Abstract
Rationale
Previous work in humans has shown that chronic cannabis users exhibit disruptions in classical eyeblink conditioning (EBC), a form of associative learning that is known to be dependent on the cerebellum. Based upon previous work in animals, it was hypothesized that these learning deficits were related to cannabinoid receptor (CB1R) downregulation. However, it remains unclear whether there is a recovery of cerebellum-dependent learning after the cessation of cannabis use.
Methods
Therefore, former cannabis users (n=10), current cannabis users (n=10), and cannabis-naïve controls (n=10), all free of DSM-IV Axis-I or -II disorders, were evaluated. A standard delay EBC procedure was utilized in which paired presentations of a conditioned stimulus (CS; e.g., tone) and a co-terminating unconditioned stimulus (US; e.g., ocular air-puff) were administered, thus eliciting a conditioned eyeblink response (CR). The primary dependent measures were percentage of CRs and CR latency across conditioning blocks.
Results
Similar to prior studies, current cannabis users exhibited marked impairments in both the acquisition and timing of CRs compared to controls. Although former cannabis users showed intact CR acquisition compared to controls, they exhibited significantly impaired (shorter) CR latencies. In both cannabis groups, UR amplitude did not differ from controls, indicating normal US processing.
Conclusions
These data suggest that a recovery of function has occurred for the learning of the CS–US association, while the accurate timing of the CR shows lasting impairments. Taken together, these results suggest that heavy cannabis use can disrupt timing-related synaptic plasticity within the cerebellum, even after the cessation of cannabis use.
Keywords: Cannabinoids, Conditioning, Cerebellum, Drug abuse, Abstinence
Introduction
Cannabis remains one of the most widely used psychoactive substances in the world (SAMHSA 2006; ONDCP 2008), and consumption rates have increased substantially during the past 25 years (UNODC 2006). The principal psychoactive constituent in cannabis, Δ9-tetrahydrocannabinol (THC) (Gaoni and Mechoulam 1971), affects the brain via the action of central cannabinoid-1 receptors (CB1R; (Devane et al. 1988; Pertwee et al. 2010). The CB1R is one of the most abundant G-protein coupled receptors in the central nervous system, with high densities in areas such as the basal ganglia, hippocampus, and cerebellar cortex (Herkenham et al. 1990; Glass et al. 1997; Pertwee 1997; Tsou et al. 1998; Pertwee 1999; Egertova and Elphick 2000; Eggan and Lewis 2007). Given the widespread distribution of CB1Rs in the mammalian brain, it is of increasing interest to fully understand its mechanism of action.
For instance, it is now well-established that chronic exposure to exogenous cannabinoids leads to behavioral and physiological tolerance in both animals (Martin et al. 2004; Gonzalez et al. 2005; Lichtman and Martin 2005) and humans (Jones et al. 1976, 1981; Haney et al. 1999; D’Souza et al. 2008; Ranganathan et al. 2008; Ramaekers et al. 2009). One likely neural mechanism contributing to cannabinoid tolerance is the downregulation of CB1Rs. Repeated administration of THC or synthetic agonists (i.e., WIN55,212-2) induces both downregulation and desensitization of CB1Rs in the mammalian brain (Rodriguez de Fonseca et al. 1994; Sim-Selley 2003; Martin et al. 2004; Gonzalez et al. 2005; Clapper et al. 2009). The time course of CB1R downregulation differs between brain areas. Areas such as the cerebral cortex and hippocampus exhibit these changes as quickly as 24 h after first administration (Romero et al. 1998). However, areas with higher densities of CB1Rs, such as the basal ganglia and cerebellum, show longer latencies to downregulation, needing at least 3 days of daily CB1R agonist administration before reductions are detectable (Romero et al. 1998; Breivogel et al. 1999). This downregulation of CB1Rs, however, does not appear to be permanent. In mice, CB1R availability, as measured by Bmax, took 14 and 7 days to recover in the hippocampus and striatum, respectively (Sim-Selley et al. 2006). Similarly, recovery of tolerance to the motor and antinociceptive effects of THC occurred 7.5 and 11.5 days, respectively, after cessation of chronic THC treatment in mice (Bass and Martin 2000). Thus, animal studies indicate that there is a recovery, at least to a degree, from CB1R downregulation.
A paucity of data exists that have examined CB1R down-regulation in humans. Villares (2007) examined the postmortem brains of six chronic cannabis users and reported reductions in [3H]SR141617A (rimonabant) binding and decreased CB1R mRNA in the caudate nucleus, putamen, accumbens nucleus, and hippocampal region; however, areas dense with CB1Rs, such as the cerebellum, were not examined in that study. While it is difficult to directly examine human CB1R levels in vivo, it may be possible to deduce whether downregulation has occurred or recovered by using neurobehavioral measures known to be dependent on normal CB1R function. In the cognitive domain, several groups have shown memory and attention deficits in long-term cannabis users. Interestingly, while these deficits persist for a few days after abstinence, they are no longer evident after 28 days of abstinence in some studies (Pope et al. 1995; Fletcher et al. 1996; Pope and Yurgelun-Todd 1996; Patrick et al. 1997, 1999; Patrick and Struve 2000; Pope et al. 2002). Conversely, other experiments suggest that these impairments persist beyond 4 weeks of abstinence (Bolla et al. 2002; Eldreth et al. 2004; Bolla et al. 2005; Pillay et al. 2008; Schweinsburg et al. 2008; Sneider et al. 2008). Thus, it remains unclear whether cannabis-induced neurobehavioral deficits persist after prolonged abstinence. Further, it is also worth noting that the above-mentioned findings are difficult to interpret, as tasks evaluating higher cognitive processes probe the integrity of distributed neocortical networks (i.e., neocortical areas contain relatively moderate CB1R densities compared with structures such as the cerebellum). Hence, tasks which examine cerebellar function may be particularly useful indices of the long-term effects of exogenous cannabinoids.
As mentioned above, CB1Rs are one of the most abundant receptors in the mammalian brain, particularly in the molecular layer of the cerebellar cortex (Herkenham et al. 1990; Glass et al. 1997; Pertwee 1997; Tsou et al. 1998; Pertwee 1999; Egertova and Elphick 2000; Eggan and Lewis 2007). These receptors have been shown to be involved in synaptic plasticity (e.g., the induction long-term depression; LTD) at granule cell–Purkinje cell synapses (Safo and Regehr 2005). This LTD is thought to be important in cerebellar-dependent learning, specifically in delay eyeblink conditioning (EBC). EBC is an associative motor learning task which involves the paired presentation of a neutral conditioned stimulus (CS), such as a tone, followed by an unconditioned stimulus (US), such as an ocular air puff. The US air puff evokes a reflexive eyeblink, the unconditioned response (UR), and after repeated CS–US paired presentations, a conditioned response (CR), a blink of the eye, forms subsequent to CS presentation. After repeated pairings, the CR is timed so that the peak eyelid closure occurs near the onset of the US (Gormezano et al. 1983). The neurocircuitry involved in EBC is well established, highly conserved across species, and includes the cerebellar cortex and anterior interpositus nucleus (for review, see (Thompson and Steinmetz 2009)).
Previous studies have demonstrated that CB1Rs within the cerebellum play a key role in the acquisition, retention, and extinction of delay EBC. Human data collected from current cannabis-using individuals have shown severely impaired learning during delay conditioning (Skosnik et al. 2008). Similarly, CB1R knockout mice and rats administered a CB1R agonist or antagonist have shown deficits in acquisition of delay EBC (Kishimoto and Kano 2006; Steinmetz and Freeman 2010). These studies all indicate that normal CB1R function is needed for optimal acquisition and retention of delay EBC. Hence, delay EBC may be a useful non-invasive method with which to probe CB1R function in humans, both in the context of chronic use and after prolonged abstinence. As discussed above, it appears that recovery of some cognitive functions occurs after abstinence from cannabis. However, there is a paucity of human data examining recovery of function within the cerebellum after cessation of cannabis exposure. The current study therefore examined cerebellum-dependent delay EBC in a sample of carefully screened current and former cannabis users.
Methods
Participants
This study was approved by the Indiana University Bloomington Human Subjects Committee. Participants were recruited from the local university community, were paid for their participation, and written informed consent was obtained from each. Former cannabis users, current cannabis users, and healthy drug-naive controls were assessed. Current cannabis users and controls were age matched (±1 year) with former cannabis users. Table 1 shows the basic demographic information as well as drug/alcohol use rates. The inclusion criteria were as follows: (1) for the former cannabis group: history of cannabis consumption (smoked joints) at the rate of at least once per week for at least 1 month prior to quitting, >60 lifetime joints, having quit at least 1 week prior to testing, no illicit substance use during the past 6 months, a negative urine toxicology screen for all drugs tested including cannabis, and no current or past DSM-IV axis I or II diagnosis except past cannabis abuse or dependence; (2) for the cannabis group: current cannabis consumption (smoked joints) at the rate of at least once per week during the past month, a positive urine toxicology screen for THC, no other illicit substance use during the past 6 months (including a negative urine toxicology screen for other illicit drugs), and no current or past DSM-IV axis I or II diagnosis except cannabis abuse or dependence; (3) for the control group: no history of illicit substance use, a negative urine toxicology screen for all drugs tested, and no history of psychiatric illness (axis I or II); (4) for all participants: ages 18–35, completion of high school education, no family history of bipolar or schizophrenia spectrum disorders, no history of cardiovascular disease, hearing problems, neurological disease, learning disability, or head injury resulting in loss of consciousness. In addition, participants were excluded if they reported consumption of more than two alcoholic drinks per day (one per day for females) or more than five drinks in a single session during the past week. The cannabis group drug-use inclusion criteria (cannabis use at least once per week; 24 h abstinence) were chosen to eliminate acute cannabis effects, while retaining neurophysiological effects from altered CB1R activity. Human studies indicate that 80–90% of the total amount of THC is excreted within 5 days, so a minimum use of once per week enabled the detection of THC metabolites.
Table 1.
Demographic and drug use histories for the current users (n=10), former users (n=10), and controls (n=10)
| Variable | Control group (n=10) | Former cannabis group (n=10) | Current cannabis user group (n=10) | Valuea | pa |
|---|---|---|---|---|---|
| Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | |||
| Age (years) | 21.2 (2.2) | 20.4 (2.3) | 20.2 (1.7) | F=0.62 | 0.55 |
| Educational level (years) | 15.2 (1.1) | 14.5 (1.2) | 14.4 (1.2) | F=1.41 | 0.26 |
| Days since last joint | 0 (0.0) | 164.0 (223.3) | 2.0 (1.3) | F=5.29 | 0.03 |
| Total cannabis use past month | 0 (0.0) | 2.3 (4.2) | 42.2 (32.5) | F=14.86 | 0.001 |
| Total cannabis use past 6 months | 0 (0.0) | 53.6 (90.7) | 281.1 (196.1) | F=10.92 | 0.004 |
| Age of first cannabis use | 0 (0.0) | 15.3 (2.8) | 15.3 (1.3) | F=0.00 | 1.00 |
| Total years of cannabis use | 0 (0.0) | 6.2 (4.4) | 4.9 (2.3) | F=0.69 | 0.42 |
| Avg. alcoholic drinks per week (past month) | 2.3 (3.5) | 4.6 (5.3) | 6.7 (4.0) | F=2.74 | 0.08 |
| Avg. number of cigarettes per day (past month) | 1.5 (4.7) | 0.3 (0.4) | 7.1 (9.4) | F=3.55 | 0.04 |
| Handedness | |||||
| Right | 10 | 8 | 9 | ||
| Left | 0 | 2 | 1 | ||
| Gender | |||||
| Male | 3 | 3 | 5 | ||
| Female | 7 | 7 | 5 |
Comparisons of cannabis use was calculated for only past and current THC groups
One-way ANOVA
Clinical interviews, questionnaires, and drug-use assessment
The Structured Clinical Interview for DSM-IV Axis I and II Disorders (SCID I and SCID II) and a locally developed substance use questionnaire were administered to determine drug use rates and histories of psychopathology. The SCID I module E and drug-use questionnaire were used to ascertain current and past diagnoses for alcohol and substance abuse and dependence. Measures of frequency, quantity, and density of cannabis consumption were determined via the interview and questionnaire for lifetime, the past 6 months, 1 month, and then for the week prior to the test session as has been described previously (Skosnik et al. 2006, 2008; Fridberg et al. 2010). Participants were instructed to consider each day of the week and indicate, for an average week, how much they consumed per drug-use occasion for each length of time assessed. Recency and density of last use was assessed using the past month and past week section of the interview. Urine screens (Q10-1, Proxam) were administered immediately preceding EBC testing in order to corroborate self-reports from the drug questionnaire and clinical interviews. The Q10-1 kit screens for cannabis (THC-COOH; 50 ng/mL sensitivity), opiates, amphetamines, cocaine, MDMA (ecstasy), tricyclic anti-depressants, phencyclidine, benzodiazepines, methamphet-amines, and barbiturates.
EBC stimuli and procedure
Participants engaged in a 108-trial delay EBC paradigm. Initially, eight US alone trials were presented, with an intertrial interval (ITI) of 15 s. Subsequently, the acquisition phase commenced consisting of ten blocks of trials (mean ITI=15 s, range=10–20 s). Each block contained nine CS–US paired trials and one CS-alone trial, which were randomly presented within the last five trials of each block. Paired CS–US trials consisted of a 400 ms, 1,000 Hz tone (80 dB SPL) with a coterminating 50 ms air puff. To maintain attention throughout the procedure, participants were asked to rate the pleasantness of neutral pictures selected from the International Affective Picture System (Lang and Greenwald, 1988). Pictures were presented for 2 s in between each trial, and participants rated the images on a scale of 1–10 using a button response pad. Eyeblinks were recorded using pairs of electromyographic (EMG) electrodes (8 mm AG/AGCl; Model TD-23; MedAssociated, St Albans, VT). The electrodes were placed on the orbicularis palpebrarum muscle below each eye, with a ground electrode on the forehead. All electrode impedances were maintained below 5 kΩ. The US consisted of a 10-p.s.i. (50 ms duration) puff of medical-grade air presented to the left eye with copper tubing affixed to eye-glass rims and placed 1 cm away from the inner canthus of the eye. Foam ear inserts were used for presentation of the CS tones (E-ARLINKFAearo Company Auditory Systems, Indianapolis, IN). EMG data were continuously recorded at 2.5 kHz with a Sensorium EPA-6 bioamplifier (highpass filter=1 Hz, 12 dB/octave; lowpass filter=300 Hz, eighth order elliptic; gain=5,000) and acquired using the software Neuroscan (v. 4.4, El Paso, TX).
EBC data processing
Individual trials were epoched (1,086 ms) from the continuous EMG data file beginning 500 ms before presentation of the CS (using Neuroscan Edit software), and high pass filtered (10 Hz, 6 dB/octave) before being rectified and smoothed using a 41-point Gaussian weighted moving average. Data were then entered into the software Data-Munch for further analysis (King and Tracy 1999; Tracy et al. 2001). For each subject, responses were recorded as blinks if the amplitude exceeded five standard deviations above the baseline (the baseline window for each trial was 125 ms before CS presentation). CRs were recorded if the blink occurred between 100 and 350 ms after CS onset (corresponding to a period beginning 250 ms before US onset). Trials in which spontaneous blinks occur within a window from 75 ms before CS presentation to 25 ms following the CS were labeled bad trials and excluded from further analysis.
Statistical analysis
The primary dependent measures for the eyeblink procedure were percent CRs, CR peak latency, CR amplitude, and UR amplitude. A repeated-measures ANOVA was used to assess the between participants effect of group (3) and the within participants effect of block (10). For the primary dependent variables of percent CRs and CR peak latency, observed power and effect sizes are reported (partial 2), where small effect sizes are less than 0.06, moderate effect sizes range from 0.06 to 0.14, and large effect sizes are greater than 0.14 (Cohen 1973). Greenhouse–Geisser corrections for non-sphericity were used where appropriate. EBC data were normally distributed, as assessed with Shapiro–Wilk tests for normality. Pearson correlations where completed to examine possible associations between EBC measures (i.e., percentage CRs) and cannabis use variables (i.e., years smoking THC). All statistical tests used an alpha level of p<0.05 to determine significance (two-tailed), and all tests were performed using the software package SPSS 19.0.
Results
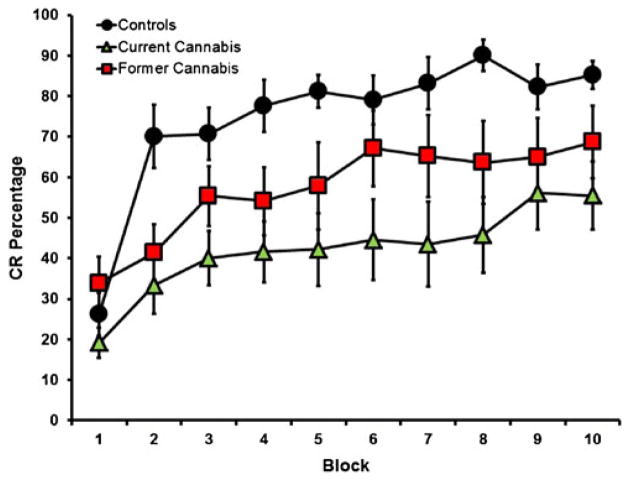
The primary dependent variables of interest were percent CRs, CR peak latencies, CR amplitudes, and UR amplitudes during paired trials across the duration of the experiment. For percent CRs (Fig. 1), the control group exhibited the largest mean percent CRs (74.53±3.86) as compared to the former cannabis users (57.22±7.04) and the current users (42.15±6.42). A repeated-measures ANOVA revealed a main effect of block [F (2,27)=18.010, p<0.001; partial η2=0.40; observed power=0.99] and a main effect of group [F (2,27)=7.31, p=0.003; partial η2=0.20; observed power=0.92]. However, there was no group × block interaction [F (9,243)=1.418, p=0.18; partial η2=0.10; observed power=0.70]. Post hoc Tukey Honestly Significant Difference (HSD) revealed significant differences between the control group and the current user group (p=0.002; Cohen’s d=0.70; observed power=0.98); however, there were no significant differences between the control group and the former user group (p=0.105; Cohen’s d=0.43; observed power=0.53) or the former user and current user groups (p=0.206; Cohen’s d=0.33; observed power=0.32). Given that the groups differed in tobacco use rates (see Table 1), the average number of tobacco uses per day (during the past month) and alcoholic drinks per week (during the past month) were included in the repeated-measures ANOVA as a covariate. After covarying for tobacco and alcohol use, the main effect of group remained significant for both tobacco [F (2,26)=5.452, p=0.01; partial η2=0.30; observed power=0.80] and alcohol use [F (2,26)=5.345, p=0.01; partial η2=0.29; observed power=0.79].
Fig. 1.
CR percentage for the current users (green triangles), former users (red squares), and controls (black circles) during ten blocks of delay eyeblink conditioning. Current users exhibited an impairment in acquisition as compared to controls, whereas former users did not differ from either the control or current using groups
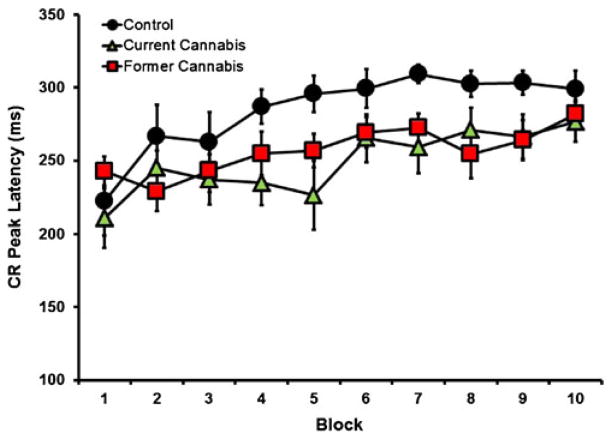
For CR peak latency (Fig. 2), there was also a main effect of block [F (9,243)=6.822, p<0.001; partial η2=0.20; observed power=0.99] and a main effect of group [F (2,27)=3.525, p=0.04; partial η2=0.21; observed power= 0.62], but there was no significant group × block interaction [F (9,243)=1.149, p=0.326; partial η2=0.08; observed power=0.63]. Post hoc Tukey HSD revealed a significant difference between the control and current using groups (p< 0.01; Cohen’s d=0.49; observed power=0.65) and for the control and former user groups (p<0.01; Cohen’s d=0.41; observed power=0.48). However, no significant differences occurred between the former and current user groups (p= 073; Cohen’s d=0.12; observed power=0.08). Tobacco and alcohol use was once again used as covariates and a main effect of group was still found for tobacco [F (2,26)=3.321, p=0.05; partial η2=0.20; observed power=0.61] and alcohol [F (2,26)=3.050, p=0.05; partial η2=0.19; observed power= 0.61].
Fig. 2.
CR peak latency for current users (green triangles), former users (red squares), and controls (black circles) during ten blocks of delay eyeblink conditioning. Current and former cannabis-using groups showed significantly (p<0.05) earlier peak latencies of the CR than control participants
For CR amplitude, there was a significant main effect of block [F (9,243)=3.429, p=0.019; partial η2=0.11; observed power=0.77], but no significant main effect of group [F (2,27)=2.127, p=0.139; partial η2=0.14; observed power= 0.40] or block × group interaction [F (9,243)=0.791, p= 0.586; partial η2=0.06; observed power=0.31]. Thus, CR amplitudes increased over the blocks for all groups, but there were no differences between the groups. No group differences were observed with UR amplitudes during paired trials [F (2,27)=0.71, p=0.41; observed power=0.42], indicating that differences were due to learning and not to UR parameters.
In order to examine whether time since last use was correlated with recovery of percent CRs or CR timing, Pearson correlations were calculated in the former cannabis users for days since last use and average percent CRs and average CR peak latency. Both average percent CRs (r=−0.025; p=0.946) and average CR peak latency (r=0.104; p=0.775) were non-significant, indicating that time since last use was not associated with recovery. Correlation coefficients were then calculated to examine whether age of first use or total years of use were associated with percent CRs for the former and current using cannabis groups. CR percent was not significantly correlated with years of first use (r=−0.041; p=0.910) or total years of use (r=−0.070; p=0.848). Similarly, CR peak latency was not correlated with either years of first use (r=0.041; p=0.869) or total years of use (r=−0.299; p= 0.733).
Discussion
To our knowledge, the current study was the first to examine cerebellar-mediated learning in former cannabis users. The significant group differences in percent CRs and CR peak latency during acquisition demonstrated that the cannabis group showed impaired learning of the conditioned eyeblink response compared to controls (Fig. 1), and shorter (less adaptive) peak CR latencies (Fig. 2). Further, while the former user group did not differ from either the control or current user groups in relation to percent CRs, significant differences were observed for CR peak latencies in comparison to controls. This study therefore replicates results from a previous report showing impaired delay EBC in current cannabis-using individuals (Skosnik et al. 2008). The present findings demonstrate that acquisition of the CR (percentage of CRs) normalizes after the cessation of cannabis use. However, CR timing (peak latency) in both the former and current cannabis-using groups was disrupted compared to cannabis-naïve controls. Therefore, these results indicate that although percentage of CRs recover after the termination of cannabis use, temporal processing during CR acquisition remains impaired following prolonged abstinence.
The role of CB1Rs during delay EBC has been previously examined in several human and animal studies. As in the current report, current cannabis use has been associated with robust decreases in percentage of CRs and altered CR timing (Skosnik et al. 2008). Skosnik et al. (2008) also demonstrated that current users showed intact URs and CS processing, indicating that the deficits in delay EBC were due to learning and not to altered CS processing or sensitization to the US. Thus, the impairment was hypothesized to be related to the downregulation of CB1Rs in the cerebellar cortex in chronic cannabis users. This was supported by a follow-up study showing that active cannabis users can accurately acquire CRs during trace conditioning, which is thought to be less cerebellar-cortical-dependent, and is mediated by more forebrain cortical structures (Edwards et al. 2008).
Animal work examining the role of cannabinoids in cerebellar function have shown similar results, thus providing converging evidence for the role of CB1Rs during the acquisition and retention of cerebellum-dependent delay EBC. For example, CB1R knockout mice exhibit impaired acquisition of delay EBC but not to trace EBC (Kishimoto and Kano 2006). Mice administered SR141716A (rimonabant) also showed impaired delay EBC but not trace EBC. The authors concluded that CB1Rs are essential for normal cerebellar learning to occur. More recently, Steinmetz and Freeman (2010) administered the CB1R agonist WIN55,212-2 and antagonist SR141716A in varying doses to rats during delay EBC. The rats exhibited a dose-dependent impairment in acquisition to both WIN55,212-2 and SR141716A. Importantly, this study showed that blink parameters were not altered by the drugs (i.e., sensitivity to the US and spontaneous blink rates). However, this study did not examine trace EBC, so it is unknown whether administration of WIN55,212-2 impairs CR acquisition during trace EBC (Steinmetz and Freeman 2010).
As discussed above, the current study showed that former cannabis users exhibited impaired CR peak latencies compared to cannabis-naïve controls, while percentage of CRs were unaffected. This dissociation may be related to the differential neurocircuitry mediating the gain and timing of CR acquisition/expression. Current theories of eyeblink conditioning hypothesize that the first step in acquisition is LTD at the granule cell–Purkinje cell synapse in the cerebellar cortex; this LTD releases the interpositus from tonic inhibition which is thought to produce learning (Mauk and Donegan 1997; Medina et al. 2000; Steinmetz and Freeman 2010). This LTD has been observed experimentally with a decrease in firing in Purkinje cells (Green and Steinmetz 2005; Jirenhed et al. 2007; Jirenhed and Hesslow 2011) and is also specific to different CS durations (Green and Steinmetz 2005; Jirenhed and Hesslow 2011). This formation of LTD at Purkinje cells has been shown to be dependent upon CB1R function (Safo and Regehr 2005). More recently, the parallel fiber–Purkinje cell synapse, in which CS information is transmitted, has been shown to be the a major contributor of LTD formation (Carey et al. 2011). As mentioned above, the role of cerebellar cortex in EBC is thought to be needed in the gain of the CR, but has also been indicated as being important for the timing of the response. Lesions to part of the cerebellar cortex following learning create significantly earlier timing of CRs (Perrett et al. 1993). In the current study, former cannabis users exhibited significantly earlier timing in comparison to controls. However, CR percentage for the former cannabis group did not differ from controls. These data would indicate that recovery of function has taken place in the part of the cerebellar cortex needed for gain of the response but not in circuits necessary for proper CR timing.
In terms of the mechanism whereby cannabis use might disrupt cerebellar circuits necessary for CR timing, several possibilities are worth mentioning. In animals, CB1R levels return to normal in the cerebellum after approximately 7–10 days. Subjects in the current study had been free of cannabis for at least 7 days (with a mean of 164 days), which, based on previous studies, appears to provide an adequate amount of time for receptor levels to return to baseline. However, the evidence for CB1R recovery has been almost exclusively examined in animals. The only study to date examining human CB1R levels in heavy cannabis users was done post-mortem, and did not examine cerebellar concentrations (Villares 2007). Thus, the in vivo time course of CB1R recovery in humans remains unknown. It is therefore plausible that heavy cannabis use causes long-term, possibly permanent alterations in the expression of neuronal CB1Rs in humans.
Another possibility relates to the age of onset of cannabis use in the current sample. The subjects in the present study started using cannabis at approximately 15 years of age, which is a critical period in human neurodevelopment. Thus, the lasting differences in CR timing could be due to structural brain changes associated with early cannabis exposure. This notion has theoretical and empirical support, as both cellular and animal studies have shown that the endogenous cannabinoid system plays a key role in neurogenesis, neural specification, neural maturation, neuronal migration, axonal elongation, and glia formation (Harkany et al. 2007, 2008a, b). Hence, adolescent cannabis use may permanently alter neuro-developmental trajectories, particularly in CB1R-rich areas such as the cerebellum. Indeed, Solowij et al. (2011) recently reported that cannabis users exhibited a 23.9% decrease in cerebellar white matter as assessed with structural MRI, a decrease that was related to the duration of cannabis exposure (Solowij et al. 2011). In another recent study, Medina et al. (2010) demonstrated that 28-day abstinent adolescent cannabis users had increased cerebellar vermis volumes, which were associated with poorer executive function. The cerebellar vermal data were interpreted as suggesting that adolescent cannabis use disrupts normal cerebellar gray matter pruning processes during development (Medina et al. 2010). Taken together, these studies suggest that early cannabis exposure can alter cerebellar structure, which may have lasting effects on synaptic plasticity and learning with the cerebellum.
Several limitations to the current study warrant further discussion. First and foremost is the cross-sectional design of the study, which makes causal inferences difficult. Hence, it remains possible that in the former and current cannabis groups, some preexisting condition contributed to both cannabis drug-seeking and altered EBC performance (e.g., past psychopathology). However, the fact that the current sample of participants had no current or past history of mental illness partly rules out this possibility. A second limitation relates to potential premorbid alterations in CB1Rs or endocannabinoids, which if evident, could contribute to altered cerebellar functioning. Since there was no way of directly assessing CB1R densities in the current sample, possible preexisting endocannabinoid differences cannot be ruled out. This limitation could be partially addressed utilizing animal models to examine the long-term effects of CB1R activation on EBC performance and receptor density. Moreover, future human work could examine EBC performance while concurrently assessing CB1R levels in current and former users via in vivo PET imaging of the CB1R (Wong et al. 2010; Herance et al. 2011). Lastly, while the effect sizes in the current study were generally large, the sample sizes were somewhat small. For example, the lack of differences between the former and current cannabis users in CR latency could be due the small effect size (Cohen’s d=0.12) of this dependent measure. Further, the absence of a correlation between length of cannabis abstinence and EBC measures could due to low statistical power, thus precluding the ability to detect such associations. Therefore, future studies should attempt to replicate these findings in a larger sample of current and former cannabis users.
In summary, the current study extends the knowledge of the role of CB1Rs during cerebellum-dependent learning and suggests that while current cannabis users exhibit impairment in both percentage of CRs and CR timing, former users of cannabis show disruptions in CR timing only. This would indicate that a recovery of function has occurred for the learning of the association between the CS and US, while the accurate timing of the CR shows lasting impairments. Taken together, these results suggest that heavy cannabis use can disrupt timing-related synaptic plasticity within the cerebellum, even after the cessation of cannabis use.
Acknowledgments
This work was supported in part by grants from NIDA 1 R21 DA023097-01A1 (PDS) and NARSAD (PDS). The authors wish to thank Peter Finn, Giri Krishnan, Jennifer Vohs, Ken Mackie, and the late Michael Walker for their input and help throughout the project.
Contributor Information
Adam B. Steinmetz, Department of Psychology, University of Iowa, Iowa City, IA 52242, USA
Chad R. Edwards, Department of Psychological and Brain Sciences, Indiana University, 1101 E. 10th St., Bloomington, IN 47405, USA
Jennifer M. Vollmer, Department of Psychiatry, Yale University School of Medicine, 300 George St., Suite 901, New Haven, CT 06511, USA
Molly A. Erickson, Department of Psychological and Brain Sciences, Indiana University, 1101 E. 10th St., Bloomington, IN 47405, USA
Brian F. O’Donnell, Department of Psychological and Brain Sciences, Indiana University, 1101 E. 10th St., Bloomington, IN 47405, USA
William P. Hetrick, Department of Psychological and Brain Sciences, Indiana University, 1101 E. 10th St., Bloomington, IN 47405, USA
Patrick D. Skosnik, Email: patrick.skosnik@yale.edu, Department of Psychiatry, Yale University School of Medicine, 300 George St., Suite 901, New Haven, CT 06511, USA. VA Connecticut Healthcare System, Building 1, 950 Campbell Avenue, West Haven, CT 06516, USA
References
- Bass CE, Martin BR. Time course for the induction and maintenance of tolerance to delta(9)-tetrahydrocannabinol in mice. Drug Alcohol Depend. 2000;60(2):113–119. doi: 10.1016/s0376-8716(99)00150-7. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, et al. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59(9):1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, et al. Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage. 2005;26(2):480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, et al. Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem. 1999;73(6):2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- Carey MR, Myoga MH, et al. Presynaptic CB1 receptors regulate synaptic plasticity at cerebellar parallel fiber synapses. J Neurophysiol. 2011;105(2):958–963. doi: 10.1152/jn.00980.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper JR, Mangieri RA, et al. The endocannabinoid system as a target for the treatment of cannabis dependence. Neuropharmacology. 2009;56(Suppl 1):235–243. doi: 10.1016/j.neuropharm.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Eta-squared and partial eta-squared in communication science. Hum Commun Res. 1973;28:473–490. [Google Scholar]
- Devane WA, Dysarz FA, 3rd, et al. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34(5):605–613. [PubMed] [Google Scholar]
- D’Souza DC, Ranganathan M, et al. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33(10):2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CR, Skosnik PD, et al. Assessment of forebrain-dependent trace eyeblink conditioning in chronic cannabis users. Neurosci Lett. 2008;439(3):264–268. doi: 10.1016/j.neulet.2008.04.102. [DOI] [PubMed] [Google Scholar]
- Egertova M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol. 2000;422(2):159–171. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17(1):175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, et al. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. NeuroImage. 2004;23(3):914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Page JB, et al. Cognitive correlates of long-term cannabis use in Costa Rican men. Arch Gen Psychiatry. 1996;53 (11):1051–1057. doi: 10.1001/archpsyc.1996.01830110089011. [DOI] [PubMed] [Google Scholar]
- Fridberg DJ, Vollmer JM, et al. Cannabis users differ from non-users on measures of personality and schizotypy. Psychiatry Res. 2011;186(1):46–52. doi: 10.1016/j.psychres.2010.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J Am Chem Soc. 1971;93(1):217–224. doi: 10.1021/ja00730a036. [DOI] [PubMed] [Google Scholar]
- Glass M, Dragunow M, et al. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77 (2):299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Cebeira M, et al. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav. 2005;81(2):300–318. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Gormezano I, Kehoe EJ, et al. Twenty years of classical conditioning with the rabbit. Prog Psychobiol Physiol Psychol. 1983;10:197–275. [Google Scholar]
- Green JT, Steinmetz JE. Purkinje cell activity in the cerebellar anterior lobe after rabbit eyeblink conditioning. Learn Mem. 2005;12 (3):260–269. doi: 10.1101/lm.89505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, et al. Abstinence symptoms following oral THC administration to humans. Psychopharmacology. 1999;141 (4):385–394. doi: 10.1007/s002130050848. [DOI] [PubMed] [Google Scholar]
- Harkany T, Guzman M, et al. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci. 2007;28(2):83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Harkany T, Keimpema E, et al. Endocannabinoid functions controlling neuronal specification during brain development. Mol Cell Endocrinol. 2008a;286(1–2 Suppl 1):S84–S90. doi: 10.1016/j.mce.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Harkany T, Mackie K, et al. Wiring and firing neuronal networks: endocannabinoids take center stage. Curr Opin Neurobiol. 2008b;18 (3):338–345. doi: 10.1016/j.conb.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herance R, Rojas S, et al. Positron emission tomographic imaging of the cannabinoid type 1 receptor system with [(11)C]OMAR ([(11) C]JHU75528): improvements in image quantification using wild-type and knockout mice. Mol Imaging. 2011 (in press) [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirenhed DA, Hesslow G. Learning stimulus intervals-adaptive timing of conditioned purkinje cell responses. Cerebellum. 2011 doi: 10.1007/s12311-011-0264-3. (in press) [DOI] [PubMed] [Google Scholar]
- Jirenhed DA, Bengtsson F, et al. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. J Neurosci. 2007;27 (10):2493–2502. doi: 10.1523/JNEUROSCI.4202-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RT, Benowitz N, et al. Clinical studies of cannabis tolerance and dependence. Ann N Y Acad Sci. 1976;282:221–239. doi: 10.1111/j.1749-6632.1976.tb49901.x. [DOI] [PubMed] [Google Scholar]
- Jones RT, Benowitz NL, et al. Clinical relevance of cannabis tolerance and dependence. J Clin Pharmacol. 1981;21(8–9 Suppl):143S–152S. doi: 10.1002/j.1552-4604.1981.tb02589.x. [DOI] [PubMed] [Google Scholar]
- King DAT, Tracy J. Datamunch: a Matlab m-file collection for the analysis of trial-based spike and behavioral data. 1999 Available from: http://www.novl.indiana.edu/Bdmunch/
- Kishimoto Y, Kano M. Endogenous cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning. J Neurosci. 2006;26(34):8829–8837. doi: 10.1523/JNEUROSCI.1236-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK. The International Affective Picture System Standardization Procedure and Initial Group Results for Affective Judgments: Technical Report 1A. The Center for Research in Psychophysiology, University of Florida; Gainesville, FL: 1988. [Google Scholar]
- Lichtman AH, Martin BR. Cannabinoid tolerance and dependence. Handb Exp Pharmacol. 2005;168:691–717. doi: 10.1007/3-540-26573-2_24. [DOI] [PubMed] [Google Scholar]
- Martin BR, Sim-Selley LJ, et al. Signaling pathways involved in the development of cannabinoid tolerance. Trends Pharmacol Sci. 2004;25(6):325–330. doi: 10.1016/j.tips.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Donegan NH. A model of Pavlovian eyelid conditioning based on the synaptic organization of the cerebellum. Learn Mem. 1997;4(1):130–158. doi: 10.1101/lm.4.1.130. [DOI] [PubMed] [Google Scholar]
- Medina JF, Nores WL, et al. Mechanisms of cerebellar learning suggested by eyelid conditioning. Curr Opin Neurobiol. 2000;10 (6):717–724. doi: 10.1016/s0959-4388(00)00154-9. [DOI] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, et al. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Res. 2010;182 (2):152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ONDCP, O. o. N. D. C. P. The Marijuana Factbook. Washington, DC 20503: Executive Office of the President; 2008. Marijuana: the greatest cause of illegal drug abuse. [Google Scholar]
- Patrick G, Struve FA. Reduction of auditory P50 gating response in marihuana users: further supporting data. Clin Electroencephalogr. 2000;31(2):88–93. doi: 10.1177/155005940003100207. [DOI] [PubMed] [Google Scholar]
- Patrick G, Straumanis JJ, et al. Early and middle latency evoked potentials in medically and psychiatrically normal daily marihuana users: a paucity of significant findings. Clin Electroencephalogr. 1997;28 (1):26–31. doi: 10.1177/155005949702800105. [DOI] [PubMed] [Google Scholar]
- Patrick G, Straumanis JJ, et al. Reduced P50 auditory gating response in psychiatrically normal chronic marihuana users: a pilot study. Biol Psychiatry. 1999;45(10):1307–1312. doi: 10.1016/s0006-3223(98)00155-3. [DOI] [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, et al. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci. 1993;13(4):1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74(2):129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6(8):635–664. [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB and CB. Pharmacol Rev. 2010;62(4):588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay SS, Rogowska J, et al. Cannabis and motor function: fMRI changes following 28 days of discontinuation. Exp Clin Psychopharmacol. 2008;16(1):22–32. doi: 10.1037/1064-1297.16.1.22. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA. 1996;275(7):521–527. [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, et al. The residual neuropsychological effects of cannabis: the current status of research. Drug Alcohol Depend. 1995;38(1):25–34. doi: 10.1016/0376-8716(95)01097-i. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, et al. Cognitive measures in long-term cannabis users. J Clin Pharmacol. 2002;42(11 Suppl):41S–47S. doi: 10.1002/j.1552-4604.2002.tb06002.x. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, et al. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol. 2009;23(3):266–277. doi: 10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, Braley G, et al. The effects of cannabinoids on serum cortisol and prolactin in humans. Psychopharmacology (Berl) 2009;203(4):737–744. doi: 10.1007/s00213-008-1422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Gorriti MA, et al. Downregulation of rat brain cannabinoid binding sites after chronic delta 9-tetrahydrocannabinol treatment. Pharmacol Biochem Behav. 1994;47 (1):33–40. doi: 10.1016/0091-3057(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Romero J, Berrendero F, et al. Time-course of the cannabinoid receptor down-regulation in the adult rat brain caused by repeated exposure to delta9-tetrahydrocannabinol. Synapse. 1998;30 (3):298–308. doi: 10.1002/(SICI)1098-2396(199811)30:3<298::AID-SYN7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48(4):647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- SAMHSA. OoA Studies. Substance Abuse and Mental Health Services Administration; Rockville: 2006. National survey on drug use and health: national findings. [Google Scholar]
- Schweinsburg AD, Nagel BJ, et al. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res. 2008;163(1):40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol. 2003;15(2):91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Schechter NS, et al. Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol Pharmacol. 2006;70(3):986–996. doi: 10.1124/mol.105.019612. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Krishnan GP, et al. Psychophysiological evidence of altered neural synchronization in cannabis use: relationship to schizotypy. Am J Psychiatry. 2006;163(10):1798–1805. doi: 10.1176/ajp.2006.163.10.1798. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Edwards CR, et al. Cannabis use disrupts eyeblink conditioning: evidence for cannabinoid modulation of cerebellar-dependent learning. Neuropsychopharmacology. 2008;33(6):1432–1440. doi: 10.1038/sj.npp.1301506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneider JT, Pope HG, Jr, et al. Differences in regional blood volume during a 28-day period of abstinence in chronic cannabis smokers. Eur Neuropsychopharmacol. 2008;18(8):612–619. doi: 10.1016/j.euroneuro.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Yucel M, et al. Cerebellar white-matter changes in cannabis users with and without schizophrenia. Psychol Med. 2011;41:2349–2359. doi: 10.1017/S003329171100050X. [DOI] [PubMed] [Google Scholar]
- Steinmetz AB, Freeman JH. Central cannabinoid receptors modulate acquisition of eyeblink conditioning. Learn Mem. 2010;17 (11):571–576. doi: 10.1101/lm.1954710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Steinmetz JE. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience. 2009;162 (3):732–755. doi: 10.1016/j.neuroscience.2009.01.041. [DOI] [PubMed] [Google Scholar]
- Tracy JA, Britton GB, Steinmetz JE. Comparison of single unit responses to tone, light, and compound conditioned stimuli during rabbit classical eyeblink conditioning. Neurobiol Learn Mem. 2001;76:253–267. doi: 10.1006/nlme.2001.4024. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, et al. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83(2):393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- UNODC. World drug report. 2006 Available at http://www.unodc.org/unodc/en/world_drug_report_2006.html.
- Villares J. Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience. 2007;145(1):323–334. doi: 10.1016/j.neuroscience.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, et al. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. NeuroImage. 2010;52(4):1505–1513. doi: 10.1016/j.neuroimage.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]