiliated with family medicine groups (mean, 2.78) and lowest for contact models (mean, 2.35). Patients with arthritis and older persons generally reported a lower assessment of chronic care.
Conclusion:
Family medicine groups represent an integrated model of PHC associated with higher levels of achievement in chronic care. Variations across PHC organizations suggest that some models are more appropriate for improving management of chronic illness.
Abstract
Objet:
Mesurer l'évaluation par les patients des soins pour les maladies chroniques et repérer les variations parmi les modèles de soins de santé primaires (SSP).
Méthode:
Nous avons recruté, auprès de 33 cliniques de SSP, 776 patients atteints de diabètes, d'insuffisance cardiaque, d'arthrite ou de maladie pulmonaire obstructive chronique. À l'aide du PACIC (Patient Assessment of Chronic Illness Care), nous avons mené des entrevues en personne, suivies d'entrevues téléphoniques douze mois plus tard. L'analyse a été faite par régression multiniveaux.
Résultats:
L'indice moyen du PACIC était bas, avec 2,5 points sur une échelle de 1 à 5. Les plus hauts indices du PACIC se trouvent chez les patients affiliés à des groupes de médecine de famille (moyenne, 2,78) et les indices les plus bas se retrouvent dans les modèles de contact (moyenne, 2,35). L'évaluation des soins chroniques est moindre, en général, chez les patients atteints d'arthrite et chez les personnes âgées.
Conclusion:
Les groupes de médecine de famille représentent un modèle intégré de SSP associé à de plus hauts niveaux d'accomplissement des soins chroniques. La variation parmi les organisations de SSP laisse voir que certains modèles sont plus appropriés pour l'amélioration de la gestion des maladies chroniques.
Aging of populations, in combination with improved treatments, leads to increased numbers of persons living with chronic or permanent illnesses (Rothenberg and Koplan 1990; McKenna et al. 1998; Glasgow et al. 1999; Le Galès-Camus et al. 2005; Yach et al. 2004). This increased prevalence leads to higher use of health and social resources (Glasgow et al. 1999). Yet, the typical medical model is not optimal for chronic disease management, especially for those with multimorbidity (Glasgow et al. 1999). Another challenge consists of increasing people's capacity to adapt and live with multiple illnesses and maintain a good quality of life (Detels and Breslow 1997). Thus, there is need for a more integrated approach to chronic disease management. The best known integrated model of prevention and management of chronic illness care is the Chronic Care Model (CCM) (Wagner et al. 2001). Implementing CCM elements has been associated with improvement in the processes and results of care and with better health outcomes (Tsai et al. 2005; Singh and Ham 2006).
In Canada, provincial and federal committees have highlighted problems related to the fragmentation of services, lack of prevention and access to care (Kirby and LeBreton 2002; Romanow 2002b). A consensus has emerged on the need for services that are accessible 24 hours a day, seven days a week, multidisciplinary teams and electronic medical records (Kirby and LeBreton 2002; Clair 2000; Romanow 2002a). As a result, new primary healthcare (PHC) organizational models have been developed and implemented.
Some community health centres have changed their organizational characteristics, such as practice size and diversity of providers, and have incorporated nurse practitioners – actions that partially explain their better performance at providing comprehensive care (Russell et al. 2009, 2010). A recent study in Ontario has found evidence that PHC delivery models are associated with higher quality of care. The study further suggests that shifting away from the traditional fee-for-service practice can be beneficial for care of chronic diseases (Liddy et al. 2011). Organizational attributes can affect processes of care and influence patient outcomes (Hogg et al. 2008; Hung et al. 2006, 2008). However, the association between organizational models and the level of assessment of chronic care has not been evaluated.
The aims of this study are to document patients' assessment of chronic illness care and to evaluate its association with various PHC organizational models. We hypothesized that new models of care that are part of current reforms of PHC in Quebec, such as family medicine groups and some existing models that conform to desirable attributes of primary care, would be associated with higher assessment of chronic care.
Methods
The present study, MaChro-1 (a contraction of maladies chroniques en première ligne, which means “chronic disease in primary care” in French), was designed to assess the association of PHC organizational models with patients' access to and utilization of healthcare, and their health status. A cohort of patients with diabetes, heart failure, arthritis or chronic obstructive pulmonary disease was recruited from participating PHC clinics in two regions of the province of Quebec. These two regions comprise a metropolitan area, suburban areas, and smaller urban and rural communities in the southwestern part of the province and cover 40% of the population of the province of Quebec. We approached 90 clinics that were known to provide services to patients living with chronic diseases. Of these, 45 agreed to participate in the study (50% participation rate), but 33 actually recruited participants (73% recruitment rate). Compared to these averages, solo providers were as likely to accept participation (53%) and subsequently recruit patients (71%). Group practices showed slightly lower rates of acceptance of participation (47%) and recruitment of patients (65%). Local community health centres were as likely to accept participation (55%) but more likely to recruit patients following acceptance (92%) as were family medicine groups (55% and 100%, respectively).
Patients were recruited (a) during a clinical encounter with their physician (n=533), (b) in the clinics' waiting room (n=226) by a trained interviewer or (c) by phone from a list of patients living with chronic diseases, provided by physicians (n=272). Specific criteria were given to clinicians in order to recruit patients with established diagnoses of the chronic disease under study. A standardized form was used for the clinician to refer patients to the research team. Figure 1 describes the recruitment of the overall study. Patients who agreed to participate were interviewed in person, at baseline, by professionally trained interviewers using validated questionnaires and were subsequently interviewed by phone at 6, 12 and 18 months. Assessment of chronic care was done at baseline and 12 months; this paper covers these two measurement points.
FIGURE 1.
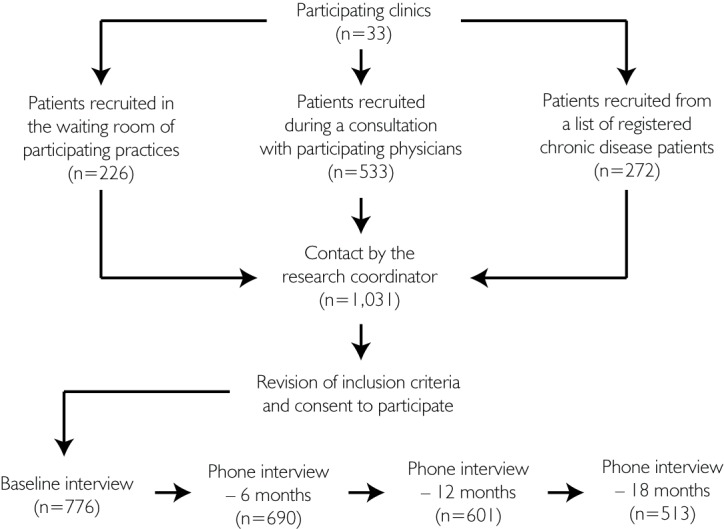
Study diagram
Participants, both clinicians and patients, gave informed consent to participate in the study. This study was approved by the Centre de recherche du centre hospitalier de l'Univeristé de Montréal Research and Ethics Committee.
In this paper, we report results based on the Patient Assessment of Chronic Illness Care instrument (PACIC) developed by Glasgow and collaborators (2005a). The PACIC is a 20-item questionnaire designed to measure quality of care from the patient's perspective. Patients rate various aspects of care on a scale from 1 (none of the time) to 5 (always) based on their experience with their care provider. The PACIC is based on the CCM and encompasses subdimensions such as patient activation, delivery system design/decision support, goal-setting, problem-solving/contextual counselling and follow-up/coordination. The PACIC score has been validated for internal consistency, and the methods suggested by the instruments' authors have been followed to calculate the scores as part of this study (Glasgow et al. 2005b). The PACIC instrument was translated into French and validated using a back-translation method; it was used in French and English, according to the preference of study participants.
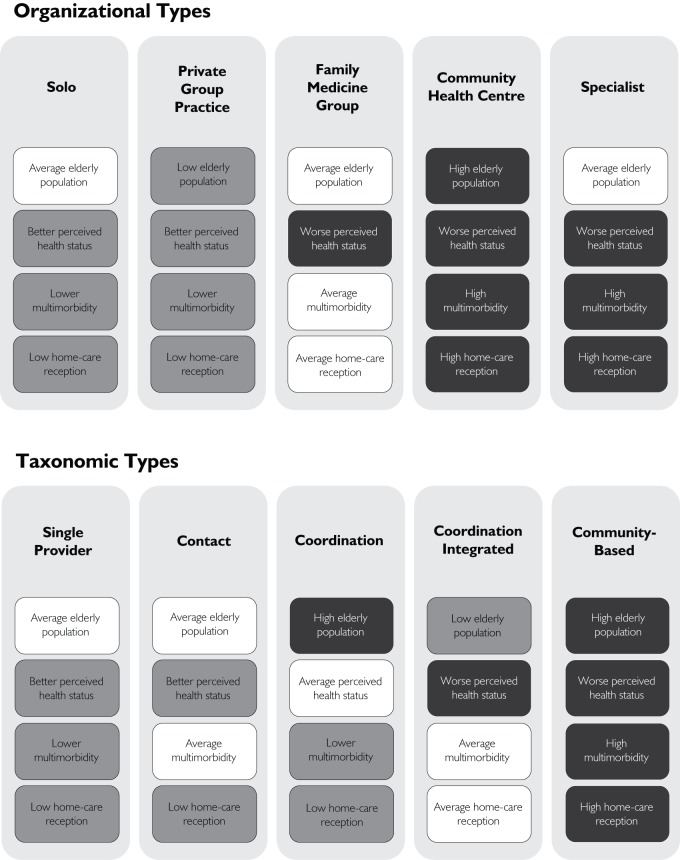
We used two classifications for PHC organizational forms. The first used a typology of PHC clinics according to their official denomination in the study context. These types were (1) solo provider (clinics where only one doctor provides care), (2) group practice (independent group of doctors), (3) family medicine groups (a group of doctors and nurses having a contractual agreement with local health authorities) and (4) community health centres (medical clinics located in a public sector health centre) (Table 1). The reader can find detailed description of these types of organizations in Provost and colleagues (2010), Pineault and colleagues (2011), Hutchison and colleagues (2011) and Lemieux and colleagues (2010).
TABLE 1.
Characteristics of organizational typology models (percentage of patients in each model shown)
| Organizational types | Definition and main characteristics |
|---|---|
| Solo provider 8% |
Medical practice where a single doctor works in privately owned clinic |
| Group Practice 34% |
Group medical practice in privately owned clinic |
| Family Medicine Group / Network 22% |
Group practice (6 to 10 doctors for 10 to 22,000 registered patients |
| Collaboration with multidisciplinary team (ex. nurses) | |
| Patients' registration based on a non-geographic roster | |
| Extended hours of services on evenings/weekends with telephone on-call services for vulnerable patients when the clinic is closed | |
| Local Community Health Centre / Family Medicine unit 36% |
Integrated to the governmental hospitals |
| Complementary primary care services provided outside of the clinic walls (community, schools, work or home | |
| Broad range of services including health promotion and social care |
Source: Levesque et al. 2010; Hutchison et al. 2010
The second classification was based on a taxonomy of PHC organizations that classifies them according to their empirical configuration of characteristics (Pineault et al. 2008; Levesque et al. 2010). This approach categorizes organizations so that it minimizes the heterogeneity within each category and maximizes the heterogeneity between categories. Five taxonomic models were identified: (1) single-provider model, (2) contact model, (3) coordination model, (4) coordination integrated model and (5) community-oriented model (Table 2). Specialty clinics were considered as an additional model of care in both typology and taxonomy analyses because 25% of patients declared that their main source of chronic care was a specialist. In addition, we conducted subanalyses using specific PHC attributes known to be associated with the management of chronic illnesses, such as the use of clinical information systems, the presence of nursing staff with or without an expanded clinical role and the linkages with specialty care through networking activities.
TABLE 2.
Characteristics of organizational taxonomic models (percentage of patients in each model shown)
| Dimensions | Professional models | Communitybased Model | ||||
|---|---|---|---|---|---|---|
| Single-provider 10% |
Contact 24% |
Coordination 5% |
Coordination integrated 27% |
|||
| Vision | Responsibility | Clienteles | Individuals who present | Clienteles | Population-Clienteles | Population-Clienteles |
| Structure | Governance | Private professional | Public | |||
| Resources | Integration | Low-low | Medium-low | Medium-low | High-high | High-average |
| Quantity and variety | Low | Medium | Medium | High | High | |
| Practice | Appointment – walk-in | Mostly by appointment | Mostly walk-in | Mostly by appointment | Mixed | Mixed |
| Scope of services | Narrow | Narrow | Average | Wide | Wide | |
Source: Provost et al. 2010; Pineault et al. 2011; Pineault et al. 2008; Levesque et al. 2010
A multilevel approach to linear regression was used to account for the nesting of the data and to determine the association of PHC types and taxonomy models with PACIC scores (level 1: repeated measures; level 2: individual patient characteristics; level 3: PHC organizations). The intercept-only model was used to determine the amount of variation attributable to each level. Significant covariates in bivariate analyses were entered in sequence in a random intercept model, starting with the lower levels. All continuous predictors were centred on their means. Statistical analyses were done using SPSS 12.0 (2003) as well as HLM 6.03 (2004).
Results
Of the 1,031 patients initially approached by their participating primary care doctor, 776 consented to participate (response rate, 75%). There were 598 participants at 12 months, corresponding to a 77.1% retention rate. Participants lost to follow-up did not differ from those who participated in both baseline and follow-up interviews with regard to type or model of PHC affiliation, but were more likely to report a greater number of co-morbid conditions at baseline and to be users of home care services.
Table 3 describes the characteristics of our sample. Mean age of participants was 67 years old, and approximately one-third were over 75 years of age. More than half were females, and almost three-quarters reported having four or more medical visits in the preceding year. Participants were unevenly sampled from the different organizational types and taxonomic models. Figure 2 provides details of the case mix of patients selected from each organizational types and taxonomic models.
TABLE 3.
Participants' characteristics at baseline (n=776)
| Dimensions | Characteristics | n | Percentage (except if specified otherwise) |
|---|---|---|---|
| Socio-demographics | Mean age | 776 | 67.13 years |
| Mean no. co-morbidities | 776 | 3.6 co-morbidities | |
| Female | 429 | 55.3 | |
| Education ≤ high school | 578 | 74.5 | |
| Health Characteristics | Diabetes | 258 | 33.2 |
| Heart failure | 150 | 19.3 | |
| Chronic arthritis | 211 | 27.2 | |
| COPD | 157 | 20.2 | |
| 6 or more co-morbidities | 195 | 25.1 | |
| Mean self-rated physical health | 776 | 2.77 / 5.00 | |
| Mean self-rated mental health | 776 | 3.07 / 5.00 | |
| Health Services Utilization (12 months) | 4 or more medical consultations | 566 | 73.0 |
| Emergency room visits | 272 | 35.1 | |
| Hospitalization | 188 | 24.2 | |
| PHC Affiliation (Types) | Solo | 66 | 8.5 |
| Group practice | 266 | 34.3 | |
| Family medicine group | 167 | 21.5 | |
| Community health centre | 277 | 35.7 | |
| PHC Affiliation (Taxonomy) | Single-provider model | 77 | 10.0 |
| Contact model | 186 | 24.0 | |
| Coordination model | 39 | 5.0 | |
| Coordination integrated model | 210 | 27.0 | |
| Community-oriented model | 264 | 34.0 |
FIGURE 2.
Case-mix composition characteristics across organizational types and taxonomic models (lower care burden in white and heavier burden in black)
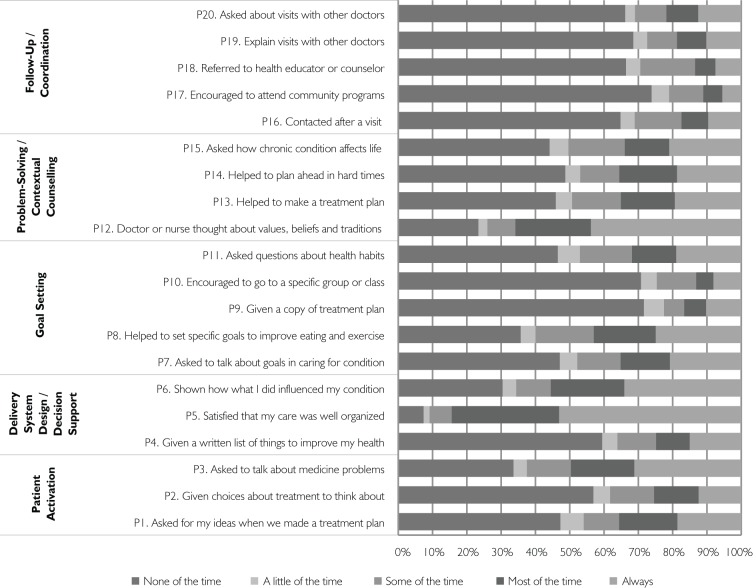
Patient assessment of chronic care was low, with an average overall score of 2.48 (SD 0.98) at baseline and 2.54 (SD 0.97) at 12 months. Figure 3a details responses to the different PACIC items at baseline. Most items received an unfavourable or average rating. For example, 69% of respondents declared that they were never encouraged to participate in a group or class related to their chronic illness, 70% were never given a copy of their treatment plan, 64% were never contacted after a visit and 49% were never questioned about their health habits.
FIGURE 3A.
PACIC results by items at baseline (n=776)
Overall PACIC scores ranged from 2.32 and 2.67 at baseline, and between 2.33 and 2.86 at the 12 months follow-up. Nonetheless, family medicine groups tended to show higher scores both at baseline and at follow-up (2.67 and 2.87), while group practices had lower scores (2.32 and 2.35, respectively).
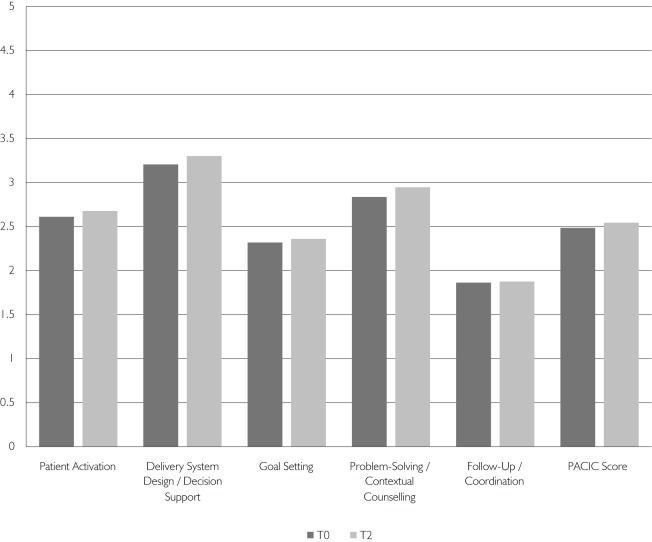
With regard to subdimensions of chronic care, items measuring follow-up/coordination had the most negative scores, while items related to patient activation and delivery system design/decision support received better scores. Figure 3b details the overall PACIC scores and subdimension scores at baseline and follow-up.
FIGURE 3B.
PACIC results by subdimensions at baseline (n=776) and 12 months (n=601)
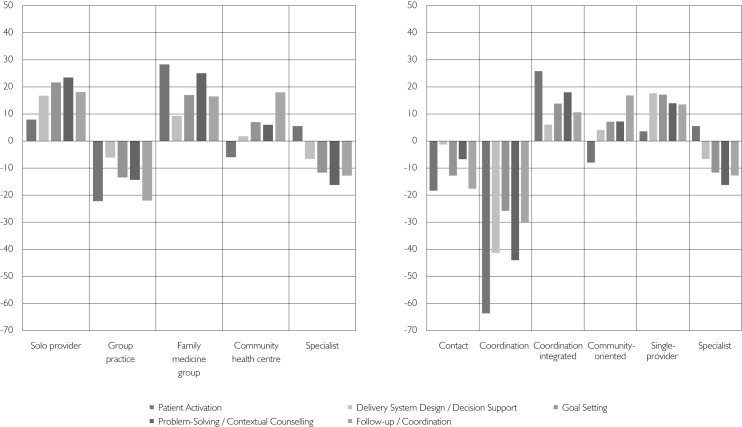
The comparison of the PACIC subscale scores across organizational types at baseline (Figure 4) suggests that specific organizational forms perform better on certain aspects of the CCM than others. Family medicine groups' and community health centres' subscale scores are consistently above the mean as opposed to group practices and specialist clinics. At 12 months, family medicine groups and community health centres are also the only PHC types to present an improvement, albeit modest, in their global PACIC scores, with respective increments of 0.2 (+7.4%) and 0.09 (+3.4%). With regard to the taxonomic models, similar results were found, with coordination-integrated and community-oriented models presenting more favourable results (data not shown).
FIGURE 4.
Average gap (%) in mean PACIC scores by subdimensions and organizational typology and taxonomic models at baseline (T0) (n=776)
Overall, multilevel modelling revealed that differences between organizations accounted for 7.05% of the observed variance in PACIC scores, while between-individual and between-interview variations accounted for 50.03% and 42.63%, respectively.
In multilevel models using PHC typology as an independent variable, patient assessment of chronic care was lower in those affiliated with group practices compared to community health centres (coefficient 0.31; 95% CI –0.54, –0.08). Other types did not significantly differ from community health centres, controlling for covariates (Table 4).
TABLE 4.
Regression models, Patient Assessment of Chronic Illness Care (PACIC) across organizational typology and taxonomy (n=776)
| Coeff. | [95% CI] | Coeff. | [95% CI] | |||
|---|---|---|---|---|---|---|
| Typology model | Taxonomic model | |||||
| Intercept | 2.39 | [2.23;2.54] | 2.51 | [2.29;2.72] | ||
| Level 3 | Models | Community Health Care (CHC) (ref.) | — | — | ||
| Solo Provider | 0.03 | [–0.24; 0.30] | –0.08 | [–0.43; 0.27] | ||
| Group Practice | –0.31 | [–0.54; –0.08] | –0.48 | [–0.76; –0.20] | ||
| Family Medicine Group (FMG) | 0.17 | [–0.08; 0.42] | –0.04 | [–0.29; 0.21] | ||
| Specialty Clinic | –0.15 | [–0.39; 0.09] | –0.56 | [–1.08; –0.04] | ||
| –0.31 | [–0.58; –0.04] | |||||
| Level 2 | Health Care Utilization | Institutional (ref.) | — | — | ||
| Ambulatory Care | 0.10 | [−0.03; 0.24] | 0.11 | [−0.03; 0.24] | ||
| Primary Diagnosis | Arthritis (ref.) | — | — | |||
| Cardiac Failure | 0.14 | [−0.02; 0.29] | 0.14 | [−0.04; 0.32] [0.27; 0.67] | ||
| Diabetes | 0.47 | [0.30; 0.64] | 0.47 | [0.15; 0.43] | ||
| COPD | 0.29 | [0.16; 0.42] | 0.29 | |||
| Self-Rated Health | Mental Health | −0.24 | [−0.53; 0.05] | −0.23 | [−0.52; 0.06] | |
| Physical Health | −0.18 | [−0.38; 0.02] | −0.18 | [−0.37; 0.01] | ||
| Age | −0.01 | [−0.02; −0.004] | −0.01 | [−0.02; −0.004] | ||
| Sex | Women | −0.02 | [−0.19; 0.15] | −0.02 | [−0.19; 0.15] | |
| Level 1 | Repeated Measure | 0.002 | [−0.03; 0.04] | 0.002 | [−0.03; 0.04] | |
Note: Numbers in bold characters identify statistically significant results.
Compared with the community-oriented model, coordination and contact models showed significantly lower mean PACIC scores. Patients seen primarily in specialist clinics also reported significantly lower PACIC scores. In the models using a taxonomic approach, before adjusting for covariates, the highest PACIC score was found in the coordination-integrated model. However, this result did not remain significant after controlling for lower-level variables in the multilevel model.
We conducted subanalyses on specific organizational attributes. Stronger linkage of PHC organizations with specialty care through networking activities was associated with higher PACIC scores (coefficient 0.28; 95% CI 0.12, 0.40). Use of clinical information systems was associated with higher PACIC scores (coefficient 0.22; 95% CI 0.142, 0.42). The presence of nursing staff with a traditional clinical role was also positively associated with PACIC scores, but this association did not reach statistical significance, nor did the presence of nurses with an expanded role (coefficient 0.24; 95% CI –0.08, 0.39 and coefficient 0.04; 95% CI –0.19, 0.27).
Interpretation/Discussion
Our results suggest that major improvements are required in chronic care in both PHC and specialist settings. In fact, patients negatively assessed most items related to the dimensions of the CCM. In general, the few studies that have reported PACIC scores show slightly higher scores than our study, with a range of 2.7 to 3.8 (Wensing et al. 2008; Schmittdiel et al. 2008; Rosemann et al. 2008).
The non-technical aspects of chronic care (such as coordination, patient activation, organization of care) that are depicted in the PACIC, which ensure integrated prevention and management of chronic illness, do not appear to be implemented in our context. This appears to be the case in all models of PHC and specialist care. A low level of assessment of components of integrated care could be a reflection of the poor organizational structure of primary care, more than problems related to the professional practice of individual doctors. While technical aspects of care are mostly driven by professional judgment and prescriptions, the domains addressed by the CCM involve broader interdisciplinary teams and diversity of expertise. In addition, current training of medical doctors and other health professionals might not promote an approach to care that recognizes the crucial role of patients in managing their own chronic illness.
New models of PHC that have emerged recently through primary care reform adopt a more systematic and integrated mode of organization that is more conducive to the provision of aspects of the CCM. PHC organizations associated with a community orientation and more coordinated care appear better suited to chronic care compared to other professional models. Single-provider models also were associated with better chronic care.
Our results also suggest that certain aspects of care are better addressed by certain types of organizations. Patients of solo providers and family medicine groups rated their care more highly with respect to patient activation, goal-setting and problem-solving/contextual counselling. This may suggest that these organizations promote a strong link between patients and providers. The picture is different for group practices and patients followed mostly in specialist clinics for their chronic care, which seem to be underperforming on most of the subdimensions. Finally, the community health centres (CHCs) achieved good results on coordination but had lower results in subdimensions related to the active participation of patients. This could reflect the organizational structure and positioning in the system of CHCs. CHCs in Quebec are integrated with other health facilities, such as hospitals, which are focused on the provision of care for patients, including those with chronic illness. This finding suggests that CHCs could be a base on which to build integrated networks promoting coordination of care for these patients. However, their size (they tend to be very big organizations) and the presence of various established administrative and management protocols could present certain structural issues that need to be overcome in order to enhance their ability to improve chronic care and accommodate individual patient needs.
Surprisingly, patients recruited in PHC clinics but reporting receiving care for their chronic illness in specialist clinics did not show high levels of assessment of chronic care. Although technical quality of care may be very good in these settings, they may lack the organizational infrastructure to address other dimensions of chronic care. In addition, a disease-specific approach could be less conducive to the provision of advice, counselling and care that relates to broad determinants of health and function for persons living with chronic illnesses. Thus, there is a need to better understand the specific characteristics of organizations that promote an integrated approach to the prevention and management of chronic care and to better assess the specific roles of PHC organizations and specialized settings. Results from our study suggest that strategic links between PHC clinics and specialty settings, presence of nursing staff and use of clinical information systems were associated with higher perceived levels of chronic care.
In addition, our study provides some knowledge about the impact of primary care organizational models, in addition to specific provider-level and patient-level influences, in a context of poorly implemented desirable attributes of primary care. There is room for optimism, because many Canadian provinces are moving away from a poorly integrated primary care system towards newer models of care. There is intensification of these efforts to transform primary care and provide more complex and integrated models that should improve patients' assessment of care.
Strengths and limitations
Our study is based on a broad sample of persons living with chronic illnesses that have an affiliation with different types of PHC settings. It is among the first to assess the impact of organizational models on the quality of chronic care received by patients. However, our study evaluated only the patient's perspective of chronic care. A look at both patient reports and technical aspects of care would provide more comprehensive information regarding innovations and improvements needed in management of chronic illnesses.
We used the PACIC, a validated instrument that assesses the elements of the chronic care model from the patient's perspective, often tested in the context of organizational interventions for single diseases. In this study, we did not test any particular intervention but used the PACIC to discern differences across PHC organizational models in the delivery of chronic care. It is the first study to report results of the association between this instrument and the global organization and configuration of primary care using a natural experiment design where healthcare reform is currently being implemented. Our results may not be generalizable to other contexts, although our use of the taxonomic classification may provide insights that may be applicable to other milieus.
We found that a relatively low level of variance was explained by organizational models; nevertheless, this does not necessarily reflect the potential impact of models in the future.
Our analyses could not control for various aspects related to individual providers. This might affect the provision of chronic care for patients. In addition, the variation between types of PHC organizations with regard to acceptance of participation in the study and, mostly, in recruiting patients may also have limited our capacity to find differences among practices.
In general, it is suggested that satisfaction and experience of care measures overestimate ratings of care, possibly because of a social desirability bias (Sitzia and Wood 1997). Thus, it is possible that the true ratings may be even lower than reported in our study. Finally, the PACIC does not control for the specific needs of people to receive such services. The time since diagnosis and the duration of affiliation with a clinic could also influence the reporting of chronic care. In addition, some clinicians might question the necessity to systematically provide all aspects of chronic care as measured by the PACIC instrument. It could be true that achieving a perfect score for all items in the scale is not feasible in clinical practice.
Conclusion
Chronic illness management represents a challenge for the Canadian healthcare system. Currently, PHC settings do not seem to be adequately addressing the challenges related to non-clinical aspects of chronic care. New organizational forms and those adopting a more systemic and integrated approach – while maintaining a strong individual bond between patients and providers – could potentially promote improvements in chronic care.
Acknowledgements
This study has benefited from the support of the Canadian Institutes of Health Research, the Institut national de santé publique du Québec, the Direction de santé publique de l'agence de la santé et des services sociaux de Montréal and the Agence de la santé et des services sociaux de la Montérégie. We received consent from each participant as well as ethics approval from the relevant institutional ethics committees. We would like to acknowledge the contributions of Caroline Dufresne, Houda Ouchêne, Jean-Louis Larochelle, Raynald Pineault and Marjolaine Hamel to this project. Jean-Frédéric Lévesque is currently a recipient of a junior clinical scientist career award from the Fonds recherche santé – Québec. Debbie Feldman is a recipient of a senior scientist award from the Fonds recherche santé – Québec.
Contributor Information
Jean-Frédéric Lévesque, Scientific Director, Institut national de santé publique du Québec, Centre de recherche du centre hospitalier de l'Université de Montréal, QC.
Debbie Ehrmann Feldman, Professor, Université de Montréal, Institut national de santé publique du Québec, Direction de santé publique de Montréal, QC.
Valérie Lemieux, Agente de planification, programmation et recherche, Direction de santé publique de Montréal, Montreal, QC.
André Tourigny, Institut national de santé publique du Québec, Centre de recherche FRSQ du CHA universitaire de Québec, Quebec, QC.
Jean-Pierre Lavoie, Researcher, Centre de santé et de services sociaux, Cavendish, Adjunct Professor, School of Social Work, McGill University, Montreal, QC.
Pierre Tousignant, Associate Professor, McGill University, Direction de santé publique de Montréal, QC.
REFERENCES
- Clair M. 2000. Commission d'étude sur les services de santé et les services sociaux (Commission Clair). Québec: Ministère de la santé et des services sociaux [Google Scholar]
- Detels R., Breslow L. 1997. “Current Scope and Concerns in Public Health.” In Detels R., Hollander M., McEwen J., Omenn G.S. (eds.), Oxford Textbook of Public Health (pp. 3–17). Los Angeles: Oxford University Press [Google Scholar]
- Glasgow R.E., Ory M.G., Klesges L.M., Cifuentes M., Fernald D.H., Green L.A. 2005a. “Practical and Relevant Self-Report Measures of Patient Health Behaviors for Primary Care Research.” Annals of Family Medicine 3: 73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow R.E., Wagner E.H., Kaplan R.M., Vinicor F., Smith L., Norman J. 1999. “If Diabetes Is a Public Health Problem, Why Not Treat It as One? A Population-Based Approach to Chronic Illness.” Annals of Behavioral Medicine 21: 159–70 [DOI] [PubMed] [Google Scholar]
- Glasgow R.E., Wagner E.H., Schaefer J., Mahoney L.D., Reid R.J., Greene S.M. 2005b. “Development and Validation of the Patient Assessment of Chronic Illness Care (PACIC).” Medical Care 43: 436–44 [DOI] [PubMed] [Google Scholar]
- HLM 6: Hierarchical Linear and Nonlinear Modeling. 2004. (Rep. No. Version 6.03). Lincolnwood, IL: Scientific Software International [Google Scholar]
- Hung D.Y., Glasgow R.E., Dickinson L.M., Froshaug D.B., Fernald D.H., Balasubramanian et al B.A. 2008. “The Chronic Care Model and Relationships to Patient Health Status and Health-Related Quality of Life.” American Journal of Preventive Medicine 35: S398–S406 [DOI] [PubMed] [Google Scholar]
- Hung D.Y., Rundall T.G., Crabtree B.F., Tallia A.F., Cohen D.J., Halpin H.A. 2006. “Influence of Primary Care Practice and Provider Attributes on Preventive Service Delivery.” American Journal of Preventive Medicine 30: 413–22 [DOI] [PubMed] [Google Scholar]
- Hutchison B., Lévesque J.-F., Strumpf E., Coyle N. 2011. “Primary Health Care in Canada: Systems in Motion.” Milbank Quarterly 89(2): 256–88 doi: 10.1111/j.1468-0009.2011.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby M.J.L., LeBreton M. 2002. La Santé des Canadiens: Le Rôle du gouvernement fédéral – Volume cinq: Principes et recommandations en vue d'une réforme: Partie I. Ottawa: Comité sénatorial permanent des affaires sociales, des sciences et de la technologie [Google Scholar]
- Le Galès-Camus C., Beaglehole R., Epping-Jordan J.E. 2005. “Preventing Chronic Diseases: A Vital Investment.” Geneva: World Health Organization [Google Scholar]
- Lemieux V., Lévesque J.-F., Ehrmann-Feldman D. 2011. “Are Primary Healthcare Organizational Attributes Associated with Patient Self-Efficacy for Managing Chronic Disease?” Healthcare Policy 6(4): e89–e105 [PMC free article] [PubMed] [Google Scholar]
- Lévesque J.-F., Pineault R., Provost S., Tousignant P., Couture A., Borgès Da Silva R., Breton M. 2010. “Assessing the Evolution of Primary Healthcare Organizations and Their Performance (2005–2010) in Two Regions of Quebec Province: Montreal and Monteregie.” BMC Family Practice 11: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddy C., Singh J., Hogg W., Dahrouge S., Taljaard M. 2011. “Comparison of Primary Care Models in the Prevention of Cardiovascular Disease – A Cross-Sectional Study.” BMC Family Practice 18(12): 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna M.T., Taylor W.R., Marks J.S., Koplan J.P. 1998. “Current Issues and Challenges in Chronic Disease Control.” In Brownson R.C., Remington P. L. (eds.), Chronic Disease Epidemiology and Control (pp. 1–26). Washington, DC: American Public Health Association [Google Scholar]
- Pineault R., Lévesque J.F., Roberge D., Hamel M., Lamarche P., Haggerty J. 2008. L'Accessibilité et la continuité des services de santé: Une étude sur la première ligne au Québec. Rapport de recherche. Montréal: Direction de santé publique de l'agence de la santé et des services sociaux de Montréal, Institut national de santé publique, Centre de recherche de l'Hôpital Charles LeMoyne [Google Scholar]
- Pineault R., Provost S., Hamel M., Couture A., Lévesque J.-F. 2011. “The Influence of Primary Healthcare Organizational Models on the Patients' Experience of Care in Different Chronic Disease Situations.” Chronic Diseases and Injuries in Canada 31(3): 109–20 [PubMed] [Google Scholar]
- Provost S., Pineault R., Lévesque J.-F., Groulx S., Baron G., Roberge D., Hamel M. 2010. “Does Receiving Clinical Preventive Services Vary across Different Types of Primary Healthcare Organizations?” Healthcare Policy 6(2): 67–83 [PMC free article] [PubMed] [Google Scholar]
- Romanow R.J. Guidé par nos valeurs: L'Avenir des soins de santé du Canada (Rapport final). Ottawa: Commission sur l'avenir des soins de santé du Canada: 2002a. [Google Scholar]
- Romanow R.J. Préparer l'avenir des soins de santé. Rapport d'étape, Commission sur l'avenir des soins de santé au Canada. Saskatoon: Santé Canada: 2002b. [Google Scholar]
- Rosemann T., Laux G., Szecsenyi J., Grol R. 2008. “The Chronic Care Model: Congruency and Predictors among Primary Care Patients with Osteoarthritis.” Quality and Safety in Health Care 17: 442–46 [DOI] [PubMed] [Google Scholar]
- Rothenberg R.B., Koplan J. P. 1990. “Chronic Disease in the 1990s.” Annual Review of Public Health 11: 267–96 [DOI] [PubMed] [Google Scholar]
- Russell G.M., Dahrouge S., Hogg W., Geneau R., Muldoon L., Tuna M. 2009. “Managing Chronic Disease in Ontario Primary Care: The Impact of Organizational Factors.” Annals of Family Medicine 7(4): 309–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell G., Dahrouge S., Tuna M., Hogg W., Geneau R., Gebremichael G. 2010. “Getting It All Done. Organizational Factors Linked with Comprehensive Primary Care.” Family Practice 27(5): 535–41 [DOI] [PubMed] [Google Scholar]
- Schmittdiel J., Mosen D.M., Glasgow R.E., Hibbard J., Remmers C., Bellows J. 2008. “Patient Assessment of Chronic Illness Care (PACIC) and Improved Patient-Centered Outcomes for Chronic Conditions.” Journal of General Internal Medicine 23: 77–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D., Ham C. 2006. “Improving Care for People with Long-Term Conditions: A Review of UK and International Frameworks.” Birmingham, UK: Health Services Management Centre NHS Institute for Innovation and Improvement [Google Scholar]
- Sitzia J., Wood N. 1997. “Patient Satisfaction: A Review of Issues and Concepts.” Social Science and Medicine 45(12): 1829–43 [DOI] [PubMed] [Google Scholar]
- SPSS Base 12.0 for Windows. 2003. Chicago: SPSS [Google Scholar]
- Tsai A.C., Morton S.C., Mangione C.M., Keeler E.B. 2005. “A Meta-analysis of Interventions to Improve Care for Chronic Illnesses.” American Journal of Managed Care 11: 478–88 [PMC free article] [PubMed] [Google Scholar]
- Wagner E.H., Austin B.T., Davis C., Hindmarsh M., Schaefer J., Bonomi A. 2001. “Improving Chronic Illness Care: Translating Evidence into Action.” Health Affairs 20: 64–78 [DOI] [PubMed] [Google Scholar]
- Wensing M., van Lieshout J., Jung H.P., Hermsen J., Rosemann T. 2008. “The Patients Assessment Chronic Illness Care (PACIC) Questionnaire in the Netherlands: A Validation Study in Rural General Practice.” BMC Health Services Research 8: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yach D., Hawkes C., Gould C.L., Hofman K.J. 2004. “The Global Burden of Chronic Diseases: Overcoming Impediments to Prevention and Control.” Journal of the American Medical Association 291: 2616–22 [DOI] [PubMed] [Google Scholar]