Abstract
Background
Trials comparing hypertension monotherapies have found either no difference or modest differences in blood pressure (BP) and cardiovascular (CV) events. However, no trial has assessed the comparative effectiveness of second-line therapy in patients whose BP was not controlled on a thiazide diuretic.
Methods and Results
Observational study conducted using a hypertension registry of adults enrolled in 3 large integrated healthcare delivery systems from 2002-2007. Patients newly started on thiazide monotherapy whose BP remained uncontrolled were observed following addition of either an ACE inhibitor or beta-blocker for subsequent BP control and CV events. Patients for whom either add-on drug was indicated or contraindicated were excluded.
After adjustment for patient characteristics and study year, BP control during the subsequent 6-18 months was comparable for the two agents (70.5% ACE, 69.0% Beta-blockers; p=0.09). Rates of incident myocardial infarction (HR 1.05, 95% CI 0.69 – 1.58) and stroke (1.01. 95% CI 0.68 – 1.52) were also similar for the ACE inhibitor and beta-blocker groups during an average of 2. 3 years of follow-up. There were also no differences in heart failure or renal function.
Conclusions
ACE inhibitors and beta-blockers are equally effective in lowering blood pressure and preventing cardiovascular events for patients whose blood pressure is not controlled on a thiazide diuretic alone and who have no compelling indication for a specific second-line agent.
Keywords: hypertension, comparative effectiveness, diuretics, cardiovascular diseases
INTRODUCTION
Hypertension affects 29% of US adults.1 There is a strong and linear association between the level of blood pressure (BP) and subsequent risk of cardiovascular events.2 Prior studies have also clearly demonstrated that hypertension treatment reduces morbidity and mortality.3
The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) study found that thiazide diuretics are efficacious for reducing blood pressure and cardiovascular events, and thiazides are currently recommended as first-line therapy for patients with essential hypertension.4,5 However, control of blood pressure to guideline-recommended levels often requires two or more agents and the optimal second-line agent for patients whose blood pressure is not adequately controlled on a thiazide alone is unknown.6 Selection of optimal add-on therapy to a thiazide diuretic was identified as a key question for which there is currently insufficient data by the National Heart Lung and Blood Institute working group on future directions in hypertension research.7
The objective of this study was to assess the comparative effectiveness of two commonly used second-line antihypertensive agents: angiotensin-converting enzyme (ACE) inhibitors and beta-blockers. We hypothesized that after controlling for baseline BP level, there would be no difference between ACE inhibitors and beta-blockers in blood pressure control at one year. Similarly, we hypothesized that there would be no difference in the incidence of myocardial infarction, stroke, congestive heart failure, or chronic kidney disease between patients receiving ACE inhibitors vs. beta-blockers as second-line therapy.
METHODS
Study Setting
The study was conducted in three large, integrated healthcare delivery systems that collectively care for over 4 million people: Kaiser Permanente Colorado, Kaiser Permanente Northern California, and HealthPartners in Minneapolis. Kaiser Permanente Colorado has over 460,000 enrollees in the Denver, CO metropolitan area and contracts with over 600 physicians to deliver care in 18 outpatient clinics. HealthPartners serves over 620,000 members in the Minneapolis, MN metropolitan area with more than 200 physicians who work in 22 clinics. Kaiser Permanente Northern California provides care to over 3.2 million members and contracts with a medical group of more than 6,000 physicians who treat patients at 39 clinics. Electronic data on longitudinal blood pressure measurements, medication dispensings, laboratory test results, diagnoses, and healthcare utilization was available from electronic health records and administrative databases at all sites dating back to January 2000. Data from each of the health plans were restructured into a common, standardized format with identical variable names, formats, and specifications and identical variable definitions, labels, and coding.
In order to confirm that algorithms designed to identify hypertensive patients were valid and the degree to which the analytic data were identical to the source data, a chart review of 450 randomly selected charts (150 from each site) was conducted of patients who had been continuously enrolled with pharmacy coverage for 12 months prior to the date of entry into the registry. To confirm that hypertension was in fact incident on the date assigned by the algorithm, the auditors examined whether there was mention of hypertension in the physician note, a hypertension diagnosis code or evidence of antihypertensive drug treatment at the visit preceding the incident date or before. Five (1%) audits showed evidence of a hypertension diagnosis and 16 (4%) audits revealed use of an antihypertensive drug for hypertension prior to the incident date, indicating 96% accuracy of this method for excluding pre-existing hypertension. Chart auditors also recorded the blood pressure values in the vital signs field or in the physician notes from the electronic medical record on that date. The blood pressure was an exact match between the analytic and source data in all 300 audits from HealthPartners and Kaiser Permanente Colorado and all 34 audits from 2007 at Kaiser Northern California. In Northern California, the electronic blood pressure data was recorded in categories prior to 2007; the electronic categorical data matched the BP in the chart in 109/116 audits (94%), for an overall agreement rate of 98%.
Study Population
The study population included all patients 18 years or older with incident hypertension during 2002-2007 who were started on an ACE inhibitor or beta-blocker after failure of initial thiazide therapy (Figure 1). To assemble this cohort we first identified all patients with a diagnosis code of hypertension during the study period. To identify those with incident hypertension, we excluded patients with prior diagnoses or treatment for hypertension based on pharmacy dispensing data. We also excluded patients who did not have continuous health plan membership with a pharmacy benefit for at least one year prior to their first hypertension diagnosis since prevalent hypertension could not be reliably excluded in this group.
Figure 1.
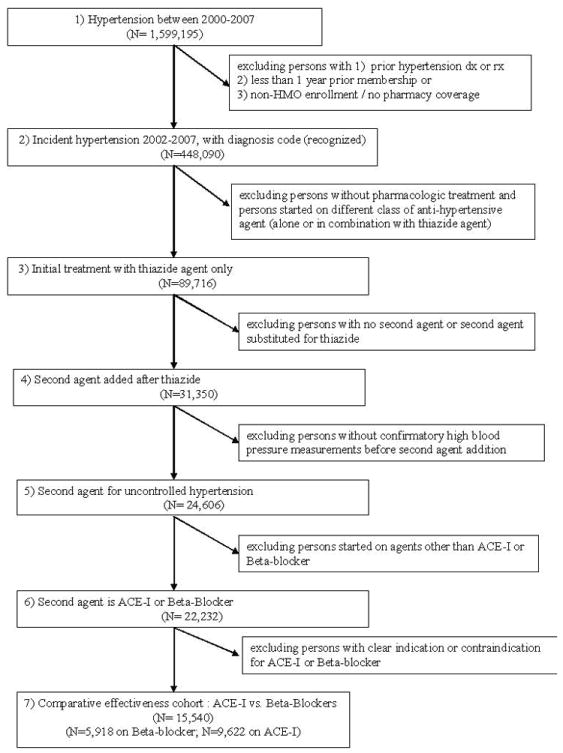
Flow chart for cohort selection
Among the patients with incident hypertension, we identified those initially treated with a thiazide diuretic as first-line therapy. We excluded patients without a pharmacy benefit, those not treated with any anti-hypertensive medication, and those started on an anti-hypertensive agent other than a thiazide diuretic (including those initiated on multiple anti-hypertensive agents or combination therapy). The vast majority of patients initiated on a thiazide were prescribed hydrochlorothiazide. The initial dose of hydrochlorothiazide was 25 mg in 63% of patients and 50 mg in 17% of patients.
Next we identified patients who were started on an ACE inhibitor or beta-blocker as second-line therapy. To ensure that the medication was prescribed for uncontrolled blood pressure, we excluded patients who did not have elevated blood pressure at the time of the addition of the second agent. We also excluded patients who were prescribed a second-line agent other than an ACE inhibitor or beta-blocker, and those who did not continue on their thiazide after the new anti-hypertensive agent was started (since the prescription of the ACE inhibitor or beta-blocker in these patients may represent a medication substitution rather than an add-on therapy).
To reduce potential confounding bias, we excluded all patients with a specific indication or contraindication for either an ACE inhibitor or beta-blocker. Patients with a history of any of the following conditions were excluded: diabetes, chronic kidney disease, asthma, albuminuria, myocardial infarction, heart failure, 2nd or 3rd degree heart block, atrial fibrillation, other arrhythmias (i.e. ventricular or atrial tachycardia), peripheral vascular disease, a history of percutaneous coronary intervention or coronary artery bypass graft procedures, cerebrovascular disease, migraine headaches, pregnancy or angioedema. The presence of these conditions was determined based on ICD-9 diagnosis codes, problem list entries, medications, and laboratory data according to pre-specified algorithms. The final analytic sample of patients with incident hypertension who were started on a second-line agent after failure of initial thiazide therapy was 9,622 on ACE inhibitors and 5,918 on beta-blockers.
Outcomes
The primary outcome of the study was blood pressure control at one year after initiation of the second-line agent. Blood pressure was considered controlled if it was below 140/90 mm Hg. The blood pressure measurement closest to one year following initiation of the second-line agent was used for this assessment. Overall, 5245 patients (3,698 in the ACE inhibitor group and 1,544 in the beta-blocker group) were excluded from the analysis of blood pressure control because they did not have a blood pressure measurement recorded in the 6-18 months following initiation of the second-line agent. Of those who were excluded due to a lack of blood pressure measurement, the majority (60%) were enrolled in health plan for less than 1 year following initiation of the 2nd line agent.
Secondary outcomes included incident cases of myocardial infarction, congestive heart failure, stroke, and chronic kidney disease following initiation of the second-line agent. The study cohort was followed for a maximum of six years with most persons followed for just over 2 years (median 2.3 years (interquartile range 1.2-3.7 years)). We also performed a sensitivity analysis looking at these outcomes within the first year. The results of the sensitivity analysis were consistent with the results of the primary analysis and therefore are not presented. Primary hospital discharge diagnoses were used to identify incident cases of myocardial infarction (ICD-9 codes 410.xx), congestive heart failure (ICD-9 codes 428.xx), and stroke (ICD-9 codes 430.xx- 434.xx, 436.xx, 852.0, 852.2, 852.4, 853.0). Both diagnosis data and laboratory measures of renal function were used to identify incident cases of chronic kidney disease. Patients with previously normal renal function were considered to have progressed to chronic kidney disease if, following initiation of second-line therapy, they had a new diagnosis of kidney disease (ICD-9 codes: 585.1-585.9) or an estimated glomerular filtration rate less than 60 ml/min/1.73 m2.
Additional outcomes assessed included the proportion of patients in each group with: a) no change (additions or substitutions) to their anti-hypertensive medication regimen; b) addition of a 3rd-line anti-hypertensive agent, and c) persistence of the 2nd-line agent at 1-year. Persistence at 1-year was reported as a dichotomous variable and a patient was considered “persistent” if they filled a prescription for the 2nd-line agent between 10-14 months after initiation of initial prescription (http://www.ispor.org/sigs/medcompliance).
Statistical Analyses
We used an intention-to-treat approach to assess the comparative effectiveness of ACE inhibitors vs. beta-blockers as second-line anti-hypertensive agents. To characterize the study population at baseline, we calculated descriptive statistics using means and standard deviations for continuous variables and compared the two groups using t-tests. For categorical variables we calculated percents and compared the two groups using chi-square tests. The proportions of patients in each group with no change to their anti-hypertensive medication regimen, addition of a 3rd-line anti-hypertensive agent, and persistence at 1-year were also compared using chi-square tests.
We used logistic regression to compare the proportion of patients in the ACE inhibitor and beta-blocker groups achieving blood pressure control at 1-year. Because the outcome of blood pressure control is not rare, we present estimated relative risks using the method of Zhang et al. instead of odds ratios.8 To adjust for differences in baseline demographic and clinical factors we performed a propensity score analysis and created inverse probability-weighted estimators.9-11 Propensity scores were created using all the covariates in Table 1. Because of changing prescription patterns over time, interactions with the initiation year of the 2nd agent and all covariates were also included in the models creating the propensity scores. Stabilized inverse probability weights were created and used to adjust for covariates in the outcome models.12 Stabilized weights reduce the possibility of large changes to estimates being caused by a few, unusual observations.13 Stabilized weights also realign weights to range 0-1+ so that the resulting sample size is comparable to the original population and standard errors are more appropriate. The consistency of ACE inhibitor vs. beta-blocker results within subgroups of site, age, gender and year was tested by interactions tests in the full model and estimated within strata effects.
Table 1.
Comparisons of persons initiated on ACE-I* vs. Beta-blocker as a second agent
| ACE-I* (N=9,622) |
Beta-blocker (N=5,918) |
p value† | ||
|---|---|---|---|---|
| Year of 2nd agent start | ||||
| 2002 | 30.8% | 69.2% | <0.001 | |
| 2003 | 39.9% | 60.1% | ||
| 2004 | 51.4% | 48.6% | ||
| 2005 | 62.8% | 37.3% | ||
| 2006 | 81.8% | 18.2% | ||
| 2007 | 85.1% | 14.9% | ||
| Adjusted for year of 2nd agent start | ||||
|
| ||||
| Age in years | 55.9 | 55.3 | 0.006 | |
| Male gender | 47.7% | 42.2% | <0.001 | |
| Mean # days on thiazide prior to 2nd agent start | 336 | 308 | <0.001 | |
| Average # of visits during year prior to thiazide initiation | 1.7 | 1.7 | 0.57 | |
| Mean Systolic BP‡ (closest measure prior to or same day as 2nd agent start) | 151.8 | 152.7 | <0.001 | |
| Mean Diastolic BP‡ (closest measure prior to or same day as 2nd agent start) | 89.0 | 90.0 | <0.001 | |
| Chronic Obstructive Pulmonary Disease | 0.6% | 0.4% | 0.09 | |
| Hyperlipidemia | 4.0% | 5.0% | 0.005 | |
| Cancer | 1.7 | 2.3 | 0.01 | |
| Dementia | 0.03% | 0.06% | 0.35 | |
| Chronic liver disease | 0.1% | 0.2% | 0.44 | |
| Depression | 13.6% | 14.5% | 0.11 | |
| Minimum of 1 year enrollment after 2nd line initiation§ (N=12,371) | 80.0% | 79.6% | 0.61 | |
| Blood pressure measured 12 months after 2nd line agent start∥ (N=10,298) | 83.7% | 83.0% | 0.36 | |
ACE-I: angiotensin-converting enzyme inhibitor
p value from chi-square test for categorical variables and t-test for continuous variables
BP: blood pressure
persons with 2nd agent start in 2007 all have < 1 year enrollment before Dec. 31 2007. (Years 2002-2006 have >94% with one year enrollment minimum)
Among persons with 1 year enrollment after 2nd line initiation
We used Cox proportional hazards model to assess the association between the specific 2nd line agent and outcomes of incident myocardial infarction, stroke, heart failure and progression to kidney disease.1-16 Stabilized inverse propensity scores were similarly used to adjust for potential confounders and differing prescription patterns over time in these models. For models predicting incident kidney disease, the closest estimated glomerular filtration rate (eGFR) measure preceding the 2nd line agent was also incorporated into the propensity model.
RESULTS
The baseline characteristics for the ACE inhibitor and beta-blocker groups are shown in Table 1. In the study cohort, beta-blockers were much more commonly prescribed in the earlier years of the study period, while ACE inhibitors were more commonly prescribed in the latter years. After adjusting for the year the 2nd line agent start, the remaining baseline patient characteristics were similar in the two groups though some statistical differences were evident in this relatively large cohort. Mean age was slightly higher for ACE inhibitor users than those on beta-blockers (55.9 vs. 55.3 years). Blood pressure was slightly higher for patients started on beta-blockers (152.7/90.0 vs. 151.8/89.0) and ACE inhibitor users were on average treated with thiazide monotherapy for longer time period before being started on a second agent. ACE inhibitor users had a higher percentage of men while beta-blocker users had a higher percentage of persons with a diagnosis of hyperlipidemia.
Table 2 presents results for the outcome of blood pressure control at 12 months. Crude results suggested a slightly higher blood pressure control rate for ACE inhibitors; however, in the adjusted models, the rates of BP control were comparable for the two agents (70.5% ACE inhibitors, 69.0% Beta-blockers; p=0.09). Interactions tests and results by subgroup strata were similar by gender, age (± 65 years), site and year (p values all >0.20). Adjusted blood pressure control rates for ACE inhibitor and beta-blocker groups by year are shown in Figure 2. This figure shows increasing blood pressure control rates in later years but comparable rates of control for ACE inhibitor vs. beta-blocker groups within each year.
Table 2.
| Estimated percentage with BP‡ controlled at 12 months | ||||
|---|---|---|---|---|
| ACE-I† | Beta-blocker (BB) | p value | RR (95% CI)§ ACE vs. BB |
|
| Univariate | 72.0% | 67.9% | <0.001 | 1.06 (1.03, 1.09) |
| Adjusted∥ | 70.5 | 69.0% | 0.09 | 1.02 (0.99, 1.05) |
closest blood pressure to 12 months after 2nd line initiation (range 6-18 months)
ACE-I: angiotensin-converting enzyme inhibitor
BP: blood pressure
estimated RR and 95% CI from logistic regression models with non-rare outcome
adjusted by inverse propensity score weights
Figure 2.
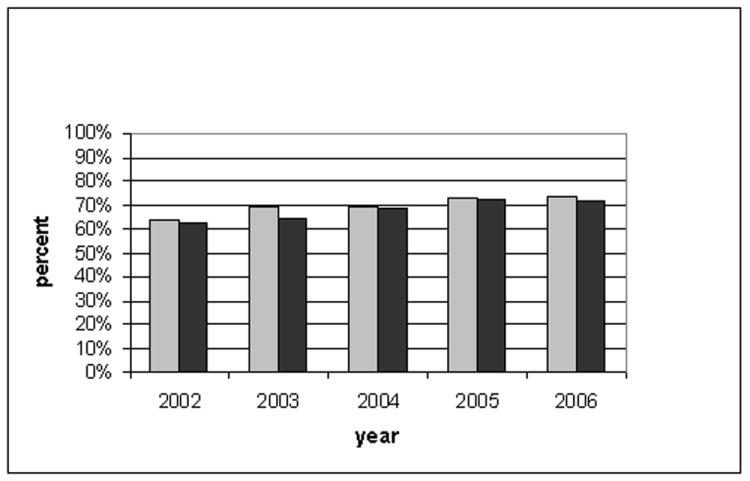
Percentage with blood pressure controlled at 12 months by year of 2nd agent start
Outcomes of hypertension sequelae (myocardial infarction, stroke, congestive heart failure, and kidney disease) are reported in Table 3. Rates of incident myocardial infarction and stroke were similar for the ACE inhibitor and beta-blocker groups as evidenced by hazard ratios close to one. Rates of incident congestive heart failure were not significantly different by second line agent, however, we had limited statistical power to find a difference due to the low incidence of congestive heart failure. There was also no difference in subsequent kidney disease in the ACE inhibitor group compared to those in the beta-blocker group (Hazard ratio= 0.95 (95% CI 0.85, 1.05)).
Table 3.
Adjusted hazard ratios* for incident myocardial infarction, stroke, chronic kidney disease, or congestive heart failure comparing ACE-I† vs. Beta-blocker second line initiation (N=15,532‡)
| Incident : | Number of events | Adjusted hazard ratio* ACE-I vs. BB§ | 95% CI | p value |
|---|---|---|---|---|
| Myocardial Infarction | 96 | 1.05 | (0.69, 1.58) | 0.83 |
| Stroke | 101 | 1.01 | (0.68, 1.52) | 0.95 |
| Congestive Heart Failure | 23 | 1.71 | (0.69, 4.23) | 0.25 |
| Chronic Kidney Disease∥ | 1,445 | 0.95 | (0.85, 1.05) | 0.33 |
adjusted by inverse propensity score weights
ACE-I: angiotensin-converting enzyme inhibitor
eight persons missing initial blood pressure measurements or comorbidity information
BB: beta-blocker
additionally adjusted for eGlomerular Filtration Rate (N=14,080)
The proportion of patients who were dispensed a new blood pressure lowering agent over a one year time frame was slightly higher for patient in the ACE inhibitor group compared to the beta-blocker group (24.0% vs. 21.9%, chi-square p=0.003). Among persons with a new anti-hypertensive agent prescribed within one year, the new agent was more often an apparent substitution (2nd agent no longer dispensed) for ACE inhibitors than beta-blockers (18.9% vs. 9.3%) and less often an addition (i.e. 2nd agent dispensed after new agent started) (5.2% ACE-inhibitors, 12.6% beta-blockers).
DISCUSSION
The objective of this study was to assess the comparative effectiveness of ACE inhibitors vs. beta-blockers as second-line therapy in patients whose blood pressure was not controlled on a thiazide diuretic alone and did not have a compelling indication for a specific second-line agent. This is an important clinical question that has not been addressed in any trials to date. Blood pressure control rates were similar when either ACE inhibitors or beta-blockers were added to a thiazide diuretic. Furthermore, rates of hypertension sequelae, including incident myocardial infarction, stroke, and kidney disease were also comparable between the ACE inhibitor and beta-blocker groups.
The findings of our study are consistent with the results of randomized controlled trials of hypertension monotherapy that have found either no difference or only a modest differences in the degree of blood pressure and cardiovascular disease risk reduction in patients treated with any of the major classes of anti-hypertensive medications.3,17 Our results are also consistent with a meta-analysis of clinical trials that concluded that each of the main classes of hypertensive agents reduce blood pressure to the same extent and the degree of blood pressure reduction alone accounts for the effect of these drugs in preventing coronary heart disease and stroke in patients with uncomplicated hypertension.18,19 The finding of a somewhat higher rate of substitution of another agent for ACE inhibitors than for beta-blockers is also consistent with the relatively high incidence of chronic dry cough with ACE inhibitors. In several blinded trials, ACE inhibitors had higher discontinuation rates than placebo or comparator drugs. 3,20,21
Most clinical trials of blood pressure lowering medications have focused on the choice of initial hypertension agent. Based on the totality of the evidence, the JNC7 guidelines recommend thiazide diuretics as initial therapy for uncomplicated hypertension, either alone or in combination with other agents.22 Two recent clinical trials (VALUE and ASCOT) explicitly tested adding different 2nd agents in a stepped care regimen.23,24 However, neither of these trials used thiazide monotherapy as the initial drug treatment. Another large trial (ACCOMPLISH) has compared two different combination therapy regimens in which both drugs were started simultaneously (ACE inhibitor plus calcium channel blocker compared to ACE inhibitor plus thiazide).25 Although this trial, published in late 2008, found a statistically significant difference in the important composite outcome of cardiovascular death, non-fatal myocardial infarction and non-fatal stroke, the trial design has been criticized for its use of low-dose hydrochlorothiazide (12.5 mg).26 In contrast, our study specifically addresses the question of optimal add-on therapy for patients whose blood pressure in not controlled by a thiazide alone (primarily moderate-dose hydrochlorothiazide 25-50 mg), a question designated as high priority by experts in hypertension research.5 Our findings that ACE inhibitors and beta-blockers are equally effective in lowering blood pressure and preventing cardiovascular events suggest that either is a reasonable choice for add-on therapy for patients not controlled on a thiazide monotherapy.
We would like to acknowledge several potential limitations of this study. Patients were not randomized to second-line anti-hypertensive therapy. The decision to choose one agent instead of another may be related to factors associated with blood pressure control or cardiovascular outcomes. To reduce the impact of potential confounding by indication bias, we restricted the study cohort to incident cases of hypertension and patients without indications or contraindications to either ACE inhibitors or beta-blockers. We also used propensity matching to compare the effectiveness of ACE inhibitors vs. beta-blockers in strata within which patients were comparable with regards to baseline co-variates and on the predicted probability of receiving each treatment. Nevertheless, despite the methodological rigor of our study design and analysis, we may not have been able to eliminate the impact of unmeasured confounding.
Additional considerations include limited generalizability due to the geographic location of study sites and selection effects related to enrollment for care in the participating health plans. However, the study populations were broadly representative of their geographic regions, and the results of subgroup analyses were consistent with the primary analysis. Due to the available sample size and follow-up period we had somewhat limited statistical power to detect differences in cardiovascular events between in the ACE inhibitor and beta-blocker group. We ascertained clinical outcomes using data captured in the electronic medical records and through insurance claims. Finally, due to the composition of health plan formularies we were unable to assess for differences in the effectiveness of individual drugs within the ACE inhibitor and beta-blocker groups and due to their low use in the participating health plans we were unable to evaluate the effectiveness of calcium channel blockers and angiotensin-receptor-blockers (ARBs) as second-line agents.
In conclusion, we found that that ACE inhibitors and beta-blockers are equally effective in lowering blood pressure and preventing cardiovascular events for patients whose blood pressure is not controlled on a thiazide diuretic alone and who have no compelling indication for a specific second-line agent. This suggests that both ACE inhibitors and beta-blockers are a reasonable choice for add-on therapy for patients with essential hypertension not controlled on a thiazide monotherapy.
WHAT IS KNOWN
The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) study found that thiazide diuretics are efficacious for reducing blood pressure and cardiovascular events, and thiazides are currently recommended as first-line therapy for patients with essential hypertension.
However, control of blood pressure to guideline-recommended levels often requires two or more agents and the optimal second-line agent for patients whose blood pressure is not adequately controlled on a thiazide alone is unknown.
WHAT THE STUDY ADDS
The objective of this study was to assess the comparative effectiveness of two commonly used second-line antihypertensive agents: angiotensin-converting enzyme (ACE) inhibitors and beta-blockers.
We found that that ACE inhibitors and beta-blockers are equally effective in lowering blood pressure and preventing cardiovascular events for patients whose blood pressure was not controlled on a thiazide diuretic alone and who have no compelling indication for a specific second-line agent.
Acknowledgments
Funding Sources: The study was funded by the National Institutes of Health, Agency for Healthcare Research and Quality (DEcIDE Program, contract #HHSA29020050033, Richard Platt, principal investigator). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the National Institutes of Health.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Prospective Studies Collaboration Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 3.Turnbull F. Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–35. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 4.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 5.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 6.Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, Black HR, Hamilton BP, Holland J, Nwachuku C, Papademetriou V, Probstfield J, Wright JT, Jr, Alderman MH, Weiss RJ, Piller L, Bettencourt J, Walsh SM ALLHAT Collaborative Research Group. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) J Clin Hypertens (Greenwich) 2002;4:393–404. doi: 10.1111/j.1524-6175.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- 7.The National Heart, Lung, and Blood Institute Working Group on Future Directions in Hypertension Treatment Trials. Major clinical trials of hypertension: what should be done next? Hypertension. 2005;46:1–6. [Google Scholar]
- 8.Zhang J, Yu KF. What’s the relative risk. A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 9.Kosuke I, van Dyk DA. Causal inference with general treatment regimes: Generalizing the propensity score. J of Am Stat Assoc. 2004;99:854–66. [Google Scholar]
- 10.Rosenbaum PR, R D. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 11.Rosenbaum PR, R D. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–24. [Google Scholar]
- 12.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45:S103–7. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 13.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Hosmer DW, Jr, L S. Applied survival analysis: regression modeling of time to event data. New York, NY: John Wiley & Sons, Inc; 1999. [Google Scholar]
- 15.Moeschberger M, Ka J. Survival analysis: Techniques for censored and truncated data. New York: Springer; 1997. [Google Scholar]
- 16.Hougaard P. Frailty models for survival data. Lifetime Data Analysis. 1995;1:255–273. doi: 10.1007/BF00985760. [DOI] [PubMed] [Google Scholar]
- 17.Wright JT, Jr, Probstfield JL, Cushman WC, Pressel SL, Cutler JA, Davis BR, Einhorn PT, Rahman M, Whelton PK, Ford CE, Haywood LJ, Margolis KL, Oparil S, Black HR, Alderman MH ALLHAT Collaborative Research Group. ALLHAT findings revisited in the context of subsequent analyses, other trials, and meta-analyses. Arch Intern Med. 2009;169:832–42. doi: 10.1001/archinternmed.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Law MR, Morris JK, Wald NJ. Use of Blood Pressure Lowering Drugs in the Prevention of Cardiovascular Disease: Meta-Analysis of 147 Randomised Trials in the Context of Expectations From Prospective Epidemiological Studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–41. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 21.Neaton J, Grimm R, Prineas R, Stamler J, Grandits G, Elmer P, Cutler J, Flack J, Schoenberger J, McDonald R, Lewis C, Liebson P. Treatment Of Mild Hypertension Study final results. JAMA. 1993;270:713–724. [PubMed] [Google Scholar]
- 22.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 23.Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, Plat F, Schork A, Smith B, Zanchetti A VALUE trial group. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–31. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 24.Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required vs. atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 25.Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. NEJM. 2008;359:2417–28. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 26.Wright JT, Jr, Probstfield JL, Cushman WC, Pressel SL, Cutler JA, Davis BR, Einhorn PT, Rahman M, Whelton PK, Ford CE, Haywood LJ, Margolis KL, Oparil S, Black HR, Alderman MH for the ALLHAT Collaborative Research Group. ALLHAT Findings Revisited in the Context of Subsequent Analyses, Other Trials, and Meta-analyses. Arch Intern Med. 2009;169:832–842. doi: 10.1001/archinternmed.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]


