TAp63α is the predominant TAp53 family member expressed in primordial follicle oocytes1 and is essential for oocyte death following genotoxic stress2. How DNA damage leads to the activation of TAp63α is poorly defined. Gonfloni et al.3 reported that inhibition of c-ABL with imatinib (Gleevec), designed to inhibit the oncogenic kinase BCR-ABL, protects oocytes from cisplatin-induced killing, and therefore proposed that c-ABL is critical for the induction of TAp63-mediated apoptosis. However, the relationships between c-ABL, TAp63 and DNA damage-induced cell death are complex4,5 and imatinib itself is actually known to promote apoptosis by inhibiting certain kinases that promote cell survival, such as the SCF receptor, c-KIT6,7. Accordingly, imatinib is used clinically not only for the treatment of BCR-ABL+ chronic myelogenous leukemia (CML) but also for c-KIT mutated Gastro-Intestinal Stromal Tumor (GIST)8. Of particular concern, c-KIT is critical for oocyte survival, with blocking antibodies to c-KIT causing follicular atresia9. We therefore independently explored the impact of imatinib on the response of oocytes to DNA-damage induced by cisplatin. We found that imatinib did not protect primordial follicle oocytes from cisplatin-induced apoptosis or prevent loss of fertility in two independent strains of mice.
For in vivo analysis, PN5 CD1 mice were treated with vehicle or imatinib (7.5 mg/kg i.p.) or cisplatin, (5 mg/kg i.p.) or both imatinib and cisplatin administered together (as in Gonfloni et al3). Ovaries were harvested at PN10 and ovarian follicles quantified using established methodology10,11 (Fig. 1A, B and Supplementary Table 1). In contrast to the data reported by Gonfloni et al3, coadministration of imatinib did not rescue primordial follicles from elimination caused by exposure to cisplatin. As Gonfloni et al did not report the primordial follicle count per se (rather, they reported on the composite of the primordial follicle count plus the larger primary follicle count), enumeration of primordial follicles in their study is unclear. We also performed a similar analysis with a second mouse strain (C57BL/6), with imatinib or vehicle administered 2 h prior to cisplatin or vehicle.As we report for the CD1 strain, no rescue of cisplatin-induced oocyte death was observed in the C57BL/6 strain (Supplementary Fig. 1A, B and Supplementary Table 1). In our studies, treatment with imatinib alone increased by nearly 5-fold the numbers of pyknotic bodies (oocytes with nuclear fragmentation, a hallmark of apoptosis) observed in ovaries in vivo (at PN10, p <0.03). This is consistent with in vivo pro-apoptotic activity of imatinib in oocytes (Supplementary Fig. 1C) and the notion that an imatinib-sensitive kinase, most likely c-KIT, is critical for the survival of female germ cells12.
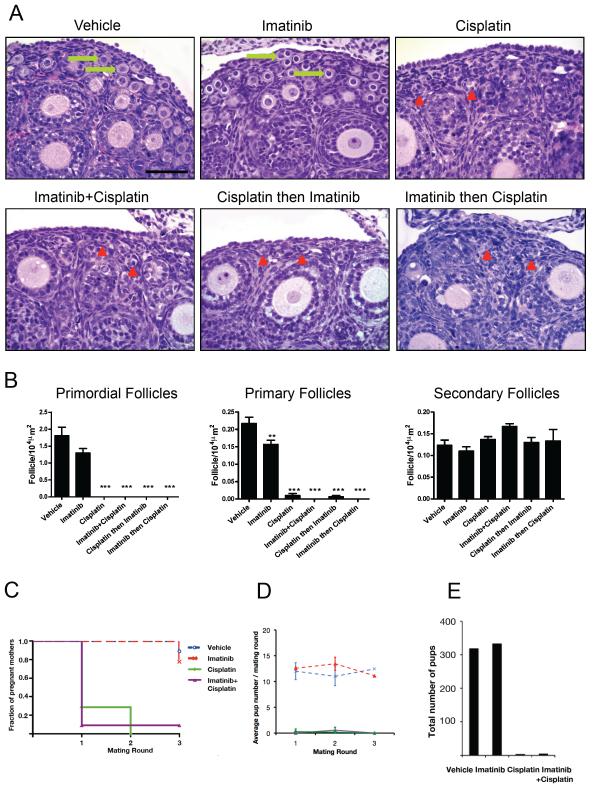
Figure 1. Pre-treatment with imatinib did not protect primordial follicle oocytes from DNA damage induced death or rescue cisplatin-induced loss of fertility in CD1 mice.
PN5 CD1 female pups were treated with vehicle (PBS), or imatinib (7.5 mg/kg i.p.), or cisplatin (5 mg/kg) or with imatinib and cisplatin administered together, or with imatinib administered 2 h prior to cisplatin, or with cisplatin administered prior to imatinib and harvested at PN10. (A) Hematoxylin and eosin staining of ovaries: vehicle-treated and imatinib-treated ovaries show numerous primordial follicles with oocytes (arrows). In all cisplatin-treated or cisplatin + different regimens of imatinib treatments, oocyte-containing primordial follicles are absent, but empty follicle-like structures lacking an oocyte are numerous (arrowheads). Scale bar indicates 50 μm. (B) Quantification of primordial, primary and secondary follicles in CD1 mice treated as above and analyzed at PN10. No differences in primary and secondary follicle numbers were observed among groups (not shown). For comparison with untreated controls: **p<0.01, ***p<0.001. n=3 ovaries per treatment group. (C-E) PN5 CD1 female pups were treated with vehicle (PBS), or imatinib (7.5 mg/kg i.p.), or cisplatin (5 mg/kg) or with imatinib and cisplatin administered together and allowed to mature. Mice commenced breeding trials at PN42 with proven wt males and the mating procedure was repeated at regular intervals (about every 5 weeks) as per Gonfloni et al3. (C) The proportion of cisplatin-treated females becoming pregnant (fraction of pregnant mothers as reported in Gonfloni et al) was not altered by co-administration of imatinib (Kaplan-Meier analysis, cisplatin vs imatinib+cisplatin p>0.7, n=8-11 mice per treatment group). (D) The average pup number per mating round was not altered by co-administration of imatinib (n=7-11 pups per breeding round per treatment). (E) The total pup number generated as a result of the breedings described above was not altered by co-administration of imatinib (n= 319, 334, 4 and 5 pups respectively).
Importantly, TUNEL staining confirmed that pre-treatment with imatinib did not cause a reduction in apoptotic cells in ovaries of mice exposed to cisplatin for 24 or 48 h (Supplementary Fig. 2). When mice treated at PN5 or PN7 were analyzed as adults at PN49 (Supplementary Table 2) or at 9-11 months of age (Supplementary Table 3), no protection against oocyte killing was observed following treatment with imatinib prior to injection of cisplatin, compared to treatment with cisplatin alone. Similar depletion of primordial follicles was observed in both cisplatin- and imatinib+cisplatin-treated ovaries at PN49 (p<0.05 both, for cisplatin-treated versus vehicle-treated ovaries and for imatinib+cisplatin versus vehicle-treated ovaries; Supplementary Fig. 3). No significant differences in depletion of primary or secondary follicles were observed following treatment with imatinib+cisplatin versus cisplatin alone (p=0.08 for both primary and secondary/antral follicles). Representative histologic sections are shown in Supplementary Fig. 4A (PN49) and 4B (9-11 months). We also performed in vitro analysis, with imatinib (10 μM) or vehicle added to whole, postnatal day (PN) 5 C57BL/6 ovary cultures for 2 h followed by exposure to cisplatin (20 μM) or vehicle. After a further 24-48 h in culture, quantification of follicles (which contain oocytes) and TUNEL staining demonstrated no protection afforded by imatinib (Supplementary Fig. 5A, B and 6 and Supplementary Table 4).
In order to study cisplatin-induced infertility, mice that had been treated at PN7 with vehicle or imatinib (7.5 mg/kg i.p.) or cisplatin, (5 mg/kg i.p.) or both imatinib and cisplatin administered together, were studied in mating rounds of approximately 5 weeks, as described in Gonfloni et al3 from the age of 6 weeks (Supplementary Table 5). The proportion of pregnant mothers and the average pup number per mating round were calculated for the CD1 strain. No difference was observed for the co-administration of imatinib with cisplatin compared with cisplatin alone (Fig. 1C, D). The total number of pups presented as in Gonfloni et al3 was no different for mice treated with imatinib and cisplatin, versus cisplatin alone (Fig. 1E and Supplementary Table 5).
In their breeding studies, Gonfloni et al3 did not provide statistical analysis in support of their assertion that imatinib preserved fertility of mice exposed to cisplatin. The most stringent measure of reproductive potential is provided by the analysis of fertility (the ability to produce a litter within 12 weeks of mating). In order to study infertility, rather than just the proportion of mice becoming pregnant within the short 5 week mating rounds described by Gonfloni, we observed breeders for a total of twelve weeks following previous delivery/mating. Histologic analysis of ovaries from adult females observed to become sterile during these breeding studies (Supplementary Table 6) confirmed the absence of viable ovarian follicles (Supplementary Fig. 4C). The proportion of females in each treatment group noted to be fertile at each round of breeding and the average number of pups per litter (i.e. once the mother became pregnant) were determined. Ovulatory follicles present beyond 6 weeks following treatment were considered to be derived from primordial follicles that survived cisplatin treatment, which indeed is not sterilizing until after 3 rounds of breeding3. In our studies, by allowing female breeders to continue mating for a total of 12 weeks post previous delivery (or until they became pregnant, whichever occurred first, either of these being considerably longer than the one week of mating time observed by Gonfloni et al3), we observed that treatment with cisplatin at PN5-7 caused a significant deficit in fertility (p=0.02 PBS vs cisplatin), with only 72% females remaining fertile by breeding round 6 compared with 100% in the vehicle-treated group. Importantly, in up to 6 rounds of breeding, we found no evidence for rescue of cisplatin-induced loss of fertility by pre-treatment with imatinib (Supplementary Fig. 6A and Supplementary Table 6). The likelihood of infertility in each treatment group was no different between females treated with imatinib pre-cisplatin, compared with cisplatin alone (Kaplan-Meier analysis imatinib pre-cisplatin versus cisplatin p> 0.3) (Supplementary Fig. 6B). Consistent with this, the average pup numbers seen at each breeding round in imatinib pre-cisplatin-treated litters were not significantly different from those seen in litters from females treated with cisplatin alone (p>0.6) (Supplementary Fig. 6A).
In conclusion, we have shown that imatinib does not protect primordial follicle oocytes from cisplatin-induced apoptosis and loss of fertility in two independent strains of mice. These results indicate that imatinib-sensitive kinases, such as c-ABL, are not required for DNA damage activated oocyte apoptosis that is mediated by TAp63. Indeed, the imatinib-sensitive kinase c-KIT is known to be critical for the survival of female germ cells9, heightening concerns about the potential effects of imatinib on oocytes and female fertility. Thus, we find no support for “a new use for imatinib, aimed at preserving oocytes of the follicle reserve during chemotherapeutic treatments” and urge caution in this regard.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/naturemedicine/.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by fellowships and grants from the National Health and Medical Research Council (NHMRC Australia; Program Grants #494802 and #257502, Fellowships JKF (#441101), KJH (#494836), CLS (#406675), AS (#461299)), TPS (#575503); the Leukemia and Lymphoma Society (New York; SCOR grant #7015), the National Cancer Institute (NIH, US; CA 80188 and CA 43540) and the Victorian Cancer Council Fellowship CLS (CRF10_20). We thank Profs JM Adams, S Cory and A Villunger for gifts of mice, E. Jansen for technical assistance, Drs G Enders and R Schultz for gifts of antibodies, and Dr M Olshansky for help with calculations. This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS.
Footnotes
AUTHOR CONTRIBUTIONS
JBK and KJH performed and planned experiments, interpreted data and wrote manuscript. MC helped with experiments and contributed data. TPS performed statistical analysis and contributed to manuscript writing. AS, JKF and CLS conceived of the study, planned experiments, interpreted data and wrote the manuscript.
CONFLICTING INTEREST STATEMENT
The authors declare that they have no competing financial interests.
JBK & KJH share equal first authorship; AS, JKF & CLS share equal senior authorship
REFERENCES
- 1.Livera G, et al. p63 null mutation protects mouse oocytes from radio-induced apoptosis. Reproduction. 2008;135:3–12. doi: 10.1530/REP-07-0054. [DOI] [PubMed] [Google Scholar]
- 2.Suh EK, et al. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624–628. doi: 10.1038/nature05337. [DOI] [PubMed] [Google Scholar]
- 3.Gonfloni S, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med. 2009;15:1179–1185. doi: 10.1038/nm.2033. [DOI] [PubMed] [Google Scholar]
- 4.Ongkeko WM, et al. Gleevec suppresses p63 expression in head and neck squamous cell carcinoma despite p63 activation by DNA-damaging agents. Laryngoscope. 2006;116:1390–1396. doi: 10.1097/01.mlg.0000225941.60901.0f. [DOI] [PubMed] [Google Scholar]
- 5.Leong CO, Vidnovic N, DeYoung MP, Sgroi D, Ellisen LW. The p63/p73 network mediates chemosensitivity to cisplatin in a biologically defined subset of primary breast cancers. J Clin Invest. 2007;117:1370–1380. doi: 10.1172/JCI30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuveson DA, et al. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene. 2001;20:5054–5058. doi: 10.1038/sj.onc.1204704. [DOI] [PubMed] [Google Scholar]
- 7.Krystal GW, Honsawek S, Litz J, Buchdunger E. The selective tyrosine kinase inhibitor STI571 inhibits small cell lung cancer growth. Clin Cancer Res. 2000;6:3319–3326. [PubMed] [Google Scholar]
- 8.Reynoso D, Trent JC. Neoadjuvant and adjuvant imatinib treatment in gastrointestinal stromal tumor: current status and recent developments. Curr Opin Oncol. 2010;22:330–335. doi: 10.1097/CCO.0b013e32833aaaad. [DOI] [PubMed] [Google Scholar]
- 9.Carlsson IB, et al. Kit ligand and c-Kit are expressed during early human ovarian follicular development and their interaction is required for the survival of follicles in long-term culture. Reproduction. 2006;131:641–649. doi: 10.1530/rep.1.00868. [DOI] [PubMed] [Google Scholar]
- 10.Kerr JB, et al. Quantification of healthy follicles in the neonatal and adult mouse ovary: evidence for maintenance of primordial follicle supply. Reproduction. 2006;132:95–109. doi: 10.1530/rep.1.01128. [DOI] [PubMed] [Google Scholar]
- 11.Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127:569–580. doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]
- 12.Hutt KJ, McLaughlin EA, Holland MK. Kit ligand and c-Kit have diverse roles during mammalian oogenesis and folliculogenesis. Mol Hum Reprod. 2006;12:61–69. doi: 10.1093/molehr/gal010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.