Abstract
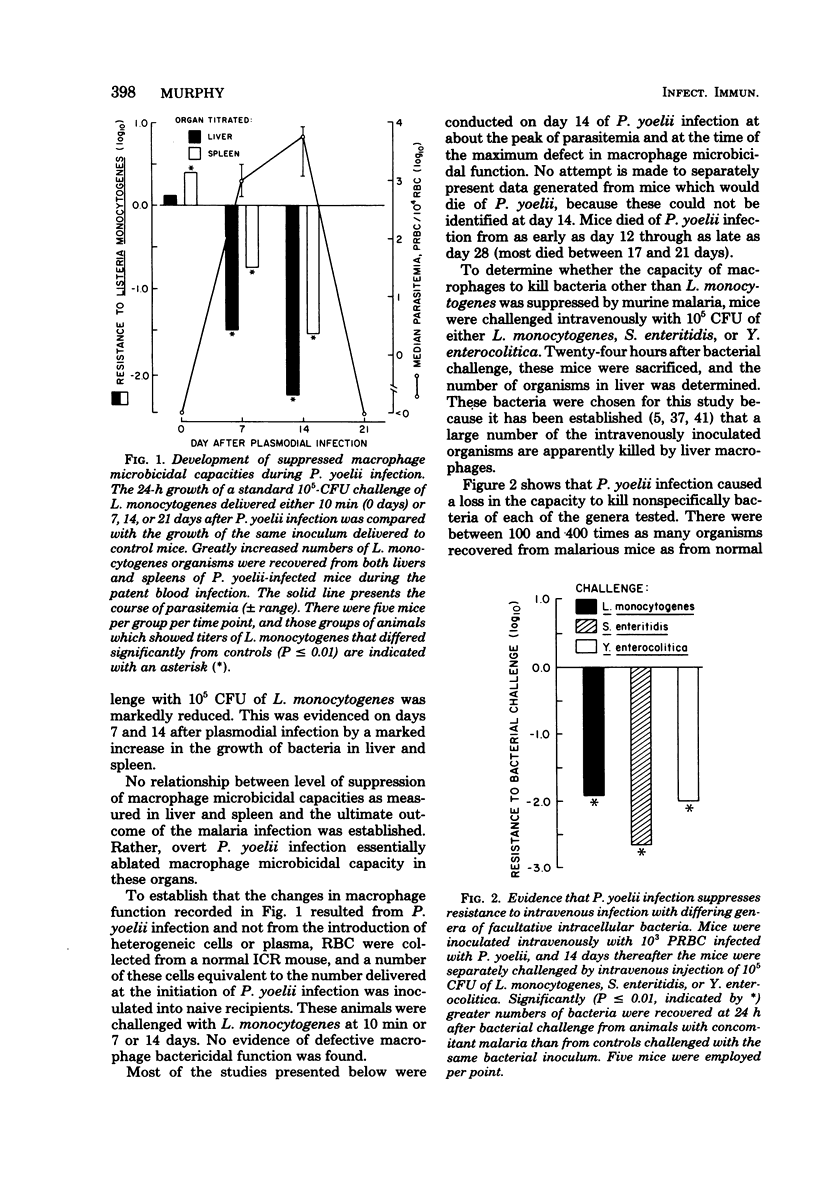
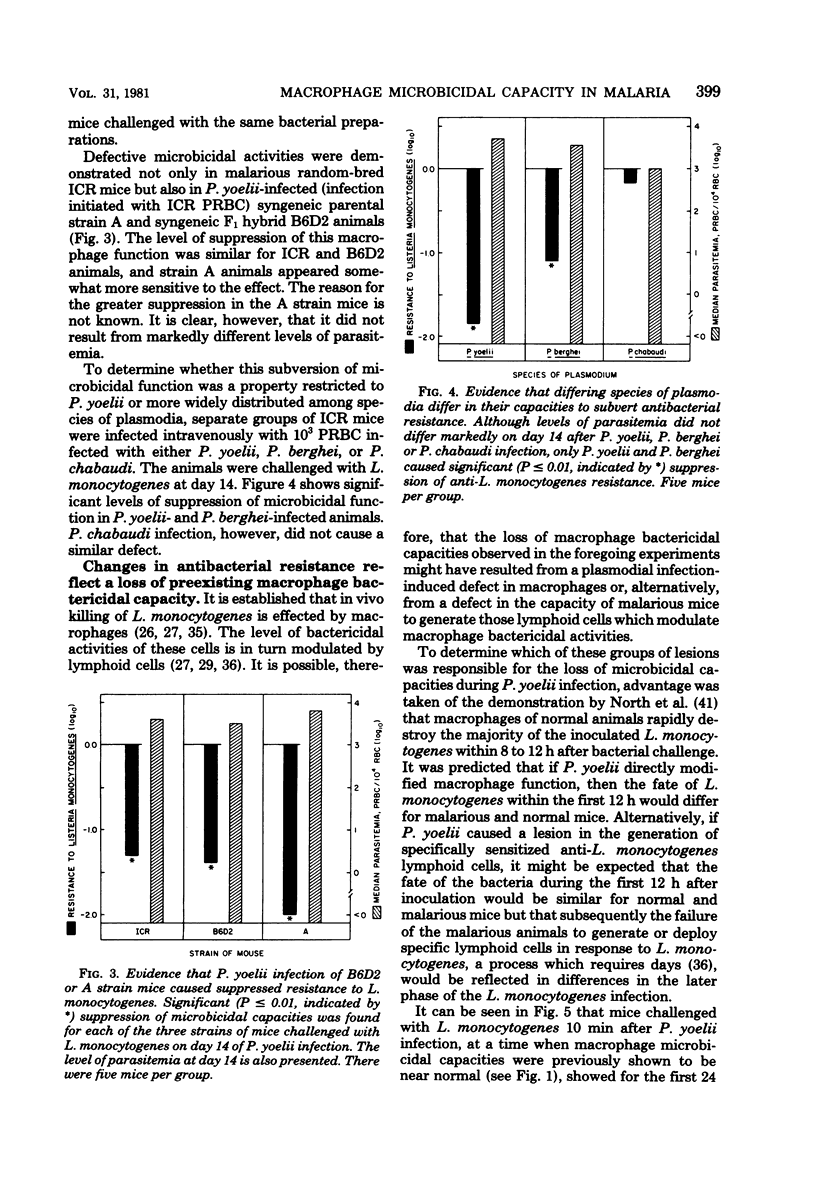
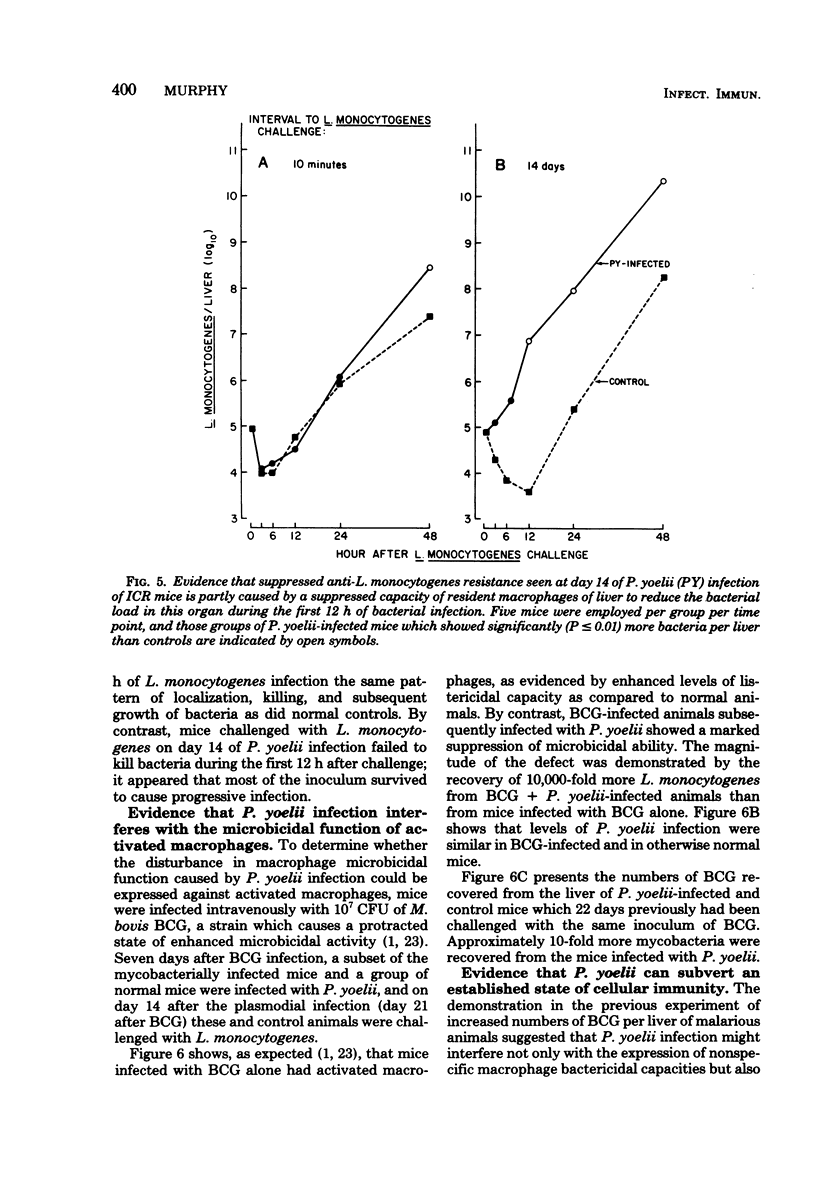
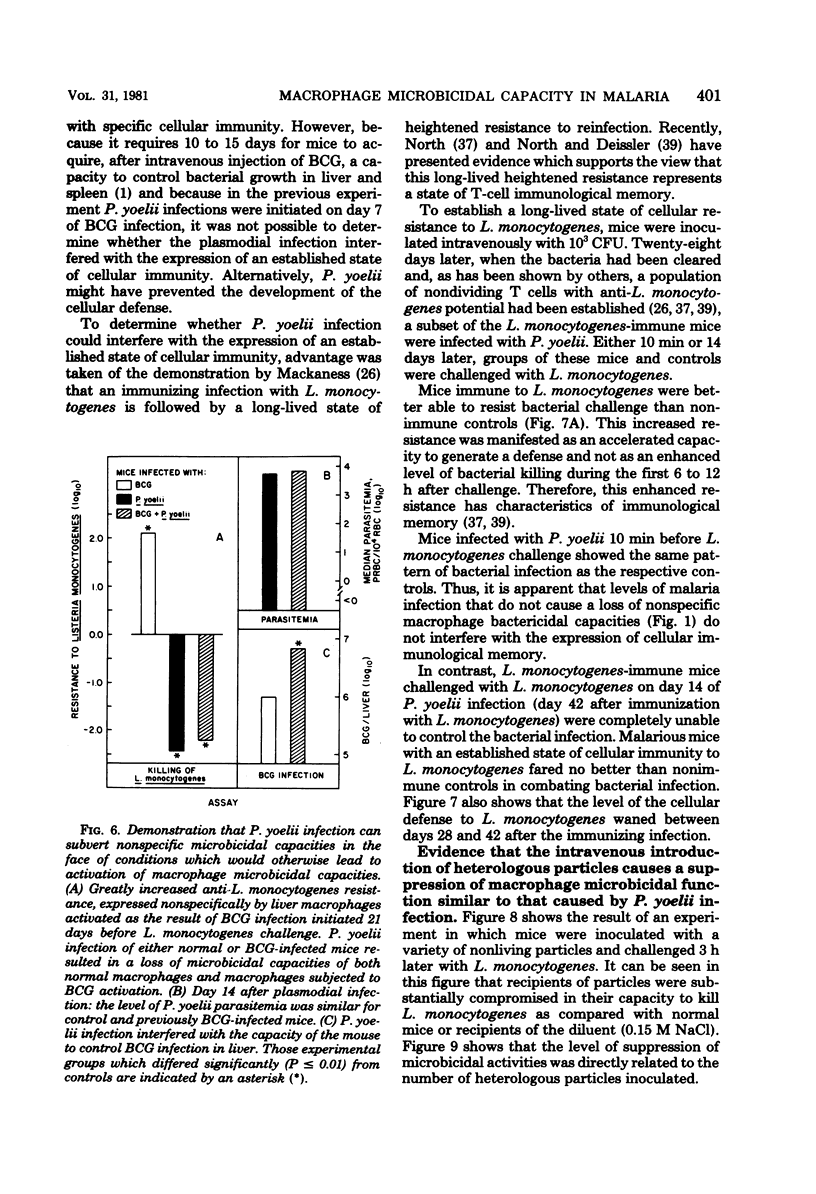
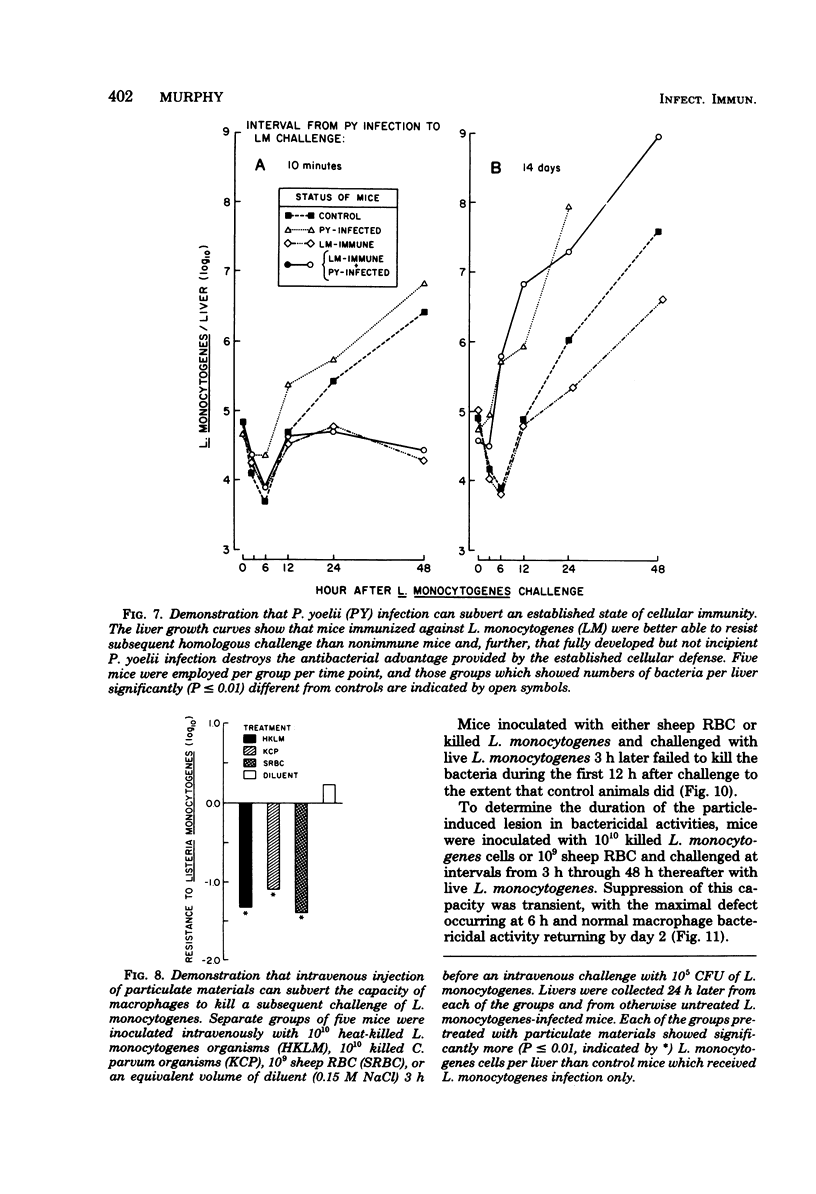
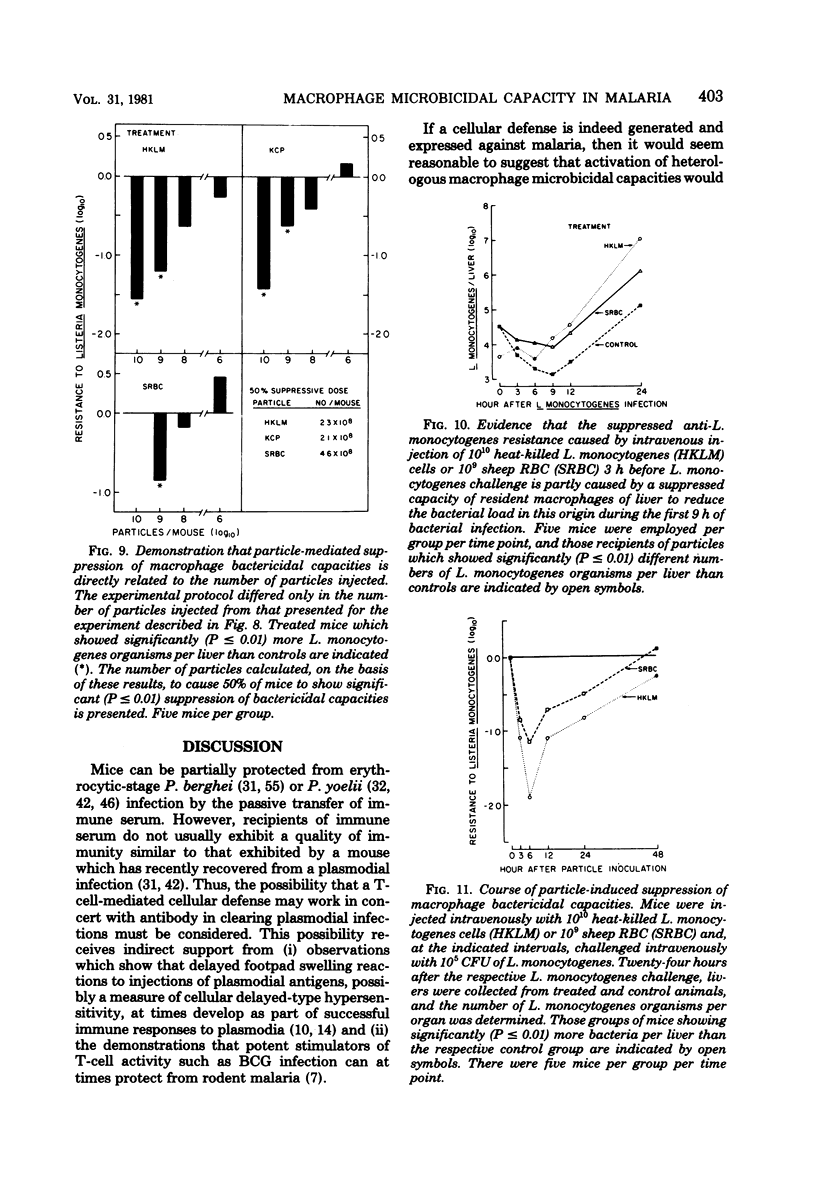
Macrophage-dependent killing of facultative intracellular bacteria was markedly impaired by overt erythrocytic Plasmodium yoelii or Plasmodium berghei infection of mice. P. yoelii infection was capable of ablating not only the macrophage microbicidal capacity of "normal" animals but also the bactericidal capacities of "activated" macrophages. The uptake by spleen and liver of an intravenous challenge of Listeria monocytogenes was not altered by plasmodial infection, but within hours of injection markedly enhanced bacterial growth was found in tissues of malarious mice. The evidence gives credence to the view that the uptake of bacteria by macrophages of malarious mice was normal but that malarious mice, unlike normal mice, were unable to kill the bacteria. The plasmodial infection-caused defect in macrophage microbicidal capacity could be partially mimicked by the intravenous injection of large numbers of nonreplicating heterologous particles (i.e., killed bacteria, sheep erythrocytes). This result suggests that the uptake of particles generated during overt erythrocytic malaria may be responsible for the malaria-associated defects in macrophage bactericidal capacity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. N., Brown I. N., Trigg P. I., Phillips R. S., Hills L. A. Immunity to malaria. II. Serological response of monkeys sensitized by drug-suppressed infection or by dead parasitized cells in Freund's complete adjuvant. Exp Parasitol. 1970 Oct;28(2):318–338. doi: 10.1016/0014-4894(70)90102-5. [DOI] [PubMed] [Google Scholar]
- Cantrell W., Elko E. E. Plasmodium berghei: phagocytic activtiy in two strains of rats. Exp Parasitol. 1976 Oct;40(2):281–285. doi: 10.1016/0014-4894(76)90092-8. [DOI] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. Experimental Yersinia enterocolitica infection in mice: kinetics of growth. Infect Immun. 1974 May;9(5):851–857. doi: 10.1128/iai.9.5.851-857.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J. S., Kreier J. P. Plasmodium berghei: adherence and phagocytosis by rat macrophages in vitro. Exp Parasitol. 1972 Feb;31(1):13–18. doi: 10.1016/0014-4894(72)90042-2. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C., Cox F. E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976 Jan 29;259(5541):309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cox F. E., Allison A. C. Protection of mice against Babesia spp. and Plasmodium spp. with killed Corynebacterium parvum. Parasitology. 1977 Feb;74(1):9–18. doi: 10.1017/s003118200004748x. [DOI] [PubMed] [Google Scholar]
- Cohen S., Butcher G. A. Properties of protective malarial antibody. Immunology. 1970 Aug;19(2):369–383. [PMC free article] [PubMed] [Google Scholar]
- Cottrell B. J., Playfair J. H., De Souza B. J. Cell-mediated immunity in mice vaccinated against malaria. Clin Exp Immunol. 1978 Nov;34(2):147–158. [PMC free article] [PubMed] [Google Scholar]
- Davies A. J., Carter R. L., Leuchars E., Wallis V., Dietrich F. M. The morphology of immune reactions in normal, thymectomized and reconstituted mice. 3. Response to bacterial antigens: salmonellar flagellar antigen and pneumococcal plysaccharide. Immunology. 1970 Dec;19(6):945–957. [PMC free article] [PubMed] [Google Scholar]
- Diggs C. L., Osler A. G. Humoral immunity in rodent malaria. II. Inhibition of parasitemia by serum antibody. J Immunol. 1969 Feb;102(2):298–305. [PubMed] [Google Scholar]
- Feldmann M., Palmer J. The requirement for macrophages in the secondary immune response to antigens of small and large size in vitro. Immunology. 1971 Oct;21(4):685–699. [PMC free article] [PubMed] [Google Scholar]
- Finerty J. F., Krehl E. P. Cyclophosphamide pretreatment and protection against malaria. Infect Immun. 1976 Oct;14(4):1103–1105. doi: 10.1128/iai.14.4.1103-1105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T. J., Kreier J. P. Demonstration of the role of cytophilic antibody in resistance to malaria parasites (Plasmodium berghei) in rats. Infect Immun. 1978 Jan;19(1):138–145. doi: 10.1128/iai.19.1.138-145.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K. W., Jr, Winkelstein J. A., Simpson T. W. Serum opsonic activity in rodent malaria: functional and immunochemical characteristics in vitro. J Immunol. 1979 Dec;123(6):2582–2587. [PubMed] [Google Scholar]
- KAYE D., HOOK E. W. THE INFLUENCE OF HEMOLYSIS OR BLOOD LOSS ON SUSCEPTIBILITY TO INFECTION. J Immunol. 1963 Jul;91:65–75. [PubMed] [Google Scholar]
- Kaye D., Gill F. A., Hook E. W. Factors influencing host resistance to Salmonella infections: the effects of hemolysis and erythrophagocytosis. Am J Med Sci. 1967 Aug;254(2):205–215. doi: 10.1097/00000441-196708000-00011. [DOI] [PubMed] [Google Scholar]
- Kaye D., Merselis J. G., Jr, Hook E. W. Influence of Plasmodium berghei infection on susceptibility to salmonella infection. Proc Soc Exp Biol Med. 1965 Dec;120(3):810–813. doi: 10.3181/00379727-120-30661. [DOI] [PubMed] [Google Scholar]
- Kitchen A. G., Di Luzio N. R. Influence of Plasmodium berghei infections on phagocytic and humoral recognition factor activity. J Reticuloendothel Soc. 1971 Mar;9(3):237–247. [PubMed] [Google Scholar]
- Krettli A. U., Nussenzweig R. Depletion of T and B lymphocytes during malarial infections. Cell Immunol. 1974 Sep;13(3):440–446. doi: 10.1016/0008-8749(74)90263-9. [DOI] [PubMed] [Google Scholar]
- Lefford M. J. The effect of inoculum size on the immune response to BCG infection in mice. Immunology. 1971 Aug;21(2):369–381. [PMC free article] [PubMed] [Google Scholar]
- Loose L. D., Trejo R., Di Luzio N. R. Impaired endotoxin detoxification as a factor in enhanced endotoxin sensitivity of malaria infected mice. Proc Soc Exp Biol Med. 1971 Jul;137(3):794–797. doi: 10.3181/00379727-137-35669. [DOI] [PubMed] [Google Scholar]
- Loose L. D., di Luzio N. R. A temporal relationship between reticuloendothelial system phagocytic alterations and antibody responses in mice infected with Plasmodium berghei (NYU-2 strain). Am J Trop Med Hyg. 1976 Mar;25(2):221–228. doi: 10.4269/ajtmh.1976.25.221. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Koster F. T., Mackaness G. B. The mediator of cellular immunity. I. The life-span and circulation dynamics of the immunologically committed lymphocyte. J Exp Med. 1971 Feb 1;133(2):389–399. doi: 10.1084/jem.133.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C. J., De Rivera V. S., Turk J. L. The immunological significance of histological changes in the spleen and liver in mouse malaria. Clin Exp Immunol. 1973 Mar;13(3):467–478. [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R. Host defenses in murine malaria: analysis of the mechanisms of immunity to Plasmodium berghei generated in response to immunization with formalin-killed blood-stage parasites. Infect Immun. 1979 Jun;24(3):707–712. doi: 10.1128/iai.24.3.707-712.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Lefford M. J. Host defenses in murine malaria: evaluation of the mechanisms of immunity to Plasmodium yoelii infection. Infect Immun. 1979 Feb;23(2):384–391. doi: 10.1128/iai.23.2.384-391.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Lefford M. J. Host defenses in murine malaria: successful vaccination of mice against Plasmodium berghei by using formolized blood parasites. Am J Trop Med Hyg. 1979 Jan;28(1):4–11. doi: 10.4269/ajtmh.1979.28.4. [DOI] [PubMed] [Google Scholar]
- Newborg M. F., North R. J. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Deissler J. F. Nature of "memory" in T-cell mediated antibacterial immunity: cellular parameters that distinguish between the active immune response and a state of "memory". Infect Immun. 1975 Oct;12(4):761–767. doi: 10.1128/iai.12.4.761-767.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Kirstein D. P. T-cell-mediated concomitant immunity to syngeneic tumors. I. Activated macrophages as the expressors of nonspecific immunity to unrelated tumors and bacterial parasites. J Exp Med. 1977 Feb 1;145(2):275–292. doi: 10.1084/jem.145.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Kirstein D. P., Tuttle R. L. Subversion of host defense mechanisms by murine tumors. I. A circulating factor that suppresses macrophage-mediated resistance to infection. J Exp Med. 1976 Mar 1;143(3):559–573. doi: 10.1084/jem.143.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Nature of "memory" in T-cell-mediated antibacterial immunity: anamnestic production of mediator T cells. Infect Immun. 1975 Oct;12(4):754–760. doi: 10.1128/iai.12.4.754-760.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974 Jul;10(1):66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B., Cottrell B. J. Protection of mice against malaria by a killed vaccine: differences in effectiveness against P. yoelii and P. berghei. Immunology. 1977 Oct;33(4):507–515. [PMC free article] [PubMed] [Google Scholar]
- Quinn T. C., Wyler D. J. Mechanisms of action of hyperimmune serum in mediating protective immunity to rodent malaria (Plasmodium berghei). J Immunol. 1979 Nov;123(5):2245–2249. [PubMed] [Google Scholar]
- Reed N. D., Jutila J. W. Immune response of congenitally thymusless mice to heterologous erythrocytes. Proc Soc Exp Biol Med. 1972 Apr;139(4):1234–1237. doi: 10.3181/00379727-139-36337. [DOI] [PubMed] [Google Scholar]
- Roberts D. W., Rank R. G., Weidanz W. P., Finerty J. F. Prevention of recrudescent malaria in nude mice by thymic grafting or by treatment with hyperimmune serum. Infect Immun. 1977 Jun;16(3):821–826. doi: 10.1128/iai.16.3.821-826.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGER I. The cellular reactions to infections with Plasmodium berghei in the white mouse. J Infect Dis. 1954 May-Jun;94(3):241–261. doi: 10.1093/infdis/94.3.241. [DOI] [PubMed] [Google Scholar]
- Sheagren J. N., Tobie J. E., Fox L. M., Wolff S. M. Reticuloendothelial system phagocytic function in naturally acquired human malaria. J Lab Clin Med. 1970 Mar;75(3):481–487. [PubMed] [Google Scholar]
- Shear H. L., Nussenzweig R. S., Bianco C. Immune phagocytosis in murine malaria. J Exp Med. 1979 Jun 1;149(6):1288–1298. doi: 10.1084/jem.149.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K., Diener E., Russell P., Armstrong W. D. The role of nonlymphoid accessory cells in the immune response to different antigens. J Exp Med. 1970 Mar 1;131(3):461–482. doi: 10.1084/jem.131.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Weidanz W. P. Malarial immunodepression in vitro: adherent spleen cells are functionally defective as accessory cells in the response to horse erythrocytes. Eur J Immunol. 1976 Nov;6(11):816–819. doi: 10.1002/eji.1830061112. [DOI] [PubMed] [Google Scholar]
- Wells R. A., Diggs C. L. Protective activity in sera from mice immunized against Plasmodium berghei. J Parasitol. 1976 Aug;62(4):638–639. [PubMed] [Google Scholar]
- Wiedanz W. P., Rank R. G. Regional immunosuppression induced by Plasmodium berghei yoelii infection in mice. Infect Immun. 1975 Jan;11(1):211–212. doi: 10.1128/iai.11.1.211-212.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler D. J., Gallin J. I. Spleen-derived mononuclear cell chemotactic factor in malaria infections: a possible mechanism for splenic macrophage accumulation. J Immunol. 1977 Feb;118(2):478–484. [PubMed] [Google Scholar]
- Zuckerman A. Recent studies on factors involved in malarial anemia. Mil Med. 1966 Sep;131(9 Suppl):1201–1216. [PubMed] [Google Scholar]



