Abstract
In vivo, nicotine in cigarette smoke induces various effects not only on the respiratory system but also the central and peripheral nerve systems, circulatory organs and digestive organs, and there is a possibility of promotion of lung tumorigenesis. The present experiment was conducted to examine histopathological changes caused by nicotine in the lung with repeated intratracheal instillation (i.t.). Six-week-old male F344 rats were administered nicotine by i.t. at doses of 0.05, 0.1 and 0.2 mg nicotine/rat every 3 weeks beginning at week 4, for up to a total of 9 times and were then sacrificed at week 30. The total number of administrations, total dose of nicotine and effective number of rats were 9 times, 0.45 mg and 5 rats and 4 times, 0.20 mg and 5 rats for the 0.05 mg nicotine/rat group; 3 times, 0.30 mg and 5 rats and 4 times, 0.40 mg and 3 rats for the 0.1 mg group; and 3 times, 0.60 mg and 3 rats for the 0.2 mg group, respectively. As a control group, 5 rats were administered 0.2 ml saline/rat 9 times. Some rats administered 0.1 and 0.2 mg nicotine suffered convulsions just after administration. Histopathologically, though proliferative changes were not observed, neutrophil infiltration, edema and fibrosis in the lung were induced by nicotine. In conclusion, repeated treatment of nicotine promoted neurologic symptoms in the acute phase, and strong inflammation in the lungs in the chronic phase, even at a low dose. Toxicity of nicotine is suggested to depend not on total dose of nicotine in the experiment but rather on repeated injury with consecutive administration.
Keywords: nicotine, lung, intratracheal instillation, toxicity, rat
Introduction
There are many chemicals including carcinogens in cigarette smoke, and at least 4000 component compounds have been described1. Of the smoking-related chemicals, nicotine is one of the major important components with toxicity. Nicotine is taken into the blood via the lungs from the inhaled smoke and binds to nicotinic acetylcholine receptors on the central and peripheral nerves2. It thereby induces various effects not only in the respiratory system but also circulatory and digestive organs3,4,5. In addition, according to a previous report, nicotine enhances proliferation, migration, and radioresistance of human malignant glioma cells through EGFR activation6. Nicotine is the addictive component of tobacco acting on neuronal nicotinic receptors (nAChRs). Functional nAChRs are also present on endothelial, hematological and epithelial cells7. Nicotine has been shown to stimulate the growth of solid tumors in vivo and to promote gastric cancer in the stomach8. Tobacco carcinogens can initiate and promote tumorigenesis, so concomitant exposure to nicotine could confer a proliferative advantage to early tumors, although there is no evidence that nicotine itself provokes cancer9.
However, there have only been few reports of in vivo toxicity and histopathological changes on aspiration of nicotine in the respiratory organs. To examine any lung toxicity induced by nicotine, it is necessary to have a system for frequent respiratory exposure. We have previously described a rat in vivo bioassay for detection of hazards due to fine particles by intratracheal instillation (i.t.)10, which can be used for risk assessment of inhaled chemicals. The i.t. method has been proposed as the most reliable route for assessing the pulmonary toxicity of particles in rodents11, although there are biologically different responses to inhalation and instillation12,13. Using this i.t. technique, the present experiment was conducted to histopathologically examine toxicity and cell proliferation caused by nicotine in the lungs by repeated i.t. administration in vivo.
Materials and Methods
Chemicals
Nicotine (chemical formula: C10H14N2 and CAS: 54-11-5) was purchased from Sigma-Aldrich (St. Louis, MO, USA) and suspended in saline (Otsuka isotonic sodium chloride solution, Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan).
Animals
Male Fischer-344/DuCrlCrj rats (4 weeks of age) purchased from Charles River Laboratories Japan, Inc. (Kanagawa, Japan) were maintained in the Division of Animal Experiments, Life Science Research Center, Kagawa University, according to the Institutional Regulations for Animal Experiments. The regulations included the best considerations for animal welfare and good practice of animal handling contributing to the replacement, refinement and reduction of animal testing (3Rs). The protocols of the experiments were approved by the Animal Care and Use Committee for Kagawa University. The animals were housed in polycarbonate cages with white wood chips for bedding and given free access to drinking water and a basal diet, CE-2 (CLEA Japan, Inc., Tokyo, Japan), under controlled conditions of humidity (60 ± 10%), lighting (12-h light/dark cycle) and temperature (24 ± 2ºC). The experiments were started after a 2-week acclimation period.
Experimental design and tissue preparation
A total of thirty 6-week-old male F344 rats were randomly separated into 6 groups of 5 rats each and scheduled to be administered nicotine by i.t.10 every 3 weeks from week 4 to 28, for a total of 9 times, and to be sacrificed at week 30. The doses of nicotine were decided to be 0.05, 0.1 and 0.2 mg nicotine/0.2 ml saline/rat based on the report that 0.2 mg nicotine/rat corresponds to a lethal dose for human adults (30–60 mg, 0.5–1.0 mg/kg body weight)14,15. The pHs of the diluted nicotine solutions (0.05, 0.1 and 0.2 mg nicotine/0.2 ml saline) were 9.22, 9.42 and 9.60, respectively (pH meter F-52, HORIBA, Ltd., Kyoto, Japan). The pH of the saline used as a vehicle and control was 6.14. During the experiment, the third or fourth administrations were not given to the high-dose group, 0.1 and 0.2 mg nicotine/rat, because of the death of some rats following nicotine administration. The final experiment protocol was therefore modified as shown in Table 1. The total numbers of administrations and effective numbers of rats were 9 times and 5 rats (Group 3) and 4 times and 5 rats (Group 2) for the 0.05 mg nicotine/rat group; 3 times and 5 rats (Group 4) and 4 times and 3 rats (Group 5) for the 0.1 mg group; and 3 times and 3 rats (Group 6) for the 0.2 mg group, respectively. As a control group (Group 1), 5 rats were administered 0.2 ml saline/rat 9 times. Total doses of nicotine per rat were 0.00 mg (Group 1), 0.20 (Group 2), 0.45 (Group 3), 0.30 (Group 4), 0.40 (Group 5) and 0.60 (Group 6).
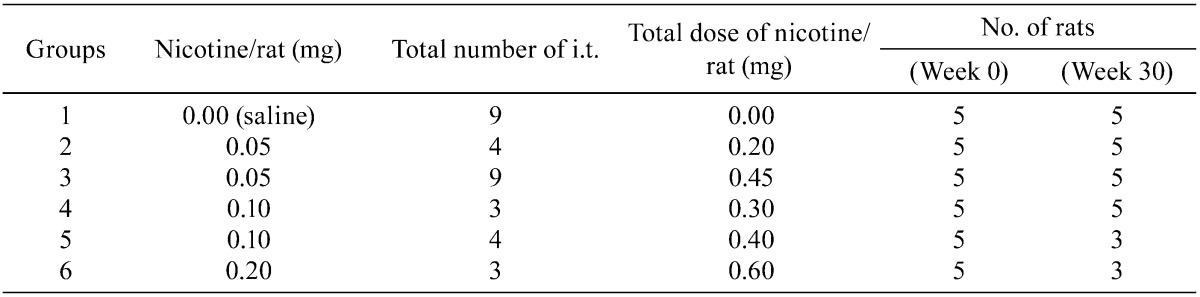
Table 1. Details of the Final Experimental Protocol.
At autopsy, the lungs, liver and kidneys were removed. The lungs were weighed including the trachea and heart first, rinsed in 10% neutral buffered formalin after excision and then infused with 10% neutral buffered formalin. The weights of the lungs were calculated by subtraction of the weight of the remaining trachea and heart. The liver and kidneys were weighed and immersed in 10% neutral buffered formalin for 3 days. Slices of organs were routinely processed for embedding in paraffin for histopathological examination of H&E-stained sections. For lungs, this was routinely performed for 2 slices of the left lobe and 1 slice each of the other lobes. Each lung lobe was examined histopathologically for neutrophil infiltration, pulmonary edema, pulmonary fibrosis, macrophage infiltration in the alveoli, restructuring of walls, granuloma16 and atelectasis. Severity for each parameter, except for atelectasis, was divided as follows: 0, no change; 1, weak; 2, moderate; and 3, severe. Severity for atelectasis was divided as follows: 0, none; 1, 1 lobe; 2, 2 lobes; and 3, more than 3 lobes.
Statistical analysis
Body and organ weights were analyzed by the Tukey-Kramer post hoc test. P values less than 0.05 were considered significant.
Results
All rats in Groups 4, 5, and 6 (0.1 or 0.2 mg nicotine/rat) laid on their backs and suffered convulsions a few seconds after each i.t. administration. This symptom continued for approximately 5 to 10 seconds. At the third or fourth administration, there were a number of mortalities, although other rats survived the acute symptoms. As noted above, due to the deaths, the experimental design was modified, and the number of administrations was modified in some groups.
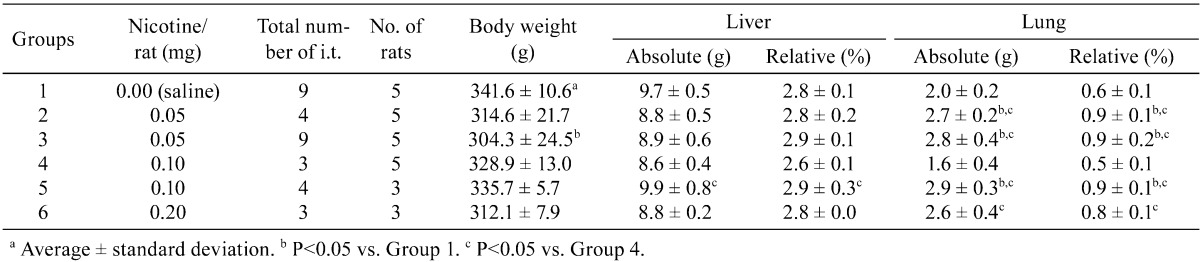
Final body and organ weights are summarized in Table 2. The body weights of the rats in Group 3 (0.05mg nicotine 9 times) showed significant decreases compared with Group 1 (control group). Absolute and relative weights of the lung were significantly increased in Groups 2, 3 and 5 compared with Group 1 (control group). Regarding liver weights, there were no significant differences compared with Group 1 (saline control group).
Table 2. Body and Organ Weights of the Rats.
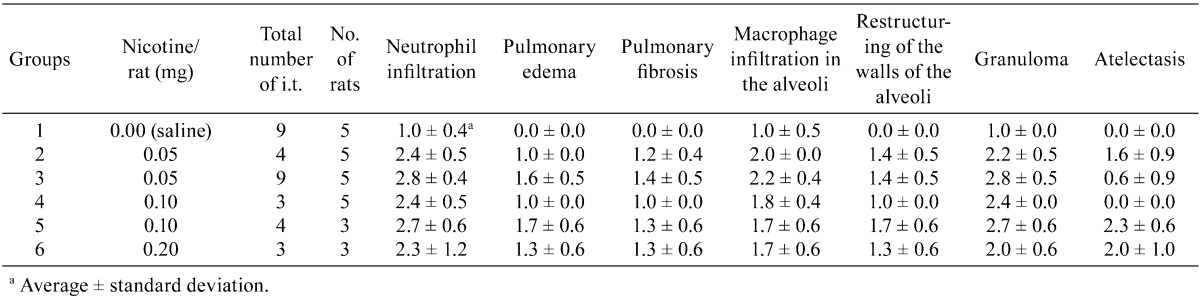
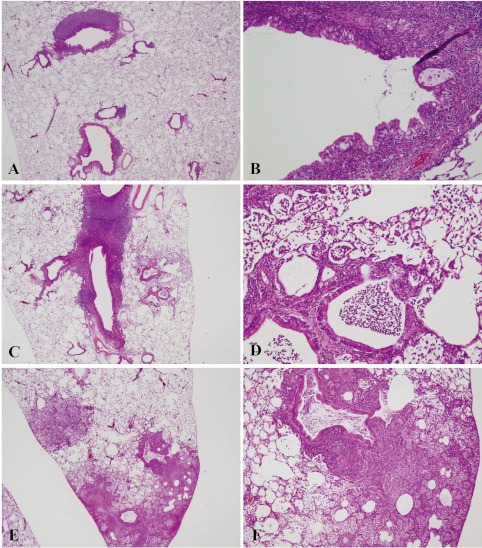
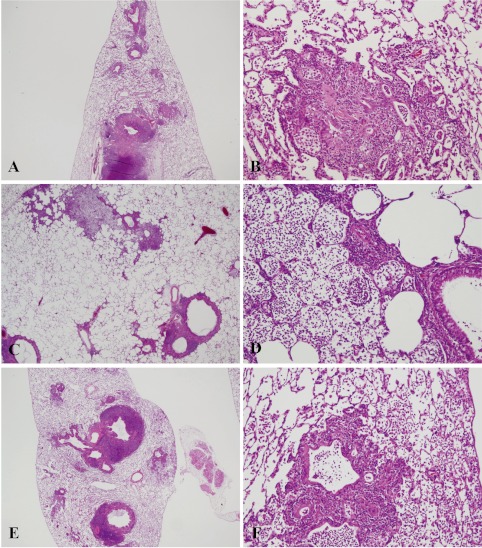
Histopathologically, the lungs of Groups 2–6 (nicotine-treated groups) showed inflammatory changes, neutrophil infiltration, pulmonary edema, pulmonary fibrosis, macrophage infiltration in the alveoli, restructuring of walls and granuloma, in all animals (incidence: 100%) (Table 3). Atelectasis was observed in Groups 2, 3, 5 and 6. No proliferative alteration of alveolar cells was apparent. The lungs of Group 3 (0.05 mg nicotine 9 times) showed the severest and most widespread inflammatory changes in all rats (Fig. 1-E and F). The inflammation in Groups 2, 4, 5 and 6 persisted until autopsy (week 30), despite the 17–20-week period between the final instillation of nicotine and autopsy. The areas of inflammation in the lungs of Groups 2, 4, 5 and 6 were localized and showed clear borders with normal areas (Fig. 1-C, Fig. 2-A, C, E).
Table 3. Scoring Indices of Histopathological Changes.
Fig. 1.
Histopathological findings for lungs in Groups 1–3. The lungs of Group 3 (0.05 mg nicotine 9 times) showed the severest inflammatory changes in all rats (E and F). Inflammation in Group 2 (0.05 mg 4 times) persisted until autopsy (week 30). The saline control group (Group 1) also demonstrated severe lymphoid cell infiltration around the bronchus, as in the other groups (Group 2–6) (A and B). A, saline control ×9 (Group 1) (magnification: ×20); B, saline control ×9 (Group 1) (×200); C, 0.05 mg nicotine ×4 (group 2) (×12.5); D, 0.05 mg nicotine ×4 (group 2) (×100); E, 0.05 mg nicotine ×9 (group 3) (×12.5); F, 0.05 mg nicotine ×9 (group 3) (×40).
Fig. 2.
Histopathological findings for lungs in Groups 4–6. Inflammation in Groups 4, 5 and 6 persisted until autopsy (week 30) with severe lymphoid cell infiltration around the bronchus (A–F). A, 0.10 mg nicotine ×3 (Group 4) (magnification: ×12.5); B, 0.10 mg nicotine ×3 (Group 4) (×100); C, 0.10 mg nicotine ×4 (Group 5) (×20); D, 0.10 mg nicotine ×4 (Group 5) (×100); E, 0.20 mg nicotine ×3 (Group 6) (×12.5); F, 0.20 mg nicotine ×3 (group 6) (×100).
All rats (Group 1-6) also showed severe lymphoid cell infiltration around the bronchus in their lungs with almost the same degree. The saline control group (Group 1) also featured severe lymphoid cell infiltration around the bronchus, but not inflammatory changes in the alveoli (Fig. 1-A, B).
In the kidneys and livers of animals treated with nicotine (Groups 2-6), no remarkable changes were observed macroscopically and histopathologically compared with the control group (Group 1).
Discussion
In the present study, rats treated with 0.1 or 0.2 mg nicotine suffered convulsions after each i.t. administration. The behavioral effects of nicotine are reported to be attributed to an action on nicotinic receptors, their over stimulation of nicotinic receptors in the brain resulting in clonic-tonic convulsions17. Damaj MI et al. reported that nicotine enhances the release of glutamate either directly or indirectly (membrane depolarization that opens L-type calcium channels) and that glutamate release in turn stimulates N-methyl-D-aspartate receptors, thus triggering a cascade of events leading to nitric oxide formation and possibly seizure18. Nicotine at a dose of 0.75 or 1.0 mg/kg body weight is reported to lead to a decrease in cortical after-discharge duration and influence seizure susceptibility, but not cause any detectable neuronal damage19.
The body weights of the rats treated nicotine tended to be decreased compared with the control group (Group 1). Furthermore, in Group 3 (0.05 mg nicotine 9 times), the decrease was significant as compared with Group 1 (control group). This decrease in body weight can be considered due to the toxicity of nicotine. The total dose of nicotine in Group 3 was 0.45 mg and was lower than that in Group 6 (0.60 mg). However, the number of administrations in Group 3 was 9, and this was the maximum number. The results suggest that the decrease in body weight depends not only on the total dose of nicotine in the experiment but also on repeated and consecutive administrations. The lung weights of the rats treated with nicotine were increased significantly compared with the control group (Group 1) but not in the group treated with 0.1 mg nicotine 3 times (Group 4). This result corresponds with inflammatory change caused by nicotine in the lungs, excluding the decrease in lung weight in Group 4.
Histopathologically, the lungs of Group 3 (0.05 mg nicotine 9 times, 0.45 mg total dose) showed the severest and most widespread inflammatory changes in all rats. Histopathological inflammation also did not solely depend on the total dose of nicotine in the experiment, and repeated and consecutive administrations correspond with a decrease in body weight. Mabley J et al. reported that intraperitoneal injection of 0.2 or 0.4 mg/kg nicotine exerts an anti-inflammatory effect in a murine model of acute lung injury induced by intratracheal lipopolysaccharide (LPS, 50 µl)20. The difference in the result of their report, exerting an anti-inflammation effect, and our experiment, inducing an inflammatory change, is suggested to be due to the difference in administration route. In the present experiment, the pHs of the diluted nicotine solutions (0.05, 0.1 and 0.2 mg nicotine/0.2 ml saline) were very alkaline at 9.22, 9.42 and 9.60, respectively (saline: 6.14). Alkaline compounds cause liquefaction necrosis, which in turn causes ongoing invasion into deeper layers of tissue21. This is the same problem that occurs with accidental drinking of lye solution, the high pH of which is associated with esophageal ulceration. Vancula EM et al. concluded that the critical pH that causes esophageal ulceration is 12.521. This is much higher than the solutions used in the present experiment. However, because the target organ is different, it is difficult to conclude that lung inflammatory changes were due to the nicotine itself or the high pH.
All rats (Groups 1–6) showed almost the same degree of severe lymphoid cell infiltration around the bronchus in their lungs, not only those treated with nicotine but also those treated with saline vehicle alone. Our previous 30-week experiment using F344 male rats also showed severe lymphoid cell infiltration around the trachea in a saline control group with 100% incidence, and this finding was reported as large granular lymphocytic lymphoma (LGL lymphoma)22. In this context, it should be mentioned that F344 rats demonstrate an incidence of over 50% of LGL lymphoma in aged animals23. Frith CH et al. concluded that lymphoid cell neoplasms in F344 rat should not be grouped with nonlymphoid neoplasms in determining the toxicity and carcinogenicity of test substances24.
CYP2A5 is reported to be involved in metabolism of nicotine and its major circulating metabolite, cotinine, in the mouse liver25. CYP2A5, a mouse cytochrome P450 monooxygenase that shows high similarities to human CYP2A6 and CYP2A13 in protein sequence and substrate specificity, is expressed in multiple tissues, including the liver, kidney, lung and nasal mucosa. In humans, CYP2A6 is the predominant enzyme responsible for 70–80% of nicotine metabolism to cotinine26,27. The much higher exposure to cotinine than to nicotine in smokers should be taken into consideration, since cotinine suppression of apoptosis may play an important role in lung tumorigenesis in vivo28. We have established a bioassay using the tobacco-specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) as an initiating carcinogen29. In the lungs of A/J female mice, the initial event in this model is reported to be formation of O6-methylguanine-DNA, a major promutagenic adduct that leads to GC>AT transitional mispairing and subsequent activation of the K-ras proto-oncogene30,31. We have previously reported inhibitory effects of 8-methoxypsoralen, a potent human CYP2A6 inhibitor, on NNK-induced lung carcinogenesis in female A/J mice32,33. Human CYP2A6 (mouse CYP2A5) might affect the metabolism of both nicotine and NNK. Therefore, it is strongly expected that human CYP2A6 inhibitors would have major effects on lung carcinogenesis after administration of nicotine and NNK.
In conclusion, repeated i.t. treatment with nicotine in the present study was associated with neurologic symptoms (convulsions) in the acute phase, and marked inflammation in the lungs in the chronic phase, even at a low dose. Toxicity of nicotine is suggested to depend not on total dose of nicotine in the experiment but rather repeated and consecutive exposure.
Acknowledgments
We thank Dr. Malcolm A. Moore for critical reading of the manuscript and Sanae Kushida for assistance in its preparation.
References
- 1.Smith CJ, Hansch C. The relative toxicity of compounds in mainstream cigarette smoke condensate. Food Chem Toxicol. 38: 637–646 2000. [DOI] [PubMed] [Google Scholar]
- 2.Hone AJ, Meyer EL, McIntyre M, McIntosh JM. Nicotinic acetylcholine receptors in dorsal root ganglion neurons include the alpha6beta4* subtype. FASEB J. 26: 917–926 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catanzaro DF, Zhou Y, Chen R, Yu F, Catanzaro SE, De Lorenzo MS, Subbaramaiah K, Zhou XK, Pratico D, Dannenberg AJ, Weksler BB. Potentially reduced exposure cigarettes accelerate atherosclerosis: evidence for the role of nicotine. Cardiovasc Toxicol. 7: 192–201 2007. [DOI] [PubMed] [Google Scholar]
- 4.Alamanda V, Singh S, Lawrence NJ, Chellappan SP. Nicotine-mediated induction of E-selectin in aortic endothelial cells requires Src kinase and E2F1 transcriptional activity. Biochem Biophys Res Commun. 418: 56–61 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Özkan KU, Ozokutan BH, Inanc F, Boran C, Kilinc M. Does maternal nicotine exposure during gestation increase the injury severity of small intestine in the newborn rats subjected to experimental necrotizing enterocolitis. J Pediatr Surg. 40: 484–488 2005. [DOI] [PubMed] [Google Scholar]
- 6.Khalil AA, Jameson MJ, Broaddus WC, Lin PS, Chung TD. Nicotine enhances proliferation, migration, and radioresistance of human malignant glioma cells through EGFR activation. Brain Tumor Pathol. 2012 [DOI] [PubMed]
- 7.Cardinale A, Nastrucci C, Cesario A, Russo P. Nicotine: specific role in angiogenesis, proliferation and apoptosis. Crit Rev Toxicol. 42: 68–89 2012. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Liu BA. Enhanced proliferation, invasion, and epithelial-mesenchymal transition of nicotine-promoted gastric cancer by periostin. World J Gastroenterol. 17: 2674–2680 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grozio A, Catassi A, Cavalieri Z, Paleari L, Cesario A, Russo P. Nicotine, lung and cancer. Anticancer Agents Med Chem. 7: 461–466 2007. [DOI] [PubMed] [Google Scholar]
- 10.Yokohira M, Takeuchi H, Yamakawa K, Saoo K, Ikeda M, Matsuda Y, Zeng Y, Hosokawa K, Maeta H, Imaida K. Establishment of a bioassay system for detection of lung toxicity due to fine particle instillation: Sequential histopathological changes with acute and subacute lung damage due to intratracheal instillation of quartz in F344 male rats. J Toxicol Pathol. 18: 13–18 2005. [Google Scholar]
- 11.Warheit DB, Brock WJ, Lee KP, Webb TR, Reed KL. Comparative pulmonary toxicity inhalation and instillation studies with different TiO2 particle formulations: impact of surface treatments on particle toxicity. Toxicol Sci. 88: 514–524 2005. [DOI] [PubMed] [Google Scholar]
- 12.Osier M, Oberdorster G. Intratracheal inhalation vs intratracheal instillation: differences in particle effects. Fundam Appl Toxicol. 40: 220–227 1997. [DOI] [PubMed] [Google Scholar]
- 13.Yokohira M, Kuno T, Yamakawa K, Hashimoto N, Ninomiya F, Suzuki S, Saoo K, Imaida K. An intratracheal instillation bioassay system for detection of lung toxicity due to fine particles in F344 rats. J Toxicol Pathol. 22: 1–10 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gosselin R, Smith R, Hodge H, Braddock J. Clinical Toxicology of Commercial Products. Williams & Wilkins, Baltimore. 311–313. 1988 [Google Scholar]
- 15.Okamoto M, Kita T, Okuda H, Tanaka T, Nakashima T. Effects of aging on acute toxicity of nicotine in rats. Pharmacol Toxicol. 75: 1–6 1994. [DOI] [PubMed] [Google Scholar]
- 16.Yokohira M, Kuno T, Yamakawa K, Hosokawa K, Matsuda Y, Hashimoto N, Suzuki S, Saoo K, Imaida K. Lung toxicity of 16 fine particles on intratracheal instillation in a bioassay model using f344 male rats. Toxicol Pathol. 36: 620–631 2008. [DOI] [PubMed] [Google Scholar]
- 17.Czuczwar M, Kis J, Czuczwar P, Wielosz M, Turski W. Nicotine diminishes anticonvulsant activity of antiepileptic drugs in mice. Pol J Pharmacol. 55: 799–802 2003. [PubMed] [Google Scholar]
- 18.Damaj MI, Glassco W, Dukat M, Martin BR. Pharmacological characterization of nicotine-induced seizures in mice. J Pharmacol Exp Ther. 291: 1284–1291 1999. [PubMed] [Google Scholar]
- 19.Riljak V, Maresova D, Pokorny J. Nicotine effects on rat seizures susceptibility and hippocampal neuronal degeneration. Neuro Endocrinol Lett. 31: 792–795 2010. [PubMed] [Google Scholar]
- 20.Mabley J, Gordon S, Pacher P. Nicotine exerts an anti-inflammatory effect in a murine model of acute lung injury. Inflammation. 34: 231–237 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vancura EM, Clinton JE, Ruiz E, Krenzelok EP. Toxicity of alkaline solutions. Ann Emerg Med. 9: 118–122 1980. [DOI] [PubMed] [Google Scholar]
- 22.Yokohira M, Hashimoto N, Yamakawa K, Suzuki S, Saoo K, Kuno T, Imaida K. Lung carcinogenic bioassay of CuO and TiO(2) nanoparticles with intratracheal instillation using F344 male rats. J Toxicol Pathol. 22: 71–78 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stromberg PC, Vogtsberger LM. Pathology of the mononuclear cell leukemia of Fischer rats. I. Morphologic studies. Vet Pathol. 20: 698–708 1983. [DOI] [PubMed] [Google Scholar]
- 24.Frith CH, Ward JM, Chandra M. The morphology, immunohistochemistry, and incidence of hematopoietic neoplasms in mice and rats. Toxicol Pathol. 21: 206–218 1993. [DOI] [PubMed] [Google Scholar]
- 25.Zhou X, Zhuo X, Xie F, Kluetzman K, Shu YZ, Humphreys WG, Ding X. Role of CYP2A5 in the clearance of nicotine and cotinine: insights from studies on a Cyp2a5-null mouse model. J Pharmacol Exp Ther. 332: 578–587 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K, Funae Y, Shimada N, Kamataki T, Kuroiwa Y. Role of human cytochrome P4502A6 in C-oxidation of nicotine. Drug Metab Dispos. 24: 1212–1217 1996. [PubMed] [Google Scholar]
- 27.Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K, Funae Y, Shimada N, Kamataki T, Kuroiwa Y. Characterization of CYP2A6 involved in 3’-hydroxylation of cotinine in human liver microsomes. J Pharmacol Exp Ther. 277: 1010–1015 1996. [PubMed] [Google Scholar]
- 28.Nakada T, Kiyotani K, Iwano S, Uno T, Yokohira M, Yamakawa K, Fujieda M, Saito T, Yamazaki H, Imaida K, Kamataki T. Lung tumorigenesis promoted by anti-apoptotic effects of cotinine, a nicotine metabolite through activation of PI3K/Akt pathway. J Toxicol Sci. 37: 555–563 2012. [DOI] [PubMed] [Google Scholar]
- 29.Yokohira M, Takeuchi H, Saoo K, Matsuda Y, Yamakawa K, Hosokawa K, Kuno T, Imaida K. Establishment of a bioassay model for lung cancer chemoprevention initiated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in female A/J mice. Exp Toxicol Pathol. 60: 469–473 2008. [DOI] [PubMed] [Google Scholar]
- 30.Peterson LA, Hecht SS. O6-methylguanine is a critical determinant of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenesis in A/J mouse lung. Cancer Res. 51: 5557–5564 1991. [PubMed] [Google Scholar]
- 31.Ronai ZA, Gradia S, Peterson LA, Hecht SS. G to A transitions and G to T transversions in codon 12 of the Ki-ras oncogene isolated from mouse lung tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and related DNA methylating and pyridyloxobutylating agents. Carcinogenesis. 14: 2419–2422 1993. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi H, Saoo K, Yokohira M, Ikeda M, Maeta H, Miyazaki M, Yamazaki H, Kamataki T, Imaida K. Pretreatment with 8-methoxypsoralen, a potent human CYP2A6 inhibitor, strongly inhibits lung tumorigenesis induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in female A/J mice. Cancer Res. 63: 7581–7583 2003. [PubMed] [Google Scholar]
- 33.Takeuchi H, Saoo K, Matsuda Y, Yokohira M, Yamakawa K, Zeng Y, Miyazaki M, Fujieda M, Kamataki T, Imaida K. Dose dependent inhibitory effects of dietary 8-methoxypsoralen on NNK-induced lung tumorigenesis in female A/J mice. Cancer Lett. 234: 232–238 2006. [DOI] [PubMed] [Google Scholar]