Abstract
Transient brain hypoxia-ischemia (HI) in neonates leads to delayed neuronal death and long-term neurological deficits. However, the underlying mechanisms are incompletely understood. Calcium-calmodulin-dependent protein kinase II (CaMKII) is one of the most abundant protein kinases in neurons and plays crucial roles in synaptic development and plasticity. This study used a neonatal brain HI model to investigate whether and how CaMKII was altered after HI and how the changes were affected by brain development. Expression of CaMKII was markedly up-regulated during brain development. After HI, CaMKII was totally and permanently depleted from the cytosol and concomitantly deposited into a Triton-insoluble fraction in neurons that were undergoing delayed neuronal death. Autophosphorylation of CaMKII-Thr286 transiently increased at 30 min of reperfusion and declined thereafter. All these changes were mild in P7 pups but more dramatic in P26 rats, consistent with the development- dependent CaMKII expression in neurons. The results suggest that long-term CaMKII depletion from the cytosolic fraction and deposition into the Triton-insoluble fraction may disable synaptic development, damage synaptic plasticity, and contribute to delayed neuronal death and long-term synaptic deficits after transient HI.
Keywords: brain development, CaMKII, neonatal hypoxia-ischemia, neuronal death, synaptic plasticity
A brief period of brain hypoxia-ischemia (HI) in neonates is able to induce delayed neuronal death and long-term neurological deficits. It is generally held that energy failure, increase in intracellular calcium and overproduction of reactive oxygen species play major roles in cell death for both immature and mature brains after HI (Hagberg 1992; Siesjö et al. 1999; Johnston et al. 2002). However, the mechanisms underlying neuronal death and neurological deficits after HI between immature and mature brains are far from identical (Hu et al. 2000; Johnston et al. 2002; Blomgren et al. 2003). First of all, immature neurons are significantly less vulnerable to hypoxia-ischemia than mature neurons, which has been attributed to the maturation of neuronal circuits (Johnston et al. 2002). Apoptosis is much more prominent in developing neurons than in adult neurons after HI (Hu et al. 2000; Gill et al. 2002; Liu et al. 2004a). The capacity to recover, or neuroplasticity after HI, appears much more robust in immature than in mature brains (Johnston 2004). Although progress has been made, it remains incompletely understood why brain maturation dictates HI outcome (Hu et al. 2000; Johnston et al. 2002; Blomgren et al. 2003; Liu et al. 2004a).
A striking role of CaMKII in the regulation of synapto-genesis and plasticity during development has been well recognized (Silva et al. 1992; Rongo and Kaplan 1999; Kennedy 2000; Frankland et al. 2001; Jourdain et al. 2003). Four distinct CaMKII subunit genes (α, β, γ and δ) have been discovered and they form various CaMKII subtypes. The α and β subunits are neuron-specific, constitute about 2% of the protein in mature brain and are highly enriched in postsynaptic densities (PSDs) (Kennedy 2000). Autophosphorylation of CaMKII-Thr286 leads to the generation of a long-lasting, Ca2+-independent autonomous activity and to its translocation from cytoplasm to PSDs (Lai et al. 1986; Molloy and Kennedy 1991; Hanson and Schulman 1992; Hudmon and Schulman 2002). Expression of both alpha and beta subunits of CaMKII is strictly developmentally dependent. The CaMKII-α and -β proteins are at very low level in the early post-natal days, markedly increase at post-natal day 10 and reach a plateau at post-natal day 30 in soluble and particulate fractions of rat forebrain (Sugiura and Yamauchi 1992). The developmental up-regulation of CaMKII correlates well with synaptogenesis during development (Taha et al. 2002).
Alterations of both CaMKIIα mRNA and protein in adult brains are observed in mature brains after ischemia (Aronowski et al. 1992; Churn et al. 1992; Morioka et al. 1992; Hu and Wieloch 1994; Hu et al. 1995; Shackelford et al. 1995; Waxham et al. 1996; Mengesdorf et al. 2002; Katsura et al. 2003). However, changes in CaMKII after injury in the developing brain have not been studied. Because CaMKII is a key molecule in regulation of brain synapse development, we studied whether and how CaMKII was altered after HI in brains as a function of developmental stage. We found that CaMKII was markedly altered after HI as a function of brain development.
Materials and methods
Materials
Leupeptin, pepstatin, aprotinin and propidium iodide were purchased from Sigma (St Louis, MO, USA). A monoclonal antibody to CaMKIIα was purchased from Affinity Bioreagents (Golden, CO, USA). Polyclonal antibodies to CaMKIIα and phospho-CaMKIIThr286 and to active caspase-3 were obtained from Cell Signaling Technology (Beverly, MA, USA). Fluorescein-labeled anti-mouse and lissamine rhodamine-labeled anti-rabbit IgG were purchased from Jackson Immuno-Research (West Grove, PA, USA). Peroxidase- linked secondary antibodies were purchased from Amersham (Piscataway, NJ, USA).
Hypoxia-ischemia animal model
Pregnant Sprague-Dawley rats were purchased from Charles River Laboratories Inc. (Wilmington, MA, USA) and housed in individual cages. The newborn rats were housed with their dams until weaning at post-natal day 21. All procedures for animal studies were approved by the Animal Care and Use Committee of the University of Miami. Brain hypoxia-ischemia (HI) was produced by a combination of ligation of the left common carotid artery (CCA) and systemic hypoxia (8% O2) (Rice et al. 1981). Briefly, post-natal rats at 7 (P7), 15 (P15) and 26 (P26) days were anesthetized with halothane (4% induction and 1% maintenance). A midline neck incision was made; thereafter the left CCA was exposed and ligated permanently. The incision was sutured and the rats were returned to their dams or original cages. Following 1 h of recovery, the rats were placed in a hypoxic chamber through which humidified 8% oxygen with the balance nitrogen flowed for 60 min for the P7 and P15 rat pup groups, and for 30 min in the P26 groups. The concentration of oxygen flowing through the hypoxic system was monitored with an Ohmeda 5120 Oxygen Monitor (Madison, WI, USA) during the experiments. The hypoxic duration in different groups of rats was chosen based on our previous experiments (Hu et al. 2000) and the literature, which showed that these periods produced a similar severity of brain damage in different age groups (Blumenfeld et al. 1992; Towfighi et al. 1997; Towfighi and Mauger 1998). The hypoxic chambers were partially submerged in a 36°C water bath to maintain normothermia during the HI periods. Under this condition, rat body temperature during HI was 37.0 ± 0.15 (n = 7). After the periods of HI, the animals were kept in the open chamber for about 30 min until they showed signs of recovery. They were then returned to their dams or cages. Sham-operated controls were subjected to the same procedures but without HI. In some cases, the contralateral (non-ischemic) sides of the brains after HI were also used as controls (Blumenfeld et al. 1992; Towfighi et al. 1997; Towfighi and Mauger 1998). The brains were collected at 30 min, 4, 12, 24 and 48 h after HI. The tissue samples for biochemical analyses were obtained by freezing the brain in situ with liquid nitrogen (Ponten et al. 1973). The rats were perfused with ice-cold 4% phosphate-buffered paraformaldehyde for confocal microscopy. The brains were sectioned with a Leica vibratome (Bensheim, Germany) at a thickness of 50 µm.
Confocal microscopy and histology
Double-label fluorescence confocal microscopy was performed on 50-µm coronal brain sections (Hu et al. 2000). The brain sections were from sham-operated control rats and rats subjected to HI followed by 30 min, 4, 24 and 48 h of recovery for all age groups. For confocal study, a monoclonal antibodies against CaMKIIα and active caspase-3 were obtained from Affinity Bioreagents (Golden, CO, USA) and from Cell Signaling Technology (Beverly, MA, USA), respectively. Brain sections were washed with phosphate-buffered saline (PBS) containing 0.1% Triton X100 (TX100) for 30 min. Non-specific binding sites were blocked in 3% BSA in PBS/0.1% TX100 for 30 min. The primary antibodies were diluted 1 : 400 in PBS/0.1% TX100 and 1% BSA. After incubation overnight at 4°C, the sections were washed in PBS containing 0.1% TX100, and were then incubated for 1 h at room temperature (25°C) with the fluorescent secondary antibodies, either fluorescein-labeled anti-rabbit or lissamine rhodamine-labeled anti-mouse diluted 1 : 200, or propidium iodide (PI) 15 µg/mL in PBS containing 1% BSA. Sections were washed several times in PBS and mounted on glass slides. The slides were analyzed on a Zeiss laser-scanning confocal microscope. Brain damage was also evaluated on brain sections stained with acid fuchsin and thionine according to the method of Auer et al. (1984). Most damaged neurons stained with fuchsin were shrunken and acidophilic.
Subcellular fractionation
Subcellular fractions were prepared from sham-operated brain tissues and from post-HI brain tissues. One sample was prepared from each pup/rat and four animals or four samples were used in each experimental group. Forebrain tissue blocks between bregma 1.70 mm and bregma −4.52 mm were collected and divided into ipsilateral and contralateral sides. Preparation of subcellular fractions was carried out either in an ice-bath or at 4°C. Brain tissue was cut into less than 1 mm3 small pieces and was homogenized with a Dounce homogenizer (25 strokes) in 10 vol. of ice-cold homogenization buffer containing 15 mm Tris base/ HCl pH 7.6, 1 mm dithiothreitol (DTT), 0.25 m sucrose, 1 mm MgCl2, 1 µg/mL pepstain A, 5 µg/mL leupeptin, 2.5 µg/mL aproptonin, 0.5 mm phenylmethylsulfonyl fluoride (PMSF), 2.5 mm EDTA, 1 mm EGTA, 0.25 m Na3VO4, 25 mm NaF and 2 mm sodium pyrophosphate. Part of the homogenate (H) was directly used for western blot analysis and the rest was further centrifuged at 800 g for 10 min to get the nuclear pellet (P1). The supernatant was then centrifuged at 10 000 g, for 10 min to obtain a crude pellet (P2) enriched with synaptosomes and mitochondria, and the resulting supernatant was further centrifuged at 165 000 g, for 1 h to get a cytosolic fraction (S3) and a microsomal fraction (P3) that contained intracellular membranes. The P2 fraction was suspended in an ice-cold homogenization buffer containing 1% TX100 and 300 mm KCl, sonicated three times each for 10 s, washed on a shaker for 1 h and then centrifuged at 10 000 g for 10 min to obtain the Triton-soluble (P2s) and -insoluble (P2p) fractions. The contents of these subcellular fractions were verified in many previous studies including our studies, demonstrating that P1 is a crude nuclear fraction, P2 contains enriched synapses and mirochondria, P3 is mainly composed of cytoskeletal proteins and crude intracellular membranes, and S3 is a cytosolic fraction (Hu and Wieloch 1994; Hu et al. 1995, 1998). Protein concentration was determined by a microbicinchoninic acid (BCA) method.
Western blot analysis
Western blot analysis was carried out on 8% sodium dodecyl sulfate – polyacrylamide gel electrophoresis (SDS–PAGE) as described previously (Hu et al. 1998). Samples for western blotting contained 25 µg of protein in the Triton-insoluble pellets (P2p) and 50 µg of protein in the Triton-soluble supernatants (P2s), 50 µg of protein in the cytosolic fraction (S3) and 50 µg of protein in P3. Four different samples prepared from four rats, one sample per rat, in each experimental group were analyzed statistically. Following electrophoresis, the proteins were transferred to an Immobilon-P membrane. The membranes were incubated overnight at 4°C with primary antibodies against CaMKIIα (1 : 5000) or phospho-CaMKII-Thr286 (1 : 1000). The membranes were then incubated with horseradish-peroxidase conjugated anti-mouse or anti-rabbit secondary antibodies for 1 h at room temperature. The blots were developed with an ECL detection method (Amersham). Mean optical intensities of immunoblot bands were evaluated with Kodak 1D image software. The data are expressed as percentage of control (mean ± SD, n = 4). One-way anova was employed to assess statistical significance (p < 0.05).
Results
HI-induced cell death
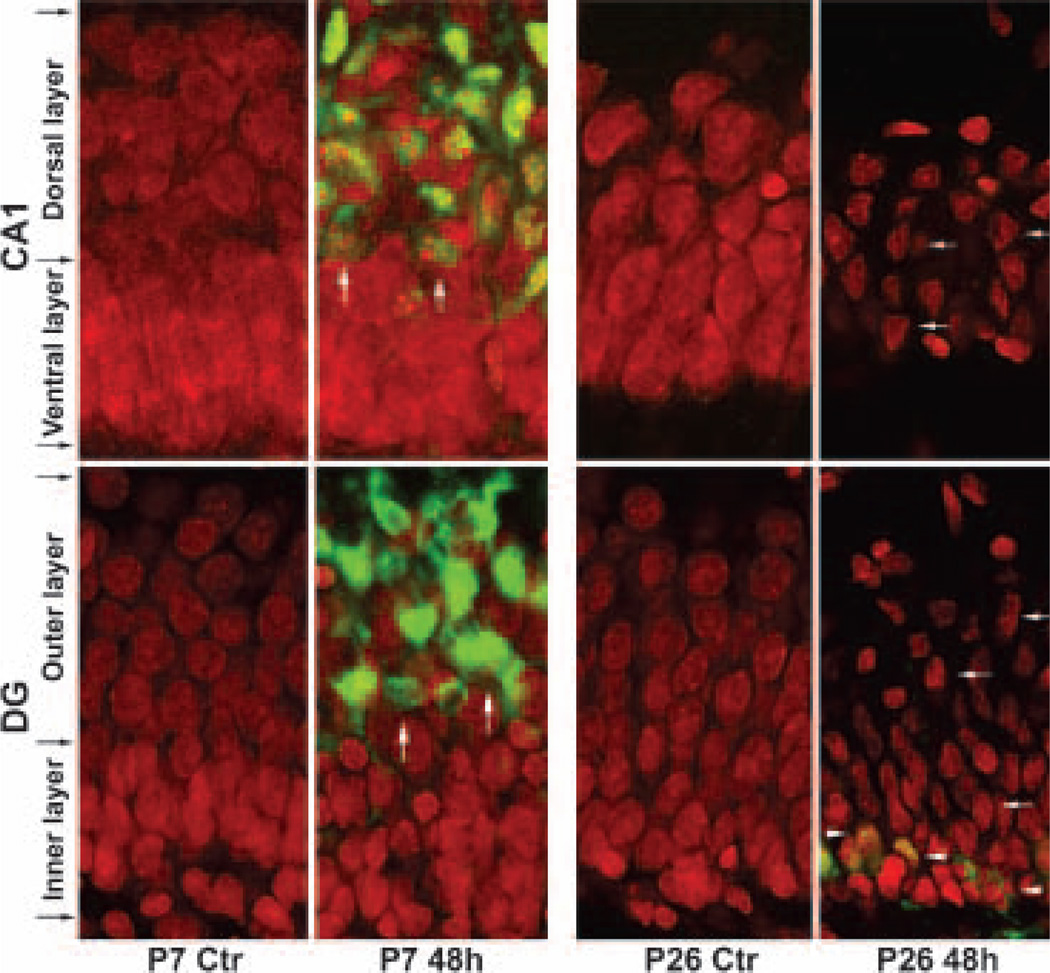
Brain sections were obtained from P7 and P26 rats subjected to either sham-operated surgery or HI followed by 48 h of recovery. The sections were double-labeled with propidium iodide (PI), together with an antibody specifically recognizing active caspase-3, and they were examined by confocal microscopy. Delayed neuronal death occurred in ischemic brain regions at about 24–48 h in both these age groups, consistent with previous reports (Blumenfeld et al. 1992; Towfighi et al. 1997; Towfighi and Mauger 1998; Hu et al. 2000; Liu et al. 2004a). In Fig. 1, the red color represents PI staining and the green color indicates active caspase-3. PI is a fluorescent dye that stains nucleic acids and is often utilized to stain necrotic cells in culture because it is unable to pass lipid membranes. In fixed brain sections, however, PI is able to penetrate into both normal and damaged cells to stain nucleic acids (Fig. 1). It appeared that there were two subpopulations of CA1 or DG neurons stained with PI in P7 sham-operated control animals; one subpopulation of neurons were densely packed in the ventral layer of the CA1 and the inner layer of the DG, and another subpopulation of neurons was loosely packed in the dorsal layer of the CA1 and the outer layer of the DG (Fig. 1, P7 Ctr, black arrows). Both subpopulations of neurons had sphere-shaped nuclei in sham-operated controls, but the nuclei were fragmented or damaged mainly in the dorsal layer of P7 CA1 and the outer layer of P7 DG neurons after HI (Fig. 1, P7 48 h, arrows). Consistently, the dorsal or outer layers of CA1 or DG neurons were highly positive for active caspase-3, indicating that caspase-3-mediated events took place in these neurons after HI (Fig. 1, P7 48 h, green color, arrows). In comparison with P7 pups, there were no differences in neuronal density in P26 CA1 and DG neuronal layers from sham-operated controls. After HI, P26 CA1 and DG neurons were severely damaged (Fig. 1, P26 48 h, arrows). However, unlike HI-injured P7 neurons that were fragmented and positive for active caspase-3, most HI-damaged P26 CA1 and DG neurons assumed a polygonal shape and were mostly negative for active caspase-3 (Fig. 1, P26 48 h, arrows). A few P26 cells in the bottom area of the DG inner layer were also positive for active caspase-3 after HI (Fig. 1, P26 48 h, arrowheads). This population of caspase-3 positive neurons was also seen in the subgranular zone of P7 pups after HI (Liu et al. 2004a). Although the lineage of these cells is uncertain, according to their distribution, these active caspase-3 positive cells might be the subgranular zone progenitor cells.
Fig. 1.
Confocal microscopic images of neurons labeled with anti-active caspase-3 antibody (green) and propidium iodide (PI, red) in hippocampal CA1 and the upper blade of DG. Brain sections were obtained from a sham-operated control rat (Ctr) or a rat subjected to 60 min of hypoxia-ischemia (HI) for P7 pups and 30 min of HI for P26 rats followed by 48 h of reperfusion. In P7 rat pups, PI stained two sublayers of CA1 and DG neurons from sham-operated controls. The CA1 dorsal layer and the DG outer layer appeared loosely packed, whereas the CA1 ventral layer and the DG inner layer were densely packed. After HI, PI-stained nuclei in the dorsal CA1 and outer DG sublayers were mostly fragmented and they were highly positive for active caspase-3 (green, white arrows), indicating that cell death took place in these neurons. In P26 rats, PI did not stain two sublayers of CA1 and DG neurons as in P7 pups. After HI, PI-stained nuclei were mostly polygon-shaped (white arrows), and mostly negative for active caspase-3. A few active caspase-3-positive cells were also found in the subgranular zone of P26 DG (arrowheads).
The pattern of hippocampal CaMKIIα-expressing neurons changes during development
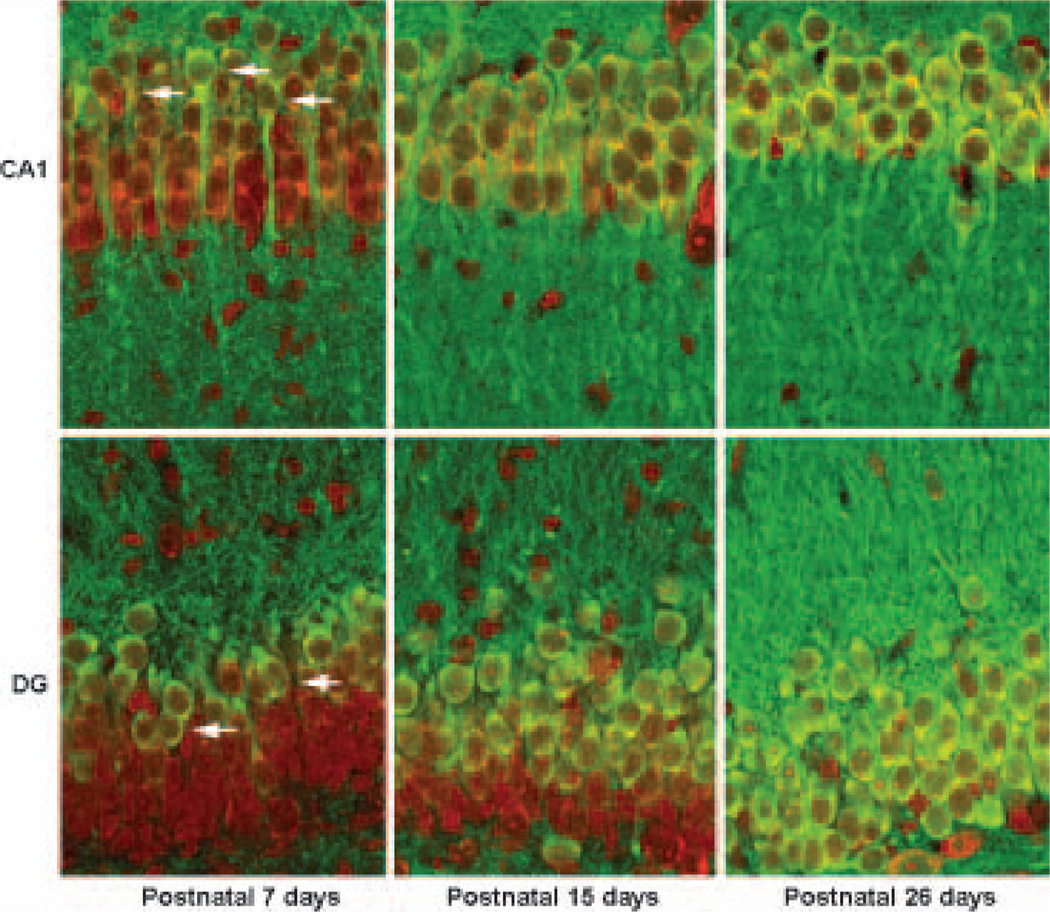
To investigate hippocampal regional CaMKIIα expression during development, we double-labeled brain sections with anti-CaMKIIα antibody and PI (Fig. 2). Brain sections were obtained from sham-control P7, P15 and P26 rats. Figure 2 shows that the neuronal somata of the CA1 dorsal and DG outer layer were labeled with both CaMKII in green and PI in red (Fig. 2, P7, arrows), whereas the neuronal somata of the CA1 ventral and DG inner layer were only labeled with PI in red (Fig. 2, P7), indicating that CaMKIIα protein (green color) was distributed mainly in the CA1 dorsal and DG outer layer neurons in P7 pups. In comparing P7 pups, the CaMKIIα-positive CA1 neurons expanded to the whole CA1 neuronal layer and to about two-thirds of the DG outer layer at P15 (Fig. 2, P15, green color). In P26 rats, virtually all CA1 and DG neuronal somata were positive for CaMKIIα (Fig. 2, P26, green color). Similarly, CaMKIIα immunoreactivity in the CA1 and DG neuropil regions was least in P7, moderate in P15 and strongest in P26 among the age groups studied (Fig. 2, CA1 and DG, green color). Quantitative analyses of development-dependent CaMKIIα expression on western blots are shown in Fig. 5(e) below.
Fig. 2.
Confocal microscopic images of hippocampal CA1 and DG neurons labeled with anti-CaMKIIα antibody (green) and PI (red). In these images, CaMKIIα-positive neurons were labeled in green and PI-stained nuclei in red, and the overlay of the green and red colors created yellow. Brain sections were obtained from sham-operated control rats. In post-natal 7 day rat pups, there were 6–7 layers of CA1 and DG neurons stained with PI (red), and CaMKIIα-positive neuronal soma were mainly located in the upper two to three CA1 and DG cell layers (arrows). In P15 rat pups, there were four to five layers of CA1 and DG neurons stained with PI (red). In P26 rats, there are three to four layers of CA1 and DG neurons stained with PI (red). Whereas only the DG outer layer was CaMKIIα-positive in P15 rats, virtually all CA1 neurons in P15 and P26 rats, and DG neurons in P26 rats, were positive for CaMKIIα (yellow).
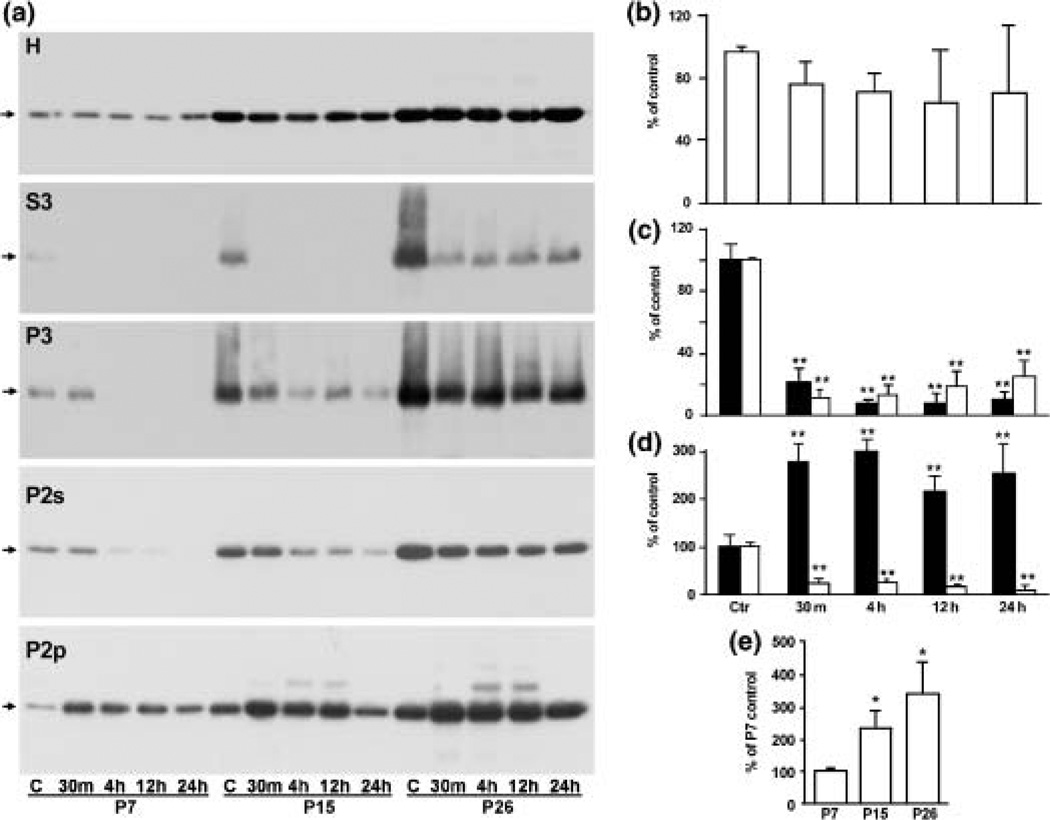
Fig. 5.
(a) Immunoblot analyses of CaMKII in the homogenates (H), microsomal (P3), cytosolic (S3) fractions, Triton-insoluble pellets (P2p) and Triton-insoluble supernatants (P2s) after HI. Samples were from rats at post-natal 7 days (P7), 15 days (P15) and 26 days (P26) subjected to either sham-operation (Ctr) or to 60 min of HI for P7 and P15 pups, and 30 min of HI for P26 rats, followed by 30 min, 4, 12 and 24 h of recovery, respectively. CaMKIIα tended to decrease in H, markedly decrease in S3, P3, and P2s, and concomitantly increase in P2p after HI. (b–d) Quantification of CaMKIIα protein levels in the P7 homogenate (b); P7 S3 (c, black column) and P7 P3 (c, white column); P7 P2p (d, black column) and P2s (d, white column) with Kodak 1D image software. Mean optical intensities of immunoblot bands are expressed as percentage of control (mean ± SD, n = 4). **p < 0.01 denotes statistically significant difference between control and post-HI conditions (one-way anova followed by Fischer’s PLSD post-hoc test). (e) Statistical analysis of development-dependent CaMKIIα changes in homogenates prepared from sham-operated control rats at P7, P15 and P26. Mean optical densities of immunoblot bands were quantified with Kodak 1D image software and expressed as percentage of P7 control group (mean ± SD, n = 4). **p < 0.01 indicates statistically significant difference among different post-natal age groups (one-way anova followed by Fischer’s PLSD post-hoc test).
Alterations of CaMKII after HI
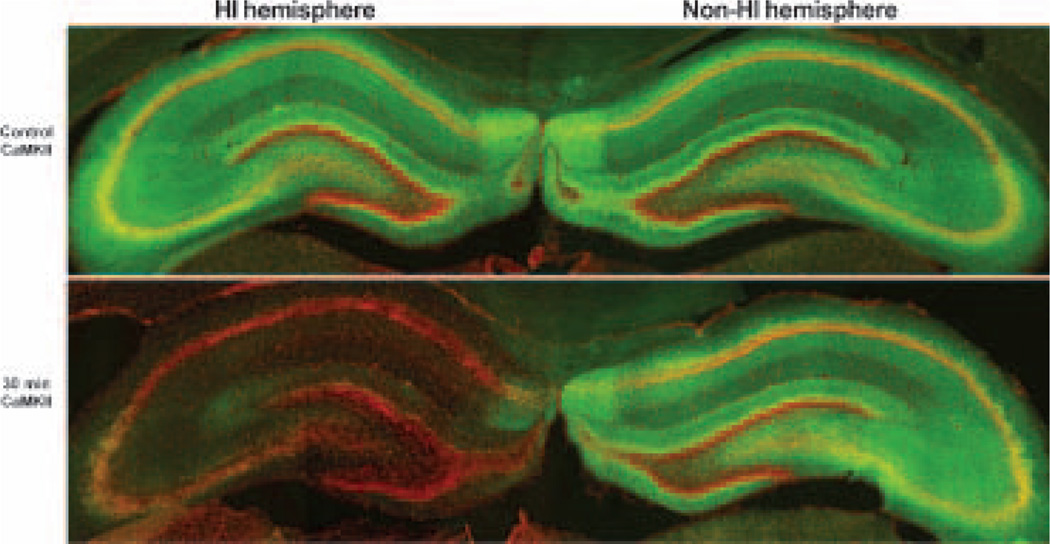
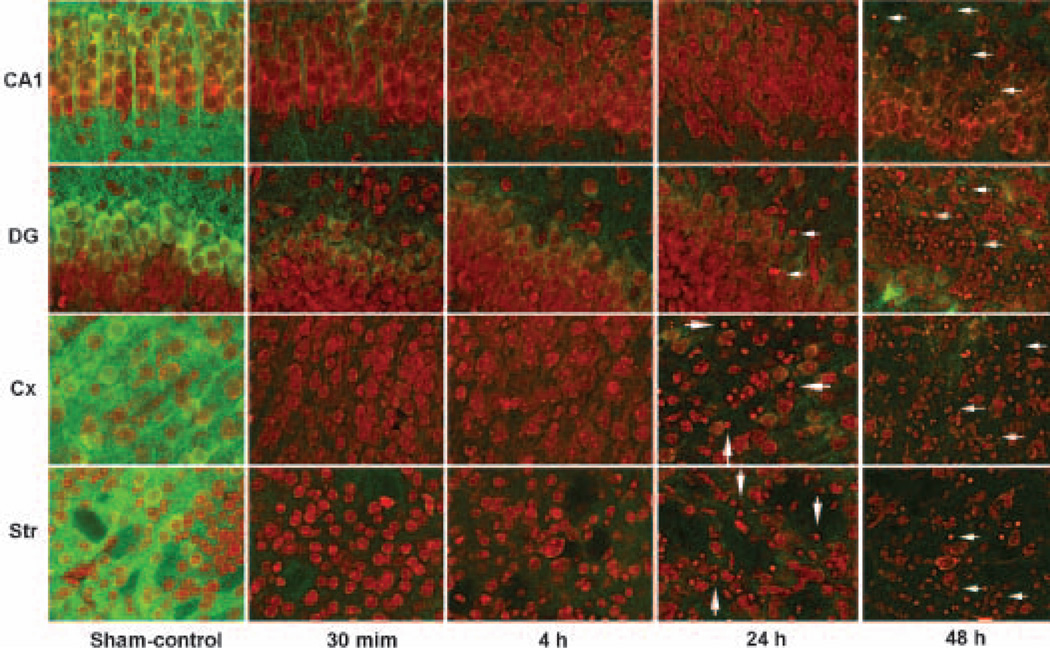
To study whether and how CaMKIIα is possibly altered after HI, we investigated CaMKIIα alterations in post-HI brains. Brain sections were obtained from P7 pups subjected to either sham-operated surgery or 60 min HI followed by 30 min, 4, 24 and 48 h of recovery. They were double-labeled with anti-CaMKIIα antibody and PI (Figs 3 and 4). CaMKIIα immunostaining was observed in the cell layers and neuropil on brain sections in sham-operated controls (Fig. 3, control CaMKII, green color). The immunoreactivity was strongest in the subiculum, followed by CA3 and DG, and then in CA1 (Fig. 3, control CaMKII, green color). Sixty minutes of HI followed by 30 min of reperfusion virtually abolished CaMKII immunoreacticity in HI-affected hippocampal regions on brain sections (Fig. 3, 30 min, HI hemisphere, green color), whereas CaMKIIα immunoreactivity was unchanged in the brain regions contralateral to HI (Fig. 3, 30 min, non-HI hemisphere, green color). CaMKII confocal microscopic images at higher magnification showed that CaMKIIα immunoreactivity was depleted in all HI-affected neurons, including hippocampal, cortical and striatal neurons (Fig. 4), but it was unchanged in the non-HI hemisphere after HI (data not shown, but see Fig. 3). The time-course study showed that depletion of CaMKII took place as early as 30 min of reperfusion and never recovered in neurons undergoing delayed neuronal death (Fig. 4, green color). There were virtually no changes in the PI staining before 24–48 h of reperfusion (Fig. 4, red color). At 24–48 h of reperfusion, some neurons in the HI-injured CA1, DG, cortex and striatum were shrunken or fragmented, indicating that neuronal death took place (Fig. 4, 24 and 48 h, red color, arrows). The morphologies of PI-stained surviving or dead neurons in post-ischemic brain sections were confirmed by pathological staining with acid fuchsin and celestin blue and by electron microscopy, as we reported previously (Liu et al. 2004a). Development-dependent CaMKII changes after HI in P15 and P26 rats were also studied by confocal microscopy. CaMKII immunoreactivity in brain sections was up-regulated in a development-dependent manner in sham-control rats as shown in Fig. 2 but, just like the CaMKII depletion after HI in P7 brains shown in Figs 3 and 4, it was also depleted after HI in P15 and P26 rats (data not shown).
Fig. 3.
Confocal microscopic images of hippocampi double-labeled with anti-CaMKIIα antibody (green) and PI (red). Brain sections were obtained from P7 rat pups subjected to either sham-operation or to 60 min HI followed by 30 min of reperfusion. PI stained hippocampal cell layers (red) and this staining was unchanged after HI. Anti-CaMKIIα immunoreactivity was located in neurons and neuropil in control, and in the non-HI-affected hemisphere after HI, but its immunoreactivity disappeared in the HI-affected hemisphere (30 min CaMKII, green).
Fig. 4.
Confocal microscopic images of CA1, DG, cortical and striatal neurons double-labeled with anti-CaMKIIα antibody (green), together with PI (red). Brain sections were obtained from P7 rat pups subjected to either sham-operated surgery or to 60 min HI followed by 30 min, 4, 24 and 48 h of reperfusion. Anti-CaMKIIα immune-reactivity was located in neurons and neuropil in control but it was persistently abolished after HI (green). At 24–48 h of reperfusion after HI, some neurons stained with PI were shrunken, indicating cell death.
Translocation and autophosphorylation of CaMKII into a Triton-insoluble fraction after HI
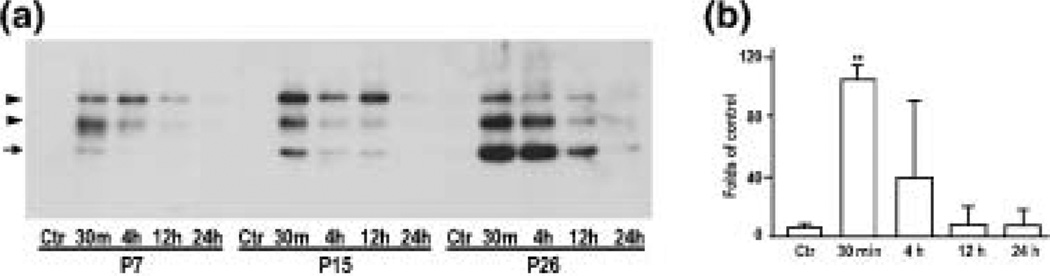
It is evident that, upon autophosphorylation of Thr286, CaMKIIα is translocated into postsynaptic densities (PSDs) (Strack et al. 1997; Yamauchi and Yoshimura 1998; Shen and Meyer 1999; Hudmon and Schulman 2002; Meng and Zhang 2002). The disappearance of CaMKIIα immunoreactivity after HI seen in Figs 3 and 4 might be due to either the net loss of CaMKIIα protein or the translocation of CaMKIIα to the dense PSDs in which the antibody cannot penetrate (Ochiishi et al. 1993). To investigate possible CaMKIIα redistribution, we prepared homogenate (H), cytosol (S3), a microsomal fraction (P3), a Triton-insoluble synaptosomal pellet (P2p) which enriched PSDs and mitochondria, and the Triton-soluble supernatant (P2s). Western blot analyses of homogenates demonstrated that, after HI, total CaMKIIα level tended to decrease although the changes were not statistically significant (Figs 5a and b). Western blot analyses of subcellular fractions showed that CaMKII was depleted from S3, P3 and P2s fractions, and was concomitantly deposited into the Triton-insoluble P2p pellets after HI (Figs 5a, c and d). The time-course study showed that CaMKIIα depletion and deposition occurred as early as 30 min of reperfusion and lasted until 24 h of reperfusion when delayed neuronal death started to take place (Figs 5a–d, also see Fig. 1). CaMKIIα translocation after HI was seen in all age groups, but the basic level of CaMKIIα was low in P7, higher in P15 and highest in P26 rats (Figs 5a and e). Consistently, 50 kDa phospho-CaMKIIα-Thr286 was undetectable in sham-operated controls in all subcellular fractions, but after HI significantly increased in the Triton-insoluble P2p fraction (Fig. 6a upper panel, arrow, and 6b). The increase in 50 kDa phospho-CaMKII-Thr286 was peaked at 30 min of reperfusion and then declined thereafter in P7 pups, whereas CaMKII-Thr286 phosphorylation was more sustained in P26 rats (Fig. 6a upper panel, arrow, and 6b). In addition to 50 kDa phospho-CaMKIIα-Thr286, two more additional protein bands with molecular sizes about 55 and 70 kDa on the blot were also labeled by the anti-phospho-CaMKII-Thr286 antibody and these bands were markedly increased after HI (Fig. 6a, arrowheads). The identities of these two proteins are unknown.
Fig. 6.
(a) Immunoblots of phospho-CaMKII-Thr286 in P2p after HI. Samples were from rats at post-natal 7 days (P7), 15 days (P15) or 26 days (P26) subjected to either sham-operation (Ctr), or to 60 min of HI for P7 and P15, or 30 min of HI for P26 rats, followed by 30 min, 4, 12 and 24 h of recovery, respectively. Arrows indicate the 50-kDa CaMKIIα band. Arrowheads indicate two unidentified proteins recognized by the phospho-CaMKII antibody. (b) Statistical analysis of the 50-kDa phospho-CaMKII-Thre286 in P2p fraction from P7 pups. Mean optical densities of the 50-kDa phospho-CaMKII-Thre286 bands were quantified with Kodak 1D image software and expressed as percentage of P7 control group (mean ± SD, n = 4). Phospho-CaMKIIThre286 was increased at 30 min and then returned to the control level at 12 h after HI. **p < 0.01 indicates statistical significance between age-matched sham-operated control groups and post-HI groups (one-way anova followed by Fischer’s PLSD post-hoc test).
Discussion
Transient HI causes delayed neuronal death in both immature and mature neurons, but immature neurons are generally much more resistant to HI relative to mature neurons (Johnston et al. 2002). The search for the mechanisms underlying neuronal vulnerability and delayed neuronal death has been extensive, but they are still incompletely understood (Siesjö 1994). In this study, we used a rat HI model to investigate CaMKII changes as a function of brain development. This study has clearly demonstrated that, after HI, CaMKII is persistently depleted from the cytoplasm and is deposited into Triton-insoluble pellets. Redistribution of CaMKII is accompanied by transient autophosphorylation of CaMKII-Thr286 as viewed by western blot analysis. The total amount of CaMKIIα protein translocated in the Tritoninsoluble fraction after HI is more in older rats than in younger pups. However, the ratio between translocated and cytosolic CaMKIIα contents after HI appears similar. This is consistent with the observation that CaMKII-expressing neurons are fewer in developmental brains than in mature brains, as shown by confocal microscopy (see Fig. 2). Thirty minutes of HI are able to cause delayed neuronal death in mature neurons, whereas 60 min is necessary to cause neuronal death in neonatal neurons in the hypoxia-ischemia model utilized in the present study. Furthermore, in neonatal brains, CaMKII-expressing neurons appear more vulnerable to HI as compared with neurons that do not express CaMKII. These novel results suggest that alterations of CaMKII may play an important role in delayed neuronal death and long-term synaptic deficits after transient HI.
Alterations of CaMKII after global ischemia in adult rat brain was reported previously (Aronowski et al. 1992; Morioka et al. 1992; Hu and Wieloch 1994; Hu et al. 1995). In striking contrast with its increase in Triton-insoluble fractions, CaMKII immunoreactivity in brain disappeared in brain sections after HI. The general CaMKII antibody used in this study is excellent for recognizing CaMKII on western blots, but does not recognize translocated CaMKII in post-ischemic brain sections. Based on an immunoelectron microscopic study demonstrating that CaMKII antibodies cannot penetrate into PSDs to reach translocated CaMKII (Ochiishi et al. 1993), it is possible that this difference between CaMKII increase in Triton-insoluble fraction on the western blot and CaMKII disappearance in brain sections after HI may be due to steric hindrance of the PSDs that prevent the antibody from reaching the translocated CaM-kinase II in PSDs. It is also plausible that the CaMKII epitopes have been modified so that this general CaMKII antibody cannot recognize the translocated CaMKII in brain sections after HI.
Development-dependent up-regulation of CaMKII in neurons appears synchronized with expression of synaptogenesis (Zou and Cline 1996; Rongo and Kaplan 1999; Xue et al. 2002). In embryonic developing neurons in the hippocampal culture, CaMKII is localized in the neuronal soma and dendrites, but not in the presynaptic terminals (Menegon et al. 2002). Postsynaptic expression of constitutively active CaMKII promotes morphological maturation of synapses and augments the amplitude of excitatory synaptic currents. Conversely, expression of a CaMKII inhibitory peptide in postsynaptic neurons interferes with synapse maturation (Kazama et al. 2003). Synaptic activation contributes to HIinduced cell death (Johnston 2002). In this study, we noticed by confocal microscopy that P7 CA1 and DG neuronal layers can be subdivided into a CaMKII-positive neuronal layer and a CaMKII-negative neuronal subfield. The former layer is loosely packed and the latter is densely packed. We failed to find published data about the post-natal development or maturation within either the CA1 or DG neuronal layers. Results from the active caspase-3 and PI confocal microscopy study (Fig. 1) suggest that CaMKII-expressing neurons appear more mature, perhaps have more synapses, and are thus more vulnerable to HI. This may be related to the changes in susceptibility to HI as a function of brain development. It has generally been proposed that the more synaptic connection, the higher the susceptibility of neurons to HI (Johnston 2002). Many previous studies have suggested that CaMKII is a key protein in regulation of synaptogenesis (Zou and Cline 1996; Rongo and Kaplan 1999; Xue et al. 2002). Therefore, the more CaMKII expression in brain tissues, the more synapses, thus the higher susceptibility of neurons to HI.
The deposition of CaMKII in the Triton-insoluble fraction after HI observed in this study is somewhat similar but far from identical to the autophosphorylation-dependent translocation of CaMKII to PSDs seen in many previous studies under physiological conditions (for reviews: Meyer and Shen 2000; Hudmon and Schulman 2002). A sequence of ATP depletion, membrane depolarization, activation of NMDA receptor and increase in intracellular calcium takes place in neurons during and after brain hypoxia-ischemia (Siesjo 1994; Johnston 2002). These events may well explain CaMKII translocation to PSDs after HI. However, translocation of CaMKII is a transient and reversible event and CaMKII should normally depart from the PSD structure and return to the cytoplasm for the reuse under physiological conditions (for reviews: Meyer and Shen 2000; Hudmon and Schulman 2002). This is not the case in neurons after HI. In addition, CaMKII-Thr286 is dephosphorylated after 30 min of reperfusion, indicating CaMKII inactivation after HI. Therefore, permanent CaMKII depletion from cytoplasm, and deposition and dephosphorylation in the Triton-insoluble fraction, are likely pathological. Accumulating evidence demonstrates that synaptic repair and plasticity require much faster turnover of PSD proteins than previously estimated (Okabe et al. 2001; Ehlers 2003). Our previous study shows that PSD proteins are dramatically unfolded after ischemia (Hu and Martone 2001; Hu et al. 2004; Liu et al. 2004b). It is therefore likely that during the process of translocation into PSDs, CaMKII may be trapped into PSD sticky unfolded proteins that are overproduced during and after HI.
HI-induced persistent CaMKII depletion and deposition should have severe consequences. CaMKII is one of the most abundant protein kinase in the nervous system, and plays a key role in synaptogenesis and synaptic plasticity (Silva et al. 1992). Several studies have reported activation of ERK1/2 and Akt after neonatal rat cerebral hypoxia-ischemia (Wang et al. 2003; Jones and Bergeron 2004). It will be interesting to study whether these protein kinases are also altered after HI as a function of brain development. It has been repeatedly demonstrated that phosphorylation of glutamate receptors by CaMKII potentiates glutamate receptormediated neurotransmission (Fang et al. 2002; Poncer et al. 2002). Therefore, early transient phosphorylation or activation of CaMKII may sensitize glutamate receptors and contribute to the vulnerability of CaMKII-expressing neurons after HI (Hagberg 1992; Johnston 2002). The more sustained phosphorylation of CaMKII-Thr286 in older rats than in younger rats is also coincident with the development-dependent increase in neuronal vulnerability after HI. Furthermore, persistent CaMKII depletion from the cytoplasm, and its inactivation in the late phase of reperfusion after HI as shown by its dephosphorylation, may bring CaMKII-mediated events to a standstill, resulting in interruption of synapse development, synaptic remodeling and plasticity. Synapse transmission is severely damaged in HI-affected brain regions (Fowler et al. 2003). Without proper synaptic connections, developing neurons may undergo apoptosis (Oppenheim 1991; Mennerick and Zorumski 2000; Lossi et al. 2002; Blomgren et al. 2003). Evidence suggests that persistent CaMKII depletion and inactivation after transient HI may contribute to synaptic dysfunction and delayed neuronal death.
Acknowledgements
The authors thank Dr Brant Watson for proofreading this manuscript. This work was supported by National Institutes of Health grants NS36810 and NS40407.
Abbreviations used
- CCA
common carotid artery
- DG
dentate gyrus
- EM
electron microscopy
- HI
hypoxia-ischemia
- P7
post-natal 7 days
- PBS
phosphate-buffered saline
- TX
Triton X100.
References
- Aronowski J, Grotta JC, Waxham MN. Ischemia-induced translocation of Ca2+/calmodulin-dependent protein kinase II: potential role in neuronal damage. J. Neurochem. 1992;58:1743–1753. doi: 10.1111/j.1471-4159.1992.tb10049.x. [DOI] [PubMed] [Google Scholar]
- Auer RN, Olsson Y, Siesjo BK. Hypoglycemic brain injury in the rat. Correlation of density of brain damage with the EEG isoelectric time: a quantitative study. Diabetes. 1984;33:1090–1098. doi: 10.2337/diab.33.11.1090. [DOI] [PubMed] [Google Scholar]
- Blomgren K, Zhu C, Hallin U, Hagberg H. Mitochondria and ischemic reperfusion damage in the adult and in the developing brain. Biochem. Biophys. Res. Commun. 2003;304:551–559. doi: 10.1016/s0006-291x(03)00628-4. [DOI] [PubMed] [Google Scholar]
- Blumenfeld KS, Welsh FA, Harris VA, Pesenson MA. Regional expression of c-fos and heat shock protein-70 mRNA following hypoxia-ischemia in immature rat brain. J. Cereb. Blood Flow Metab. 1992;12:987–995. doi: 10.1038/jcbfm.1992.136. [DOI] [PubMed] [Google Scholar]
- Churn SB, Yaghmai A, Povlishock J, Rafiq A, DeLorenzo RJ. Global forebrain ischemia results in decreased immunoreactivity of calcium/calmodulin-dependent protein kinase II. J. Cereb. Blood Flow Metab. 1992;12:784–793. doi: 10.1038/jcbfm.1992.109. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat. Neurosci. 2003;10:10–15. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Fang L, Wu J, Lin Q, Willis WD. Calcium-calmodulindependent protein kinase II contributes to spinal cord central sensitization. J. Neurosci. 2002;22:4196–4204. doi: 10.1523/JNEUROSCI.22-10-04196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JC, Gervitz LM, Hamilton ME, Walker JA. Systemic hypoxia and the depression of synaptic transmission in rat hippocampus after carotid artery occlusion. J. Physiol. 2003;550:961–972. doi: 10.1113/jphysiol.2003.039594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, O’Brien C, Ohno M, Kirkwood A, Silva AJ. Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature. 2001;411:309–3013. doi: 10.1038/35077089. [DOI] [PubMed] [Google Scholar]
- Gill R, Soriano M, Blomgren K, et al. Role of caspase-3 activation in cerebral ischemia-induced neurodegeneration in adult and neonatal brain. J. Cereb. Blood Flow Metab. 2002;22:420–430. doi: 10.1097/00004647-200204000-00006. [DOI] [PubMed] [Google Scholar]
- Hagberg H. Hypoxic-ischemic damage in the neonatal brain: excitatory amino acids. Dev. Pharmacol. Ther. 1992;18:139–144. [PubMed] [Google Scholar]
- Hanson PI, Schulman H. Neuronal Ca2+/calmodulindependent protein kinases. Annu. Rev. Biochem. 1992;61:559–601. doi: 10.1146/annurev.bi.61.070192.003015. [DOI] [PubMed] [Google Scholar]
- Hu BR, Martone ME. In: Changes in postsynaptic densities after brain ischemia, in Maturation Phenomenon in Cerebral Ischemia IV. Bazan N, Ito M, editors. 2001. pp. 93–102. [Google Scholar]
- Hu BR, Wieloch T. Tyrosine phosphorylation and activation of mitogen-activated protein kinase in the rat brain following transient cerebral ischemia. J. Neurochem. 1994;62:1357–1367. doi: 10.1046/j.1471-4159.1994.62041357.x. [DOI] [PubMed] [Google Scholar]
- Hu BR, Kamme F, Wieloch T. Alterations of Ca2+/calmodulin- dependent protein kinase II and its messenger RNA in the rat hippocampus following normo- and hypothermic ischemia. Neuroscience. 1995;68:1003–1016. doi: 10.1016/0306-4522(95)00213-3. [DOI] [PubMed] [Google Scholar]
- Hu BR, Park M, Martone ME, Fischer WH, Ellisman MH, Zivin JA. Assembly of proteins to postsynaptic densities after transient cerebral ischemia. J. Neurosci. 1998;18:625–633. doi: 10.1523/JNEUROSCI.18-02-00625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BR, Liu CL, Ouyang Y, Blomgren K, Siesjo BK. Involvement of caspase-3 in cell death after hypoxia-ischemia declines during brain maturation. J. Cereb. Blood Flow Metab. 2000;20:1294–1300. doi: 10.1097/00004647-200009000-00003. [DOI] [PubMed] [Google Scholar]
- Hu BR, Martone ME, Liu CL. In: Protein aggregation, unfolded protein response and delayed neuronal death after brain ischemia, in maturation phenomenon, in Cerebral Ischemia V. Buchan A, Ito M, editors. 2004. pp. 225–237. [Google Scholar]
- Hudmon A, Schulman H. Neuronal CA2+/calmodulindependent protein kinase II: the role of structure and autoregulation in cellular function. Annu. Rev. Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Clinical disorders of brain plasticity. Brain Dev. 2004;26:73–80. doi: 10.1016/S0387-7604(03)00102-5. [DOI] [PubMed] [Google Scholar]
- Johnston MV, Nakajima W, Hagberg H. Mechanisms of hypoxic neurodegeneration in the developing brain. Neuroscientist. 2002;8:212–220. doi: 10.1177/1073858402008003007. [DOI] [PubMed] [Google Scholar]
- Jones NM, Bergeron M. Hypoxia-induced ischemic tolerance in neonatal rat brain involves enhanced ERK1/2 signaling. J. Neurochem. 2004;89:157–167. doi: 10.1111/j.1471-4159.2004.02324.x. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Fukunaga K, Muller D. Calcium/calmodulindependent protein kinase II contributes to activity-dependent filopodia growth and spine formation. J. Neurosci. 2003;23:10 645–10 649. doi: 10.1523/JNEUROSCI.23-33-10645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura KI, Kurihara J, Watanabe M, Takahashi K, Katayama Y. FK506 attenuates the post-ischemic perturbation of protein kinases and tyrosine phosphorylation in the gerbil hippocampal CA1 sectors. Acta Neurochir. Suppl. 2003;86:113–116. doi: 10.1007/978-3-7091-0651-8_25. [DOI] [PubMed] [Google Scholar]
- Kazama H, Morimoto-Tanifuji T, Nose A. Postsynaptic activation of calcium/calmodulin-dependent protein kinase II promotes coordinated pre- and postsynaptic maturation of Drosophila neuromuscular junctions. Neuroscience. 2003;117:615–625. doi: 10.1016/s0306-4522(02)00923-5. [DOI] [PubMed] [Google Scholar]
- Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290:750–754. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- Lai Y, Nairn AC, Greengard P. Autophosphorylation reversibly regulates the Ca2+/calmodulin-dependence of Ca2+/ calmodulin-dependent protein kinase II. Proc. Natl Acad. Sci. USA. 1986;83:4253–4257. doi: 10.1073/pnas.83.12.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CL, Siesjö BK, Hu BR. Pathogenesis of hippocampal neuronal death after hypoxia-ischemia changes during brain development. Neuroscience. 2004a;127:113–123. doi: 10.1016/j.neuroscience.2004.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CL, Martone ME, Hu BR. Protein ubiquitination in postsynaptic densities following transient cerebral ischemia. J. Cereb. Blood Flow Metab. 2004b doi: 10.1097/01.WCB.0000136706.77918.21. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossi L, Mioletti S, Merighi A. Synapse-independent and synapse-dependent apoptosis of cerebellar granule cells in postnatal rabbits occur at two subsequent but partly overlapping developmental stages. Neuroscience. 2002;112:509–523. doi: 10.1016/s0306-4522(02)00112-4. [DOI] [PubMed] [Google Scholar]
- Menegon A, Verderio C, Leoni C, Benfenati F, Czernik AJ, Greengard P, Matteoli M, Valtorta F. Spatial and temporal regulation of Ca2+/calmodulin-dependent protein kinase II activity in developing neurons. J. Neurosci. 2002;22:7016–7026. doi: 10.1523/JNEUROSCI.22-16-07016.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Zhang G. Autophosphorylated calcium/calmodulin-dependent protein kinase II alpha induced by cerebral ischemia immediately targets and phosphorylates N-methyl-D-aspartate receptor subunit 2B (NR2B) in hippocampus of rats. Neurosci. Lett. 2002;333:59–63. doi: 10.1016/s0304-3940(02)00961-8. [DOI] [PubMed] [Google Scholar]
- Mengesdorf T, Althausen S, Mies G, Olah L, Paschen W. Phosphorylation state, solubility, and activity of calcium/calmodulin- dependent protein kinase II alpha in transient focal ischemia in mouse brain. Neurochem. Res. 2002;27:477–484. doi: 10.1023/a:1019844518704. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. Neural activity and survival in the developing nervous system. Mol. Neurobiol. 2000;22:41–54. doi: 10.1385/MN:22:1-3:041. [DOI] [PubMed] [Google Scholar]
- Meyer T, Shen K. In and out of the postsynaptic region: signalling proteins on the move. Trends Cell Biol. 2000;10:238–244. doi: 10.1016/s0962-8924(00)01764-5. [DOI] [PubMed] [Google Scholar]
- Molloy SS, Kennedy MB. Autophosphorylation of type II Ca2+/calmodulin-dependent protein kinase in cultures of postnatal rat hippocampal slices. Proc. Natl Acad. Sci. USA. 1991;88:4756–4760. doi: 10.1073/pnas.88.11.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka M, Fukunaga K, Yasugawa S, Nagahiro S, Ushio Y, Miyamoto E. Regional and temporal alterations in Ca2+/ calmodulin-dependent protein kinase II and calcineurin in the hippocampus of rat brain after transient forebrain ischemia. J. Neurochem. 1992;58:1798–1809. doi: 10.1111/j.1471-4159.1992.tb10056.x. [DOI] [PubMed] [Google Scholar]
- Ochiishi T, Sugiura H, Yamauchi T. Characterization and autophosphorylation of Ca2+/calmodulin-dependent protein kinase in the postsynaptic density of the rat forebrain. Brain Res. 1993;610:97–107. doi: 10.1016/0006-8993(93)91222-e. [DOI] [PubMed] [Google Scholar]
- Okabe S, Urushido T, Konno D, Okado H, Sobue K. Rapid redistribution of the postsynaptic density protein PSD-Zip45 (Homer 1c) and its differential regulation by NMDA receptors and calcium channels. J. Neurosci. 2001;21:9561–9571. doi: 10.1523/JNEUROSCI.21-24-09561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW. Cell death during development of the nervous system. Annu. Rev. Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Poncer JC, Esteban JA, Malinow R. Multiple mechanisms for the potentiation of AMPA receptor-mediated transmission by alpha-Ca2+/calmodulin-dependent protein kinase II. J. Neurosci. 2002;22:4406–4411. doi: 10.1523/JNEUROSCI.22-11-04406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponten U, Ratcheson RA, Salford LG, Siesjo BK. Optimal freezing conditions for cerebral metabolites in rats. J. Neurochem. 1973;21:1127–1138. doi: 10.1111/j.1471-4159.1973.tb07567.x. [DOI] [PubMed] [Google Scholar]
- Rice JE, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rats. Ann. Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Rongo C, Kaplan JM. CaMKII regulates the density of central glutamatergic synapses in vivo . Nature. 1999;402:195–199. doi: 10.1038/46065. [DOI] [PubMed] [Google Scholar]
- Shackelford DA, Yeh RY, Hsu M, Buzsaki G, Zivin JA. Effect of cerebral ischemia on calcium/calmodulin-dependent protein kinase II activity and phosphorylation. J. Cereb. Blood Flow Metab. 1995;15:450–461. doi: 10.1038/jcbfm.1995.56. [DOI] [PubMed] [Google Scholar]
- Siesjö BK. Calcium-mediated processes in neuronal degeneration. Ann. NY Acad. Sci. 1994;747:140–161. doi: 10.1111/j.1749-6632.1994.tb44406.x. [DOI] [PubMed] [Google Scholar]
- Siesjö BK, Elmer E, Janelidze S, Keep M, Kristian T, Ouyang YB, Uchino H. Role and mechanisms of secondary mitochondrial failure. Acta Neurochir. (Suppl.)(Wien) 1999;73:7–13. doi: 10.1007/978-3-7091-6391-7_2. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium–calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Strack S, Choi S, Lovinger DM, Colbran RJ. Translocation of autophosphorylated calcium/calmodulin-dependent protein kinase II to the postsynaptic density. J. Biol. Chem. 1997;272:13 467–13 470. doi: 10.1074/jbc.272.21.13467. [DOI] [PubMed] [Google Scholar]
- Sugiura H, Yamauchi T. Developmental changes in the levels of Ca2+/calmodulin-dependent protein kinase II alpha and beta proteins in soluble and particulate fractions of the rat brain. Brain Res. 1992;593:97–104. doi: 10.1016/0006-8993(92)91269-k. [DOI] [PubMed] [Google Scholar]
- Taha S, Hanover JL, Silva AJ, Stryker MP. Autophosphorylation of alphaCaMKII is required for ocular dominance plasticity. Neuron. 2002;36:483–491. doi: 10.1016/s0896-6273(02)00966-2. [DOI] [PubMed] [Google Scholar]
- Towfighi J, Mauger D. Temporal evolution of neuronal changes in cerebral hypoxia-ischemia in developing rats: a quantitative light microscopic study. Brain Res. Dev. Brain Res. 1998;109:169–177. doi: 10.1016/s0165-3806(98)00077-7. [DOI] [PubMed] [Google Scholar]
- Towfighi J, Mauger D, Vannucci RC, Vannucci SJ. Influence of age on the cerebral lesions in an immature rat model of cerebral hypoxia-ischemia: a light microscopic study. Brain Res. Dev Brain Res. 1997;100:149–160. doi: 10.1016/s0165-3806(97)00036-9. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhu C, Qiu L, Hagberg H, Sandberg M, Blomgren K. Activation of ERK1/2 after neonatal rat cerebral hypoxiaischaemia. J. Neurochem. 2003;86:351–362. doi: 10.1046/j.1471-4159.2003.01838.x. [DOI] [PubMed] [Google Scholar]
- Waxham MN, Grotta JC, Silva AJ, Strong R, Aronowski J. Ischemia-induced neuronal damage: a role for calcium / calmodulin-dependent protein kinase II. J. Cereb. Blood Metab. 1996;16:1–6. doi: 10.1097/00004647-199601000-00001. [DOI] [PubMed] [Google Scholar]
- Xue J, Li G, Bharucha E, Cooper NG. Developmentally regulated expression of CaMKII and iGluRs in the rat retina. Brain Res. Dev. Brain Res. 2002;138:61–70. doi: 10.1016/s0165-3806(02)00460-1. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Yoshimura Y. Phosphorylation-dependent reversible translocation of Ca2+/calmodulin-dependent protein kinase II to the postsynaptic densities. Life Sci. 1998;62:1617–1621. doi: 10.1016/s0024-3205(98)00117-9. [DOI] [PubMed] [Google Scholar]
- Zou DJ, Cline HT. Control of retinotectal axon arbor growth by postsynaptic CaMKII. Prog. Brain Res. 1996;108:303–312. doi: 10.1016/s0079-6123(08)62548-0. [DOI] [PubMed] [Google Scholar]