Abstract
Background
This study aimed to investigate the possibility of isolating mesenchymal stem cells (MSCs) from human thigh adipose tissue and the ability of human thigh adipose stem cells (HTASCs) to differentiate into hepatocytes.
Methods
The adipose-derived stem cells (ADSCs) were isolated from thigh adipose tissue. Growth factors, cytokines, and hormones were added to the collagen coated dishes to induce the undifferentiated HTASCs to differentiate into hepatocyte-like cells. To confirm the experimental results, the expression of hepatocyte-specific markers on undifferentiated and differentiated HTASCs was analyzed using reverse transcription polymerase chain reaction and immunocytochemical staining. Differentiation efficiency was evaluated using functional tests such as periodic acid schiff (PAS) staining and detection of the albumin secretion level using enzyme-linked immunosorbent assay (ELISA).
Results
The majority of the undifferentiated HTASCs were changed into a more polygonal shape showing tight interactions between the cells. The differentiated HTASCs up-regulated mRNA of hepatocyte markers. Immunocytochemical analysis showed that they were intensely stained with anti-albumin antibody compared with undifferentiated HTASCs. PAS staining showed that HTASCs submitted to the hepatocyte differentiation protocol were able to more specifically store glycogen than undifferentiated HTASCs, displaying a purple color in the cytoplasm of the differentiated HTASCs. ELISA analyses showed that differentiated HTASCs could secrete albumin, which is one of the hepatocyte markers.
Conclusions
MSCs were islolated from human thigh adipose tissue differentiate to heapatocytes. The source of ADSCs is not only abundant abdominal adipose tissue, but also thigh adipose tissue for cell therapy in liver regeneration and tissue regeneration.
Keywords: Thigh, Adipose tissue, Stem cells, Hepatocytes, Cell Differentiation
INTRODUCTION
The liver is one of the most important organs for the survival of living organisms. It performs a wide range of functions including detoxification, protein synthesis, and the production of numerous biochemicals. Malfunction or dysfunction of hepatocytes leads, in many cases, to incurable liver diseases. Until now, orthotopic liver transplantation has been the only available treatment for patients with end-stage liver diseases. However, the use of this procedure is limited by the availability of donor organs. Hepatocyte transplantation could be employed as an alternative therapeutic approach to whole liver transplantation for liver failure. Recent studies have suggested that stem cell-derived hepatocytes could be utilized as therapeutic hepatocytes [1-5].
Bone marrow-derived mesenchymal stem cells (BM-MSCs), which possess characteristics such as self-renewal, pluripotency, proliferation, longevity, and differentiation, could be one of the sources for transplantable and therapeutic cells [6,7]. However, traditional bone marrow procurement procedures may be distressing to patients and yield a low number of MSCs when performed. Therefore, many researchers have investigated alternative stem cell sources for replacing BM-MSCs.
Adipose tissue commonly obtained using liposuction during plastic surgery is an attractive source of human MSCs, because it is a safe and abundant tissue in the body compared with other tissues. The stem cells in adipose tissue that are capable of differentiating into functional hepatocyte-like cells have phenotypic characteristics similar to BM-MSCs [8,9]. Several studies have reported the differentiation of hepatocyte-like cells from human adipose tissue-derived mesenchymal stem cells [1-3]. Seo et al. [4] demonstrated for the first time that human adipose tissue-derived mesenchymal stem cells differentiate into hepatocyte-like cells upon treatment with a hepatocyte growth factor (HGF), oncostatin M (OSM), and dimethyl sulfoxide (DMSO). These cells expressed albumin (ALB), α-fetoprotein (AFP), and other hepatocyte-specific markers during hepatic differentiation and increased in low-density lipoprotein (LDL)-uptake and urea production. In most of the reports on adipose-derived stem cells (ADSCs), the source of the adipose tissue used has been abundant abdominal adipose tissue. However, thigh adipose tissue that is commonly obtained using liposuction in plastic surgery can also be used as a source of ADSCs.
In this study, MSCs were isolated from human thigh adipose tissue and characterized for morphology, growth potency, phenotypic profile, and the capacity of differentiating into hepatic lineages. In addition, the ability of human thigh adipose stem cells (HTASCs) to differentiate into hepatocytes was evaluated.
METHODS
Hepatic differentiation protocol
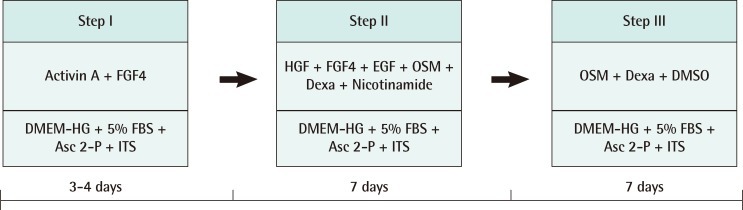
Passages of 3 to 7.5×103 cells were plated onto a collagen coated 48-well culture plate, and hepatic differentiation was then performed over a period of three weeks using a three-step protocol (Fig. 1). At step I for the initiation, the cells were cultured for 4 days in serum-deprived Dulbecco's modified Eagle's medium with high glucose (DMEM-HG) supplemented with 100 µM L-ascorbic acid 2-phosphate (Asc 2-P) and 1×insulin-transferrin-selenium (ITS) consisting of 10 mg/mL insulin, 0.55 µg/mL transferrin, 0.67 µg/mL sodium selenite, 50 ng/mL activin A, and 20 ng/mL fibroblast growth factor 4 (FGF4). At step II for the induction, they were cultured for 7 days in DMEM with low glucose (LG) supplemented with 10% fetal bovine serum (FBS), 100 µM A2P, 1 µM dexamethasone, 10 mM nicotinamide, and 1×ITS consisting of 20 ng/mL HGF, 20 ng/mL FGF4, 20 ng/mL epidermal growth factor (EGF), and 20 ng/mL OSM. During step III for the maturation, the cells were treated with DMEM-LG supplemented with 10% FBS, 100 µM Asc 2-P, 20 ng/mL OSM, 1 µM dexamethasone, 1% DMSO, and 1× ITS for 7 days.
Fig. 1.
The steps of differentiation
The steps of human thigh adipose stem cells differentiation into hepatocytes. FGF4, fibroblast growth factor 4; DMEM-HG, Dulbecco's modified Eagle's medium with high glucose; FBS, fetal bovine serum; Asc 2-P, L-ascorbic acid 2-phosphate; ITS, insulin-transferrin-selenium; HGF, hepatocyte growth factor; EGF, epidermal growth factor; OSM, oncostatin M; DMSO, dimethyl sulfoxide.
Reverse transcription-polymerase chain reaction
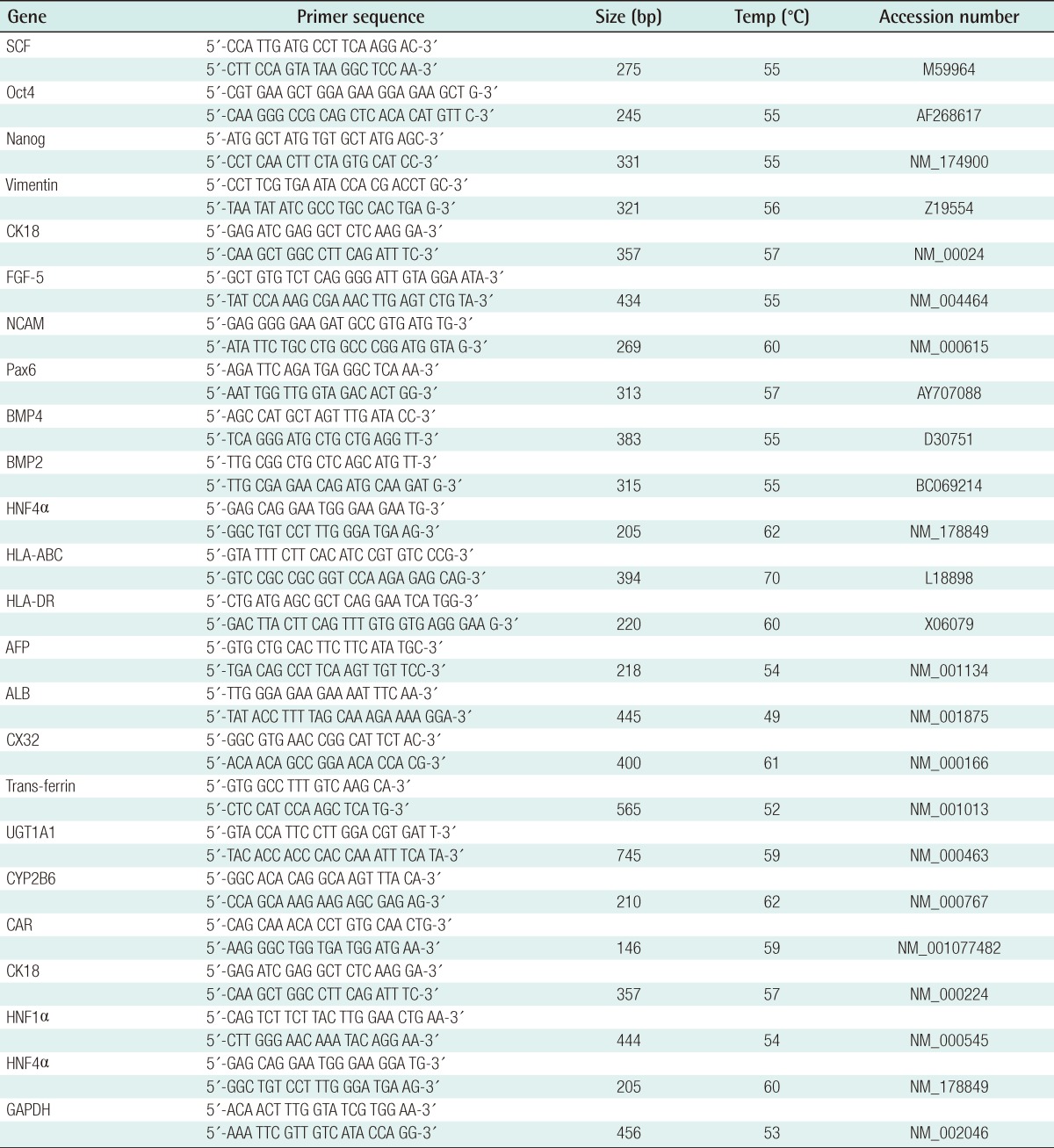
Total RNA was isolated from undifferentiated or differentiated HTASCs using RNAiso kit (Takara, Otsu, Japan). The amount of RNA was assessed by measuring the ratio of absorbance at 260 nm to that at 280 nm (>1.8). Reverse transcription polymerase chain reaction (RT-PCR) was carried out using a thermal cycler (GeneAmp PCR system 2400, Perkin-Elmer, Boston, MA, USA). A total of 7.5 µg (1 µg/µL) RNA was reverse-transcribed using the following RT mixture: 25 mM MgCl2, 10×PCR buffer, 2.5 mM dNTPs mixture, 0.5 mg/mL oligo (d)T15, 40 U RNase inhibitor, and 20 U AMV-RT. The RT reaction was allowed to occur for 60 minutes at 42℃, and the resultant cDNA was subjected to PCR amplification, using upstream and downstream primers of hepatocyte-specific genes as shown in Table 1. PCR was performed using the following PCR mixture: 25 mM MgCl2, 10×PCR buffer, 2.5 U Thermus aquaticus (Taq) polymerase, and 100 pM sense and antisense primers. Amplification was performed for 35 cycles. Annealing temperature was depending on the species of primers. The PCR products were mixed with 6×loading buffer (0.25% bromophenol blue, 0.25% xylene cyanol, and 40% sucrose) and separated on 2% agarose gels. After electrophoresis, the gels were stained with ethidium bromide. DNA signals on the gel were imaged under ultraviolet light using a Bioprofile Image Analysis System (UV Viewing Cabinet, Vilber Lourmat, Marne la Vallee, France).
Table 1.
Primers used for the RT-PCR analysis for the stem cells and hepatocyte-related genes
RT-PCR, reverse transcription-polymerase chain reaction.
Immunocytochemical analysis
The immunophenotype of the cells was determined using a stain kit (Vector Laboratory, Burlington, CA, USA) according to the manufacturer's instructions. After the cells were cultured onto 8-well culture dishes, they were washed with Dulbecco's phosphate buffered saline (DPBS) three times and fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) for 1 hour at 4℃. They were washed with PBS and then permeabilized with 0.5% Triton X-100 in DPBS for 10 minutes at room temperature. After several washings, the cells were incubated in 3% hydrogen peroxide for 15 minutes to quench the endogenous peroxidase activities. They were then rinsed with DPBS and incubated in a blocking solution containing 2.5% horse serum for 30 minutes at room temperature with gentle shaking. The cells were incubated with mouse monoclonal antibodies against human albumin (ALB, 1:100), which had been diluted in 0.5% horse serum. After incubation with the primary antibodies for 17 hours at 4℃ with gentle shaking, the cells were washed and rinsed with DPBS. They were incubated with a biotinylated universal antibody horse anti-rabbit/mouse IgG for 30 minutes at room temperature. The cells were rinsed several times, and then incubated with avidin-biotin complex (ABC) reagent conjugated streptavidin for 10 minutes at room temperature. Immunoreactivity was visualized utilizing 3,3'-diaminobenzidine tetrahydrochloride (DAB). The color reaction was stopped with water. Finally, they were counterstained with Mayer's hematoxylin for 1 minute, and then observed under a light microscope (Zeiss, Oberkochen, Germany).
Albumin secretion by enzyme-linked immunosorbent assay (ELISA)
After 18 days of culture in hepatocyte differentiation media, the cells were transferred to starvation media deprived of serum, and the supernatant was harvested 20 hours later. The human albumin concentration in the supernatant was determined using the Human Albumin ELISA Quantitation Kit (Exocell Inc., Philadelphia, PA, USA) according to the manufacturer's instructions. Serial dilution of a stock solution of albumin was used to create a standard curve. Samples were analyzed in duplicate under each condition.
Periodic acid schiff (PAS) staining of glycogen deposits
To visualize cellular deposits of glycogen molecules, cultured cells were fixed in 4% paraformaldehyde in PBS for 1 hour at 4℃ and rinsed with PBS several times. They were permeabilized with 0.1% Triton X-100 in PBS for 10 minutes. The cells were then oxidized in periodic acid for 20 minutes at room temperature and rinsed three times in deionized H2O (dH2O). After treating the cells with 0.5% sodium bisulfite in 0.05 N HCl for 10 minutes, the cells were counterstained with Mayer's hematoxylin for 1 minute, rinsed in dH2O, and observed for the purple staining indicative of the presence of glycogen using a light microscope.
Statistical analysis
Statistical analyses of this data were performed using the t-test. P-values <0.05 were considered statistically significant. All results were derived from at least three independent experiments.
RESULTS
In vitro differentiation of HTASCs into hepatocyte-like cells
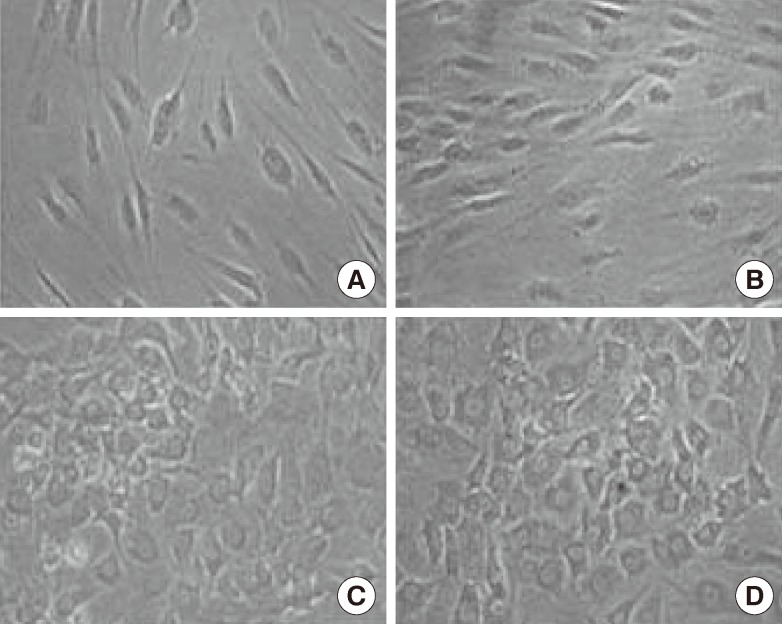
Growth factors, cytokines, and hormones were added to the collagen coated dishes to induce the undifferentiated HTASCs to differentiate into hepatocyte-like cells. During step I for initiation, cell morphology was not greatly changed by the treatment of activin A and FGF4 in the serum-deprived media. When the cells were cultured in the step II media containing HGF, OSM, and dexamethasone for 7 days, the cells gradually changed from a fibroblast-like morphology to a round or polygonal shape. Finally, the cells were cultured in the step III media including OSM, dexamethasone, and DMSO for more than 7 days for maturation; the majority of the cells changed into a more polygonal shape showing tight interactions between the cells (Fig. 2).
Fig. 2.
Morphological features
Morphological features following the steps of hepatogenic-differentiated human thigh adipose stem cells (×100). In stage II, the cells' morphology was gradually changed to a round or polygonal shape. The majority of the cells were changed into a more polygonal shape showing tight interactions between the cells. (A) Human thigh adipose stem cells (0 day), (B) Stage I (4 days), (C) Stage II (11 days), and (D) Stage III (18 days).
Gene expression patterns of HTASC-derived hepatocyte-like cells
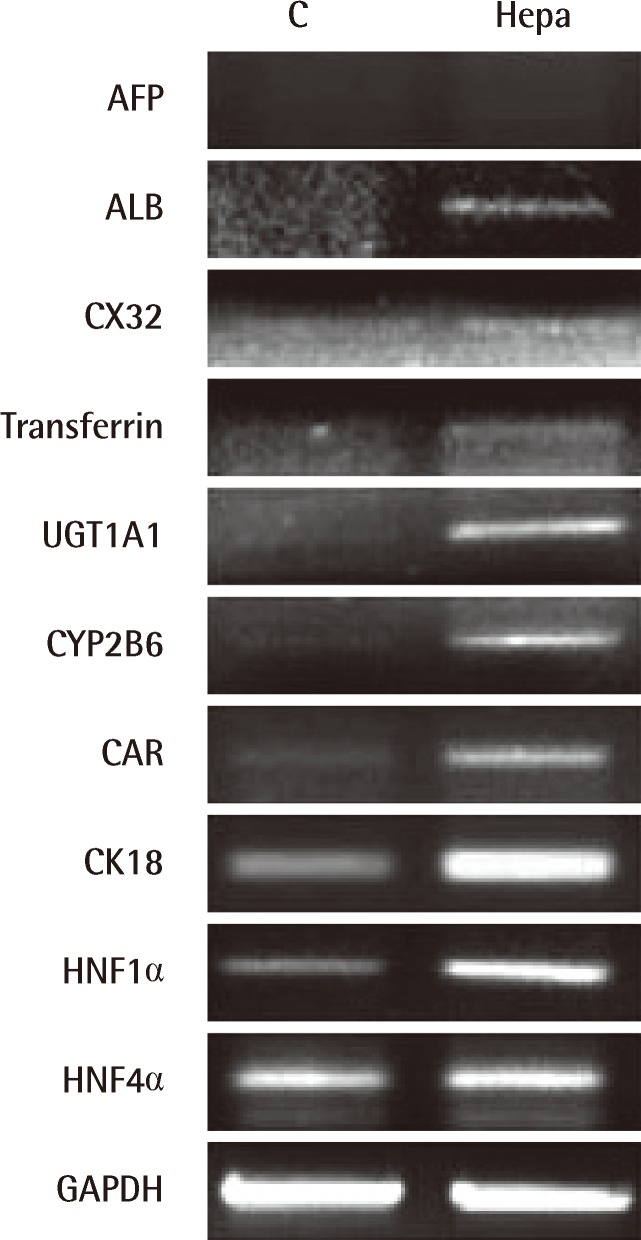
To confirm the above results, the expression of hepatocyte-specific markers on undifferentiated and differentiated HTASCs was analyzed using RT-PCR and immunocytochemical staining. Undifferentiated cells expressed mRNA for CK18, HNF1α, and HNF4α, but not ALB, CX32, transferrin, UGT1A1, CYP2B6, or CAR. In contrast, differentiated HTASCs expressed or up-regulated mRNA for ALB, CX32, transferrin, UGT1A1, CYP2B6, CAR, CK18, HNF1α, and HNF4α (Fig. 3). Immunocytochemical analysis showed that they were intensely stained with an anti-albumin antibody compared with undifferentiated HTASCs (Fig. 4).
Fig. 3.
The expression of hepatocyte-specific markers
Expression of hepatocyte-specific markers on undifferentiated (C) and differentiated (Hepa) human thigh adipose stem cells; (Hepa) expressed or up-regulated mRNA for albumin (ALB), CX32, transferrin, UGT1A1, CYP2B6, CAR, CK18, HNF1α, and HNF4α.
Fig. 4.
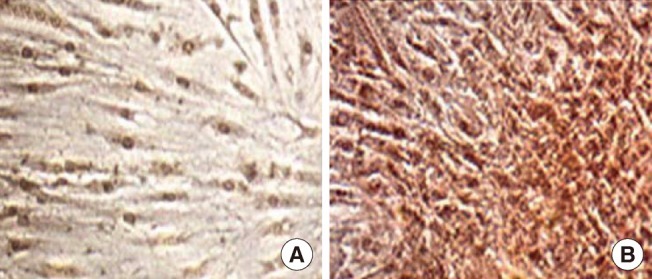
Immunocytochemical analysis
Immunocytochemical analysis was performed using an anti-albumin antibody on undifferentiated (A) and differentiated (B) human thigh adipose stem cells (HTASCs) (×100). Differentiated HTASCs were intensely stained with anti-albumin antibody compared with undifferentiated HTASCs.
Functional characterization of HTASCderived hepatocyte-like cells
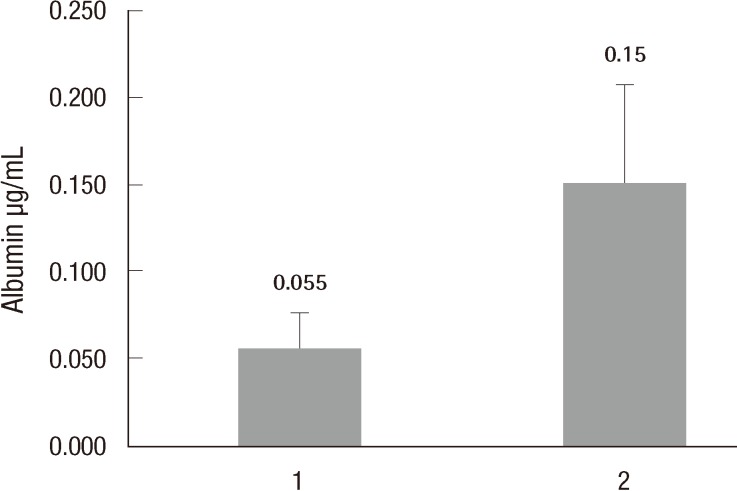
Differentiation efficiency was also evaluated using functional tests such as PAS staining and detecting of albumin secretion level using ELISA. PAS staining showed that HTASCs submitted to the hepatocyte differentiation protocol were able to more specifically store glycogen compared with undifferentiated HTASCs, which displayed a purple color in the cytoplasm of differentiated HTASCs (Fig. 5). ELISA analyses showed that differentiated HTASCs had a significantly higher secreted albumin (0.15±0.057 µg/mL) than undifferentiated HTASCs (0.05±0.021 µg/mL, P<0.05) (Fig. 6), suggesting that differentiated HTASCs could secrete albumin, which is one of the hepatocyte markers.
Fig. 5.
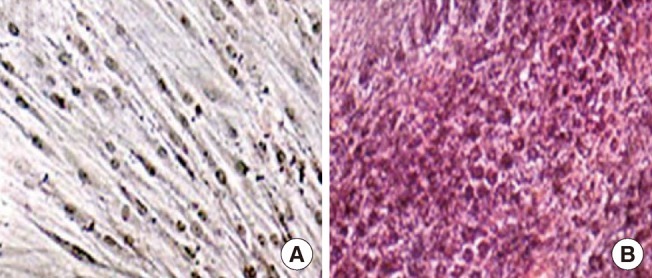
Functional analysis by PAS staining
Functional analysis of hepatogenic differentiated human thigh adipose stem cells (HTASCs). Glycogen storage of the cells was detected with periodic acid schiff (PAS) on undifferentiated (A) and differentiated (B) HTASCs (×100). PAS staining showed that HTASCs submitted to the hepatocyte differentiation protocol were able to more specifically store glycogen than were undifferentiated HTASCs.
Fig. 6.
Functional analysis using ELISA
Functional analysis of hepatogenic differentiated human thigh adipose stem cells (HTASCs). The albumin secretion level was detected using enzyme-linked immunosorbent assay (ELISA) on undifferentiated (1) and differentiated (2) HTASCs. ELISA analyses showed that differentiated HTASCs could secrete albumin, which is one of the hepatocyte markers.
DISCUSSION
Regenerative medicine involves the combination of cells and scaffold materials that can be loaded with bioactive factors, ideally resulting in the regeneration or replacement of lost or damaged tissues and organs. Multiple cell sources have been investigated for their possible applicability in tissue engineering. Stem cells that characterize self-renewal and differentiation into multi-lineaged cells are being investigated for tissue engineering. Embryonic stem cells are the most potent stem cells. However, their use is controversial and has major ethical implications [10]. MSCs can be obtained from adults and are widely used because of their differentiation potential. In addition to bone marrow, periosteum [11] and muscle [12] also appear to be sources of MSCs. Particularly, adipose tissue that is commonly obtained using liposuction in plastic surgery is an attractive source of human MSCs because it is a safe and abundant tissue in the body compared with other tissues. In addition, the Korea Food and Drug Administration has granted permission to use self-adipose-derived stem cells for transplantation using a minimum process.
Noteably, the fact that plastic surgeons can obtained abundant adipose tissue by liposuction makes the study of ADSCs very convenient. Therefore, many researchers use abundant abdominal adipose tissue as a stem cell source. However, although studies on hepatocyte differentiation using abdominal ADSCs have been reported, reports on hepatocyte differentiation using thigh ADCS have not been documented. Therefore, ADSCs were isolated from human thigh adipose tissue in this study and their morphology, growth potency, phenotypic profile, and capacity for differentiating into various lineages were characterized. In addition, the ability of HTASCs to differentiate into hepatocytes was evaluated.
To recapitulate liver development, a three-step protocol with sequential addition of growth factors (FGF4, EGF, and HGF), cytokines (activin A, OSM), hormones (dexamethasone and insulin), nicotinamide, and DMSO was used in this study [13,14]. Activin signaling at a level threefold above baseline dictates that the mesendoderm will move toward the endodermal cell fate [15]. HGF was originally identified and cloned as a morphogenic activator for a wide variety of cells and is known to play an essential role in the development and regeneration of the liver [5]. The foregut endoderm is induced to the hepatocyte lineage by the FGF family produced by the adjacent cardiac mesoderm, which are required to induce a hepatic fate and not the default pancreatic fate [16]. It has been reported that the treatment of cells with OSM, a member of the interleukin-6 cytokine family, increased the cell size of hepatocytes and enhanced cell differentiation and formation of bile canaliculi [17]. Although OSM alone had weak effects on hepatocyte functions, albumin secretion and CYP450 activity were greatly enhanced when combined with nicotinamide or DMSO [18]. DMSO has been known to maintain the functions of adult hepatocytes in vitro [13]. It has been reported that nicotinamide and DMSO remarkably enhanced the emergence of small hepatocytes, and that OSM also synergistically enhanced the selective growth of small hepatocytes and inhibited the growth of blood cell populations [18].
After differentiation, the cells were observed for the morphological changes, gene expression pattern, and functional activities of hepatocyte-like cells derived from HTASCs. Similar to other studies using ADSCs from abdominal adipose tissue [1,4], these cells displayed a hepatocyte-like morphology such as a polygonal shape and cytoplasmic granulation with a more prominent nucleus. The cells also expressed hepatocyte-specific genes including ALB, CX32, transferrin, UGT1A1, CYP2B6, CAR, CK18, HNF1α, and HNF4α, which was consistent with the gene expression pattern from abdominal ADSCs in the report by Seo et al. [4]. Furthermore, these cells exhibited hepatocyte functions such as storage of glycogen, and expression and secretion of albumin, a major protein produced by the hepatocytes. Schmelzer et al. [19] examined the gene expression profile of hepatic cells ranging from the stem cell stage to the mature functional stage. They showed that hepatic stem cells expressed high levels of CK19, low levels of ALB, and no AFP or adult liver-specific proteins. In contrast, hepatoblasts expressed low levels of CK19, elevated levels of ALB, high levels of AFP, and low levels of adult liver-specific proteins.
Taken together, this study successfully isolated human thigh ADSCs, differentiated them into endodermal hepatocytes, and confirmed that the hepatocytes were functional. Therefore, the source of ADSCs is not only abundant abdominal adipose tissue, but also thigh adipose tissue for cell therapy in liver regeneration and tissue regeneration.
Footnotes
This article was presented as a poster at the 69th Congress of the Korean Society of Plastic and Reconstructive Surgeons on November 11-13, 2011 in Seoul, Korea.
No potential conflict of interest relevant to this article was reported.
References
- 1.Banas A, Teratani T, Yamamoto Y, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- 2.Talens-Visconti R, Bonora A, Jover R, et al. Human mesenchymal stem cells from adipose tissue: differentiation into hepatic lineage. Toxicol In Vitro. 2007;21:324–329. doi: 10.1016/j.tiv.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 4.Seo MJ, Suh SY, Bae YC, et al. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258–264. doi: 10.1016/j.bbrc.2004.12.158. [DOI] [PubMed] [Google Scholar]
- 5.Oh SH, Miyazaki M, Kouchi H, et al. Hepatocyte growth factor induces differentiation of adult rat bone marrow cells into a hepatocyte lineage in vitro. Biochem Biophys Res Commun. 2000;279:500–504. doi: 10.1006/bbrc.2000.3985. [DOI] [PubMed] [Google Scholar]
- 6.Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood) 2001;226:507–520. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]
- 7.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 8.Lee RH, Kim B, Choi I, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi Y, Sekiya I, Yagishita K, et al. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 10.Dresser R. Ethical issues in embryonic stem cell research. JAMA. 2001;285:1439–1440. [PubMed] [Google Scholar]
- 11.Nakahara H, Goldberg VM, Caplan AI. Culture-expanded human periosteal-derived cells exhibit osteochondral potential in vivo. J Orthop Res. 1991;9:465–476. doi: 10.1002/jor.1100090402. [DOI] [PubMed] [Google Scholar]
- 12.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita T, Miyajima A. Cytokine regulation of liver development. Biochim Biophys Acta. 2002;1592:303–312. doi: 10.1016/s0167-4889(02)00323-3. [DOI] [PubMed] [Google Scholar]
- 14.Michalopoulos GK, Bowen WC, Mule K, et al. HGF-, EGF-, and dexamethasone-induced gene expression patterns during formation of tissue in hepatic organoid cultures. Gene Expr. 2003;11:55–75. doi: 10.3727/000000003108748964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLean AB, D'Amour KA, Jones KL, et al. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. doi: 10.1634/stemcells.2006-0219. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz RE, Reyes M, Koodie L, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazaro CA, Croager EJ, Mitchell C, et al. Establishment, characterization, and long-term maintenance of cultures of human fetal hepatocytes. Hepatology. 2003;38:1095–1106. doi: 10.1053/jhep.2003.50448. [DOI] [PubMed] [Google Scholar]
- 18.Sakai Y, Jiang J, Kojima N, et al. Enhanced in vitro maturation of fetal mouse liver cells with oncostatin M, nicotinamide, and dimethyl sulfoxide. Cell Transplant. 2002;11:435–441. [PubMed] [Google Scholar]
- 19.Schmelzer E, Wauthier E, Reid LM. The phenotypes of pluripotent human hepatic progenitors. Stem Cells. 2006;24:1852–1858. doi: 10.1634/stemcells.2006-0036. [DOI] [PubMed] [Google Scholar]