Abstract
The tobacco (Nicotiana tabacum) cultivar Xanthi-nc (genotype NN) produces high levels of salicylic acid (SA) after inoculation with the tobacco mosaic virus (TMV). Gaseous methyl salicylate (MeSA), a major volatile produced in TMV-inoculated tobacco plants, was recently shown to be an airborne defense signal. Using an assay developed to measure the MeSA present in tissue, we have shown that in TMV-inoculated tobacco plants the level of MeSA increases dramatically, paralleling increases in SA. MeSA accumulation was also observed in upper, noninoculated leaves. In TMV-inoculated tobacco shifted from 32 to 24°C, the MeSA concentration increased from nondetectable levels to 2318 ng/g fresh weight 12 h after the temperature shift, but subsequently decreased with the onset of the hypersensitive response. Similar results were observed in plants inoculated with Pseudomonas syringae pathovar phaseolicola, in which MeSA levels were highest just before the hypersensitive response-induced tissue desiccation. Transgenic NahG plants unable to accumulate SA also did not accumulate MeSA after TMV inoculation, and did not show increased resistance to TMV following MeSA treatment. Based on the spatial and temporal kinetics of its accumulation, we conclude that tissue MeSA may play a role similar to that of volatile MeSA in the pathogen-induced defense response.
Tobacco (Nicotiana tobaccum L. cv Xanthi-nc) plants carrying the N gene produce high levels of SA after inoculation with TMV. An increase in the tissue content of SA follows the appearance of the virus-induced HR (Malamy et al., 1990). The correlation between SA accumulation and pathogen resistance has also been shown in cucumber (Metraux et al., 1990; Rasmussen et al., 1991), tomato (Hammond-Kosack et al., 1996), and Arabidopsis (Delaney et al., 1995). Following the primary infection, the inoculated plant becomes resistant to subsequent pathogen attack, both locally and systemically, in pathogen-free leaves. This phenomenon, called SAR, has attracted scientific interest for more than 60 years (Chester, 1933). The dependence of SAR on SA accumulation was shown in transgenic NahG tobacco plants expressing the salicylate hydroxylase, which accumulate little or no SA and do not exhibit SAR (Gaffney et al., 1993).
While investigating the movement and distribution of SA in TMV-inoculated tobacco, we discovered that the plants evolve large amounts of gaseous MeSA (Shulaev et al., 1997). In contrast, healthy or mechanically wounded plants did not evolve any detectable amounts of MeSA. Application of gaseous MeSA to healthy tobacco plants increased the expression of the PR-1 gene and TMV resistance (measured as the size reduction of the TMV-induced lesions). The amount of MeSA produced by TMV-inoculated tobacco plants was sufficient to induce PR-1 gene expression and resistance in healthy plants receiving air from the headspace of enclosed TMV-inoculated plants.
The accumulation of SA in tissue exposed to MeSA gas and labeling studies using [14C]SA and MeSA suggest that MeSA is synthesized from SA and acts by being converted back to SA in the target tissues. MeSA is not the only metabolite of SA found in plants. Large amounts of glucosyl SA accumulate in and around the HR lesions (Enyedi et al., 1992; Malamy et al., 1992). However, indirect evidence suggests that glucosyl SA is neither mobile nor biologically active and may function as a storage form of SA (Enyedi et al., 1992; Malamy et al., 1992). In contrast, data indicate that MeSA may serve as an airborne signal involved in inter- and intra-plant communication during the pathogen infection (Shulaev et al., 1997).
The fact that MeSA is naturally produced by a number of plants is well known (Wilson et al., 1987; Hamilton-Kemp et al., 1988; Hayden and Clough, 1990; Loughrin et al., 1993); however, the suggestion that gaseous MeSA serves as an inducible signal for resistance in tobacco prompted further investigation of this compound. MeSA, also known as oil of wintergreen, is a volatile liquid at room temperature (boiling point 220–224°C). Whereas previous work examined the effects of MeSA vapor (Shulaev et al., 1997), the work presented here investigates the production and role of nongaseous MeSA in tobacco plants using an analytical method developed for this purpose. In addition, this study provides further evidence that the conversion of MeSA to SA is required for the biological activity of this compound.
MATERIALS AND METHODS
Plant Material and Inoculation with Pathogens
Wild-type and transgenic NahG (gift from J. Ryals, CIBA-Geigy, NC) tobacco (Nicotiana tabacum L. cv Xanthi-nc, NN genotype) seeds were germinated and grown as described previously (Yalpani et al., 1991). Fully expanded leaves of 6- to 8-week-old seedlings were inoculated with TMV strain U1 (5 μg per leaf) in 5 mm potassium phosphate buffer, pH 7.0, or mock inoculated with buffer by gently rubbing the leaves with carborundum, as described previously (Yalpani et al., 1991). Plants were kept under continuous illumination provided by incandescent and cool-white fluorescent lamps (200 μmol m−2 s−1). HR was induced by incubating TMV-inoculated plants at 32°C and then shifting them to 24°C after 4 d, as described previously (Malamy et al., 1992).
For bacterial inoculation, overnight cultures of Pseudomonas syringae pv phaseolicola (NPS3121) (Lindgren et al., 1986) (a gift from R. Mittler, Rutgers University, New Brunswick, NJ) were infiltrated into the abaxial surface of tobacco leaves using a sterile plastic syringe. Following the inoculation, 18-mm leaf discs immediately surrounding the infiltrated areas were sampled and analyzed.
For experiments with gaseous MeSA, 5-week-old wild-type and transgenic NahG tobacco plants were enclosed in 4-L gas-tight glass jars containing an upright cotton swab. The jars were opened daily and liquid MeSA was applied to the cotton swab to produce a concentration of 250 μg L−1 in the headspace after evaporation. After 6 d of exposure to MeSA, leaves from one subset of plants were excised for SA and PR-1 determination. Another subset of plants was virus inoculated on all fully expanded leaves (see above), and the diameter of TMV-induced lesions was measured 72 h postinoculation with a low-power microscope.
Determination of SA
Total SA (the sum of free and glucosyl SA) was extracted and quantified as described previously (Enyedi et al., 1992). Leaf tissue samples (0.5 g fresh weight) were frozen in liquid nitrogen, ground to a fine powder, and sequentially extracted with 90 and 100% methanol. The combined methanolic extracts were vacuum dried and the pellets were resuspended in 5 mm sodium acetate buffer, pH 5.5, containing 80 units/g fresh weight β-glucosidase (Sigma). Following enzymatic hydrolysis (90 min at 37°C), the reaction was stopped with the addition of 10% TCA. The supernatant was partitioned with ethyl acetate:cyclopentane:isopropanol (100:99:1, v/v). SA was determined by fluorescence (excitation 301 nm, emission 412 nm) after separation on a C18 reverse-phase HPLC column (Waters). The column was maintained at 40°C and equilibrated in 0.5% glacial acetic acid:methanol (75:25, v/v) with a flow rate of 1.5 mL min−1. Three minutes after injection, a methanol gradient (25–60%) was applied over 7 min, after which the methanol concentration was returned to 25%. All data were corrected for recovery using spiked samples.
Determination of MeSA
Leaf tissue samples (0.5 g fresh weight) were frozen and ground in liquid nitrogen. The resulting powder was resuspended in 2.5 mL of 100 mm phosphate buffer, pH 7.0, containing 0.125 μg mL−1 vanillin as an internal standard. After 10 min of sonication and centrifugation at 3000g, the supernatant was loaded onto a 3-mL C18 column (Bakerbond SPE, J.T. Baker) preconditioned with 3 mL of 100% methanol and 3 mL of phosphate buffer added sequentially. MeSA was eluted with 2.5 mL of 80% methanol. The methanolic fraction was further diluted with water to 40% methanol (approximately 5 mL total volume), and loaded onto a second 3-mL C18 column preconditioned with 3 mL of 100% methanol and 3 mL of 40% methanol added sequentially. MeSA was eluted with 2.5 mL of 100% methanol. The volume of column eluent was reduced to 0.5 mL by partial drying under vacuum (SpeedVac, Savant Instruments, Holbrook, NY). The recovery of MeSA was 55 to 75%.
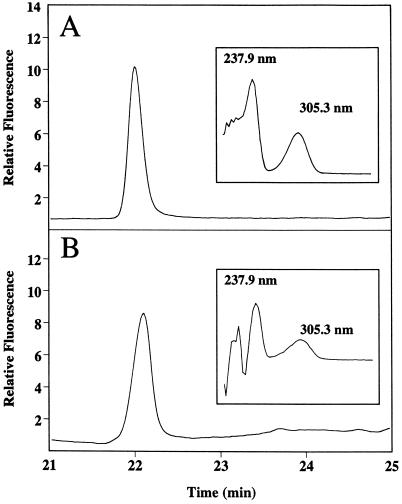
Vanillin and MeSA were detected and quantified by online UV absorption at 280 nm (model 486 tunable absorbance detector, Waters) and fluorescence detection at an excitation of 292 nm and an emission of 360 nm (model 474 scanning fluorescence detector, Waters), respectively. The samples (30-μL volume) were injected (model 717-Plus autosampler, Waters), and the compounds were separated on a C8 reverse-phase HPLC column (Symmetry Shield RP8, Waters) maintained at 40°C. MeSA was separated during a 35-min acetonitrile (10–90%):1.2% acetic acid (90–10%) gradient (flow rate of 1 mL min−1) maintained by HPLC pumps (model 510, Waters). Under these conditions the retention time for MeSA was approximately 22.04 min (Fig. 1, A and B). This procedure gave the detection limit of 200 ng/mL of MeSA (6 ng per injection). An absorption spectrum of an authentic MeSA standard, obtained using a photodiode array detector (model 996, Waters), was almost identical to that of a compound that coeluted with authentic MeSA. GC-MS analysis of TMV-inoculated tobacco leaf extracts demonstrated directly the presence of a compound with the retention time and fragmentation pattern of authentic MeSA (data not shown).
Figure 1.
Identification of MeSA in leaf extracts of TMV-inoculated tobacco. HPLC-elution profile (fluorescence at 360 nm upon excitation at 292 nm) of authentic MeSA standard (A) and of leaf extract of TMV-inoculated tobacco obtained 12 h after temperature shift (B). Insets, UV-absorption spectra of the corresponding MeSA peaks from A and B.
RNA Isolation and Analysis
Total RNA was isolated using the guanidine thiocyanate and phenol-chloroform method (Chomczynski and Sacchi, 1987). RNA (10 μg) was separated by electrophoresis, transferred to nylon membrane (Zeta-Probe, Bio-Rad), and hybridized according to the manufacturer's protocols. PR-1 transcripts were detected with a corresponding 32P-labeled tobacco cDNA probe (a gift from R. Smith, Rutgers University, New Brunswick, NJ) prepared by specific priming. As a control for loading and transfer efficiency, blots were stripped and rehybridized with an 18S gene probe.
RESULTS
MeSA Production in TMV-Inoculated Tobacco Leaves
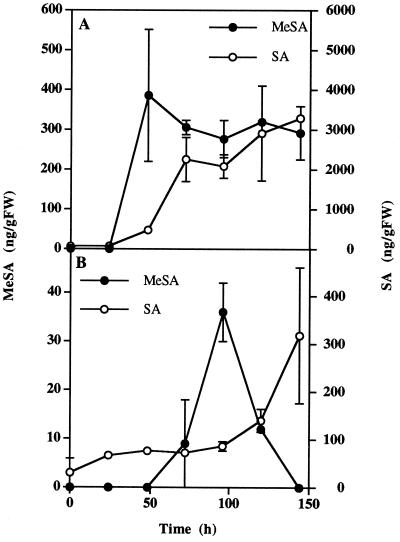
Approximately 48 h after tobacco leaves were inoculated with TMV, HR lesions developed around the site of inoculation. At that time the SA concentration increased 8-fold and continued to accumulate in the inoculated leaf (Fig. 2A). MeSA accumulation was also first detected at the time of HR appearance and reached a maximum of 386 ng g−1 fresh weight. MeSA levels remained relatively steady from 48 to 96 h postinoculation.
Figure 2.
Accumulation of MeSA and SA in tobacco plants after TMV inoculation of a single leaf. Eight-week-old tobacco plants were inoculated on the third leaf from the bottom. MeSA and SA accumulation was analyzed in the inoculated leaf (A) and in the leaf located directly above it (leaf 8) (B). Each point is the mean ± se of three replicates. The experiment was repeated twice with similar results. FW, Fresh weight.
SA content increases systemically after TMV inoculation (Malamy et al., 1990), which at least partially explains its signaling role in SAR. This systemic SA increase is particularly pronounced in the leaf located directly above the inoculated leaf (Shulaev et al., 1995). To investigate the possible systemic mobility of MeSA, we measured the concentration of SA and MeSA in the leaf directly above the TMV-inoculated leaf. As expected, the SA concentration in the uninoculated leaf gradually increased, reaching a maximum of 318 ng g−1 fresh weight at 144 h postinoculation (Fig. 2B). The uninoculated leaf also showed a significant but transient increase in nonvolatile MeSA content. Immediately following inoculation, MeSA concentration was below detection levels but reached 36 ng g−1 fresh weight 96 h after the leaf below was inoculated with TMV. MeSA concentration returned to below detection levels at 144 h postinoculation. In most other experiments the MeSA concentration in the systemic leaf did not show such a rapid decline and remained elevated for a longer period of time (data not shown).
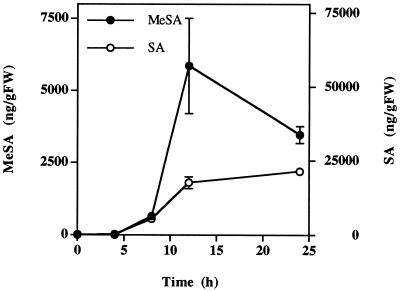
Xanthi-nc tobacco plants inoculated with TMV do not develop HR lesions and do not accumulate SA when they are placed at 32°C following TMV inoculation (Kassanis, 1952; Malamy et al., 1992). Within 24 h of being returned to 24°C, inoculated plants respond with massive tissue necrosis and a dramatic accumulation of SA in the tissues to which the virus has spread. We used the temperature-shift experiment to amplify the biochemical changes associated with the HR response and MeSA production. MeSA concentration increased significantly as early as 8 h after temperature shift, and reached a maximum of 5858 ng g−1 fresh weight after 12 h, just before the tissue started to collapse (Fig. 3). From 12 to 24 h the concentration of MeSA decreased in parallel with tissue desiccation during massive necrosis. However, tissue SA continued to accumulate throughout the 24-h period.
Figure 3.
Accumulation of MeSA and SA in TMV-inoculated tobacco leaves after temperature shift. Tobacco plants were inoculated with TMV on a fully expanded leaf and kept at 32°C for 96 h. At time 0, the temperature was lowered to 24°C. Each point is the mean ± se of three replicates. The experiment was repeated twice with similar results. FW, Fresh weight.
MeSA Production in P. syringae-Inoculated Tobacco Leaves
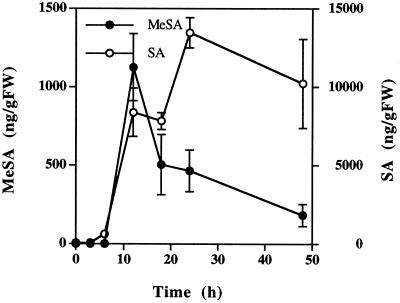
To establish whether the production of MeSA in tobacco is specific for TMV, we measured MeSA accumulation in tobacco leaves inoculated with the bacterial pathogen P. syringae pv phaseolicola, which induces strong HR lesions around the site of infiltration 12 to 18 h postinoculation. Total SA concentration in the vicinity of the lesions reached 13,466 ng g−1 fresh weight after 24 h (Fig. 4). Following the increase in tissue SA, a large increase in MeSA concentration of 1,126 ng g−1 fresh weight was detectable 12 h postinoculation. However, by 18 h, when necrotic HR lesions had developed, the concentration of MeSA decreased to 504 ng g−1 fresh weight. In contrast, tissue desiccation did not substantially reduce SA content.
Figure 4.
Accumulation of MeSA and SA in tobacco leaves inoculated with P. syringae pv. phaseolicola. One fully expanded leaf per plant was inoculated with a strain that induces the HR. The infiltrated tissue was harvested with a cork borer at the times indicated. Each point is the mean ± se of three replicates. The experiment was repeated with similar results. FW, Fresh weight.
Effect of MeSA on Transgenic NahG Tobacco Plants
In the previous experiments we observed that MeSA accumulation paralleled or followed an increase in tissue SA (Figs. 2, 3, and 4). To investigate whether SA accumulation is required for MeSA production, we used transgenic tobacco plants expressing the salicylate hydroxylase gene (nahG) from Pseudomonas putida, which converts SA to catechol (Gaffney et al., 1993). NahG tobacco plants, which are unable to accumulate SA, and wild-type plants were inoculated with TMV and kept at 32°C for 4 d. In contrast to the wild type, TMV inoculation of NahG tobacco plants did not cause SA or MeSA accumulation (Table I). Even though the leaf tissue of both wild-type and NahG plants became necrotic approximately 12 h after the temperature shift, the amounts of SA in NahG plants remained close to basal level, whereas MeSA was below the limit of detection.
Table I.
SA and MeSA concentration in TMV-inoculated wild-type and NahG tobacco plants after temperature shift
| Plant | SA
|
MeSA
|
||
|---|---|---|---|---|
| 0 h | 12 h | 0 h | 12 h | |
| ng g−1 fresh wt | ||||
| Wild type | 38 ± 5 | 12,600 ± 3,110 | nda | 2318 ± 110 |
| NahG | 59 ± 25 | 63 ± 2 | nd | nd |
Tobacco plants were inoculated with TMV and incubated at 32°C for 96 h. At time 0, the temperature was lowered to 24°C. Tissue was sampled 0 and 12 h after temperature shift. Each value is the mean of three replicates ± se. The experiment was repeated with similar results.
nd, Not detected.
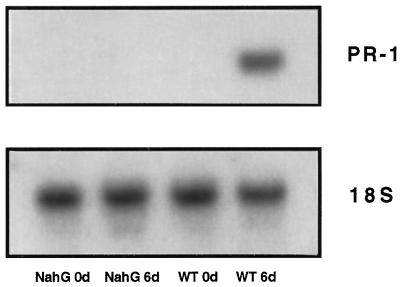
Gaseous MeSA was shown to induce PR-1 transcripts and to reduce the size of TMV-induced lesions in tobacco, presumably through conversion to SA (Shulaev et al., 1997). To further test this hypothesis, we investigated whether MeSA can induce PR-1 transcripts and TMV resistance at low SA levels by exposing NahG plants for 6 d to 250 μg L−1 of gaseous MeSA. MeSA treatment of NahG plants resulted in only a 1.9-fold increase in leaf SA levels compared with a 193-fold increase in the wild type (Table II). In addition, the diameter of the TMV lesions on NahG leaves exposed to MeSA was 2.1 times greater than those on wild-type tobacco leaves, an indication of decreased TMV resistance. Moreover, MeSA treatment did not induce PR-1 transcripts in NahG plants, in contrast to its dramatic effect in the wild-type plants (Fig. 5).
Table II.
Effect of MeSA on SA concentration and TMV-lesion size in wild-type and NahG tobacco plants
| Plant | SA
|
Lesion Diameter | |
|---|---|---|---|
| d 0 | d 6 | ||
| ng g−1 fresh wt | mm | ||
| Wild type | 34 ± 1 | 6546 ± 683 | 0.852 ± 0.04 |
| NahG | 45 ± 4 | 84 ± 17 | 1.785 ± 0.08 |
Tobacco plants were enclosed in gas-tight glass chambers to which 250 μg L−1 MeSA was supplied daily. After 6 d of incubation the plants were sampled and analyzed for SA content. Each value is the mean ± se of three replicates. Plants were then inoculated with TMV, and the diameter of the TMV-induced lesions was measured 72 h postinoculation. Data represent the mean diameter ± se of at least 30 lesions per plant. The experiment was repeated with similar results. The diameters of TMV lesions in untreated wild-type and NahG plants were 1.328 ± 0.09 and 1.800 ± 0.11 mm, respectively.
Figure 5.
PR-1 gene expression in wild-type (WT) and transgenic NahG tobacco plants exposed to gaseous MeSA. Five-week-old tobacco plants were enclosed in gas-tight glass chambers containing 250 μg L−1 MeSA. After the 6-d incubation period, fully expanded leaves were removed from plants and analyzed for PR-1 gene transcription. Total RNA was isolated and the PR-1 transcript was detected by hybridization with a cDNA probe (top). A probe for rRNA (18S) was used to demonstrate equal RNA loading (bottom).
DISCUSSION
We previously demonstrated that gaseous MeSA produced in TMV-inoculated tobacco leaves acts as an airborne defense signal involved in the communication between infected and healthy plants (Shulaev et al., 1997). The amounts of gaseous MeSA produced after the infection were sufficient to induce expression of PR-1 proteins and TMV resistance in nearby healthy plants.
SA glucoside, which is believed to be biologically inactive and immobile, was previously identified as a major metabolite of SA in TMV-infected tobacco tissue (Enyedi et al., 1992; Hennig et al., 1993; Edwards, 1994; Lee et al., 1995). Since MeSA is a volatile liquid at room temperature, earlier steps (such as drying) used in tissue extraction prevented researchers from detecting MeSA as another important metabolite of SA. Measurements of MeSA in the air around virus-inoculated plants provided the first assessment, but underestimated the total MeSA production in the inoculated tissue. Thus, we have developed a method for measuring MeSA in plant tissues (Fig. 1). Our results demonstrate that large amounts of nonvolatile MeSA accumulate in the TMV-inoculated tissue around the time of its appearance in the gas phase (Shulaev et al., 1997).
The amounts of endogenous MeSA in pathogen-inoculated tobacco leaves represent about 10 to 20% of the total SA detected in nontemperature-shifted plants (Figs. 2 and 4), and approximately 30% in temperature-shifted tobacco. Since a substantial portion of MeSA escapes as a vapor, the total amounts of MeSA produced in inoculated leaves should represent an even larger fraction of total SA production. It is important to mention that the induction of MeSA production is not specific to TMV. P. syringae pv phaseolicola, a bean pathogen that is incompatible in tobacco (Lindgren at al., 1986), also caused dramatic MeSA accumulation, indicating that MeSA production is not pathogen specific.
It was shown earlier that MeSA is made from SA and acts by being converted back to SA (Shulaev et al., 1997). Transgenic tobacco plants expressing the salicylate hydroxylase gene nahG are unable to accumulate SA (Gaffney et al., 1993; Friedrich et al., 1995) and provided a useful tool to test the biochemical mechanisms of SA production and action. Since NahG plants inoculated with TMV were unable to accumulate detectable amounts of MeSA (Table I), it is likely that MeSA is synthesized from SA. In addition, the observation that MeSA could not induce TMV resistance (measured as smaller HR lesions) (Table II) and PR-1 gene expression in NahG plants (Fig. 5) suggests that MeSA acts by being converted to SA. However, these results can also be explained by MeSA serving as a substrate for the salicylate hydroxylase. Another indication that SA is a precursor of MeSA comes from Nicotiana glutinosa × debneyi, a tobacco hybrid that contains constitutively high levels of SA (Yalpani et al., 1993). The MeSA concentration in healthy leaves (n = 9) of this hybrid is 480 ± 248 ng g−1 fresh weight, suggesting that the high levels of SA give rise to high levels of MeSA.
In inoculated leaves MeSA accumulation always paralleled the accumulation of endogenous SA (Figs. 2–4), consistent with the proposed synthesis of MeSA from SA. However, in the postinoculation period SA concentration increased continually and MeSA concentration decreased as HR lesions developed (Figs. 2–4). This observation agrees with our previously published observation that the highest production of gaseous MeSA is reached at the onset of tissue desiccation (Shulaev et al., 1997). Here we suggest that the differences in the kinetics of MeSA and SA accumulation can be at least partially explained by the increased volatilization of MeSA facilitated by tissue desiccation during HR-associated necrosis.
It has been previously suggested that SA may not be the only signal involved in SAR (Rasmussen et al., 1991; Vernooij et al., 1994; Shulaev et al., 1995), although it was shown to be required for the establishment of SAR (Gaffney et al., 1993). The observation that MeSA accumulation was detected in the healthy tobacco leaves located above the inoculated leaf (Fig. 2B) suggests that MeSA may function as a translocatable form of SA, into which it can be readily converted in the target tissue. Therefore, like the SA precursor benzoic acid (Shulaev et al., 1995), MeSA may play at least an accessory role in SAR signaling. We have detected MeSA in phloem exudates of TMV-inoculated leaves after a temperature shift, using a previously established exudate-collection technique (Yalpani et al., 1991; data not shown). Therefore, phloem translocation of nongaseous MeSA may complement its aerial spread and provide an early warning defense system during SAR. However, relatively low levels of MeSA compared with SA argue for a supplemental rather than an exclusive role for MeSA in SAR signaling. On the other hand, MeSA vapor may be the sole signal inducing defense responses in an adjacent plant. Further elucidation of MeSA biosynthesis and the cloning of MeSA biosynthetic enzymes may enable us to manipulate MeSA and SA levels in plants, thereby enhancing inter- and intra-plant resistance to pathogens.
Abbreviations:
- HR
hypersensitive response
- MeSA
methyl salicylate
- PR protein
pathogenesis-related protein
- SA
salicylic acid
- SAR
systemic acquired resistance
- TMV
tobacco mosaic virus
Footnotes
This research was funded by grant no. 96-35304-3874 from the U.S. Department of Agriculture. Additional support was provided by the New Jersey Agricultural Experiment Station and the New Jersey Commission for Science and Technology.
LITERATURE CITED
- Chester KS. The problem of acquired physiological immunity in plants. Q Rev Biol. 1933;8:129–154. [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidine thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Delaney TP, Friedrich L, Ryals J. Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc Natl Acad Sci USA. 1995;92:6602–6606. doi: 10.1073/pnas.92.14.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. Conjugation and metabolism of salicylic acid in tobacco. J Plant Physiol. 1994;143:609–614. [Google Scholar]
- Enyedi AJ, Yalpani N, Silverman P, Raskin I. Localization, conjugation, and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc Natl Acad Sci USA. 1992;89:2480–2484. doi: 10.1073/pnas.89.6.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich L, Vernooij B, Gaffney T, Morse A, Ryals J. Characterization of tobacco plants expressing a bacterial salicylate hydroxylase gene. Plant Mol Biol. 1995;29:959–968. doi: 10.1007/BF00014969. [DOI] [PubMed] [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessman H, Ryals J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- Hamilton-Kemp TR, Andersen RA, Rodriguez JG, Loughrin JH, Patterson CG. Strawberry foliage headspace vapor components at periods of susceptibility and resistance to Tetranuchys urticae. Koch. J Chem Ecol. 1988;14:789–798. doi: 10.1007/BF01018773. [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Silverman P, Raskin I, Jones JDG. Race-specific elicitors of Cladosporium fulvum induce changes in cell morphology and the synthesis of ethylene and salicylic acid in tomato plants carrying the corresponding Cf disease resistance gene. Plant Physiol. 1996;110:1381–1394. doi: 10.1104/pp.110.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden WJ, Clough J. Methyl salicylate secretory cells in roots of Viola arvensis and V. rafinesqui (Violaceae) Castanea. 1990;55:65–70. [Google Scholar]
- Hennig J, Malamy J, Grynkiewicz G, Indulski J, Klessig DF. Interconversion of the salicylic acid signal and its glucoside in tobacco. Plant J. 1993;4:593–600. doi: 10.1046/j.1365-313x.1993.04040593.x. [DOI] [PubMed] [Google Scholar]
- Kassanis B. Some effects of high temperature on the susceptibility of plants to infection with viruses. Ann Appl Biol. 1952;39:358–369. [Google Scholar]
- Lee H, Leon J, Raskin I. Biosynthesis and metabolism of salicylic acid. Proc Natl Acad Sci USA. 1995;92:4076–4079. doi: 10.1073/pnas.92.10.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren PB, Peet RC, Panopoulus NJ. Gene cluster of Pseudomonas syringae pv phaseolicola controls pathogenicity of bean plants and hypersensitivity on nonhost plants. J Bacteriol. 1986;168:512–522. doi: 10.1128/jb.168.2.512-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughrin JH, Hamilton-Kemp TR, Burton HR, Andersen RA. Effect of diurnal sampling on the headspace composition of detached Nicotiana suaveolens flowers. Phytochemistry. 1993;30:1417–1419. [Google Scholar]
- Malamy J, Carr JP, Klessig DF, Raskin I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to tobacco mosaic virus. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Malamy J, Hennig J, Klessig DJ. Temperature-dependent induction of salicylic acid and its conjugate during the resistance response to tobacco mosaic virus infection. Plant Cell. 1992;4:1002–1004. doi: 10.1105/tpc.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metraux JP, Signer H, Ryals J, Ward E, Wyss-Benz M, Gaudin J, Raschdorf K, Blum W, Inverardi B. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science. 1990;250:1004–1006. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- Rasmussen JB, Hammerschmidt R, Zook MN. Systemic induction of salicylic acid accumulation in cucumber after inoculation with Pseudomonas syringae pv syringae. Plant Physiol. 1991;97:1342–1347. doi: 10.1104/pp.97.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulaev V, Leon J, Raskin I. Is salicylic acid a translocated signal of systemic acquired resistance in tobacco? Plant Cell. 1995;7:1691–1701. doi: 10.1105/tpc.7.10.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulaev V, Silverman P, Raskin I. Airborne signaling by methyl salicylate in plant pathogen resistance. Nature. 1997;385:718–721. [Google Scholar]
- Vernooij B, Friedrich L, Morse A, Reist R, Kolditz-Jawhar R, Ward E, Uknes S, Kessman H, Ryals J. Salicylic acid is not the translocated signal responsible for inducing systemic acquired resistance but is required in signal transduction. Plant Cell. 1994;6:959–965. doi: 10.1105/tpc.6.7.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C, Franklin JD, Otto BE. Fruit volatiles inhibitory to Monilinia fructicola and Botrytis cinerea. Plant Dis. 1987;71:316–319. [Google Scholar]
- Yalpani N, Shulaev V, Raskin I. Endogenous salicylic acid levels correlate with accumulation of pathogenesis-related proteins and virus resistance in tobacco. Phytopathology. 1993;83:702–708. [Google Scholar]
- Yalpani N, Silverman P, Wilson TMA, Kleier DA, Raskin I. Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in virus-infected tobacco. Plant Cell. 1991;3:809–818. doi: 10.1105/tpc.3.8.809. [DOI] [PMC free article] [PubMed] [Google Scholar]