Abstract
Pregnane xenobiotic receptor (PXR) is an orphan nuclear receptor that regulates the metabolism of endobiotics and xenobiotics. PXR is promiscuous and unique in that it is activated by a diverse group of xenochemicals, including therapeutic anticancer drugs and naturally-occurring endocrine disruptors. PXR has been predominantly studied to understand its regulatory role in xenobiotic clearance in liver and intestine via induction of drug metabolizing enzymes and drug transporters. PXR, however, is widely expressed and has functional implications in other normal and malignant tissues, including breast, prostate, ovary, endometrium and bone. The differential expression of PXR and its target genes in cancer tissues has been suggested to determine the prognosis of chemotherapeutic outcome. In addition, the emerging evidence points to the implications of PXR in regulating apoptotic and antiapoptotic as well as growth factor signaling that promote tumor proliferation and metastasis. In this review, we highlight the recent progress made in understanding the role of PXR in cancer, discuss the future directions to further understand the mechanistic role of PXR in cancer, and conclude with the need to identify novel selective PXR modulators.
Keywords: PXR, Cancer, Drug resistance, Endocrine disruption, Dog
1. Introduction
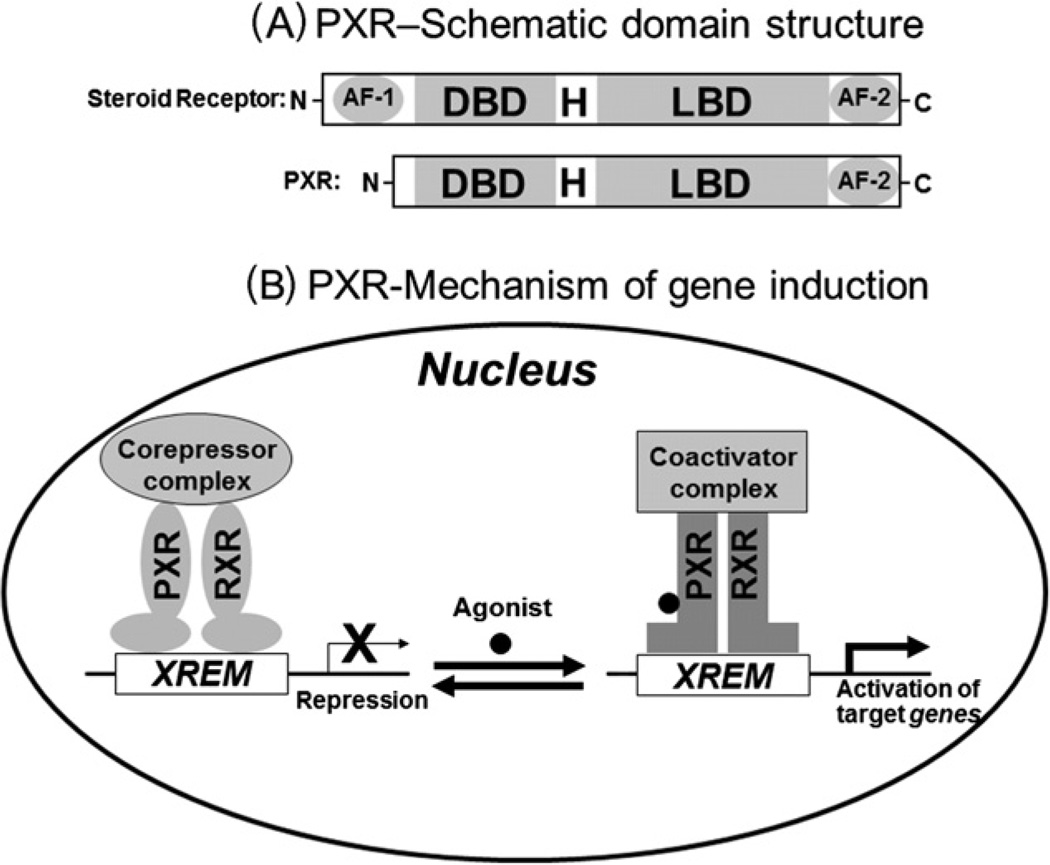
PXR is a member of the nuclear receptor superfamily of ligand-activated transcription factors that regulate the expression of their target genes by binding to the gene’s promoter (Fig. 1B). PXR is an orphan nuclear receptor that is activated by binding to various chemically and structurally distinct endobiotics and xenobiotics [1,2], including clinically-used chemotherapeutic drugs (tamoxifen, doxorubicin, taxol, and vincristine) [3–5] and environmental chemicals (bisphenol A) [6,7]. Similar to steroid receptors, PXR protein contains a DNA-binding domain (DBD), a hinge region (H), a ligand-binding domain (LBD), a ligand-dependent transactivation function 2 (AF-2) (Fig. 1A). In contrast to most nuclear receptors, PXR does not have a ligand-independent activation function 1 domain (AF-1) (Fig. 1A).
Fig. 1.
PXR structure and mechanism of target gene induction. (A) A schematic comparison of the domain structures of a steroid receptor and PXR. AF-1, activation function 1; DBD, DNA binding domain; H, hinge region; LBD, ligand binding domain; AF-2, transactivation function 2. (B) A current model of PXR-mediated gene regulation. PXR functions as heterodimer with retinoid X receptor (RXR). Agonist binding induces a dissociation of co-repressors, recruitment of co-activators and contributes to chromatin remodeling and transcriptional activation. XREM, xenobiotic responsive enhancer module.
In the absence of an agonist, PXR is associated with transcriptional corepressors such as nuclear receptor corepressor 1 (NCoR1) and NCoR2 [1,8–10], which mediate repression of PXR basal transcription activity through the recruitment of histone deacetylases [10] (Fig. 1B). Agonists such as rifampicin, SR12813, pregnenolone 16α carbonitrile (PCN), anticancer drugs, and environmental chemicals [1–7] bind to PXR and induce conformational changes that lead to dissociation of corepressors and recruitment of coactivators such as steroid receptor coactivator 1 (SRC-1) and SRC-3 which have intrinsic histone acetyltransferase activity, (Fig. 1B) [1,8–10], resulting in chromatin remodeling and subsequent transcriptional activation [1]. Ligand-bound PXR binds to the promoter of its target gene as a heterodimer with retinoid X receptor α (RXRα), and the heterodimer can form in the absence of the promoter [11].
PXR regulates proliferation of both cancer and non-cancer cells. PXR is essential for normal progression of liver regeneration [12] and activation of PXR induces hepatic proliferation or inhibits apoptosis through multiple mechanisms [13–16]. On the other hand, PXR induces osteoclast apoptosis [17–19], suggesting that the mechanisms for regulation of cell proliferation by PXR are tissue and cell-specific. Similarly, in cancer cells, PXR regulates cell growth in a variety of cancer tissues (e.g., colon, ovarian, prostate, endometrial, osteosarcoma) [20–38] through multiple mechanisms. Additionally, PXR is involved in regulating metastasis of cancer cells [26,32,35].
PXR also alters therapeutic response of cancer cells to anticancer drugs by regulating the expression of drug metabolizing enzymes and drug transporters. This is evident in a variety of cancers, including breast, prostate, ovarian, colon and endometrial cancer [20–42]. Furthermore, PXR regulates the expression of the proteins involved in apoptosis and antiapoptosis in these cancers,which further contributes to altered tumor growth and drug sensitivity [24,30–32,43]. In this review, we summarize the recent progress made to comprehend the role of PXR in tumor development and progression, as well as in anticancer drug resistance, of both reproductive and non-reproductive tissue cancers.
2. Role of PXR in cancer
2.1. Breast cancer
The expression of PXR in human breast tissue appears to be driven by the cellular context. This is evident by differential expression of PXR in normal or cancerous tissue [20–24,39]. For instance, while some studies reported PXR expression exclusively in cancerous tissues [21,22], another study [20] reported PXR expression in both cancerous and adjacent normal tissues. On the other hand, the other report provided evidence for higher PXR expression in cancerous tissue than in adjacent normal tissue [23]. Notably, PXR expression was found to be higher in invasive stage than in early phase of breast cancer patients [21]. Likewise, higher PXR expression was found to be related to breast cancer progression [22]. These observations indicate that PXR expression may be context-specific and may play a role in development and progression of breast cancer.
Several reports show that PXR can induce cell proliferation in human breast cancer cells [20–23,39] through multiple cancer cell mediated mechanisms. While organic anion-transporting polypeptide-A (OATP-A) was found to be expressed exclusively in breast carcinoma cells [21], higher expression of organic anion-transporting polypeptide 1A2 (OATP1A2) was observed in the cancerous tissue than in adjacent normal tissue [23]. In the latter study, activation of PXR in T47-D breast cancer cells with rifampicin upregulated the expression of OATP1A2 and OATP1A2-mediated estrogen uptake as well as enhanced estrogen-dependent cell proliferation. Conversely, inhibition of PXR with A-792611 or knockdown of PXR with siRNA, resulted in downregulation of OATP1A2 expression as well as OATP1A2-mediated estrogen uptake. These observations support a possible role for PXR in breast tumor growth by enhancing the uptake of estrogens via OATP, thereby increasing intracellular levels of estrogens that activate estrogen receptor (ER).
In addition, it is interesting to note a significant correlation between PXR expression and ER status in breast cancer. Miki et al. [21] noticed a positive correlation, with higher PXR labeling index in ER-positive tumors. Additionally, higher PXR expression was found to be positively associated with lymph node status, histologic grade, Ki-67 proliferation marker, and p450 aromatase (estrogen synthase) expression in ER-positive cases [21]. In contrast, Dotzlaw et al. [20] and Conde et al. [22] found an inverse relationship between PXR expression and ER status, that is, the level of PXR expression was higher in ER-negative tumors. Even in the context of inverse relationship, PXR could contribute to growth in breast cancer cells because of the fact that estrogen binds and activates PXR, suggesting that estrogen may act through PXR in ER-negative tumors, thereby, inducing growth. Notably, in an MMTVneu mouse model of breast cancer, there is a marked increase in PXR-mediated estriol-induced mammary tumors [44]. These data support the role for PXR in inducing breast tumors through multiple mechanisms. The overall implications of these data show a significant trend supporting the anti-apoptotic role for PXR in breast cancer.
However, a study by Verma et al. [24] provided contrary evidence showing that PXR induces apoptosis in breast cancer cells. Activation of PXR with rifampicin, tamoxifen, anandamide, clotrimazole and nifedipine inhibited the proliferation of MCF-7 and ZR-75-I human breast cancer cells by inducing cell cycle arrest at the G1/S phase followed by apoptosis [24]. Likewise, overexpression of constitutively active PXR decreased the growth of MCF-7 cells [24]. Conversely, PXR knockdown using the targeted siRNA blocked PXR activation-induced apoptosis of MCF-7 cells, suggesting that PXR activation can control the growth of and induce apoptosis in breast cancer cells [24]. This study further demonstrated that PXR activation resulted in the up-regulation of inducible nitric oxide synthase (iNOS) and NO-dependent up-regulation of p53 and p53 target genes; P21, BAX and PUMA, with eventual cell arrest and apoptosis of breast carcinoma cells.
In addition to its role in tumor growth and progression, PXR has implications in breast cancer drug resistance via induction of drug metabolizing enzymes/drug transporters [20–23,39]. For instance, in MDA-MB-231 and MCF-7 human breast cancer cells, preactivation of PXR by SR12813, led to an increased resistance to Taxol as well as an increased expression of CYP3A4 and MDR1 [39]. Conversely, knockdown of PXR using small hairpin RNA (shRNA) sensitized MDA-MB-231 and MCF-7 cells to the treatment of Taxol, vinblastine or tamoxifen [39]. In another study [21], MDR1 was found to be expressed only in human breast carcinoma cells but not in non-neoplastic cells. Finally, it is interesting to note that higher nuclear PXR expression was positively correlated with the cases that presented resistance to conventional treatments and that metastasized later, suggesting that overexpression of nuclear PXR may be considered as a potential poor prognostic indicator in breast cancer [22]. The overall implications of these data support that PXR confers resistance toward chemotherapy in breast cancer.
2.2. Endometrial cancer
PXR has implications in endometrial cancer growth and drug response. Variable levels of PXR expression has been noticed exclusively in human endometrial cancer tissues but not in normal tissues [34]. Additionally, the cancer tissues with higher PXR expression showed higher expression of CYP3A4 [34], suggesting that PXR may play a role in both tumor growth and anticancer drug resistance. Indeed, Masuyama et al. [35] showed that downregulation of PXR by small interfering RNA (siRNA) in HEC-1 human endometrial cancer cells decreased the expression of both CYP3A4 and MDR1 and enhanced growth inhibition and apoptosis in the presence of paclitaxel and cisplatin, indicating that downregulation of PXR sensitizes endometrial cancer cells to chemotherapeutics. Conversely, overexpression of PXR led to significant decrease in cell growth inhibition and apoptosis in the presence of paclitaxel and cisplatin, suggesting that overexpression of PXR promotes tumor growth as well as increases resistance of endometrial cancer cells to chemotherapeutics.
2.3. Prostate cancer
Differential expression of PXR was observed in human prostate tissues, with higher PXR expression in cancerous tissues when compared with normal tissues [25,40]. PXR expression was also detected in PC-3, LNCaP and DU145 human prostate cancer cell lines [25]. In PC3 cells, activation of PXR with SR12813 enhanced the expression of both CYP3A4 and MDR1 and increased the resistance of PC-3 cells to anticancer drugs, paclitaxel and vinblastine, suggesting that PXR activation confers prostate cancer cells with increased resistance toward chemotherapy. On the other hand, the targeted knockdown of PXR using shRNA increased the sensitivity of PC3 cells to paclitaxel and vinblastine, suggesting that downregulation of PXR sensitizes prostate cancer cells toward chemotherapy. Conversely, another study [40] reported that higher PXR expression was correlated with increased survival rate of prostate cancer patients, suggesting that PXR may be a strong prognostic indicator of favorable outcomes and a therapeutic target in prostate cancer.
2.4. Ovarian cancer
There is clinical evidence to support the role of PXR in ovarian tumor aggressiveness and drug resistance. Gupta et al. [26] showed that PXR was expressed in human ovarian cancer tissues and was overexpressed in SKOV-3 and OVCAR-8 human ovarian cancer cell lines. In SKOV-3 cells, activation of PXR with rifampicin induced the expression of CYP3A4 and UGT1A1. In addition, PXR activation induced the proliferation of SKOV-3 cells in vitro and SKOV-3 mouse xenografts in vivo. Furthermore, PXR activation in SKOV-3 cells conferred resistance to anticancer drugs paclitaxel, ixabepilone and SN-38, indicating that activation of PXR induces both tumor growth and chemoresistance in ovarian cancer cells. Yue et al. [27] observed a significant negative relationship between PXR protein status and clinical outcome in patients with ovarian cancer, that is, PXR-positive status is negatively correlated with disease free survival as well as overall survival. Furthermore, PXR was found to be a significant risk factor for both disease-free survival and overall survival, suggesting that PXR may be a useful marker to identify ovarian cancer patients at risk of tumor recurrence or death.
2.5. Colon or colorectal cancer
Several studies have demonstrated that PXR promotes growth and metastasis as well as drug resistance in colon/colorectal cancer. Wang et al. [32] showed that PXR activation induced proliferation, invasion, and migration of LS174T human colon cancer cells in vitro and mouse xenografts of LS174T cells. In contrast, PXR knockdown inhibited proliferation and metastasis to liver from spleen, suggesting that PXR activation can enhance tumor growth and metastasis. Notably, the same group also demonstrated that PXR induced growth factor FGF19 [45] signaling to promote growth and metastasis of LS174 cells. Another study [30] showed that PXR activation inhibited deoxycholic acid (DCA)-induced apoptosis in HCT116 human colorectal cancer cells as well as staurosporine-induced apoptosis in LS180 human colon adenocarcinoma cells. The antiapoptotic effect of PXR was found to be associated with upregulation of multiple antiapoptotic genes including BAG3, BIRC2, and MCL-1. On the other hand, the expression of proapoptotic genes including BAK1 and P53 was downregulated, suggesting that PXR activation prevents induction of apoptosis in the colon cancer cells similar to the findings observed in hepatocytes [43]. It was also shown that PXR activation sensitizes human colon cancer cells to oxidative stress [31], which may have implications in the growth and promotion of colon cancer cells.
The other study [33], however, reported that the expression of PXR was lost or greatly diminished in many colon tumors, and that overexpression of PXR significantly inhibited the proliferation of HT29 human colon cancer cells. In addition, PXR significantly suppressed HT29 xenograft tumor growth in mice as a consequence of inhibited cancer cell proliferation, resulting from cell cycle arrest at G0/G1 phase accompanied by elevated p21 expression and inhibited E2F1 expression. Although this report supports anti-proliferative role for PXR, the overall trend supports proliferative, metastatic and antiapoptotic role for PXR in sporadic colon cancer. More recent literature is supportive of these findings as human PXR agonists increase metabolic clearance of 1a, 25-OH Vitamin D3 thereby attenuating any protective effects of this hormone in LS180 cells [46]. Similarly, the PXR promoter is significantly silenced by methylation in normal colon cells as opposed to tumor cells [47].
Anti-cancer drugs, including PXR activator doxorubicin, induced MDR1 expression in LS180 cells [28]. Activation of PXR with rifampicin decreased intracellular accumulation of doxorubicin and reduced the sensitivity of LS180 cells to the cytotoxic effect of doxorubicin, suggesting that anticancer drugs induce chemoresistance by activation of PXR [28]. Similarly, activation of overexpressed PXR in LS174T cells induced CYP3A4 expression and increased chemoresistance to irinotecan (CPT-11) and SN38 [29]. Conversely, knockdown of overexpressed PXR with shRNA reduced CYP3A4 induction and reversed chemoresistance to SN38, suggesting that PXR expression in colorectal cancer cells could interfere with the sensitivity and metabolism of drugs used in the treatment of colorectal cancer.
2.6. Other cancers
PXR also has implications in the growth/chemoresistance of other cancers [48–50]. For example, in osteosarcoma, PXR activation reduced the therapeutic effectiveness of etoposide, suggesting that PXR activation confers chemoresistance in osteosarcoma [38]. In Barrett’s esophagus patients, higher nuclear PXR expression was detected in high-grade dysplasia than in low-grade dysplasia, suggesting that PXR may have a role in neoplastic progression in Barrett’s esophagus [51]. Similarly, PXR expression was markedly higher in esophageal squamous carcinoma tissue compared with non-neoplastic esophagus [52]. However, higher nuclear PXR expression was also correlated with favorable clinical outcome of the patients with esophageal squamous carcinoma, suggesting that PXR might serve as a prognostic indicator in human esophageal squamous cell carcinoma [52]. These observations support a context-specific role for PXR in esophageal cancer.
3. Future directions
Consistent with the role of PXR in cancer, numerous mechanisms may be involved in PXR-mediated tumor growth or drug response. Identifying all the mechanisms will be critical to systematically dissect the role of PXR in tumor progression or suppression as well as chemoresistance or chemosensitivity. For example, PXR activation induces steatosis and many induced lipogenic pathway targets like fatty acid transporter CD36 are indeed implicated in malignancy [53–55]. Future studies need to be focused on comprehensively identifying the PXR target genes with oncogenic or tumor suppressor nature in a cell/context-specific manner [56]. It is equally important to investigate how PXR is regulated in a cell or context-specific manner in cancer. It is important to note that PXR has been shown to be regulated by epigenetic mechanisms including noncoding RNAs [57], promoter methylation [47,58], and protein arginine methyl transferase 1 [59]. However, it is unknown whether PXR regulates epigenetic mechanisms and investigation of such mechanisms will be useful to therapeutically target PXR in PXR expressing cancers.
PXR activation has been shown to disrupt endocrine homeostasis [60,61]. More importantly, several environmental endocrine disrupting chemicals activate PXR at clinically relevant concentrations [6,7,62–80]. Additionally, recent studies indicate a growing correlation between high exposure to endocrine disruptors (e.g., bisphenol A, xenoestrogens, polycyclic aromatic hydrocarbons) and cancer risk or drug response [75,81–87]. Although, the molecular pathways governing the tissue-specific phenotypes mediated by chronic exposure to endocrine disruptors are varied, it is clear that some important effects are mediated via PXR. Therefore, it is vital to understand the role of PXR in linking environmental chemicals to cancer. We expect to see rigorous scientific investigations focused on establishing a relationship between environmental chemicals that activate PXR (i.e., endocrine disruptors) and cancer [88].
PXR exhibits species specificity at both the ligand-binding and signaling-cascade levels [89,90]. PXR humanized mouse is a very useful animal model to address species specificity at the ligand-binding level [13,91]. However, no animal model is available to address species specificity at the signaling-pathway level. An immunodeficient mouse with induced-cancer is also a very useful model to study the role of PXR in cancer. However, it is possible that the signaling pathways in induced-cancer mice models could be considerably different from spontaneously developed natural-cancers in humans. Dog PXR has been shown to be similar to human PXR in terms of ligand-binding, that is, dog PXR is activated by both rifampicin and SR 12813 but not PCN [92]. Moreover, dogs live in the same environment as humans and harbor naturally-occurring cancers in many ways identical or similar to naturally-occurring human cancers. Therefore, dog may be included as an intermediate model to study the role of PXR in cancer. Dog cancer models may prove particularly valuable to study the carcinogenic effects of environmental chemicals that activate PXR to induce cancer growth and chemoresistance in humans [75,81–87]. Recently, there is an increasing awareness in the scientific community about the significance of dog cancer models as a powerful tool for advancing comparatively oncology studies [93,94]. It is now practically feasible to incorporate dog models for comparative oncology studies and is largely because of the availability of canine genome sequence and comparative oncology consortium tissue bank [93,94].
It has been shown for several nuclear receptors that they induce a phenotype that is tissue and context (isoform) specific. For example, ERRα [95,96], LXR [97], DAX-1 [98], NOR-1 [99] and CAR [100–103], induce cell proliferation and inhibit apoptosis in a variety of tissue systems. However, other receptors like Nurr77 [104,105], LRH-1 [106], SHP [107], PPARγ [108–111] and FXR [112] induce cell cycle arrest and apoptosis. Isoform specific effects are distinct, in that, ERRβ [113] and ERRγ [114] unlike ERRα, induce growth arrest in prostate cancer cells. Tissue specific effects of the same receptor, for example, LXR [97], is observed in smooth muscle cells versus breast cancer. In the latter tissue, LXR induces apoptosis as a consequence of estrogen deprivation [115]. Similarly, another receptor, TR-3 induces cell growth in lung cancer cells through MEKK activation [116] but induces apoptosis in prostate cancer cells through up-regulation of E2F1 [117]. PPARγ [108–111] induces apoptosis in a variety of cancer cell lines but PPARδ [118] induces cell proliferation through cyclin E1-dependent mechanisms. This effect of PPARδ [119] is controversial as other reports suggest that the receptor inhibits cell proliferation in other cell lines (e.g., keratinocytes). The differential effects of PXR observed in different cancer tissues might be contributed by different isoforms/alternative splice variants of PXR [20,22,120].
There are at least three PXR isoforms (i.e. PXR.1, PXR.2 and PXR.3) defining PXR transcripts in mammalian cells [120–123]. These isoforms exhibit differential expression, ligand binding affinity and transcriptional activity. For example, the mouse PXR variant orthologous to human PXR.3 was less well activated than mouse PXR.1 by dexamethasone [1]. Likewise, human PXR.2, exhibits significantly diminished ligand-activated transcriptional activity because ligands do not bind the LBD of PXR.2 effectively [123]. Two studies provided the evidence for differential expression of PXR.1 and PXR.2 in human breast cancer [20,22]. Notably, Dotzlaw et al. [20] reported that MCF cells, with a low metastatic potential, expressed PXR.1 but not PXR.2 mRNA. However, MDAMB-231 cells, with a high metastatic potential, showed highest levels of both PXR.1 and PXR.2 mRNA [20]. These observations are tempting to speculate that differential expression of PXR isoforms might influence breast cancer progression. Tumor-specific regulation of isoforms or splice variants of some proteins has been reported to have very significant functional consequences [124]. Indeed, the spliced murine PXR, mPXRΔ171–211, exhibits repressive action rather than activation [125]. Identifying isoforms and spliced variants in human tumors and non-tumor tissues would be a critical first step towards defining the importance of the isoforms and variants of PXR in human cancer.
In keeping with this theme, spliced variants of PXR might favor selective binding to coregulators in these tissues. Thus a complete definition of coregulator expression and binding to PXR in specific tissues (e.g., tumors) would also be needed to comprehensively understand the effects of PXR activation. For example, in breast cancer, while it has been noted that PXR expression portends a favorable prognosis [24], another report suggests that nuclear localization of both PXR and RXR-alpha portends a poor prognosis [22].
Furthermore, it is now well known that PXR undergoes posttranslational modifications that affect its activity [90,126–128], and that it is possible that even though PXR is expressed in some tumors, it may not be active and be activated by ligands (the converse could also be true). Receptor cross-talk (e.g., PXR and AhR or LXR/FXR or CAR) should also be given consideration, especially for those receptors that share common ligands (e.g., T1317 activates both PXR and LXR). Indeed, some receptors like AhR have been shown to phenocopy the effect of PXR in tumors [129,130]. In this regard, the study of PXR in tumor immunology might also be of interest, given that endogenous AhR activation by constitutive tryptophan catabolism suggests that there could be potential endobiotics that might trigger PXR activation or repression in vivo.
The other important aspect for the study of these receptors in cancers is tissue-context. For example, defining the role of PXR in sporadic tumors might not necessarily signify a unifying role of this receptor in cancer. For example, there are many subsets of tumors where receptors function in an opposing manner – in inflammatory bowel induced colon cancer, loss of adenomatous polyposis coli is a late event, while p53 mutations occur early in its pathogenesis [131,132]. The opposite is true for sporadic colon tumors. Indeed, many transcription factors have dual roles that are context dependent (e.g., GADD45beta, c-Myc) [133–135]. The same receptor may function in opposing manner in different tissues (e.g., androgen receptor in breast and prostate cancer as well as within subtypes of prostate cancer) [136]. Similarly, in vitro studies for apoptosis, senescence, autophagy or other forms of cell death like necroptosis, might also not replicate in vivo effects [137–139]. Thus a complete examination of both in vitro and in vivo effects is warranted in a tissue and context specific manner.
Finally, the tissue specific phenotypes of PXR may vary considerably. For example, it remains unclear to what extent PXR induces metabolic phenotypes (e.g., lipogenesis, gluconeogenesis) in tissues other than liver [140]. Indeed, PXR’s effect on cancer specific metabolism is unknown. Lipogenesis has been associated with cancer development [141] and drug resistance [142,143]. Species specificity of phenotypes must also be clarified (e.g., hepatic hypertrophy versus hyperplasia) [14] to determine whether humans are indeed subject to similar phenotypes [144].
In order to achieve these goals, significantly better reagents are needed for the study of PXR protein and protein modifications. The commercially available antibodies require significant optimization and quality control. Better antibodies for immunoprecipitation and immunoblots are required. Furthermore, somatic (genetic) deletion models for PXR and other receptors would also be welcome additions towards controls. Thus significant more work is needed to fully define all aspects of PXR function in tumors; however, the pace of research is progressing and as better reagents are available, we should be able to define its role.
4. Conclusion
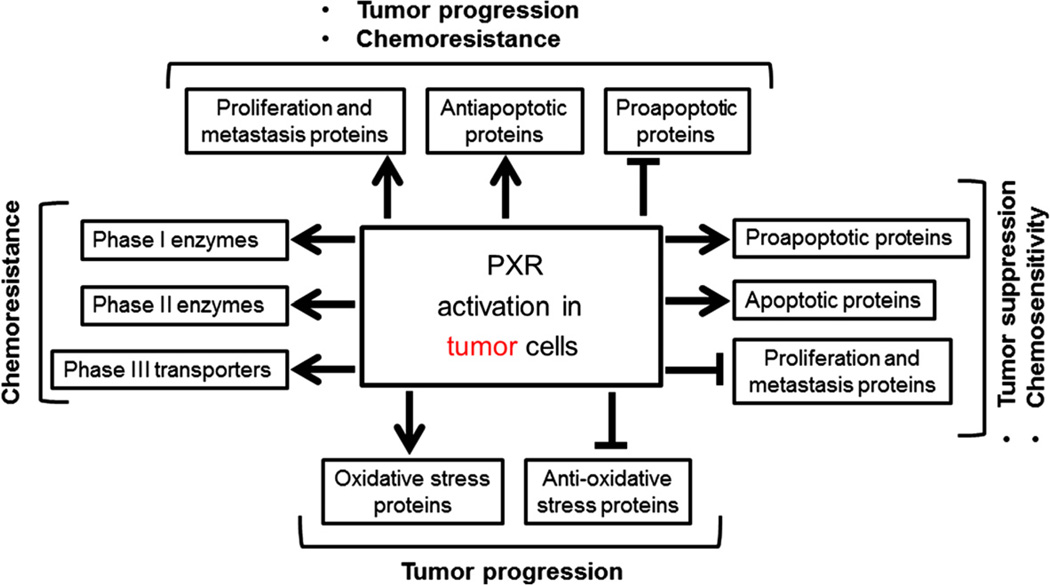
The role of PXR in malignancy and chemoresistance may be tissue/context specific. For example, while PXR induces prostate cancer drug resistance, it has also been shown to be a favorable prognostic marker [36,40]. Likewise, in breast cancer cells, while PXR induces apoptosis, [24], it has also been shown to induce proliferation [21–23]. Similarly, in Barrett’s esophagus and esophageal cancer, it remains controversial as to whether PXR predicts for poor or good prognosis [51,52,145]. Similarly, in colon cancer, depending on the cell type, PXR activation may induce cell cycle arrest [33,146]. Several mechanisms have been proposed for PXR-mediated effects in cancer and include regulation of the genes involved in cell proliferation, metastasis, proapoptosis, apoptosis, antiapoptosis, drug metabolism, drug transport, and endocrine homeostasis as well as regulation of reactive oxygen species system (Fig. 2) [20–43].
Fig. 2.
Consequences of PXR activation in tumor cells. Note that these PXR-mediated events are context and cell-specific depending on the cancer tissue. Arrows represent induction or activation; blunt arrows represent inhibition or repression.
PXR has been proposed as a therapeutic target in treating several human diseases including cancer. Depending on the cancer tissue or cellular context, PXR activation or inhibition has been shown to exert anticancer phenotypes (i.e. sensitize the cancer cells to anticancer drugs, prevent induction of drug resistance, induce apoptosis or reduce proliferation, invasion and migration of the cancer cells). Currently, there are several small molecules [1–5,29,41,147–154] available to either activate or inhibit PXR function in cancer cells. However, there are no selective PXR modulators, which are expected to display differential activities compared to the natural ligands and retain tissue-selective agonist or antagonist activity. Therefore, identification of selective novel small molecule modulators of PXR will be tremendously beneficial to improve the therapeutic outcomes of PXR expressing cancers. Synthetic ligands for PXR can be identified through high throughput screening approaches. The lead structures can be optimized further to adjust the properties of the compounds to appropriately modulate the activities of PXR. Identification of such novel, tissue-selective synthetic modulators, with minimal or no unwanted side activities, would prove extremely valuable to treat PXR expressing cancers.
Acknowledgements
This work was supported by Auburn University Start-up Funds (S.R.P), Auburn University Animal Health and Disease Research Grant (S.R.P) and Auburn University-Intramural Grant (S.R.P). Additional support included Damon Runyon Lilly Clinical Investigator Award, NCI CA 127231, CA127231-03S1 (S.M). The authors apologize to all researchers whose relevant contributions were not cited due to space limitations.
References
- 1.Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J. Clin. Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai PB, Nallani SC, Sane RS, Moore LB, Goodwin BJ, Buckley DJ, Buckley AR. Induction of cytochrome P450 3A4 in primary human hepatocytes and activation of the human pregnane X receptor by tamoxifen and 4- hydroxytamoxifen. Drug Metab. Dispos. 2002;30:608–612. doi: 10.1124/dmd.30.5.608. [DOI] [PubMed] [Google Scholar]
- 4.Mani S, Huang H, Sundarababu S, Liu W, Kalpana G, Smith AB, Horwitz SB. Activation of the steroid and xenobiotic receptor (human pregnane X receptor) by nontaxane microtubule-stabilizing agents. Clin. Cancer Res. 2005;11:6359–6369. doi: 10.1158/1078-0432.CCR-05-0252. [DOI] [PubMed] [Google Scholar]
- 5.Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat. Med. 2001;7:584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- 6.Barrett JR. BPA and PXR activation: human receptor is affected, mouse receptor is not. Environ. Health Perspect. 2012;120:A122. doi: 10.1289/ehp.120-a122a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sui Y, Ai N, Park SH, Rios-Pilier J, Perkins JT, Welsh WJ, Zhou C. Bisphenol A and its analogues activate human pregnane X receptor. Environ. Health Perspect. 2012;120:399–405. doi: 10.1289/ehp.1104426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding X, Staudinger JL. Induction of drug metabolism by forskolin: the role of the pregnane X receptor and the protein kinase a signal transduction pathway. J. Pharmacol. Exp. Ther. 2005;312:849–856. doi: 10.1124/jpet.104.076331. [DOI] [PubMed] [Google Scholar]
- 9.Ding X, Staudinger JL. Repression of PXR-mediated induction of hepatic CYP3A gene expression by protein kinase C. Biochem. Pharmacol. 2005;69:867–873. doi: 10.1016/j.bcp.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DR, Li CW, Chen LY, Ghosh JC, Chen JD. Regulation and binding of pregnane X receptor by nuclear receptor corepressor silencing mediator of retinoid and thyroid hormone receptors (SMRT) Mol. Pharmacol. 2006;69:99–108. doi: 10.1124/mol.105.013375. [DOI] [PubMed] [Google Scholar]
- 11.Noble SM, Carnahan VE, Moore LB, Luntz T, Wang H, Ittoop OR, Stimmel JB, Davis-Searles PR, Watkins RE, Wisely GB, LeCluyse E, Tripathy A, McDonnell DP, Redinbo MR. Human PXR forms a tryptophan zipper-mediated homodimer. Biochemistry. 2006;45:8579–8589. doi: 10.1021/bi0602821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai G, He L, Bu P, Wan YJ. Pregnane X receptor is essential for normal progression of liver regeneration. Hepatology. 2008;47:1277–1287. doi: 10.1002/hep.22129. [DOI] [PubMed] [Google Scholar]
- 13.Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, Neuschwander-Tetri BA, Brunt EM, Guzelian PS, Evans RM. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406:435–439. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
- 14.Elcombe CR, Elcombe BM, Foster JR, Chang SC, Ehresman DJ, Butenhoff JL. Hepatocellular hypertrophy and cell proliferation in Sprague-Dawley rats from dietary exposure to potassium perfluorooctanesulfonate results from increased expression of xenosensor nuclear receptors PPARalpha and CAR/PXR. Toxicology. 2012;293:16–29. doi: 10.1016/j.tox.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Elcombe CR, Elcombe BM, Foster JR, Chang SC, Ehresman DJ, Noker PE, Butenhoff JL. Evaluation of hepatic and thyroid responses in male Sprague Dawley rats for up to eighty-four days following seven days of dietary exposure to potassium perfluorooctanesulfonate. Toxicology. 2012;293:30–40. doi: 10.1016/j.tox.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Elcombe CR, Elcombe BM, Foster JR, Farrar DG, Jung R, Chang SC, Kennedy GL, Butenhoff JL. Hepatocellular hypertrophy and cell proliferation in Sprague-Dawley rats following dietary exposure to ammonium perfluorooctanoate occurs through increased activation of the xenosensor nuclear receptors PPARalpha and CAR/PXR. Arch. Toxicol. 2010;84:787–798. doi: 10.1007/s00204-010-0572-2. [DOI] [PubMed] [Google Scholar]
- 17.Igarashi M, Yogiashi Y, Mihara M, Takada I, Kitagawa H, Kato S. Vitamin K induces osteoblast differentiation through pregnane X receptor-mediated transcriptional control of the Msx2 gene. Mol. Cell. Biol. 2007;27:7947–7954. doi: 10.1128/MCB.00813-07. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Kameda T, Miyazawa K, Mori Y, Yuasa T, Shiokawa M, Nakamaru Y, Mano H, Hakeda Y, Kameda A, Kumegawa M. Vitamin K2 inhibits osteoclastic bone resorption by inducing osteoclast apoptosis. Biochem. Biophys. Res. Commun. 1996;220:515–519. doi: 10.1006/bbrc.1996.0436. [DOI] [PubMed] [Google Scholar]
- 19.Tabb MM, Sun A, Zhou C, Grun F, Errandi J, Romero K, Pham H, Inoue S, Mallick S, Lin M, Forman BM, Blumberg B. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J. Biol. Chem. 2003;278:43919–43927. doi: 10.1074/jbc.M303136200. [DOI] [PubMed] [Google Scholar]
- 20.Dotzlaw H, Leygue E, Watson P, Murphy LC. The human orphan receptor PXR messenger RNA is expressed in both normal and neoplastic breast tissue. Clin. Cancer Res. 1999;5:2103–2107. [PubMed] [Google Scholar]
- 21.Miki Y, Suzuki T, Kitada K, Yabuki N, Shibuya R, Moriya T, Ishida T, Ohuchi N, Blumberg B, Sasano H. Expression of the steroid and xenobiotic receptor and its possible target gene, organic anion transporting polypeptide-A, in human breast carcinoma. Cancer Res. 2006;66:535–542. doi: 10.1158/0008-5472.CAN-05-1070. [DOI] [PubMed] [Google Scholar]
- 22.Conde I, Lobo MV, Zamora J, Perez J, Gonzalez FJ, Alba E, Fraile B, Paniagua R, Arenas MI. Human pregnane X receptor is expressed in breast carcinomas, potential heterodimers formation between hPXR and RXR-alpha. BMC Cancer. 2008;8:174. doi: 10.1186/1471-2407-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer zu Schwabedissen HE, Tirona RG, Yip CS, Ho RH, Kim RB. Interplay between the nuclear receptor pregnane X receptor and the uptake transporter organic anion transporter polypeptide 1A2 selectively enhances estrogen effects in breast cancer. Cancer Res. 2008;68:9338–9347. doi: 10.1158/0008-5472.CAN-08-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma S, Tabb MM, Blumberg B. Activation of the steroid and xenobiotic receptor, SXR, induces apoptosis in breast cancer cells. BMC Cancer. 2009;9:3. doi: 10.1186/1471-2407-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Tang Y, Wang MT, Zeng S, Nie D. Human pregnane X receptor and resistance to chemotherapy in prostate cancer. Cancer Res. 2007;67:10361–10367. doi: 10.1158/0008-5472.CAN-06-4758. [DOI] [PubMed] [Google Scholar]
- 26.Gupta D, Venkatesh M, Wang H, Kim S, Sinz M, Goldberg GL, Whitney K, Longley C, Mani S. Expanding the roles for pregnane X receptor in cancer: proliferation and drug resistance in ovarian cancer. Clin. Cancer Res. 2008;14:5332–5340. doi: 10.1158/1078-0432.CCR-08-1033. [DOI] [PubMed] [Google Scholar]
- 27.Yue X, Akahira J, Utsunomiya H, Miki Y, Takahashi N, Niikura H, Ito K, Sasano H, Okamura K, Yaegashi N. Steroid and Xenobiotic Receptor (SXR) as a possible prognostic marker in epithelial ovarian cancer. Pathol. Int. 2010;60:400–406. doi: 10.1111/j.1440-1827.2010.02546.x. [DOI] [PubMed] [Google Scholar]
- 28.Harmsen S, Meijerman I, Febus CL, Maas-Bakker RF, Beijnen JH, Schellens JH. PXR-mediated induction of P-glycoprotein by anticancer drugs in a human colon adenocarcinoma-derived cell line. Cancer Chemother. Pharmacol. 2010;66:765–771. doi: 10.1007/s00280-009-1221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raynal C, Pascussi JM, Leguelinel G, Breuker C, Kantar J, Lallemant B, Poujol S, Bonnans C, Joubert D, Hollande F, Lumbroso S, Brouillet JP, Evrard A. Pregnane X Receptor (PXR) expression in colorectal cancer cells restricts irinotecan chemosensitivity through enhanced SN-38 glucuronidation. Mol. Cancer. 2010;9:46. doi: 10.1186/1476-4598-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou J, Liu M, Zhai Y, Xie W. The antiapoptotic role of pregnane X receptor in human colon cancer cells. Mol. Endocrinol. 2008;22:868–880. doi: 10.1210/me.2007-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong H, Singh SV, Singh SP, Mu Y, Lee JH, Saini SP, Toma D, Ren S, Kagan VE, Day BW, Zimniak P, Xie W. Orphan nuclear receptor pregnane X receptor sensitizes oxidative stress responses in transgenic mice and cancerous cells. Mol. Endocrinol. 2006;20:279–290. doi: 10.1210/me.2005-0205. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Venkatesh M, Li H, Goetz R, Mukherjee S, Biswas A, Zhu L, Kaubisch A, Wang L, Pullman J, Whitney K, Kuro-o M, Roig AI, Shay JW, Mohammadi M, Mani S. Pregnane X receptor activation induces FGF19-dependent tumor aggressiveness in humans and mice. J. Clin. Invest. 2011;121:3220–3232. doi: 10.1172/JCI41514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouyang N, Ke S, Eagleton N, Xie Y, Chen G, Laffins B, Yao H, Zhou B, Tian Y. Pregnane X receptor suppresses proliferation and tumourigenicity of colon cancer cells. Br. J. Cancer. 2010;102:1753–1761. doi: 10.1038/sj.bjc.6605677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masuyama H, Hiramatsu Y, Kodama J, Kudo T. Expression and potential roles of pregnane X receptor in endometrial cancer. J. Clin. Endocrinol. Metab. 2003;88:4446–4454. doi: 10.1210/jc.2003-030203. [DOI] [PubMed] [Google Scholar]
- 35.Masuyama H, Nakatsukasa H, Takamoto N, Hiramatsu Y. Down-regulation of pregnane X receptor contributes to cell growth inhibition and apoptosis by anticancer agents in endometrial cancer cells. Mol. Pharmacol. 2007;72:1045–1053. doi: 10.1124/mol.107.037937. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Nie D. Pregnane X receptor and its potential role in drug resistance in cancer treatment. Recent Pat Anticancer Drug Discov. 2009;4:19–27. doi: 10.2174/157489209787002498. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Tang Y, Guo C, Wang J, Boral D, Nie D. Nuclear receptors in the multidrug resistance through the regulation of drug-metabolizing enzymes and drug transporters. Biochem. Pharmacol. 2012;83:1112–1126. doi: 10.1016/j.bcp.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mensah-Osman EJ, Thomas DG, Tabb MM, Larios JM, Hughes DP, Giordano TJ, Lizyness ML, Rae JM, Blumberg B, Hollenberg PF, Baker LH. Expression levels and activation of a PXR variant are directly related to drug resistance in osteosarcoma cell lines. Cancer. 2007;109:957–965. doi: 10.1002/cncr.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Tang Y, Chen S, Nie D. Regulation of drug resistance by human pregnane X receptor in breast cancer. Cancer Biol. Ther. 2009;8:1265–1272. doi: 10.4161/cbt.8.13.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujimura T, Takahashi S, Urano T, Tanaka T, Zhang W, Azuma K, Takayama K, Obinata D, Murata T, Horie-Inoue K, Kodama T, Ouchi Y, Homma Y, Inoue S. Clinical significance of steroid and xenobiotic receptor and its targeted gene CYP3A4 in human prostate cancer. Cancer Sci. 2012;103:176–180. doi: 10.1111/j.1349-7006.2011.02143.x. [DOI] [PubMed] [Google Scholar]
- 41.Chen T. Overcoming drug resistance by regulating nuclear receptors. Adv. Drug Deliv. Rev. 2010;62:1257–1264. doi: 10.1016/j.addr.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harmsen S, Meijerman I, Beijnen JH, Schellens JH. The role of nuclear receptors in pharmacokinetic drug–drug interactions in oncology. Cancer Treat. Rev. 2007;33:369–380. doi: 10.1016/j.ctrv.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Zucchini N, de Sousa G, Bailly-Maitre B, Gugenheim J, Bars R, Lemaire G, Rahmani R. Regulation of Bcl-2 and Bcl-xL anti-apoptotic protein expression by nuclear receptor PXR in primary cultures of human and rat hepatocytes. Biochim. Biophys. Acta. 2005;1745:48–58. doi: 10.1016/j.bbamcr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Acevedo R, Parnell PG, Villanueva H, Chapman LM, Gimenez T, Gray SL, Baldwin WS. The contribution of hepatic steroid metabolism to serum estradiol and estriol concentrations in nonylphenol treated MMTVneu mice and its potential effects on breast cancer incidence and latency. J. Appl. Toxicol.: JAT. 2005;25:339–353. doi: 10.1002/jat.1078. [DOI] [PubMed] [Google Scholar]
- 45.Desnoyers LR, Pai R, Ferrando RE, Hotzel K, Le T, Ross J, Carano R, D’Souza A, Qing J, Mohtashemi I, Ashkenazi A, French DM. Targeting FGF19 inhibits tumor growth in colon cancer xenograft and FGF19 transgenic hepatocellular carcinoma models. Oncogene. 2008;27:85–97. doi: 10.1038/sj.onc.1210623. [DOI] [PubMed] [Google Scholar]
- 46.Zheng XE, Wang Z, Liao MZ, Lin YS, Shuhart MC, Schuetz EG, Thummel KE. Human PXR-mediated induction of intestinal CYP3A4 attenuates 1alpha,25-dihydroxyvitamin D(3) function in human colon adenocarcinoma LS180 cells. Biochem. Pharmacol. 2012;84:391–401. doi: 10.1016/j.bcp.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Habano W, Gamo T, Terashima J, Sugai T, Otsuka K, Wakabayashi G, Ozawa S. Involvement of promoter methylation in the regulation of Pregnane X receptor in colon cancer cells. BMC Cancer. 2011;11:81. doi: 10.1186/1471-2407-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kodama S, Negishi M. Pregnane X receptor PXR activates the GADD45beta gene, eliciting the p38 MAPK signal and cell migration. J. Biol. Chem. 2011;286:3570–3578. doi: 10.1074/jbc.M110.179812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rioja J, Bandres E, Rosell Costa D, Rincon A, Lopez I, Zudaire Bergera JJ, Garcia Foncillas J, Gil MJ, Panizo A, Plaza L, Rioja LA, Berian Polo JM. Association of steroid and xenobiotic receptor (SXR) and multidrug resistance 1 (MDR1) gene expression with survival among patients with invasive bladder carcinoma. BJU International. 2011;107:1833–1838. doi: 10.1111/j.1464-410X.2010.09653.x. [DOI] [PubMed] [Google Scholar]
- 50.Rioja Zuazu J, Bandres Elizalde E, Rosell Costa D, Rincon Mayans A, Zudaire Bergera J, Gil Sanz MJ, Rioja Sanz LA, Garcia Foncillas J, Berian Polo JM. Steroid and xenobiotic receptor (SXR), multidrug resistance gene (MDR1) and GSTs, SULTs and CYP polymorphism expression in invasive bladder cancer, analysis of their expression and correlation with other prognostic factors. Actas Urol. Esp. 2007;31:1107–1116. doi: 10.1016/s0210-4806(07)73772-5. [DOI] [PubMed] [Google Scholar]
- 51.van de Winkel A, van Zoest KP, van Dekken H, Moons LM, Kuipers EJ, van der Laan LJ. Differential expression of the nuclear receptors farnesoid X receptor (FXR) and pregnane X receptor (PXR) for grading dysplasia in patients with Barrett’s oesophagus. Histopathology. 2011;58:246–253. doi: 10.1111/j.1365-2559.2011.03743.x. [DOI] [PubMed] [Google Scholar]
- 52.Takeyama D, Miki Y, Fujishima F, Suzuki T, Akahira J, Hata S, Miyata G, Satomi S, Sasano H. Steroid and xenobiotic receptor in human esophageal squamous cell carcinoma: a potent prognostic factor. Cancer Sci. 2010;101:543–549. doi: 10.1111/j.1349-7006.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuriki K, Hamajima N, Chiba H, Kanemitsu Y, Hirai T, Kato T, Saito T, Matsuo K, Koike K, Tokudome S, Tajima K. Increased risk of colorectal cancer due to interactions between meat consumption and the CD36 gene A52C polymorphism among Japanese. Nutr. Cancer. 2005;51:170–177. doi: 10.1207/s15327914nc5102_7. [DOI] [PubMed] [Google Scholar]
- 54.Moya M, Gomez-Lechon MJ, Castell JV, Jover R. Enhanced steatosis by nuclear receptor ligands: a study in cultured human hepatocytes and hepatoma cells with a characterized nuclear receptor expression profile. Chem. Biol. Interact. 2010;184:376–387. doi: 10.1016/j.cbi.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 55.Zhan Y, Ginanni N, Tota MR, Wu M, Bays NW, Richon VM, Kohl NE, Bachman ES, Strack PR, Krauss S. Control of cell growth and survival by enzymes of the fatty acid synthesis pathway in HCT-116 colon cancer cells. Clin. Cancer Res. 2008;14:5735–5742. doi: 10.1158/1078-0432.CCR-07-5074. [DOI] [PubMed] [Google Scholar]
- 56.Azuma K, Urano T, Watabe T, Ouchi Y, Inoue S. PROX1 suppresses vitamin K-induced transcriptional activity of Steroid and Xenobiotic Receptor. Genes to Cells: Devoted Mol. Cell. Mech. 2011;16:1063–1070. doi: 10.1111/j.1365-2443.2011.01551.x. [DOI] [PubMed] [Google Scholar]
- 57.Takagi S, Nakajima M, Mohri T, Yokoi T. Post-transcriptional regulation of human pregnane X receptor by micro-RNA affects the expression of cytochrome P450 3A4. J. Biol. Chem. 2008;283:9674–9680. doi: 10.1074/jbc.M709382200. [DOI] [PubMed] [Google Scholar]
- 58.Misawa A, Inoue J, Sugino Y, Hosoi H, Sugimoto T, Hosoda F, Ohki M, Imoto I, Inazawa J. Methylation-associated silencing of the nuclear receptor 1I2 gene in advanced-type neuroblastomas, identified by bacterial artificial chromosome array-based methylated CpG island amplification. Cancer Res. 2005;65:10233–10242. doi: 10.1158/0008-5472.CAN-05-1073. [DOI] [PubMed] [Google Scholar]
- 59.Xie Y, Ke S, Ouyang N, He J, Xie W, Bedford MT, Tian Y. Epigenetic regulation of transcriptional activity of pregnane X receptor by protein arginine methyltransferase 1. J. Biol. Chem. 2009;284:9199–9205. doi: 10.1074/jbc.M806193200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhai Y, Pai HV, Zhou J, Amico JA, Vollmer RR, Xie W. Activation of pregnane X receptor disrupts glucocorticoid and mineralocorticoid homeostasis. Mol. Endocrinol. 2007;21:138–147. doi: 10.1210/me.2006-0291. [DOI] [PubMed] [Google Scholar]
- 61.Zhang B, Cheng Q, Ou Z, Lee JH, Xu M, Kochhar U, Ren S, Huang M, Pflug BR, Xie W. Pregnane X receptor as a therapeutic target to inhibit androgen activity. Endocrinology. 2010;151:5721–5729. doi: 10.1210/en.2010-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chaturvedi NK, Kumar S, Negi S, Tyagi RK. Endocrine disruptors provoke differential modulatory responses on androgen receptor and pregnane and xenobiotic receptor: potential implications in metabolic disorders. Mol. Cell. Biochem. 2010;345:291–308. doi: 10.1007/s11010-010-0583-6. [DOI] [PubMed] [Google Scholar]
- 63.Kumar S, Jaiswal B, Negi S, Tyagi RK. Cross-talk between androgen receptor and pregnane and xenobiotic receptor reveals existence of a novel modulatory action of anti-androgenic drugs. Biochem. Pharmacol. 2010;80:964–976. doi: 10.1016/j.bcp.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 64.Casabar RC, Das PC, Dekrey GK, Gardiner CS, Cao Y, Rose RL, Wallace AD. Endosulfan induces CYP2B6 and CYP3A4 by activating the pregnane X receptor. Toxicol. Appl. Pharmacol. 2010;245:335–343. doi: 10.1016/j.taap.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 65.Cooper BW, Cho TM, Thompson PM, Wallace AD. Phthalate induction of CYP3A4 is dependent on glucocorticoid regulation of PXR expression. Toxicol. Sci. 2008;103:268–277. doi: 10.1093/toxsci/kfn047. [DOI] [PubMed] [Google Scholar]
- 66.Coumoul X, Diry M, Barouki R. PXR-dependent induction of human CYP3A4 gene expression by organochlorine pesticides. Biochem. Pharmacol. 2002;64:1513–1519. doi: 10.1016/s0006-2952(02)01298-4. [DOI] [PubMed] [Google Scholar]
- 67.DeKeyser JG, Laurenzana EM, Peterson EC, Chen T, Omiecinski CJ. Selective phthalate activation of naturally occurring human constitutive androstane receptor splice variants and the pregnane X receptor. Toxicol. Sci. 2011;120:381–391. doi: 10.1093/toxsci/kfq394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hurst CH, Waxman DJ. Environmental phthalate monoesters activate pregnane X receptor-mediated transcription. Toxicol. Appl. Pharmacol. 2004;199:266–274. doi: 10.1016/j.taap.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 69.Jacobs MN, Nolan GT, Hood SR. Lignans, bacteriocides and organochlorine compounds activate the human pregnane X receptor (PXR) Toxicol. Appl. Pharmacol. 2005;209:123–133. doi: 10.1016/j.taap.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 70.Kojima H, Sata F, Takeuchi S, Sueyoshi T, Nagai T. Comparative study of human and mouse pregnane X receptor agonistic activity in 200 pesticides using in vitro reporter gene assays. Toxicology. 2011;280:77–87. doi: 10.1016/j.tox.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 71.Kretschmer XC, Baldwin WS. CAR and PXR: xenosensors of endocrine disrupters? Chem Biol. Interact. 2005;155:111–128. doi: 10.1016/j.cbi.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 72.Lemaire G, Mnif W, Pascussi JM, Pillon A, Rabenoelina F, Fenet H, Gomez E, Casellas C, Nicolas JC, Cavailles V, Duchesne MJ, Balaguer P. Identification of new human pregnane X receptor ligands among pesticides using a stable reporter cell system. Toxicol. Sci. 2006;91:501–509. doi: 10.1093/toxsci/kfj173. [DOI] [PubMed] [Google Scholar]
- 73.Masuyama H, Hiramatsu Y, Kunitomi M, Kudo T, MacDonald PN. Endocrine disrupting chemicals, phthalic acid and nonylphenol, activate Pregnane X receptor-mediated transcription. Mol. Endocrinol. 2000;14:421–428. doi: 10.1210/mend.14.3.0424. [DOI] [PubMed] [Google Scholar]
- 74.Medina-Diaz IM, Arteaga-Illan G, de Leon MB, Cisneros B, Sierra-Santoyo A, Vega L, Gonzalez FJ, Elizondo G. Pregnane X receptor-dependent induction of the CYP3A4 gene by o, p′-1,1,1,-trichloro-2,2-bis (p-chlorophenyl) ethane. Drug Metab. Dispos. 2007;35:95–102. doi: 10.1124/dmd.106.011759. [DOI] [PubMed] [Google Scholar]
- 75.Mikamo E, Harada S, Nishikawa J, Nishihara T. Endocrine disruptors induce cytochrome P450 by affecting transcriptional regulation via pregnane X receptor. Toxicol. Appl. Pharmacol. 2003;193:66–72. doi: 10.1016/j.taap.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 76.Mnif W, Pascussi JM, Pillon A, Escande A, Bartegi A, Nicolas JC, Cavailles V, Duchesne MJ, Balaguer P. Estrogens and antiestrogens activate hPXR. Toxicol. Lett. 2007;170:19–29. doi: 10.1016/j.toxlet.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 77.Takeshita A, Koibuchi N, Oka J, Taguchi M, Shishiba Y, Ozawa Y. Bisphenol-A, an environmental estrogen, activates the human orphan nuclear receptor, steroid and xenobiotic receptor-mediated transcription. Eur. J. Endocrinol./European Federation of Endocrine Societies. 2001;145:513–517. doi: 10.1530/eje.0.1450513. [DOI] [PubMed] [Google Scholar]
- 78.Wyde ME, Bartolucci E, Ueda A, Zhang H, Yan B, Negishi M, You L. The environmental pollutant 1,1-dichloro-2,2-bis (p-chlorophenyl)ethylene induces rat hepatic cytochrome P450 2B and 3A expression through the constitutive androstane receptor and pregnane X receptor. Mol. Pharmacol. 2003;64:474–481. doi: 10.1124/mol.64.2.474. [DOI] [PubMed] [Google Scholar]
- 79.Wyde ME, Kirwan SE, Zhang F, Laughter A, Hoffman HB, Bartolucci-Page E, Gaido KW, Yan B, You L. Di-n-butyl phthalate activates constitutive androstane receptor and pregnane X receptor and enhances the expression of steroid-metabolizing enzymes in the liver of rat fetuses. Toxicol. Sci. 2005;86:281–290. doi: 10.1093/toxsci/kfi204. [DOI] [PubMed] [Google Scholar]
- 80.Yang D, Wang X, Chen YT, Deng R, Yan B. Pyrethroid insecticides: isoform-dependent hydrolysis, induction of cytochrome P450 3A4 and evidence on the involvement of the pregnane X receptor. Toxicol. Appl. Pharmacol. 2009;237:49–58. doi: 10.1016/j.taap.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dairkee SH, Seok J, Champion S, Sayeed A, Mindrinos M, Xiao W, Davis RW, Goodson WH. Bisphenol A induces a profile of tumor aggressiveness in high-risk cells from breast cancer patients. Cancer Res. 2008;68:2076–2080. doi: 10.1158/0008-5472.CAN-07-6526. [DOI] [PubMed] [Google Scholar]
- 82.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.LaPensee EW, Ben-Jonathan N. Novel roles of prolactin and estrogens in breast cancer: resistance to chemotherapy. Endocr. Relat. Cancer. 2010;17:R91–107. doi: 10.1677/ERC-09-0253. [DOI] [PubMed] [Google Scholar]
- 84.LaPensee EW, LaPensee CR, Fox S, Schwemberger S, Afton S, Ben-Jonathan N. Bisphenol A and estradiol are equipotent in antagonizing cisplatin-induced cytotoxicity in breast cancer cells. Cancer Lett. 2010;290:167–173. doi: 10.1016/j.canlet.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takeshita A, Inagaki K, Igarashi-Migitaka J, Ozawa Y, Koibuchi N. The endocrine disrupting chemical, diethylhexyl phthalate, activates MDR1 gene expression in human colon cancer LS174T cells. J. Endocrinol. 2006;190:897–902. doi: 10.1677/joe.1.06664. [DOI] [PubMed] [Google Scholar]
- 86.Xu X, Dailey AB, Talbott EO, Ilacqua VA, Kearney G, Asal NR. Associations of serum concentrations of organochlorine pesticides with breast cancer and prostate cancer in U.S. adults. Environ. Health Perspect. 2010;118:60–66. doi: 10.1289/ehp.0900919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu H, Zheng J, Xiao X, Zheng S, Dong K, Liu J, Wang Y. Environmental endocrine disruptors promote invasion and metastasis of SK-N-SH human neuroblastoma cells. Oncol. Rep. 2010;23:129–139. [PubMed] [Google Scholar]
- 88.Soto AM, Sonnenschein C. Environmental causes of cancer: endocrine disruptors as carcinogens. Nat. Rev. Endocrinol. 2010;6:363–370. doi: 10.1038/nrendo.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lichti-Kaiser K, Xu C, Staudinger JL. Cyclic AMP-dependent protein kinase signaling modulates pregnane X receptor activity in a species-specific manner. J. Biol. Chem. 2009;284:6639–6649. doi: 10.1074/jbc.M807426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pondugula SR, Dong H, Chen T. Phosphorylation and protein–protein interactions in PXR-mediated CYP3A repression. Exp. Opin. Drug Metab. Toxicol. 2009;5:861–873. doi: 10.1517/17425250903012360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma X, Shah Y, Cheung C, Guo GL, Feigenbaum L, Krausz KW, Idle JR, Gonzalez FJ. The PREgnane X receptor gene-humanized mouse: a model for investigating drug–drug interactions mediated by cytochromes P450 3A. Drug Metab. Dispos. 2007;35:194–200. doi: 10.1124/dmd.106.012831. [DOI] [PubMed] [Google Scholar]
- 92.Moore LB, Maglich JM, McKee DD, Wisely B, Willson TM, Kliewer SA, Lambert MH, Moore JT. Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol. Endocrinol. 2002;16:977–986. doi: 10.1210/mend.16.5.0828. [DOI] [PubMed] [Google Scholar]
- 93.Gordon I, Paoloni M, Mazcko C, Khanna C. The Comparative Oncology Trials Consortium: using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLoS Med. 2009;6:e1000161. doi: 10.1371/journal.pmed.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khanna C, Lindblad-Toh K, Vail D, London C, Bergman P, Barber L, Breen M, Kitchell B, McNeil E, Modiano JF, Niemi S, Comstock KE, Ostrander E, Westmoreland S, Withrow S. The dog as a cancer model. Nat. Biotechnol. 2006;24:1065–1066. doi: 10.1038/nbt0906-1065b. [DOI] [PubMed] [Google Scholar]
- 95.Ao A, Wang H, Kamarajugadda S, Lu J. Involvement of estrogen-related receptors in transcriptional response to hypoxia and growth of solid tumors. Proc. Nat. Acad. Sci. USA. 2008;105:7821–7826. doi: 10.1073/pnas.0711677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stein RA, Chang CY, Kazmin DA, Way J, Schroeder T, Wergin M, Dewhirst MW, McDonnell DP. Estrogen-related receptor alpha is critical for the growth of estrogen receptor-negative breast cancer. Cancer Res. 2008;68:8805–8812. doi: 10.1158/0008-5472.CAN-08-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Birrell MA, De Alba J, Catley MC, Hardaker E, Wong S, Collins M, Clarke DL, Farrow SN, Willson TM, Collins JL, Belvisi MG. Liver X receptor agonists increase airway reactivity in a model of asthma via increasing airway smooth muscle growth. J. Immunol. 2008;181:4265–4271. doi: 10.4049/jimmunol.181.6.4265. [DOI] [PubMed] [Google Scholar]
- 98.Garcia-Aragoncillo E, Carrillo J, Lalli E, Agra N, Gomez-Lopez G, Pestana A, Alonso J. DAX1, a direct target of EWS/FLI1 oncoprotein, is a principal regulator of cell-cycle progression in Ewing’s tumor cells. Oncogene. 2008;27:6034–6043. doi: 10.1038/onc.2008.203. [DOI] [PubMed] [Google Scholar]
- 99.Martinez-Gonzalez J, Rius J, Castello A, Cases-Langhoff C, Badimon L. Neuron-derived orphan receptor-1 (NOR-1) modulates vascular smooth muscle cell proliferation. Circ. Res. 2003;92:96–103. doi: 10.1161/01.es.0000050921.53008.47. [DOI] [PubMed] [Google Scholar]
- 100.Baskin-Bey ES, Huang W, Ishimura N, Isomoto H, Bronk SF, Braley K, Craig RW, Moore DD, Gores GJ. Constitutive androstane receptor (CAR) ligand, TCPOBOP, attenuates Fas-induced murine liver injury by altering Bcl-2 proteins. Hepatology. 2006;44:252–262. doi: 10.1002/hep.21236. [DOI] [PubMed] [Google Scholar]
- 101.Huang W, Zhang J, Washington M, Liu J, Parant JM, Lozano G, Moore DD. Xenobiotic stress induces hepatomegaly and liver tumors via the nuclear receptor constitutive androstane receptor. Mol. Endocrinol. 2005;19:1646–1653. doi: 10.1210/me.2004-0520. [DOI] [PubMed] [Google Scholar]
- 102.Oliver JD, Roberts RA. Receptor-mediated hepatocarcinogenesis: role of hepatocyte proliferation and apoptosis. Pharmacol. Toxicol. 2002;91:1–7. doi: 10.1034/j.1600-0773.2002.910101.x. [DOI] [PubMed] [Google Scholar]
- 103.Yamamoto Y, Negishi M. The antiapoptotic factor growth arrest and DNA-damage-inducible 45 beta regulates the nuclear receptor constitutive active/androstane receptor-mediated transcription. Drug Metab. Dispos. 2008;36:1189–1193. doi: 10.1124/dmd.108.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen YL, Jian MH, Lin CC, Kang JC, Chen SP, Lin PC, Hung PJ, Chen JR, Chang WL, Lin SZ, Harn HJ. The induction of orphan nuclear receptor Nur77 expression by n-butylenephthalide as pharmaceuticals on hepatocellular carcinoma cell therapy. Mol. Pharmacol. 2008;74:1046–1058. doi: 10.1124/mol.107.044800. [DOI] [PubMed] [Google Scholar]
- 105.Kolluri SK, Zhu X, Zhou X, Lin B, Chen Y, Sun K, Tian X, Town J, Cao X, Lin F, Zhai D, Kitada S, Luciano F, O’Donnell E, Cao Y, He F, Lin J, Reed JC, Satterthwait AC, Zhang XK. A short Nur77-derived peptide converts Bcl-2 from a protector to a killer. Cancer Cell. 2008;14:285–298. doi: 10.1016/j.ccr.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Botrugno OA, Fayard E, Annicotte JS, Haby C, Brennan T, Wendling O, Tanaka T, Kodama T, Thomas W, Auwerx J, Schoonjans K. Synergy between LRH-1 and beta-catenin induces G1 cyclin-mediated cell proliferation. Mol. Cell. 2004;15:499–509. doi: 10.1016/j.molcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 107.He N, Park K, Zhang Y, Huang J, Lu S, Wang L. Epigenetic inhibition of nuclear receptor small heterodimer partner is associated with and regulates hepatocellular carcinoma growth. Gastroenterology. 2008;134:793–802. doi: 10.1053/j.gastro.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 108.Chearwae W, Bright JJ. PPARgamma agonists inhibit growth and expansion of CD133+ brain tumour stem cells. Br. J. Cancer. 2008;99:2044–2053. doi: 10.1038/sj.bjc.6604786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Elrod HA, Sun SY. PPARgamma and apoptosis in cancer. PPAR Res. 2008;2008:704165. doi: 10.1155/2008/704165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garcia-Bates TM, Bernstein SH, Phipps RP. Peroxisome proliferator-activated receptor gamma overexpression suppresses growth and induces apoptosis in human multiple myeloma cells. Clin. Cancer Res. 2008;14:6414–6425. doi: 10.1158/1078-0432.CCR-08-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nagata D, Yoshihiro H, Nakanishi M, Naruyama H, Okada S, Ando R, Tozawa K, Kohri K. Peroxisome proliferator-activated receptor-gamma and growth inhibition by its ligands in prostate cancer. Cancer Detect. Prev. 2008;32:259–266. doi: 10.1016/j.cdp.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 112.Swales KE, Korbonits M, Carpenter R, Walsh DT, Warner TD, Bishop-Bailey D. The farnesoid X receptor is expressed in breast cancer and regulates apoptosis and aromatase expression. Cancer Res. 2006;66:10120–10126. doi: 10.1158/0008-5472.CAN-06-2399. [DOI] [PubMed] [Google Scholar]
- 113.Yu S, Wong YC, Wang XH, Ling MT, Ng CF, Chen S, Chan FL. Orphan nuclear receptor estrogen-related receptor-beta suppresses in vitro and in vivo growth of prostate cancer cells via p21(WAF1/CIP1) induction and as a potential therapeutic target in prostate cancer. Oncogene. 2008;27:3313–3328. doi: 10.1038/sj.onc.1210986. [DOI] [PubMed] [Google Scholar]
- 114.Yu S, Wang X, Ng CF, Chen S, Chan FL. ERRgamma suppresses cell proliferation and tumor growth of androgen-sensitive and androgen-insensitive prostate cancer cells and its implication as a therapeutic target for prostate cancer. Cancer Res. 2007;67:4904–4914. doi: 10.1158/0008-5472.CAN-06-3855. [DOI] [PubMed] [Google Scholar]
- 115.Gong H, Guo P, Zhai Y, Zhou J, Uppal H, Jarzynka MJ, Song WC, Cheng SY, Xie W. Estrogen deprivation and inhibition of breast cancer growth in vivo through activation of the orphan nuclear receptor liver X receptor. Mol. Endocrinol. 2007;21:1781–1790. doi: 10.1210/me.2007-0187. [DOI] [PubMed] [Google Scholar]
- 116.Kolluri SK, Bruey-Sedano N, Cao X, Lin B, Lin F, Han YH, Dawson MI, Zhang XK. Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol. Cell. Biol. 2003;23:8651–8667. doi: 10.1128/MCB.23.23.8651-8667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mu X, Chang C. TR3 orphan nuclear receptor mediates apoptosis through up-regulating E2F1 in human prostate cancer LNCaP cells. J. Biol. Chem. 2003;278:42840–42845. doi: 10.1074/jbc.M305594200. [DOI] [PubMed] [Google Scholar]
- 118.Zeng L, Geng Y, Tretiakova M, Yu X, Sicinski P, Kroll TG. Peroxisome proliferator-activated receptor-delta induces cell proliferation by a cyclin E1-dependent mechanism and is up-regulated in thyroid tumors. Cancer Res. 2008;68:6578–6586. doi: 10.1158/0008-5472.CAN-08-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Borland MG, Foreman JE, Girroir EE, Zolfaghari R, Sharma AK, Amin S, Gonzalez FJ, Ross AC, Peters JM. Ligand activation of peroxisome proliferator-activated receptor-beta/delta inhibits cell proliferation in human HaCaT keratinocytes. Mol. Pharmacol. 2008;74:1429–1442. doi: 10.1124/mol.108.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lamba V, Yasuda K, Lamba JK, Assem M, Davila J, Strom S, Schuetz EG. PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol. Appl. Pharmacol. 2004;199:251–265. doi: 10.1016/j.taap.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 121.Gardner-Stephen D, Heydel JM, Goyal A, Lu Y, Xie W, Lindblom T, Mackenzie P, Radominska-Pandya A. Human PXR variants and their differential effects on the regulation of human UDP-glucuronosyltransferase gene expression. Drug Metab. Dispos. 2004;32:340–347. doi: 10.1124/dmd.32.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tompkins LM, Sit TL, Wallace AD. Unique transcription start sites and distinct promoter regions differentiate the pregnane X receptor (PXR) isoforms PXR 1 and PXR 2. Drug Metab. Dispos. 2008;36:923–929. doi: 10.1124/dmd.107.018317. [DOI] [PubMed] [Google Scholar]
- 123.Lin YS, Yasuda K, Assem M, Cline C, Barber J, Li CW, Kholodovych V, Ai N, Chen JD, Welsh WJ, Ekins S, Schuetz EG. The major human pregnane X receptor (PXR) splice variant, PXR.2, exhibits significantly diminished ligand-activated transcriptional regulation. Drug Metab. Dispos. 2009;37:1295–1304. doi: 10.1124/dmd.108.025213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Naor D, Nedvetzki S, Golan I, Melnik L, Faitelson Y. CD44 in cancer. Crit. Rev. Clin. Lab. Sci. 2002;39:527–579. doi: 10.1080/10408360290795574. [DOI] [PubMed] [Google Scholar]
- 125.Matic M, Corradin AP, Tsoli M, Clarke SJ, Polly P, Robertson GR. The alternatively spliced murine pregnane X receptor isoform, mPXR(delta171-211) exhibits a repressive action. Int. J. Biochem. Cell Biol. 2010;42:672–682. doi: 10.1016/j.biocel.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 126.Biswas A, Pasquel D, Tyagi RK, Mani S. Acetylation of pregnane X receptor protein determines selective function independent of ligand activation. Biochem. Biophys. Res. Commun. 2011;406:371–376. doi: 10.1016/j.bbrc.2011.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pondugula SR, Brimer-Cline C, Wu J, Schuetz EG, Tyagi RK, Chen T. A phosphomimetic mutation at threonine-57 abolishes transactivation activity and alters nuclear localization pattern of human pregnane×receptor. Drug Metab. Dispos. 2009;37:719–730. doi: 10.1124/dmd.108.024695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Staudinger JL, Xu C, Biswas A, Mani S. Post-translational modification of pregnane×receptor. Pharmacol. Res.: Off. J. Ital. Pharmacol. Soc. 2011;64:4–10. doi: 10.1016/j.phrs.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gramatzki D, Pantazis G, Schittenhelm J, Tabatabai G, Kohle C, Wick W, Schwarz M, Weller M, Tritschler I. Aryl hydrocarbon receptor inhibition downregulates the TGF-beta/Smad pathway in human glioblastoma cells. Oncogene. 2009;28:2593–2605. doi: 10.1038/onc.2009.104. [DOI] [PubMed] [Google Scholar]
- 130.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 131.Rhodes JM, Campbell BJ. Inflammation and colorectal cancer: IBD-associated and sporadic cancer compared. Trends Mol. Med. 2002;8:10–16. doi: 10.1016/s1471-4914(01)02194-3. [DOI] [PubMed] [Google Scholar]
- 132.Viennot S, Deleporte A, Moussata D, Nancey S, Flourie B, Reimund JM. Colon cancer in inflammatory bowel disease: recent trends, questions and answers. Gastroenterol. Clin. Biol. 2009;33(Suppl. 3):S190–S201. doi: 10.1016/S0399-8320(09)73154-9. [DOI] [PubMed] [Google Scholar]
- 133.Gao M, Guo N, Huang C, Song L. Diverse roles of GADD45alpha in stress signaling. Curr. Protein Pept. Sci. 2009;10:388–394. doi: 10.2174/138920309788922216. [DOI] [PubMed] [Google Scholar]
- 134.Prochownik EV. C-Myc: linking transformation and genomic instability. Curr. Mol. Med. 2008;8:446–458. doi: 10.2174/156652408785747988. [DOI] [PubMed] [Google Scholar]
- 135.Zhang Y, Chen L, Yang S, Fang D. E2F1: a potential negative regulator of hTERT transcription in normal cells upon activation of oncogenic c-Myc. Med. Sci. Monit.: Int. Med. J. Exp. Clin. Res. 2012;18:RA12–RA15. doi: 10.12659/MSM.882192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bluemn EG, Nelson PS. The androgen/androgen receptor axis in prostate cancer. Curr. Opin. Oncol. 2012;24:251–257. doi: 10.1097/CCO.0b013e32835105b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lebovitz CB, Bortnik SB, Gorski SM. Here, there be dragons: charting autophagy-related alterations in human tumors. Clin. Cancer Res. 2012;18:1214–1226. doi: 10.1158/1078-0432.CCR-11-2465. [DOI] [PubMed] [Google Scholar]
- 138.Reimann M, Schmitt CA, Lee S. Non-cell-autonomous tumor suppression: oncogene-provoked apoptosis promotes tumor cell senescence via stromal crosstalk. J. Mol. Med. (Berl.) 2011;89:869–875. doi: 10.1007/s00109-011-0770-2. [DOI] [PubMed] [Google Scholar]
- 139.Saab R. Senescence and pre-malignancy: how do tumors progress? Semin Cancer Biol. 2011;21:385–391. doi: 10.1016/j.semcancer.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 140.Wang YM, Ong SS, Chai SC, Chen T. Role of CAR and PXR in xenobiotic sensing and metabolism. Exp. Opin. Drug Metab. Toxicol. 2012;8:803–817. doi: 10.1517/17425255.2012.685237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bowlby SC, Thomas MJ, D’Agostino RB, Jr, Kridel SJ. Nicotinamide phosphoribosyl transferase (nampt) is required for de novo lipogenesis in tumor cells. PLoS ONE. 2012;7:e40195. doi: 10.1371/journal.pone.0040195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, Van Veldhoven PP, Waltregny D, Daniels VW, Machiels J, Vanderhoydonc F, Smans K, Waelkens E, Verhoeven G, Swinnen JV. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70:8117–8126. doi: 10.1158/0008-5472.CAN-09-3871. [DOI] [PubMed] [Google Scholar]
- 143.Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:358–365. doi: 10.1097/01.mco.0000232894.28674.30. [DOI] [PubMed] [Google Scholar]
- 144.Hall AP, Elcombe CR, Foster JR, Harada T, Kaufmann W, Knipple A, Kutttler K, Malarkey DE, Maronpot RR, Nishikawa A, Nolte T, Schulte A, Strauss V, York MJ. Liver Hypertrophy: A Review of Adaptive (Adverse and Non-adverse) Changes–Conclusions from the 3rd International ESTP Expert Workshop. Toxicol. Pathol. 2012 doi: 10.1177/0192623312448935. [DOI] [PubMed] [Google Scholar]
- 145.van de Winkel A, Menke V, Capello A, Moons LM, Pot RG, van Dekken H, Siersema PD, Kusters JG, van der Laan LJ, Kuipers EJ. Expression, localization and polymorphisms of the nuclear receptor PXR in Barrett’s esophagus and esophageal adenocarcinoma. BMC Gastroenterol. 2011;11:108. doi: 10.1186/1471-230X-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhuang W, Jia Z, Feng H, Chen J, Wang H, Guo Y, Meng C. The mechanism of the G0/G1 cell cycle phase arrest induced by activation of PXR in human cells. Biomed. Pharmacother = Biomedecine & pharmacotherapie. 2011;65:467–473. doi: 10.1016/j.biopha.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 147.Chen T. Nuclear receptor drug discovery. Curr. Opin. Chem. Biol. 2008;12:418–426. doi: 10.1016/j.cbpa.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 148.Chen Y, Tang Y, Robbins GT, Nie D. Camptothecin attenuates cytochrome P450 3A4 induction by blocking the activation of human pregnane X receptor. J. Pharmacol. Exp. Ther. 2010;334:999–1008. doi: 10.1124/jpet.110.168294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Healan-Greenberg C, Waring JF, Kempf DJ, Blomme EA, Tirona RG, Kim RB. A human immunodeficiency virus protease inhibitor is a novel functional inhibitor of human pregnane X receptor. Drug Metab. Dispos. 2008;36:500–507. doi: 10.1124/dmd.107.019547. [DOI] [PubMed] [Google Scholar]
- 150.Huang H, Wang H, Sinz M, Zoeckler M, Staudinger J, Redinbo MR, Teotico DG, Locker J, Kalpana GV, Mani S. Inhibition of drug metabolism by blocking the activation of nuclear receptors by ketoconazole. Oncogene. 2007;26:258–268. doi: 10.1038/sj.onc.1209788. [DOI] [PubMed] [Google Scholar]
- 151.Venkatesh M, Wang H, Cayer J, Leroux M, Salvail D, Das B, Wrobel JE, Mani S. In vivo and in vitro characterization of a first-in-class novel azole analog that targets pregnane X receptor activation. Mol. Pharmacol. 2011;80:124–135. doi: 10.1124/mol.111.071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang H, Huang H, Li H, Teotico DG, Sinz M, Baker SD, Staudinger J, Kalpana G, Redinbo MR, Mani S. Activated pregnenolone X-receptor is a target for ketoconazole and its analogs. Clin. Cancer Res. 2007;13:2488–2495. doi: 10.1158/1078-0432.CCR-06-1592. [DOI] [PubMed] [Google Scholar]
- 153.Wang H, Li H, Moore LB, Johnson MD, Maglich JM, Goodwin B, Ittoop OR, Wisely B, Creech K, Parks DJ, Collins JL, Willson TM, Kalpana GV, Venkatesh M, Xie W, Cho SY, Roboz J, Redinbo M, Moore JT, Mani S. The phytoestrogen coumestrol is a naturally occurring antagonist of the human pregnane X receptor. Mol. Endocrinol. 2008;22:838–857. doi: 10.1210/me.2007-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhou C, Poulton EJ, Grun F, Bammler TK, Blumberg B, Thummel KE, Eaton DL. The dietary isothiocyanate sulforaphane is an antagonist of the human steroid and xenobiotic nuclear receptor. Mol. Pharmacol. 2007;71:220–229. doi: 10.1124/mol.106.029264. [DOI] [PubMed] [Google Scholar]