Abstract
Increased incidence of adrenal pheochromocytoma is frequently encountered in rat carcinogenicity studies. In some of the studies, the finding is judged to be due to a rat-specific mechanism of carcinogenesis caused by a disturbance of calcium homeostasis. However, direct evidence that the proliferation of chromaffin cells in the adrenal medulla is induced solely by hypercalcemia is not available. In this study, calcium gluconate was intravenously infused for 7 days to rat chromaffin cells by a tail cuff method, and cumulative labeling with bromodeoxyuridine (BrdU) was carried out to evaluate the proliferative activity. The serum calcium concentration was dose-dependently increased, and a high calcium concentration was stably sustained from day 2 to 7. In the adrenal medulla, BrdU-positive chromaffin cells increased in the calcium gluconate-treated animals, and the BrdU-labeling index increased in a dose-dependent manner. In addition, an increased BrdU-labeling index of chromaffin cells was shown to correlate with the serum calcium concentration. Our results demonstrate that hypercalcemia directly enhances the proliferative activity of chromaffin cells and that the proliferative activity is correlated with the serum calcium concentration.
Keywords: cell proliferation activity, adrenal glands, chromaffin cell, hypercalcemia, rat, bromodeoxyuridine
Rodent carcinogenicity studies are widely conducted as bioassays for human safety assessment. Treatment-related increases in the incidence of tumors are often observed in such studies. A large number of compounds induce pheochromocytoma originating from chromaffin cells of the adrenal medulla in rodent carcinogenicity studies. Such compounds include a wide range of chemicals such as metal compounds, hydrocarbons, amines, dyestuffs, pesticides and pharmaceuticals1. Mechanisms that cause pheochromocytoma in experimental animals include uncoupling of mitochondrial respiration, hypoxia, disturbance of the hypothalamic endocrine axis and disturbance of calcium homeostasis1.
When assessing the risk of carcinogenicity, it must be taken into consideration that positive results in a rodent carcinogenicity study cannot always be extrapolated to humans. Indeed, several tumors observed in rodent carcinogenicity studies, such as carcinoid tumors of the stomach induced by prolonged suppression of acid secretion, thyroid follicular cell tumors associated with enzyme induction and kidney tumors related to α2μ-globulin nephropathy, show no carcinogenic risk in humans2. Among them, adrenal pheochromocytoma is commonly enhanced in rodent carcinogenicity studies when triggered by disturbance of calcium homeostasis, and its tumorigenesis has been concluded to have no carcinogenic risk in human1, 3,4,5. For instance, high doses of slowly or poorly absorbed sugars or sugar alcohols cause disturbance of calcium homeostasis by increasing calcium absorption from the small intestine in rats and thereby induces adrenal pheochromocytoma3. Vitamin D and Vitamin D-derivative compounds also cause adrenal pheochromocytomas in rats due to disturbance of calcium homeostasis4, 5. The positive results in these carcinogenicity studies are judged to be caused by rat-specific mechanisms of carcinogenesis activated by a non-genotoxic mechanism. Although disturbance of calcium homeostasis is strongly indicated to be a promotive cause of adrenal pheochromocytoma, compounds that cause the increase of this tumor have various biological functions other than their influence on calcium homeostasis, and direct evidence that proliferation of chromaffin cells in the adrenal medulla is induced solely by hypercalcemia is not available as far as we know.
We therefore induced a disturbance in calcium homeostasis by hypercalcemia in rats without using chemical compounds that are known to induce pheochromocytoma in rodent carcinogenicity studies, and examined the proliferative activity of chromaffin cells in the adrenal medulla. A sustained high serum calcium concentration was induced in rats by intravenous infusion of calcium gluconate (GACa) for 7 days, and 5-bromo-2’-deoxyuridine (BrdU) cumulative labeling was carried out to evaluate the proliferative activity of chromaffin cells in the adrenal medulla. All animal experiments were approved by the Ethical Committee for Treatment of Laboratory Animals at Chugai Pharmaceutical Co., Ltd.
In this study, 21 eight-week-old male Sprague-Dawley rats were purchased from Japan SLC, Inc. (Shizuoka, Japan). The animals were housed in cages in an animal room maintained at a temperature of 24 ± 2˚C and a humidity of 55 ± 10%, with 14 to 16 air changes per hour and a 14-hour light and 10-hour dark cycle, and were fed pelleted chow (CE-2; Clea Japan, Inc., Tokyo, Japan) and tap water ad libitum.
At 9 weeks of age, a polyurethane catheter (0.6-mm ID × 0.9-mm OD, Access Technologies, Skokie, IL, USA) was inserted from the femoral vein to the posterior vena cava under anesthesia by a tail cuff method. The swivel was connected via polyethylene PE-50 tubing (Becton, Dickinson and Company, Sparks, MD, USA) with a 23-gauge needle to a 0.22 μm filter (Millipore Japan Co., Ltd., Tokyo, Japan), which was connected to a 50-ml syringe. The detailed procedures for preparing a rat model for intravenous infusion were reported by Asanuma et al.6. After surgery, the rats were infused with saline at 1.0 ml/head/hour until the start of this study at 11 weeks of age.
In order to set the dose levels of GACa, we carried out a preliminary study. In the preliminary study, all animals infused at dose levels of 60 mg/ml (5,760 mg/kg/day) were found dead or were sacrificed in moribund condition from 1 to 3 days after the start of administration of GACa. At dose levels of 10 mg/ml (960 mg/kg/day), a high serum calcium concentration was not sustained. On the other hand, rats infused at dose levels of 20 mg/ml (1,920 mg/kg/day) and 40 mg/ml (3,840 mg/kg/day) showed a stable increase in serum calcium concentration, which was sustained for a period of 7 days. On the basis of these preliminary results, dose levels of 20 mg/ml (1,920 mg/kg/day) and 40 mg/ml (3,840 mg/kg/day) were selected.
In this study, 14 rats were divided into 2 treatment groups (n=7) and subjected to administration of GACa (Wako Pure Chemical Industries, Ltd., Osaka, Japan) at dose levels of either 20 mg/ml (1,920 mg/kg/day) or 40 mg/ml (3,840 mg/kg/day). Each dose of GACa was prepared using saline as a vehicle. The remaining 7 rats were used as the control group and administered saline. All rats were infused with GACa or saline at 4 ml/kg/hour for a period of 7 days.
The proliferative activity of chromaffin cells in the adrenal glands was determined by cumulative labeling with BrdU. BrdU labeling was carried out according to the method described by Suzuki et al.7. At day 0 (first dosing day), mini-osmotic pumps (Alza Co., Palo Alto, CA, USA) filled with BrdU (Sigma Chemical Co., St. Louis, MO, USA) were implanted into the peritoneal cavity in rats under anesthesia, and the rats were then injected with 240 µg of BrdU per hour for 7 days before sacrifice.
All animals were weighed on days 0 (before dosing), 1, 3 and 7. During the infusion period, clinical signs of all animals were observed every day. Blood was taken from the jugular vein to examine the serum calcium concentration on days 0 (before dosing), 1, 2, 3 and 6. Approximately 24 hours of cumulative pooled urine was collected to measure urine volume and urinary calcium excretion. Urinary samples were collected on days 2 and 6. Measurement of the serum calcium concentration and urinary calcium excretion was determined by the OCPC method.
Animals were sacrificed by exsanguination from the abdominal artery under anesthesia. At necropsy, the adrenal glands and kidneys were removed from each animal, and the adrenal glands were weighed. The specimens were fixed in 10% neutral-buffered formalin for 24 hours. After fixation, the tissues were embedded into paraffin according to the standard method. Thin sections from the paraffin blocks were prepared and used for HE and immunohistochemical staining.
BrdU-incorporated nuclei were immunohistochemically identified using anti-BrdU antibody as described previously7. Immunohistochemical staining was performed according to the LSAB method (Dako LSAB kit, Dako, Carpinteria, CA, USA) using an anti-BrdU monoclonal antibody (Amersham, Bucks, UK). The immunoreaction was visualized by a peroxidase-diaminobenzidine reaction. The sections were finally counterstained lightly with hematoxylin. To evaluate the proliferative activity of chromaffin cells in the adrenal glands, analysis was conducted on 500 cells for each animal. The BrdU labeling index was determined by the following formula: (BrdU-positive nuclei count) ÷ 500 (nuclei count) × 100.
Group mean values and standard deviations were calculated for body weight, serum calcium concentration, urinary calcium excretion, adrenal glands weight and BrdU labeling index. These data were analyzed for statistical significance of any differences between the control group and each drug-treated group using the Student’s or Aspin-Welch’s t-test at concentrations of 1% and 5%. To analyze the correlation between serum calcium concentration and BrdU-labeling index, the average calcium concentration of each animal on days 2 and 6 was calculated, and then the significance of the correlation value between the 2 parameters was tested using the Pearsonʼs correlation coefficient test with the Mini StatMate software (ATMS Co., Ltd., Tokyo, Japan). Difference with a P value less than 0.05 were considered to be significant.
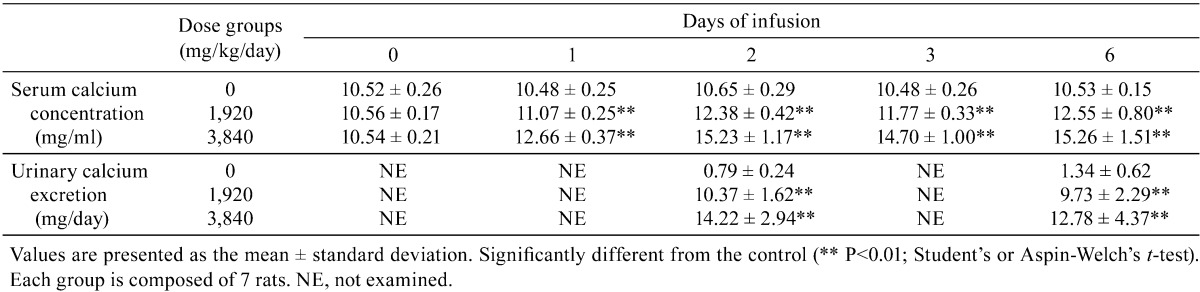
The serum calcium concentration was dose-dependently increased on day 1, and a high and stable calcium concentration was sustained from day 2 to the end of the administration period in the GACa-treated groups (Table 1). Urinary calcium excretion was dose-dependently increased on both days 2 and 6 in the GACa-treated groups (Table 1). It is well known that maintaining hypercalcemia by bolus administration of calcium solutions is difficult because of the rapid excretion of calcium in urine. In this study, however, we intravenously infused GACa to rats by a tail cuff method, successfully induced a dose-dependent increase in the serum concentration and urinary excretion of calcium and were able to sustain a high and stable serum calcium level during the administration period.
Table 1. Changes in Serum Calcium Concentration and Urinary Calcium Excretion.
No deaths or treatment-related clinical signs were observed in any dose group. There was suppression of body weight gain in the 3,840-mg/kg/day group from day 1 that continued throughout the study (Table 2).
Table 2. Body Weight and Adrenal Glands Weight.
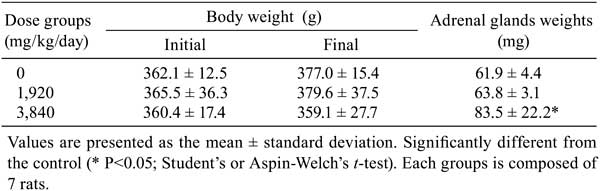
At necropsy, enlargement of the adrenal glands and white foci of the kidneys were seen in 2/7 rats in the 3,840-mg/kg/day group. No macroscopic abnormality was observed in the 1,920-mg/kg/day group. In the kidney, the histopathological changes in the 3,840-mg/kg/day group were characterized by slight nephrocalcinosis, which coincided with the white foci observed at necropsy. No histopathological findings were observed in the 1,920-mg/kg/day group. In the adrenal glands, organ weight was significantly increased in the 3,840-mg/kg/day group in addition to the enlargement observed at necropsy. Histopathologically, slight hypertrophy of cortical cells was observed in the 3,840-mg/kg/day group. In addition, BrdU-positive cortical cells also slightly increased. These findings were considered to coincide with an increased adrenal gland weight (Table 2). There were no histopathological findings in the adrenal medulla in any dose group.
In summary, the influence on the rats of infusion of GACa for 7 days was characterized by sustained hypercalcemia with a slight suppression of body weight, slight mineralization in the kidneys and no marked deterioration of clinical signs. Therefore, we considered that the changes in the adrenal cortex were appropriately evaluated in this study without the interference of secondary effects such as marked deterioration of general condition. In addition, it was indicated that intravenous infusion of calcium preparations to rats by a tail cuff method is highly useful and valuable for inducing sustained hypercalcemia and evaluating the influence of that state on the organs and tissues in rats.
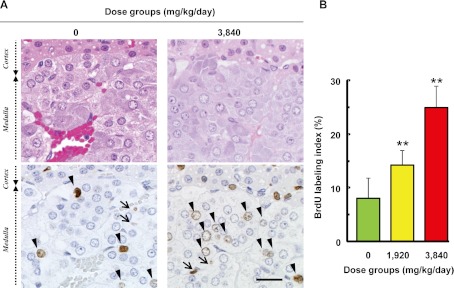
In the adrenal medulla, BrdU-positive nuclei were found in the chromaffin cells and endothelial cells. BrdU-positive chromaffin cells were sporadically localized in the medulla of both the control and GACa-treated groups, and an increase in BrdU positivity was clearly identified in the GACa-treated groups. On the other hand, BrdU-positivity of endothelial cells was smaller (approximately 5%) compared to that of chromaffin cells in the control group, and the increase in BrdU-positive endothelial cells was histopathologically marginal in the GACa-treated groups. Therefore, morphometric analysis using BrdU labeling was conducted for chromaffin cells in this study. The number of BrdU-positive chromaffin cells increased in the animals of the GACa-treated groups compared with the animals of the control group (Fig. 1A). The BrdU labeling index was significantly and dose-dependently increased in the GACa-treated groups (Fig. 1B). These results indicated proliferation of chromaffin cells in the GACa-treated groups, although there were no histopathological findings in the adrenal medulla in any dose group. We considered that this was due to the short term of this study. Moreover, cumulative labeling with BrdU enables the observation of any cell that passes through the S-phase and consequently might increase the sensitivity of detection of cell proliferation compared with histopathological evaluation.
Fig. 1.
Stimulation of adrenal chromaffin cell proliferation in response to the serum calcium concentration in rats. A: HE staining (upper) and immunohistochemical staining of BrdU (lower) in the adrenal medulla of rats treated with or without GACa. BrdU-positive cells are found in the chromaffin cells (arrowhead) and endothelial cells (arrow), and BrdU-positive chromaffin cells are more frequently found in the GACa-treated groups than in the control group (0 mg/kg/day). Bar = 20 µm. B: The labeling index of the chromaffin cells in the adrenal medulla for each animal was calculated using the formula (BrdU-positive nuclei count) ÷ 500 (nuclei count) × 100. Values represent group means ± SD. Significant differences from the control are indicated with asterisks (** P<0.01). The BrdU labeling index was significantly and dose-dependently increased in the GACa-treated groups.
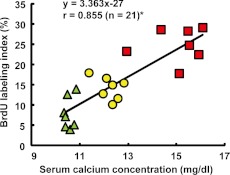
A significant correlation was noted between the serum calcium concentration and BrdU labeling index of chromaffin cells (Fig. 2). In this study, sustained hypercalcemia induced by intravenous infusion of GACa was demonstrated to increase the proliferative activity of chromaffin cells in the adrenal medulla. Moreover, the proliferative activity of chromaffin cells was correlated with the serum calcium concentration.
Fig. 2.
Serum calcium concentration and BrdU labeling index correlation. Each point represents the value for an individual animal. The functional equation is a regression equation. r = correlation coefficient. * P<0.05: Significance of the correlation of the serum calcium concentration and BrdU labeling index. Control group, closed triangles; 1,920 mg/kg/day group, closed circles; 3,840 mg/kg/day group, closed squares.
One of the carcinogenic mechanisms in non-genotoxic carcinogens is thought to be based on enhancement of cell proliferation, such as direct enhancement and/or enhancement of cell proliferation as part of a regenerative process following tissue damage due to cytotoxic effects8. In the present study, we demonstrated that an increased high serum calcium concentration enhanced the proliferative activity of the chromaffin cells in the adrenal medulla. However, necrosis/apoptosis and regeneration of chromaffin cells were not observed. Thus, we concluded that hypercalcemia directly enhances the proliferative activity of chromaffin cells in the adrenal medulla and that the continuous enhancement of cell proliferation by hypercalcemia can lead to adrenal pheochromocytoma in rats. The detailed mechanism that stimulates the proliferation of chromaffin cells in the adrenal medulla by hypercalcemia is not clear as yet. It is thought that calcium ion-dependent intracellular signalling regulates the activity of tyrosine hydroxylase and thus the synthesis of catecholamines in the adrenal medulla1. Because hypercalcemia enhances the synthesis of catecholamines, continuous synthesis may cause chromaffin cells to function excessively and proliferate9, 10.
In conclusion, GACa was intravenously infused into rats by a tail cuff method, and a high serum calcium concentration was successfully sustained. The results of this study indicate that hypercalcemia directly stimulates the proliferation of chromaffin cells in the adrenal medulla of rats. Additionally, the proliferative activity of chromaffin cells is correlated with of serum calcium concentration. Our results will be highly useful in assessing adrenal toxicity, especially for interpreting the increased incidence of adrenal pheochromocytoma in rat carcinogenicity studies.
Acknowledgments
We would like to thank Ms. Yayoi Takai at Chugai Research Institute for Medical Science, Inc. for technical assistance.
References
- 1.Greim H, Hartwig A, Reuter U, Richter-Reichhelm HB, Thielmann HW. Chemically induced pheochromocytomas in rats: mechanisms and relevance for human risk assessment. Crit Rev Toxicol. 39: 695–718 2009. [DOI] [PubMed] [Google Scholar]
- 2.Alison RH, Capen CC, Prentice DE. Neoplastic lesions of questionable significance to humans. Toxicol Pathol. 22: 179–186 1994. [DOI] [PubMed] [Google Scholar]
- 3.Tischler AS, Powers JF, Downing JC, Riseberg JC, Shahsavari M, Ziar J, McClain RM. Vitamin D3, lactose, and xylitol stimulate chromaffin cell proliferation in the rat adrenal medulla. Toxicol Appl Pharmacol. 140: 115–123 1996. [DOI] [PubMed] [Google Scholar]
- 4.Ikezaki S, Nishikawa A, Furukawa F, Tanakamaru Z, Nakamura H, Mori H, Hirose M. Influences of long-term administration of 24R, 25-dihydroxyvitamin D3, a vitamin D3 derivative, in rats. J Toxicol Sci. 24: 133–139 1999. [DOI] [PubMed] [Google Scholar]
- 5.Tischler AS, Powers JF, Pignatello M, Tsokas P, Downing JC, McClain RM. Vitamin D3-induced proliferative lesions in the rat adrenal medulla. Toxicol Sci. 51: 9–18 1999. [DOI] [PubMed] [Google Scholar]
- 6.Asanuma K, Komatsu S, Sakurai T, Takai R, Chiba S. Total parenteral nutrition using continuous intravenous infusion via the posterior vena cava in rats. J Toxicol Sci. 31: 139–147 2006. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki M, Adachi K, Sugimoto T, Nakayama H, Doi K. Application of flash and cumulative labeling with bromodeoxyuridine for analysis of cell kinetics of bone cells and chondrocytes in young growing rats. J Toxicol Pathol. 11: 65–68 1998. [Google Scholar]
- 8.Pitot HC, Dragan YP. Chemical carcinogenesis. In: Casarett & Doull’s Toxicology, 6th ed. Klaassen CD (ed). McGraw-Hill, New York. 241-319. 2001 [Google Scholar]
- 9.Baer A. Sugars and adrenomedullary proliferative lesions: the effects of lactose and various polyalcohols. J Am Coll Toxicol. 7: 71–81 1988. [Google Scholar]
- 10.Lynch BS, Tischler AS, Capen C, Munro IC, McGirr LM, McClain RM. Low digestible carbohydrates (polyols and lactose): significance of adrenal medullary proliferative lesions in the rat. Regul Toxicol Pharmacol. 23: 256–297 1996. [DOI] [PubMed] [Google Scholar]