SUMMARY
We show functional-anatomical organization of motion direction in mouse dorsal lateral geniculate nucleus (dLGN) using the first two-photon calcium imaging of dense populations in thalamus. Surprisingly, the superficial 75μm region contains anterior and posterior direction-selective neurons (DSLGNs) intermingled with non-direction-selective neurons, while upward and downward-selective neurons are nearly absent. Unexpectedly, the remaining neurons encode both anterior and posterior directions, forming horizontal motion-axis selectivity. A model of random wiring consistent with these results makes quantitative predictions about the connectivity of direction-selective retinal ganglion cell (DSRGC) inputs to the superficial dLGN. DSLGNs are more sharply tuned than DSRGCs. These results suggest dLGN maintains and sharpens retinal direction selectivity, and integrates opposing DSRGC subtypes in a functional-anatomical region, perhaps forming a novel feature representation for horizontal-axis motion, contrary to dLGN being a simple relay. Furthermore, they support recent conjecture that cortical direction and orientation selectivity emerge in part from a previously undescribed motion-selective retinogeniculate pathway.
INTRODUCTION
Visual motion perception depends on the computation of direction of motion from spatiotemporal luminance patterns. It is widely believed that these computations emerge de novo in the cortex, independently of retinogeniculate direction-selective (DS) inputs (Hubel and Wiesel, 1961; Peterson et al., 2004). This view persists in spite of the fact that motion is also computed in the retina (Wei et al., 2010; Briggman et al., 2011), where subtypes of direction-selective retinal ganglion cells (DSRGCs) encode each of four cardinal directions (On-Off cells) or three distinct directions (On cells). These cells have long been believed to serve purely subcortical pathways and mediate reflexive behaviors (Oyster and Barlow, 1967), but not to supply input to cortex.
Recent evidence has begun to challenge the assumption of separate retinal and cortical visual motion pathways in the mouse (Huberman et al., 2009; Kim et al., 2010; Rochefort et al., 2011). During early development, cortical direction and orientation selective neurons prefer cardinal directions similar to the direction preferences of some On-Off DSRGCS (Rochefort et al., 2011). After this initial period, direction and orientation tuning evolve into the adult form, characterized by the existence of neurons preferring all directions. This compelling result suggests for the first time the possibility that direction selectivity that is computed in the retina may strongly influence cortical direction and orientation tuning via a pathway through the dorsal lateral geniculate nucleus (dLGN). However, a functional DS pathway from retina to dLGN to cortex has not been shown in any species. It also remains largely unknown what motion computations, if any, are performed in the dLGN.
Recently, it was shown that at least two On-Off DSRGC subtypes and one novel Off DSRGC type terminate their axons at different depths within the mouse dLGN (Kim et al., 2008; Huberman et al., 2009; Kim et al., 2010; Rivlin-Etzion et al., 2011; Kay et al., 2011), raising the possibility that there may be a laminar organization of distinct direction preferences in dLGN. Based on the pattern of axon terminals, posterior direction selectivity may be limited to the superficial ~75 μm of dLGN and upward and downward direction selectivity may be restricted to deeper dLGN. However, it is not entirely clear from these anatomical studies whether these projections overlap with each other. Furthermore, the projections of anterior and upward On-Off DSRGCs, as well as a multitude of other cell types, have not been traced. Predictions regarding the existence of a laminar organization of direction selectivity in dLGN are further limited by unknown circuit parameters such as whether the relevant dLGN neurons sample from retinal inputs across layers versus near their cell bodies, and the degree to which direction selectivity is preserved across the retinogeniculate synapse. Surprisingly, a thorough electrophysiological study did not report DS or On-Off responses in the mouse dLGN (Grubb and Thompson, 2003), bringing into question whether direction selectivity is maintained and relayed at all in mouse dLGN. Although, it is possible that stimulus parameters and analysis criteria of this previous study did not identify DS neurons. Moreover, a functional-anatomical organization of direction tuning has not been shown in any species, despite the rare observation of direction-selective lateral geniculate neurons (DSLGNs) in rats and rabbits (Levick et al., 1969; Montero and Brugge, 1969; Stewart et al., 1971; Fukuda et al., 1979). However, the electrophysiological recording methods used by these studies may not have been able to distinguish the precise depths of a sufficient number of recorded neurons, especially given their rarity in the population (~5–10%), and potential proximity of some of these neurons to the most superficial layers of dLGN.
Here, we directly examine the functional-anatomical organization of direction tuning in the superficial 75 μm of mouse dLGN using the first two-photon calcium imaging of dense populations in the thalamus. This dense sampling of neurons in the superficial dLGN allowed us to characterize the direction tuning and precise anatomical location relative to the dLGN surface and border with the lateral posterior nucleus (LP) of dozens to hundreds of neurons simultaneously. These advantages of the imaging method allowed us to determine the functional-anatomical organization of motion direction information in the superficial dLGN.
RESULTS
Two-Photon Population Calcium Imaging of Visual Responses in Superficial Mouse dLGN
In order to determine the functional organization of direction tuning in the superficial mouse dLGN, we developed a method for in vivo two-photon calcium imaging of neuronal visual responses in the superficial region (≤ 75 μm deep from the surface) of mouse dLGN. To our knowledge, these studies yield the first simultaneous physiological measurements of populations of anatomically identified thalamic neurons (Figure 1). For calcium dye loading, Oregon Green Bapta-1 AM (OGB) was injected into the dLGN of C57/Bl6 mice (Figure 1A). To test for direction selectivity in the dLGN, we presented drifting square-wave gratings of 12 equally-spaced directions at a speed known to stimulate DSRGCs (Weng et al., 2005; Kim et al., 2008, 2010; Huberman et al., 2009; Yonehara et al., 2009) (i.e., 25 deg/s, 0.01 cpd). Five repeats of each stimulus and a blank gray stimulus were presented in random order to the animal while visually-evoked calcium responses were recorded in up to dozens of neurons simultaneously at a known depth in the dLGN, reflecting the underlying changes in firing rate of each neuron (Figures 1C–1E and 2). This method allows even rare neuron subtypes to be detected, and each neuron’s precise location to be mapped anatomically within the dLGN.
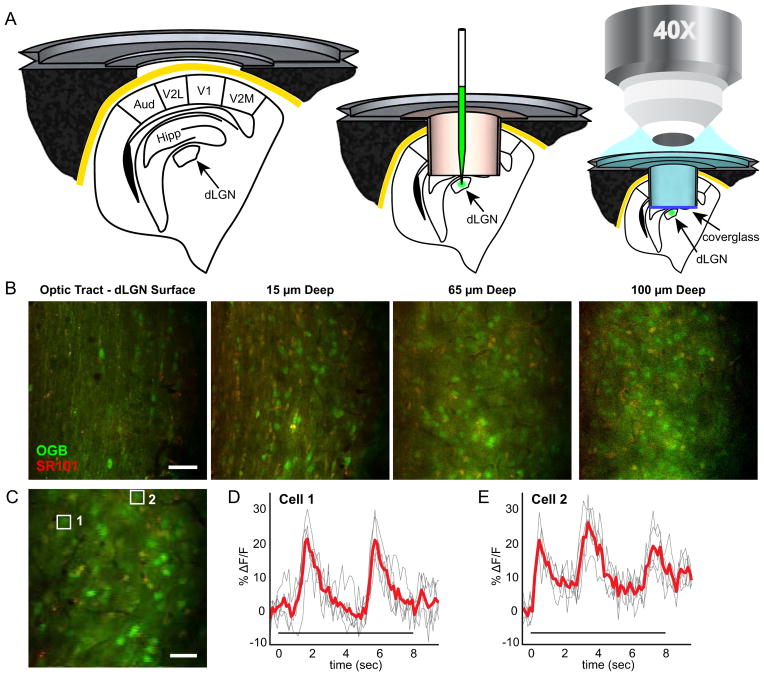
Figure 1. Two-Photon Calcium Imaging of Visual Responses in the Mouse dLGN.
(A) Surgery and calcium dye loading procedure as described in Experimental Procedures. Metal frame and tube cross sections, as well as anatomy (Paxinos, G. and Franklin, K. B. J., 2001) are drawn to scale. The microscope objective drawing is not to scale. (B), Images at multiple depths in the dLGN (Movie S1). Lateral is up, anterior is right. (C), Example field of view used for imaging visual responses. (D–E), Change in fluorescence over time (ΔF/F) for neurons indicated by white boxes in (C). Cell 1 (F1 = 7.6 ± 0.4% F/F) responds after Cell 2 (F1 = 5.9 ± 0.7% F/F) indicating slightly shifted positions of their receptive fields relative to the same grating stimulus. Fourier magnitude is unaffected by these shifts in phase (Figure S1). Red line indicates mean over five trials; each trial is a gray line. Stimulus time indicated by bar under waveforms. Scale bars: (B), 50 μm (C), 25 μm.
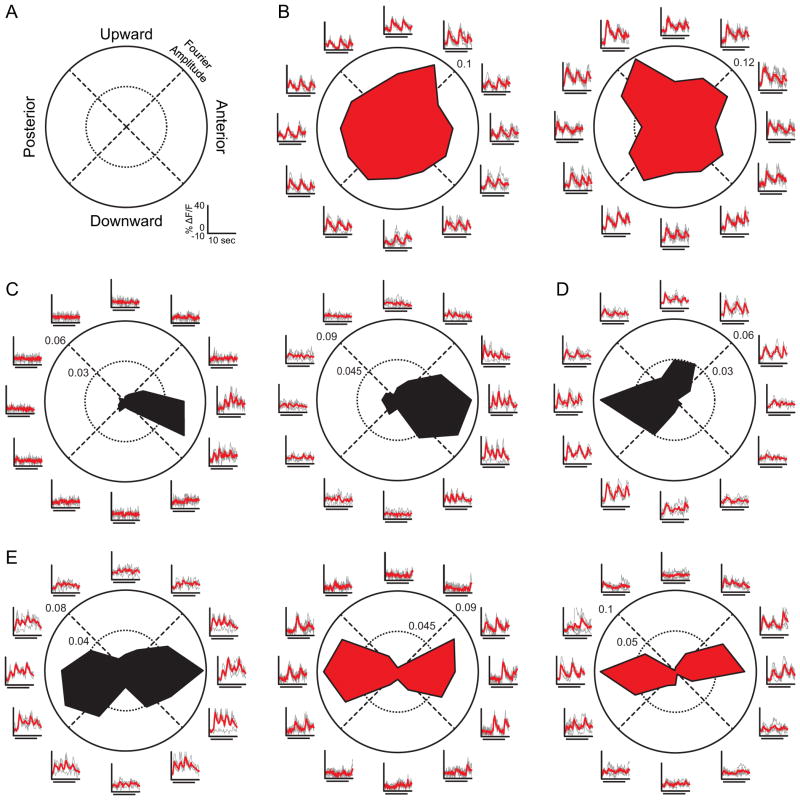
Figure 2. Direction and Axis Selectivity in the dLGN.
(A), Polar plot legend for (B–E), with directions in visual coordinates. Scale bars for fluorescence change (ΔF/F) and time in (B–E) are shown in lower right. (B–E) Examples of non-direction-selective dLGN neurons (B), anterior DS neurons (C), posterior DS neuron (D), and axis-selective neurons (E). Polar plots represent the magnitude of F1 (red) or F2 (black, On-Off) response to each grating direction. Axes outside of the circle show the fluorescence time series, in units of percent change in fluorescence, in response to each direction of the grating. Individual trials (gray) are overlaid with the mean time series (red), where stimulus time (8 seconds) is indicated by bar under waveforms as in (Figure 1D–1E).
Many neurons responded robustly and reliably to at least one direction of the drifting grating, characterized by a time-locked increase in fluorescence to the period of the drifting grating (n = 353, ΔF/F amplitude at F1 or F2 > 2.5% and circular T2 test p < 0.05; Figure S1). We used the modulation of the fluorescence signal at the temporal frequency of the grating (0.25 Hz, F1) or at twice the temporal frequency of the grating (0.5 Hz, F2) as the measure of neuronal responsiveness. The F1 modulation corresponds to either the onset (On) or offset (Off) of each bar of light passing through a cell’s receptive field, while the F2 modulation corresponds to both the onset and offset (On-Off) of each bar of light. Importantly, since the OGB signal attenuates higher frequencies, a large, detected F2 modulation represents an even stronger than recorded modulation, increasing confidence in On-Off designations. Likewise, an apparently low F2 modulation leaves characterization of On-Off ambiguous or not possible. We computed the direction-selectivity index (DSI) and axis-selectivity index (ASI) of each responsive neuron in our sample. Neurons with high DSI values (DSI > 0.5) responded preferentially to a single direction of the grating. Neurons with high ASI values (ASI > 0.5) responded preferentially to gratings drifting along a single axis of motion, responding selectively to gratings drifting in either opposing direction along a motion axis at a single orientation. The majority of neurons were not selective for motion in a particular direction or along a particular axis (n = 320/353, Figure 2B, DSI < 0.5 and ASI < 0.5). These responses are consistent with the circular direction tuning curves typical of dLGN neurons (Hubel and Wiesel, 1961). These findings demonstrate that the superficial dLGN is far from a purely DS layer.
Anterior and Posterior Direction Selectivity in Superficial Mouse dLGN
Conversely, 18 of the visually responsive cells in the dataset were strongly and consistently direction selective (example cells Figures 2C, 2D and 3A, DSI > 0.5, Hotelling T2 test p < 0.05). The proportion of DSLGNs observed is highly significant compared to chance (shuffled trials, p < 10−6, see Supplemental Experimental Procedures). The majority of neurons in our dataset responded to both the onset and offset of each bar of light moving through their receptive field (n = 10/18), defining their receptive fields as On-Off and strongly suggesting that they receive driving input from On-Off RGCs. The remaining neurons could not be definitively characterized as either On, Off or On-Off. We next tested for functional organization of preferred direction in the superficial dLGN population, based on our predictions from DSRGC projections. Unexpectedly, the majority of DSLGNs were strongly selective for the anterior direction (n = 11/18, including one near the anterior-downward border, Figures 2C and 3A), and the majority of these neurons were On-Off direction selective (n = 8/11). Another population of DSLGNs was selective for the posterior direction (n = 5/18, including one near the posterior-downward border), corroborating known posterior DSRGC projections to the superficial layer. At least one of these neurons could be defined with On-Off responses (Figure 2D), perhaps reflecting the variety of On-Off response types inherent to that population (Huberman et al., 2009; Rivlin-Etzion et al., 2011), and the attenuation of higher frequencies in the calcium signal. Only one neuron was selective for upward motion, and one for downward motion (Figure 3A), consistent with rare arborization of On-Off downward and Off upward DSRGC axons in the superficial dLGN layer (Kim et al., 2010). These results strongly predict a previously unknown retinogeniculate projection of On-Off anterior DSRGCs to the superficial dLGN region. Furthermore, insofar as On-Off upward DSRGCs project to dLGN, they are likely to project to deep rather superficial layers.
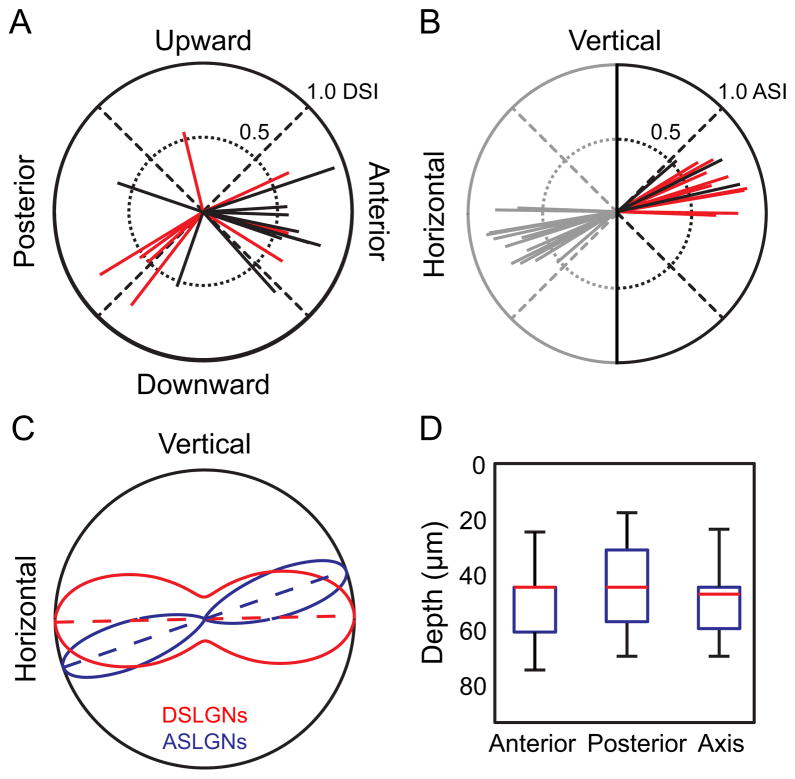
Figure 3. The Superficial dLGN is Selective for Horizontal Motion.
(A), Each vector on polar plot indicates a DS neuron. Direction of vector indicates direction preference. (B), Each vector indicates an axis-selective neuron. Direction of vector indicates axis preference. Vectors are reflected (gray) for display purposes, and represent the same data as black and red vectors. (A–B), Length of vectors indicates level of direction selectivity (DSI) or axis selectivity (ASI), using the max-null metric (Supplemental Experimental Procedures). Data for all neurons in the dataset are shown in Figure S2, using the resultant metric and the Hotelling T2 test, for all values of DSI and ASI. Black lines indicate On-Off response (F2 modulation) and red lines indicate F1 modulation. (C), Maximum likelihood fit of axial circular Gaussian distributions to the observed populations of direction- and axis-selective neurons from (A–B). Curves represent the axial Gaussian model’s probability of observing a direction- (red) or axis-selective (blue) neuron with a given preferred direction or preferred axis. Dotted lines indicate preferred axis for each population, and curves are normalized to equalize the maximum probability density for visualization. Both populations prefer axes representing horizontal motion. (D), Depth of neuron populations in dLGN dataset depending on stimulus selectivity. Whiskers are complete depth range, boxes are 25th to 75th percentile, and the red line is the median depth. Anterior-, posterior- and axis-selective neurons overlap locations in depth within the superficial ~75 μm of dLGN.
Overall, the preferred directions of DSLGNs in the superficial 75 μm of the dLGN were distributed along a single axis (Figure 3C, axial Rayleigh test, p < 0.05, unimodal Rayleigh test n.s.) corresponding to horizontal motion (fitted distribution < 2° from horizontal axis). It is important to note that the axial Raleigh test is significant (p < 0.05) for DSI thresholds less than 0.5 and greater than 0.22 for neurons which show a consistent direction bias or “sensitivity” (Hotelling T2 test, p < 0.05), suggesting that direction selectivity in the population lies on a continuum (Figure S2A). Interestingly, anterior DSLGNs (aDSLGNs) were intermingled in depth with posterior DSLGNs (pDSLGNs) within the superficial 75 μm of the dLGN (Figure 3D). The mean tuning widths of pDSLGNs and aDSLGNs were indistinguishable from each other (t-test n.s.), and were more sharply tuned for direction than reported for DSRGCs [mean width at half-maximum = 76 ± 7° (SE) for DSLGNs compared to 115° reported for DSRGCs (Elstrott et al., 2008), t-test p < 0.05]. Firing rate to OGB signal transformations are linear at low firing rates (Kerlin et al., 2010; LeChasseur et al., 2011), suggesting that the sharper tuning curves we observe are not artifacts and represent sharpening of direction tuning in the dLGN [see also (Levick et al., 1969)]. These results suggest that the dLGN both maintains and sharpens retinal direction tuning in a subset of neurons and contains a preferred direction-biased superficial region. Intriguingly, the DS neurons in this region overwhelmingly encode opposite directions along a single axis of motion.
Horizontal Axis Selectivity
This surprising functional organization of opposing direction tuning prompted us to next investigate whether the dLGN integrates across opposing directions of motion to form axis-of-motion-selective neurons within the same region, in contrast to the role of the dLGN as a simple relay of segregated functional channels. In support of this hypothesis, 15 of the visually responsive neurons were highly selective for a particular axis of motion, at a single orientation of the grating (Figures 2E and 3B, ASI > 0.5). The proportion of ASLGNs observed is also significantly different from chance (shuffled trials, p < 10−6, see Supplemental Experimental Procedures). The preferred axis of motion of these neurons was also overwhelmingly biased towards a single axis (axial Rayleigh test, p < 0.05, unimodal Rayleigh test n.s.), corresponding to horizontal motion (Figure 3C). The axial Raleigh test is significant (p < 0.05) for all ASI thresholds less than 0.5 for neurons which show a consistent axial bias or “sensitivity” (Hotelling T2 test, p < 0.05), suggesting that like direction selectivity, axis selectivity in the population lies on a continuum (Figure S2B). The preferred motion axis for axis-selective neurons was not significantly different than the axis for DS neurons (Watson-Williams test; fitted distribution < 20° from horizontal axis). Furthermore, axis-selective lateral geniculate neurons (ASLGNs), pDSLGNs and aDSLGNs were intermingled in depth within the superficial 75 μm of the dLGN (Figure 3D; one-way ANOVA n.s.). ASLGNs, like DSLGNs, were more sharply tuned than DSRGCs [mean width at half-maximum = 61 ± 2° (SE) for ASLGNs compared to 115° reported for DSRGCs (Elstrott et al., 2008), t-test p < 0.05]. Three of these neurons could be defined as On-Off cells. Cell 1 in Figure 2E shows On-Off responses in one such neuron. The similarity in response characteristics of ASLGNs and DSLGNs suggests that they may receive common, retinal input. This is further supported by parameters of the retinogeniculate circuit, as discussed below.
Random Wiring Model
DSLGNs and ASLGNs in the superficial region both have strong and statistically significant preferences for the same horizontal axis of motion. This suggests that anterior and posterior but generally not upward or downward DS inputs are likely to synapse in the superficial dLGN and that ASLGNs may arise from the integration of opposing DS inputs as a result of either specific connectivity mechanisms or random sampling from local axon terminals (random wiring). In order to ask whether random wiring alone within the superficial region can explain our findings in a way that is consistent with previous experimental results (which would suggest that specific connectivity mechanisms may not be necessary), we developed a simple statistical model of the inputs to the superficial dLGN.
In the random wiring model, neurons receive multiple independent inputs that are anterior DS, posterior DS, or non DS. The random wiring model is constrained by the previous experimental observation that mouse dLGN neurons receive one to three strong inputs from the retina [with probabilities: one input (p1), two inputs (p2), and three inputs (p3 = 1−p1+p2)], from which they derive their stimulus selectivity (Cleland et al., 1971a, 1971b; Mastronarde, 1987, 1992; Usrey et al., 1999; Chen and Regehr, 2000). Importantly, the basic results of the model are robust against the addition of dLGN neurons which receive more than three strong retinal inputs. The model assumes that input from DSRGCs must be nearly pure to generate a DSLGN or ASLGN, since linear summation of inputs only produces direction or axis selectivity (i.e., 0.5 DSI/ASI) if over 90% of the inputs to a cell are of the required type(s). To simulate random wiring in the model, the probability of input to a dLGN neuron from a given type of RGC is equal to the total proportion of input to superficial dLGN belonging to that RGC type (f). We assume that the fractions of input to superficial dLGN of either anterior or posterior DSRGCs are equal, and that upward and downward DSRGCs do not project to the superficial region, yielding 2f for the total fraction of DS input.
Together, these assumptions define a set of equations for the probability of each possible type of cell (Table S1). The sum of probabilities for observing DSLGNs with one, two, or three inputs in the model is equal to the total fraction of DSLGNs, p(DS). Similar reasoning applies to ASLGNs with two or three inputs, yielding p(AS) (see Supplemental Experimental Procedures for a full derivation). In the model, not all values for p(DS) and p(AS) are possible given random wiring; however the range of possibilities is large (Figure 4B, light gray region). Cleland et al. 1971a performed paired RGC-LGN recordings in cats and found that very few dLGN neurons (8.8%, 5/57) had a single RGC input which accounted for all of its recorded spikes. This provides bounds on the likely fraction of dLGN neurons receiving only one driving RGC input (p1 = 0.038–0.19, 95% C.I. using the Wilson interval for binomial variables with 5/57 single input LGN cells). Applying these bounds to p1 limits the possible solutions for fractions of ASLGNs and DSLGNs which are consistent with the random wiring model (dark gray region of Figure 4B). The experimentally observed fractions of ASLGNs [p(AS) = 0.043, binomial 95% C.I. 0.026–0.069] and DSLGNs [p(DS) = 0.051, binomial 95% C.I. 0.033–0.079] in our dataset (red region of Figure 4B) is consistent with the previous data on the limits on p1 (fall within the bounded region). These results suggest that random wiring is a valid mechanism for yielding the fractions of DSLGNs, ASLGNs and non-selective neurons in the superficial dLGN without violating previous results on the fraction of LGN neurons driven by a single input.
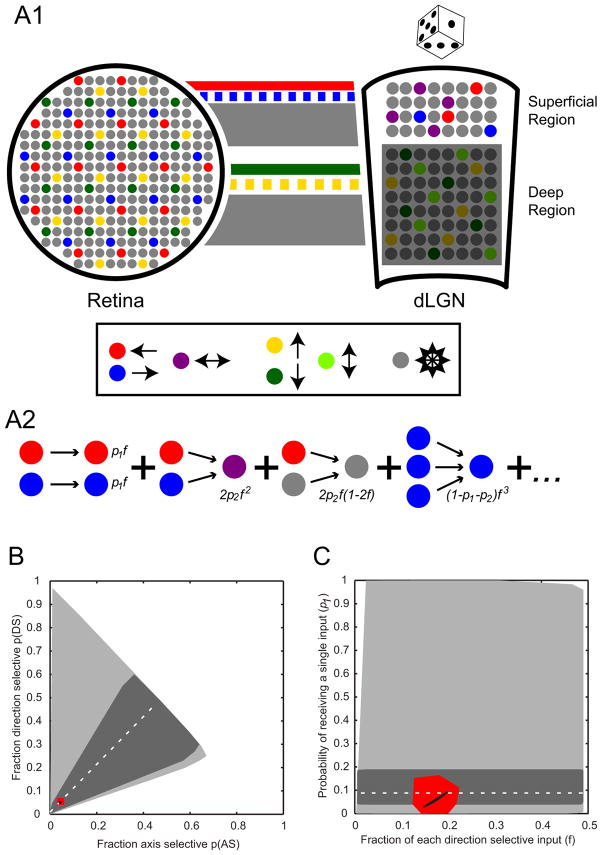
Figure 4. Wiring Model Between Retina and dLGN.
(A1) (Left) Schematic representation of mosaics of retinal ganglion cells. Each color represents a different On-Off DSRGC cell type: posterior (red), anterior (blue), upward (yellow), downward (green). Non-direction-selective neurons are gray. (Right, “Superficial Region”) Organization of dLGN showing the superficial dLGN region containing intermingled populations of posterior (red) and anterior (blue) DSLGNs as well as horizontal ASLGNs (purple) and non-direction-selective neurons (gray) as revealed by the current study. (Right, “Deep Region”) Predictions for deeper dLGN, including intermingled upward (yellow) and downward (green) DSLGNs as well as vertical ASLGNs (light green). This region is grayed out because its functional organization remains unknown. Lines between retina and dLGN schematics represent RGC axons. Color conventions are same as rest of figure. The thickness of the lines indicates predicted fraction (f) of overall input from our random wiring model. Solid red and green lines represent known projection patterns of posterior and downward DSRGCs, respectively, whereas dashed blue and yellow lines represent predicted projection patterns of anterior and upward DSRGCs made by the current study. Our random wiring model demonstrates that concentrated, laminar projection patterns of opposing DSRGCs can yield the fractions of DSLGNs and ASLGNs we observe in superficial dLGN given locally random wiring. (A2) Basic probabilistic theory of the model which assumes dLGN neurons receive one (probability = p1), two (p2) or three inputs (1-p1-p2) from retina that drive their selectivity, including a variable for the fraction of anterior and posterior direction selective input (2f). Some examples of individual probabilities are shown (see Table S1 and Supplemental Experimental Procedures). (B, C) Results of the model. (B) All possible ASLGN and DSLGN fractions based on the model without further constraints (light gray area). The fraction of purely single input neurons from Cleland et al., (1971a) (95% binomial C.I. from Wilson interval: dark gray area 0.038 < p1 < 0.19, actual value: dotted line p1 = 0.088) constrains the plausible range of ASLGNs and DSLGNs. The observed fractions of ASLGNs and DSLGNs in our study (95% binomial C.I. from Wilson interval: red area 0.026 < ASLGN fraction < 0.069, 0.033 < DSLGN fraction < 0.079, actual value: black dot ASLGN fraction = 0.043, DSLGN fraction = 0.051) falls within this plausible range. (C) Possible p1 and f values (by varying across all values of p2) corresponding to the differing constraints in (B): unconstrained model (light gray region), constraining p1 to be consistent with the fraction of purely single input neurons from Cleland et al (1971a) (95% C.I.: dark gray region, actual value: dotted line), or constraining the model to be consistent with the experimentally observed ASLGN and DSLGN fractions in this study (95% C.I.: red region, actual value: black curve).
The random wiring model thus defines equations for two experimentally determined values [probability of ASLGN, p(AS), and probability of DSLGN, p(DS)] using three variables (f, p1, p2), leaving one free variable. We varied p2 in order to find the family of solutions for p1 and 2f which satisfy the observed values for p(DS) and p(AS) (Figure 4C, black curve with red region indicating confidence intervals). In order for random wiring to explain the experimentally observed axis and DS cell fractions, the model predicts that the total fraction of DS input (2f) to the superficial dLGN must be between 29 and 39% of the total RGC inputs (25–45% including 95% C.I). The model also predicts that the probability of a dLGN neuron receiving a single, driving retinal input (p1) is between 0.028 and 0.092 (0–0.167 for the set of p1 values from the 95% C.I. of AS and DS fractions, see Supplemental Experimental Procedures). Importantly, the ranges of 2f and p1 are likely to be much narrower in actuality given that they are based here on the extreme solutions of the model (e.g., p2 = 0), which are very unlikely to occur in the actual circuit. As discussed below, our experimental results, combined with the results of our random wiring model and previous studies suggest that selective connectivity mechanisms are not required in this circuit beyond concentrated anterior and posterior DS input to the superficial dLGN region. Furthermore, the model’s results given our data make specific predictions about the wiring statistics of DSLGNs and ASLGNs.
DISCUSSION
These results demonstrate a novel functional organization of opposing direction information in the superficial region of mouse dLGN. Unexpectedly, the representation of motion information is segregated in terms of horizontal from vertical motion information, but integrated in terms of combining opposing directions along the same horizontal axis within a majority of non-direction-selective neurons in the same region. These dLGN functional cell types likely arise primarily from synaptic integration of retinal inputs (see Supplemental Discussion).
Accounting for known properties of the retinogeniculate circuit, our results suggest that dLGN can maintain, sharpen and integrate retinal information pathways. Moreover, all of these functions can be accomplished via locally random wiring and do not require uniform functional lamination, as our model shows. Since dLGN provides the majority of sensory input to primary visual cortex, and given the remarkably similar direction preference tuning between retina, dLGN and cortex [present study; (Huberman et al., 2009; Rochefort et al., 2011)], it is likely that direction tuning first computed in the retina is manipulated by the dLGN and then relayed to cortex. This previously undiscovered pathway may supply motion information to cortex to help derive cortical direction and orientation selectivity. This may indicate a separate mechanism for generating direction and orientation selectivity compared to classic models (Hubel and Wiesel, 1961, 1962; Ferster and Miller, 2000; Peterson et al., 2004). Still, like retina, the dLGN likely only represents specific axes of motion, and thus cortex must derive tuning for intermediate directions via additional circuit mechanisms. Future studies will be necessary to reveal whether the retinogeniculate pathway is necessary and sufficient to initiate direction and/or orientation tuning in cortex during development, and what roles the pathway plays in cortical computations, perception and behavior in the adult.
The pattern of direction tuning in superficial dLGN is in agreement with superficially restricted projections of posterior DSRGCs (Huberman et al., 2009), and deeply restricted projections of On-Off downward and Off upward DSRGCs (Kim et al., 2010; Kay et al., 2011). Our results suggest that regardless of whether projections of these different DSRGCs overlap, functional segregation is achieved in dLGN. This also strongly implies that DSLGNs sample retinal inputs near their cell bodies, despite having dendrites which likely span across layers, consistent with what has been observed more generally for dLGN relay neurons (Hamos et al., 1987; Sherman and Guillery, 1998). Furthermore, the results strongly predict projections of On-Off anterior DSRGCs to superficial dLGN and On-Off upward DSRGCs to deep and not superficial dLGN. Similarly, anterior DSRGCs may avoid projections to deep layers, following the pattern of posterior DSRGCs. This suggests a striking model of functional organization in which the cardinal axes of visual motion are separated in the dLGN (Figure 4A1). In potential support of this hypothesis, two extracellular recording studies in rats found a similar proportion of DSLGNs compared to the present study, but that >80% of the DSLGNs in their samples preferred motion in vertical-axis directions (Montero and Brugge, 1969; Fukuda et al., 1979), indicating that dLGN encodes vertical directions. These studies did not report precise depths of their recordings, perhaps because of limitations of their methods and the rarity of DSLGNs, but it is likely that their methods tended to sample from deep dLGN and may have largely missed superficial cells. As imaging technologies improve to provide access to deeper dLGN and more DSRGC cell type projections are labeled and characterized, the precise organization of deeper dLGN, and a more complete understanding of potential laminar organization may be revealed. Organizing opposing directions together and separating orthogonal axes in distinct layers represents an unprecedented functional organization for dLGN and may provide advantages for computing higher-order motion parameters.
Surprisingly, we observed neurons that encode an axis of motion matching the opposing preferences of DS neurons in the same dLGN region. We see two main possibilities for how this overlap in selectivity arises—either ASLGNs integrate opposing direction-selective retinal ganglion cell type inputs to form a new response class or ASLGNs receive direct input from an undiscovered axis-selective retinal ganglion cell type and relay that information. The latter hypothesis is most consistent with the view of the dLGN as a simple relay from retina to cortex. Interestingly, if this pathway exists it may suggest further specificity of RGC projections based on motion axis preference, for example if vertical axis cells are found in deeper dLGN. However, while axis-selective retinal ganglion cells have been found in the rabbit’s visual streak, they are nearly absent in the rabbit’s peripheral retina (Oyster, 1968) and have not been described previously in the rodent retina, which has no visual streak. Moreover, while the persistent view has been that the dLGN only relays retinal information and does not generate novel feature selectivity, the current results present the first observed instance of overlapping and opposing information channels in a single dLGN region, and thus the potential for direct integration of retinal pathways, for example as evaluated by our random wiring model. Interestingly, one previous study suggested potential for rare mixing of RGC type inputs in dLGN to yield intermediate tuning properties of X and Y cells in the cat (Mastronarde, 1992), suggesting that similar mechanisms may be involved in other species and cell types. However, the present results represent the first indication that dLGN may integrate retinal information to form a novel feature selectivity. Regardless of whether axis selectivity first arises in retina or dLGN, the importance of this pathway may be further pronounced if axis-selective inputs influence orientation selectivity in some neurons in the cortex.
Integration of opposing direction preferences by ASLGNs could result from either selective connectivity between DSRGCs and ASLGNs, for example favored by developmental mechanisms, or could occur by chance if connections are non-specific between retina and thalamus, given that incoming axonal arbors of opposing DSRGC types likely overlap spatially within superficial dLGN, as predicted by our results. Future studies are necessary to determine how axis selectivity develops in dLGN. In order to test whether our results are consistent with the generation of ASLGNs by chance integration of DSRGC afferents with opposing direction preferences, we generated a simple model based on random retinogeniculate wiring.
In this model, dLGN neurons receive one to three driving retinal inputs (Chen and Regehr, 2000) randomly distributed according to the fraction of DS inputs from the retina. The random wiring model predicts that an overwhelming majority (81–100%) of dLGN neurons receive more than one driving input from RGCs in order to produce the proportions of ALSGNs and DSLGNs we observe in the superficial dLGN (~4% and ~5% respectively). This is consistent with previous studies which have reported that ~91% of relay neurons receive driving inputs from more than one RGC (Cleland et al., 1971a). The model also predicts that a relatively large fraction of RGC input to superficial dLGN is direction selective (> 25%), which is similar the total fraction of RGCs that are On-Off DS (20–36%, based on anatomical estimates from Huberman et al., 2009), consistent with the notion that potentially all anterior and posterior DSRGC input to dLGN projects superficially and that other directions project deeper, maintaining the overall fraction of DS input to dLGN across depths. The random wiring model demonstrates that integration can result by chance from convergence of relatively common direction-selective inputs and give rise to the representation of motion we observed. This suggests a developmental mechanism for establishing local concentrations (i.e., lamination) of incoming fibers of specific direction preference, but does not require selective targeting on a single cell basis to generate ASLGNs and maintain direction selectivity in dLGN. If the conditions of the model are not met physiologically, selective wiring between DSRGCs and ASLGNs may be necessary to generate ASLGNs in the absence of direct axis-selective input.
Regardless of the mechanism, the juxtaposition of horizontal axis- and anterior-posterior direction-selectivity within the same region suggests a novel computational role for the superficial dLGN. By both sharpening and integrating direction information within a functional organization, the dLGN appears to not merely relay direction information from the retina to cortex, but instead organizes and manipulates that information before projecting it downstream. Future studies examining direct functional connectivity analyzed from the retina to thalamus to cortex, as well as of local interneuron circuits within dLGN may shed light on the mechanisms underlying these computations. For example, whether sharpening of direction tuning in dLGN results from nonlinear postsynaptic summation (Carandini et al., 2007) or precisely targeted feedforward inhibition (Wang et al., 2011) remains unknown. The methods developed and demonstrated here in combination with other methods are likely to aid these studies. Furthermore, the influence of these computations and the functional-anatomical organization of direction and motion axis information in the dLGN on visual cortical processing, development and behavior remain intriguing, open questions.
EXPERIMENTAL PROCEDURES
in vivo preparation
All experiments involving living animals were approved by the Salk Institute’s Institutional Animal Care and Use Committee. C57Bl/6 mice were anesthetized with isoflurane (1–1.5%). A custom metal frame was mounted to the skull (Figure 1). A craniotomy was made and the exposed cortex, including much of visual cortex, and hippocampus were aspirated, exposing the thalamus. Oregon Green Bapta-1 AM (OGB) and sulforhodamine 101 (SR101) were injected with 150 ms pulses every 15 sec for 15 min at 200 and 400 μm below the dLGN surface. A tube with a glass coverslip was inserted and filled with ACSF. OGB-loaded neurons were imaged through the tube with a two-photon microscope. For visual stimulation, chlorprothixene (1 mg/kg, IM) was administered and isoflurane was lowered to 0.3–0.5%. More details and visual stimulation parameters can be found in the Supplemental Experimental Procedures.
Data Analysis
Regions of interest (ROI) were drawn around each cell in each field of view, glia were excluded using SR101 labeling, and pixels were averaged within each ROI. Calcium signal modulations were measured as relative change in fluorescence over time compared to a prestimulus baseline (ΔF/F). Fourier transforms were taken of the signals during the stimulus period, at the first and second harmonic frequencies of the grating to measure the response of the cell to each direction of the grating. Direction selectivity was calculated by both max-null and circular variance metrics. See Supplemental Experimental Procedures for more details and statistics.
Random Wiring Model
A full derivation of the model can be found in the Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank the Callaway lab for helpful discussions and technical assistance. We also thank D. Kleinfeld for helpful discussions and D. Dombeck for imaging advice. We acknowledge support from NIH grants EY010742; EY022577 (E.M.C.), 1F30DC010541-01 (A.P.K.) and EY019821 (I.N.) and the Gatsby Charitable Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Briggman KL, Helmstaedter M, Denk W. Wiring specificity in the direction-selectivity circuit of the retina. Nature. 2011;471:183–188. doi: 10.1038/nature09818. [DOI] [PubMed] [Google Scholar]
- Carandini M, Horton JC, Sincich LC. Thalamic filtering of retinal spike trains by postsynaptic summation. J Vis. 2007;7:20.1–11. doi: 10.1167/7.14.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28:955–966. doi: 10.1016/s0896-6273(00)00166-5. [DOI] [PubMed] [Google Scholar]
- Cleland BG, Dubin MW, Levick WR. Simultaneous recording of input and output of lateral geniculate neurones. Nature New Biol. 1971a;231:191–192. doi: 10.1038/newbio231191a0. [DOI] [PubMed] [Google Scholar]
- Cleland BG, Dubin MW, Levick WR. Sustained and transient neurones in the cat’s retina and lateral geniculate nucleus. J Physiol (Lond) 1971b;217:473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstrott J, Anishchenko A, Greschner M, Sher A, Litke AM, Chichilnisky EJ, Feller MB. Direction selectivity in the retina is established independent of visual experience and cholinergic retinal waves. Neuron. 2008;58:499–506. doi: 10.1016/j.neuron.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D, Miller KD. Neural mechanisms of orientation selectivity in the visual cortex. Annu Rev Neurosci. 2000;23:441–471. doi: 10.1146/annurev.neuro.23.1.441. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Sumitomo I, Sugitani M, Iwama K. Receptive-field properties of cells in the dorsal part of the albino rat’s lateral geniculate nucleus. Jpn J Physiol. 1979;29:283–307. doi: 10.2170/jjphysiol.29.283. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Thompson ID. Quantitative characterization of visual response properties in the mouse dorsal lateral geniculate nucleus. J Neurophysiol. 2003;90:3594–3607. doi: 10.1152/jn.00699.2003. [DOI] [PubMed] [Google Scholar]
- Hamos JE, Van Horn SC, Raczkowski D, Sherman SM. Synaptic circuits involving an individual retinogeniculate axon in the cat. J Comp Neurol. 1987;259:165–192. doi: 10.1002/cne.902590202. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Integrative action in the cat’s lateral geniculate body. J Physiol (Lond ) 1961;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol (Lond ) 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. Genetic Identification of an On-Off Direction- Selective Retinal Ganglion Cell Subtype Reveals a Layer-Specific Subcortical Map of Posterior Motion. Neuron. 2009;62:327–334. doi: 10.1016/j.neuron.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay JN, De la Huerta I, Kim IJ, Zhang Y, Yamagata M, Chu MW, Meister M, Sanes JR. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J Neurosci. 2011;31:7753–7762. doi: 10.1523/JNEUROSCI.0907-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlin AM, Andermann ML, Berezovskii VK, Reid RC. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron. 2010;67:858–871. doi: 10.1016/j.neuron.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Meister M, Sanes JR. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J Neurosci. 2010;30:1452–1462. doi: 10.1523/JNEUROSCI.4779-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–482. doi: 10.1038/nature06739. [DOI] [PubMed] [Google Scholar]
- LeChasseur Y, Dufour S, Lavertu G, Bories C, Deschênes M, Vallée R, De Koninck Y. A microprobe for parallel optical and electrical recordings from single neurons in vivo. Nat Methods. 2011;8:319–325. doi: 10.1038/nmeth.1572. [DOI] [PubMed] [Google Scholar]
- Levick WR, Oyster CW, Takahashi E. Rabbit lateral geniculate nucleus: sharpener of directional information. Science. 1969;165:712–714. doi: 10.1126/science.165.3894.712. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN. Two classes of single-input X-cells in cat lateral geniculate nucleus. II. Retinal inputs and the generation of receptive-field properties. J Neurophysiol. 1987;57:381–413. doi: 10.1152/jn.1987.57.2.381. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN. Nonlagged relay cells and interneurons in the cat lateral geniculate nucleus: receptive-field properties and retinal inputs. Vis Neurosci. 1992;8:407–441. doi: 10.1017/s0952523800004934. [DOI] [PubMed] [Google Scholar]
- Montero VM, Brugge JF. Direction of movement as the significant stimulus parameter for some lateral geniculate cells in the rat. Vision Research. 1969;9:71–88. doi: 10.1016/0042-6989(69)90032-7. [DOI] [PubMed] [Google Scholar]
- Oyster CW. The analysis of image motion by the rabbit retina. The Journal of Physiology. 1968;199:613. doi: 10.1113/jphysiol.1968.sp008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyster CW, Barlow HB. Direction-selective units in rabbit retina: distribution of preferred directions. Science. 1967;155:841. doi: 10.1126/science.155.3764.841. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic Press; 2001. [Google Scholar]
- Peterson MR, Li B, Freeman RD. The derivation of direction selectivity in the striate cortex. J Neurosci. 2004;24:3583–3591. doi: 10.1523/JNEUROSCI.5398-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Zhou K, Wei W, Elstrott J, Nguyen PL, Barres BA, Huberman AD, Feller MB. Transgenic mice reveal unexpected diversity of on-off direction-selective retinal ganglion cell subtypes and brain structures involved in motion processing. J Neurosci. 2011;31:8760–8769. doi: 10.1523/JNEUROSCI.0564-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort NL, Narushima M, Grienberger C, Marandi N, Hill DN, Konnerth A. Development of direction selectivity in mouse cortical neurons. Neuron. 2011;71:425–432. doi: 10.1016/j.neuron.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators”. Proc Natl Acad Sci USA. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DL, Chow KL, Masland RH. Receptive-field characteristics of lateral geniculate neurons in the rabbit. J Neurophysiol. 1971;34:139–147. doi: 10.1152/jn.1971.34.1.139. [DOI] [PubMed] [Google Scholar]
- Usrey WM, Reppas JB, Reid RC. Specificity and strength of retinogeniculate connections. J Neurophysiol. 1999;82:3527–3540. doi: 10.1152/jn.1999.82.6.3527. [DOI] [PubMed] [Google Scholar]
- Wang X, Vaingankar V, Sanchez CS, Sommer FT, Hirsch JA. Thalamic interneurons and relay cells use complementary synaptic mechanisms for visual processing. Nat Neurosci. 2011;14:224–231. doi: 10.1038/nn.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Hamby AM, Zhou K, Feller MB. Development of asymmetric inhibition underlying direction selectivity in the retina. Nature. 2010;469:402–406. doi: 10.1038/nature09600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S, Sun W, He S. Identification of ON-OFF direction-selective ganglion cells in the mouse retina. J Physiol (Lond ) 2005;562:915–923. doi: 10.1113/jphysiol.2004.076695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara K, Ishikane H, Sakuta H, Shintani T, Nakamura-Yonehara K, Kamiji NL, Usui S, Noda M. Identification of retinal ganglion cells and their projections involved in central transmission of information about upward and downward image motion. PLoS ONE. 2009;4:e4320. doi: 10.1371/journal.pone.0004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.