Abstract
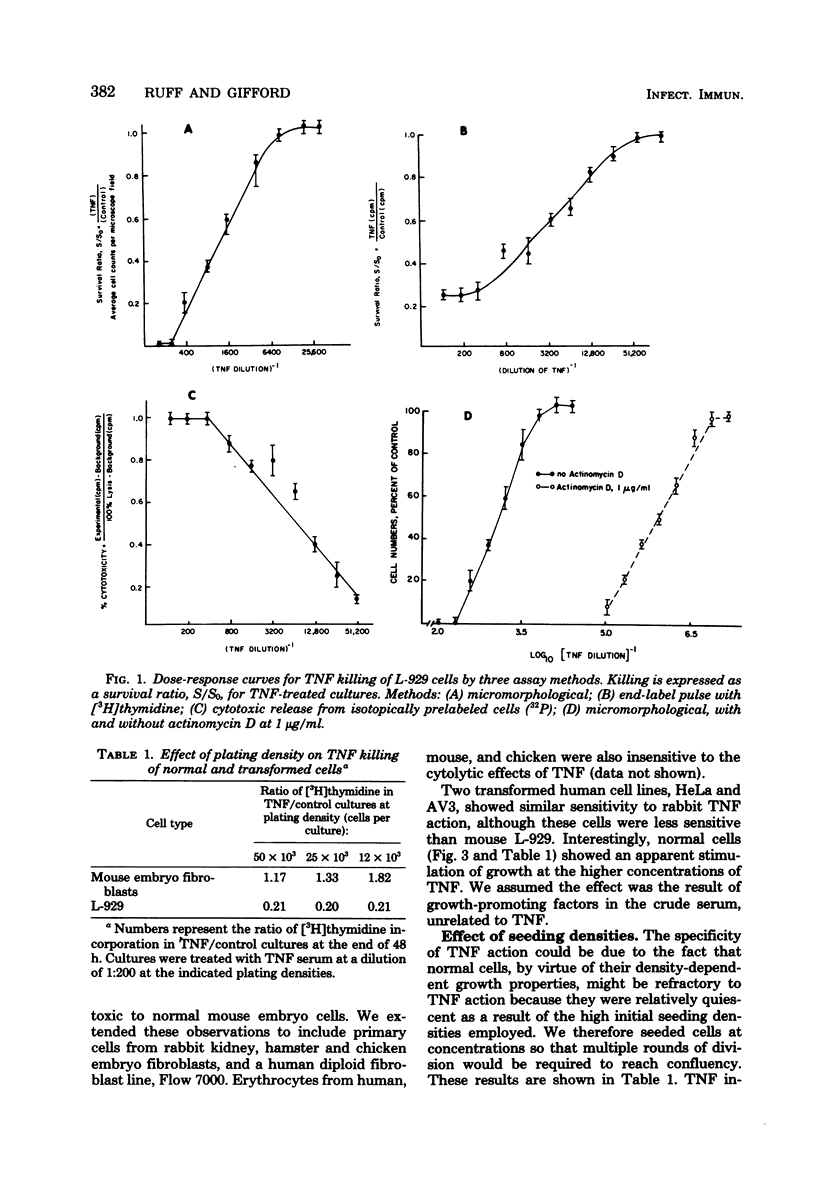
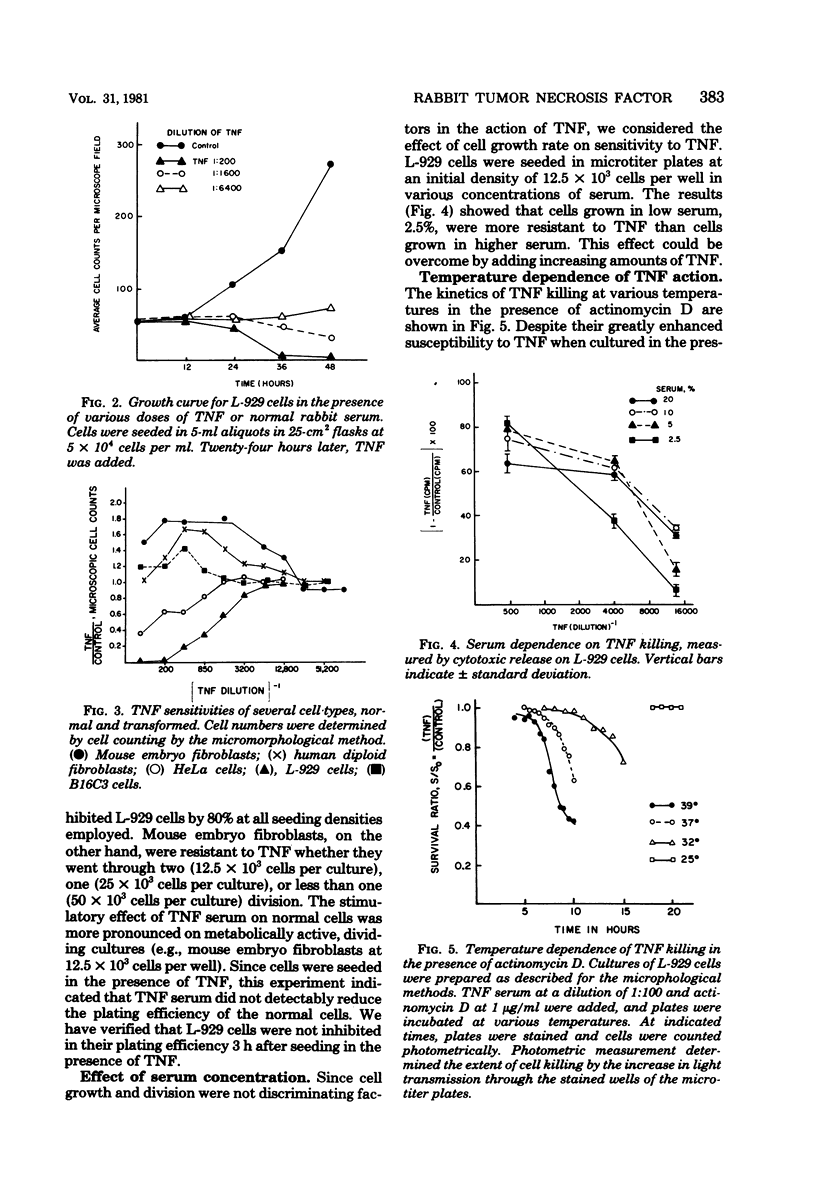
Rabbit tumor necrosis factor (TNF) was examined for effects on normal and transformed cells in culture. Several assays for killing of L-929 cell targets were developed, and their sensitivities were compared. Normal cells were not killed by TNF, and the discrimination between normal and transformed cells was shown not to be due to a cell cycle-dependent mechanism. TNF killing of L-929 cells was delayed for 10 to 12 h and thereafter showed concentration and time-dependent increases in cytolysis. Actinomycin D or cycloheximide treatment of L-929 cells resulted in an enhancement of the rate of cell killing as well as a shortening of the preceding lag period. TNF killing of L-929 cells was temperature dependent; cells were considerably more resistant to lysis at 25 degrees C and showed enhanced killing at 39 degrees C as compared to 37 degrees C controls. The slope of the dose curve showed less than single-hit kinetics. A model for cell killing whose general features incorporate both the specificity and catalytic properties of an enzymatic reaction is proposed for TNF action.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikes M., Klein G. Quantitative studies of antigen expression in cultured murine lymphoma cells. I. Cell-surface antigens in "Asynchronous" cultures. J Natl Cancer Inst. 1972 Dec;49(6):1599–1606. doi: 10.1093/jnci/49.6.1599. [DOI] [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Currie G. A., Basham C. Activated macrophages release a factor which lyses malignant cells but not normal cells. J Exp Med. 1975 Dec 1;142(6):1600–1605. doi: 10.1084/jem.142.6.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion L. D., Blalock J. E., Gifford G. E. Vitamin A-induced density-dependent inhibition of L-cell proliferation. J Natl Cancer Inst. 1977 Mar;58(3):795–801. doi: 10.1093/jnci/58.3.795. [DOI] [PubMed] [Google Scholar]
- Gifford G. E., Koch A. L. The interferon dose response curve and its possible significance. J Theor Biol. 1969 Feb;22(2):271–283. doi: 10.1016/0022-5193(69)90005-8. [DOI] [PubMed] [Google Scholar]
- Helson L., Green S., Carswell E., Old L. J. Effect of tumour necrosis factor on cultured human melanoma cells. Nature. 1975 Dec 25;258(5537):731–732. doi: 10.1038/258731a0. [DOI] [PubMed] [Google Scholar]
- Hessinger D. A., Daynes R. A., Granger G. A. Binding of human lymphotoxin to target-cell membranes and its relation to cell-mediated cytodestruction. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3082–3086. doi: 10.1073/pnas.70.11.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. J., Granger G. A. An improved in vitro assay for lymphotoxin. Cell Immunol. 1972 Jan;3(1):144–149. doi: 10.1016/0008-8749(72)90235-3. [DOI] [PubMed] [Google Scholar]
- Kunitomi G., Rosenau W., Burke G. C., Goldberg M. L. Alteratons in RNA synthesis in lymphotoxin-treated target cells. Am J Pathol. 1975 Aug;80(2):249–260. [PMC free article] [PubMed] [Google Scholar]
- Lies R. B. Requirement for a serum component in cytotoxin ("lymphotoxin")-mediated target cell lysis. Cell Immunol. 1974 Oct;14(1):155–159. doi: 10.1016/0008-8749(74)90181-6. [DOI] [PubMed] [Google Scholar]
- Matthews N. Tumour-necrosis factor from the rabbit. II. Production by monocytes. Br J Cancer. 1978 Aug;38(2):310–315. doi: 10.1038/bjc.1978.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N., Watkins J. F. Tumour-necrosis factor from the rabbit. I. Mode of action, specificity and physicochemical properties. Br J Cancer. 1978 Aug;38(2):302–309. doi: 10.1038/bjc.1978.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor. Clin Bull. 1976;6(3):118–120. [PubMed] [Google Scholar]
- Ostrove J. M., Gifford G. E. Stimulation of RNA synthesis in L-929 cells by rabbit tumor necrosis factor. Proc Soc Exp Biol Med. 1979 Mar;160(3):354–358. doi: 10.3181/00379727-160-40449. [DOI] [PubMed] [Google Scholar]
- Rosenau W., Goldberg M. L., Burke G. C. Early biochemical alterations induced by lymphotoxin in target cells. J Immunol. 1973 Oct;111(4):1128–1135. [PubMed] [Google Scholar]
- Ruff M. R., Gifford G. E. Purification and physico-chemical characterization of rabbit tumor necrosis factor. J Immunol. 1980 Oct;125(4):1671–1677. [PubMed] [Google Scholar]
- Sawada J., Osawa T. Morphological microassay and kinetics of the cytotoxicity of guinea pig lymphotoxin. Jpn J Exp Med. 1977 Apr;47(2):87–92. [PubMed] [Google Scholar]
- Walker S. M., Lucas Z. J. Cytotoxic activity of lymphocytes. II. Studies on mechanism of lymphotoxin-mediated cytotoxicity. J Immunol. 1972 Dec;109(6):1233–1244. [PubMed] [Google Scholar]
- Williams T. W., Granger G. A. Lymphocyte in vitro cytotoxicity: mechanism of lymphotoxin-induced target cell destruction. J Immunol. 1969 Apr;102(4):911–918. [PubMed] [Google Scholar]


