Abstract
Many cyanobacteria produce cyanotoxins, which has been well documented from freshwater environments but not investigated to the same extent in marine environments. Cyanobacteria are an obligate component of the polymicrobial disease of corals known as black band disease (BBD). Cyanotoxins were previously shown to be present in field samples of BBD and in a limited number of BBD cyanobacterial cultures. These toxins were suggested as one of the mechanisms contributing to BBD-associated coral tissue lysis and death. In this work, we tested nine cyanobacterial isolates from BBD and additionally nine isolated from non-BBD marine sources for their ability to produce toxins. The presence of toxins was determined using cell extracts of laboratory grown cyanobacterial cultures using ELISA and the PP2A assay. Based on these tests, it was shown that cyanobacterial toxins belonging to the microcystin/nodularin group were produced by cyanobacteria originating from both BBD and non-BBD sources. Several environmental factors that can be encountered in the highly dynamic microenvironment of BBD were tested for their effect on both cyanobacterial growth yield and rate of toxin production using two of the BBD isolates of the genera Leptolyngbya and Geitlerinema. While toxin production was the highest under mixotrophic conditions (light and glucose) for the Leptolyngbya isolate, it was highest under photoautotrophic conditions for the Geitlerinema isolate. Our results show that toxin production among marine cyanobacteria is more widespread than previously documented, and we present data showing three marine cyanobacterial genera (Phormidium, Pseudanabaena, and Spirulina) are newly identified as cyanotoxin producers. We also show that cyanotoxin production by BBD cyanobacteria can be affected by environmental factors that are present in the microenvironment associated with this coral disease.
Introduction
Black band disease (BBD) is one of the most important diseases that contribute to coral decline in the wider Caribbean [62], the Indo-Pacific [55], the Red Sea [1], and the Great Barrier Reef [16]. The disease spreads across healthy coral tissue as a distinctive dark-colored band at a rate between 3 mm and 1 cm per day [49]. The rate of disease migration depends on different environmental factors that include elevated nutrients, temperature, and light intensity [6, 60], with rates twice as fast in the summer months. It has recently been shown that between temperature and elevated nutrients, the latter is the most important in enhancing BBD progression [47].
BBD is comprised of a complex microbial consortium [39, 42] consisting of non-heterocystous filamentous cyanobacteria [41, 48], sulfide-oxidizing bacteria [21, 38], sulfate-reducing bacteria [5, 59], and various heterotrophic bacteria [14, 19, 50, 51]. Even though the disease was originally described in the 1970s [4], our knowledge of the etiology of the disease is still limited. No primary pathogen has been identified, and it has been proposed that BBD is a polymicrobial disease [42].
The filamentous cyanobacteria in BBD form the physical matrix of the BBD mat similar to other cyanobacteria-dominated microbial mats. Originally, Antonius [4] identified BBD cyanobacteria as belonging to the species Oscillatoria submembranacea. More recently, it was determined that BBD cyanobacteria are taxonomically diverse, and the dominant Caribbean BBD cyanobacterium was newly characterized as Phormidium corallyticum [48]. Use of light and scanning electron microscopy revealed the presence of several BBD cyanobacterial genera such as Schizothrix and Spirulina along with Phormidium [17, 49]. However, a more comprehensive insight into cyanobacterial composition in BBD was not available until the use of molecular techniques. These techniques included denaturing gradient gel electrophoresis (DGGE) with the use of 16S rRNA gene targeted universal [14] and cyanobacterial specific primers [31], cloning and sequencing of the 16S rRNA gene [19, 50, 51], and terminal restriction fragment length polymorphism [20]. According to these data, BBD cyanobacterial composition and diversity vary between seasons [5] and geographical locations [20]. It has also been shown that the same cyanobacterial sequences can be found in BBD of different coral species and widely divergent regions [20, 31]. By using 16S rRNA gene cloning and sequencing, it was shown that cyanobacteria in BBD are most closely related to genera that include Oscillatoria, Lyngbya, Geitlerinema, Leptolyngbya, and Phormidium [14, 31, 50].
Assessment of BBD cyanobacterial physiology in terms of potential contribution to the etiology of BBD was not possible until BBD cyanobacteria were isolated into culture. The first cyanobacterium that was isolated from BBD was a strain originally identified as P. corallyticum [38] and later re-identified by use of molecular methods as belonging to Geitlerinema (accession no. DQ151461) [36]. Our lab [31, 32] isolated nine additional BBD cyanobacterial strains into culture and confirmed their presence in BBD by using DGGE. The isolated strains were identified by 16S rRNA gene sequencing as members of the genera Geitlerinema, Leptolyngbya, and Oscillatoria and were demonstrated to have the ability to photosynthesize under anaerobic conditions in the presence of sulfide [31, 32]. This appears to be an important ecological feature that enables these cyanobacteria to thrive in the sulfide-rich environment of BBD [7], as sulfide is normally toxic to oxygenic photo-autotrophs, including most cyanobacteria [12].
The virulence factors of BBD have not been fully identified and characterized, although some have been proposed. Rützler et al. [49] first speculated that “a toxic exudate is the cause of histolysis”. Richardson et al. [39] have shown that exposure of coral fragments to 0.5 mM sulfide (a level measured in intact BBD using sulfide-sensitive microelectrodes [7]) and anoxia causes coral tissue death in the amount of time it would take a typical BBD infection to pass a fixed point. Recently, we have shown that cultures of cyanobacteria isolated from BBD as well as BBD field samples produce the cyanotoxin microcystin [43] and that exposure of apparently healthy coral fragments to purified microcystin elicits toxic effects that include degradation of coral tissue and zooxanthellae [44]. Microcystin is a cyclic heptapeptide [8] that specifically affects hepatic cells [22] but it can also affect other (non-hepatic) cells through its protein phosphatase inhibition activity [28]. Microcystin production is common among freshwater cyanobacteria [54]; however, there are limited data on cyanotoxin production by marine cyanobacteria [10, 37]. In this study, we compare cyanotoxin production in cultures of cyanobacteria from BBD and those from non-BBD marine sources. Additionally, in order to determine whether the dynamic environmental conditions within BBD can affect toxin production, BBD cyanobacterial cultures were subjected to different growth conditions and toxin production was assessed.
Materials and Methods
Organisms
Unialgal cyanobacterial cultures investigated in this study were isolated from samples collected either from BBD or non-BBD sources as indicated in Table 1. The tested strains were either new isolates or were the subject of research performed earlier [31, 32]. The new isolates were obtained from enrichment cultures, which were developed by inoculating BG11 marine medium [45] with freshly collected BBD mat or other cyanobacterial field samples and incubating at 26°C under fluorescent cool-white light (Philips, F34T12/CW/RS/EW) at an intensity of 30 μE m−2 s−1. The isolation of motile filamentous species was performed by inoculating BG11 agar plates with pigmented biomass accumulated in enrichment cultures and then transferring individual filaments from the agar surface [11]. BG11 is a nutrient-rich medium that is appropriate for enrichment of BBD cyanobacteria since the BBD environment is itself nutrient rich due to active coral tissue lysis. We also used this medium, along with ASN III [45], for isolation of non-BBD cyanobacteria. Unicellular and filamentous non-gliding species were isolated by homogenizing enrichment cultures in a blender and filtering the diluted samples through membrane filters (pore size 0.45 μm), which were then placed on BG11 agar and incubated at low light intensity (15 μE m−2 s−1) until visible colonies appeared. Maintenance of cultures was performed in BG11 medium at 26°C under a 12:12 h light/dark fluorescent light regime with an intensity of 15–30 μE m−2 s−1.
Table 1.
Cyanobacterial isolates and their taxonomic identification based on 16S rRNA gene sequencing and their source of origin
| Isolate | Closest relative | Similarity % | GenBank accession no. | Location and source (coral host sp. or environment) | References |
|---|---|---|---|---|---|
| BBD isolates | |||||
| BBD 1991 | Geitlerinema | 99 | DQ151461 | Florida Keys, Montastraea annularis | [36, 41] |
| HS 217 | Geitlerinema | 99 | EF110974 | Bahamas, Siderastrea siderea | [31, 60] |
| HS 223 | Geitlerinema | 99 | DQ680351 | Bahamas, Siderastrea siderea | [31, 60] |
| W-1 | Geitlerinema | 99 | EF154084 | Florida Keys, Siderastrea siderea | [31] |
| FLK BBD1 | Leptolyngbya | 98 | EF110975 | Florida Keys, Montastraea annularis | [31, 43, 60] |
| Phil 2b-2 | Leptolyngbya | 98 | EF372581 | Philippines, Porites lutea | [31] |
| 102a-1 | Leptolyngbya | 97 | EU743966 | Florida Keys, Dendrogyra cylindrus | This work |
| 102d-1 | Leptolyngbya | 97 | EU743968 | Florida Keys, Montastraea annularis | This work |
| 96-4 | Spirulina | 93 | EU743969 | Florida Keys, Montastraea annularis | This work |
| Non-BBD isolates | |||||
| 10 | Geitlerinemaa | N/A | FJ232377 | Florida Keys, mat on Montastraea cavernosa | This work |
| Alg | Leptolyngbyab | N/A | N/A | Florida Keys, mat on sediment | This work |
| HS26 | Leptolyngbyaa | N/A | FJ232376 | Florida Keys, mat away from BBD | This work |
| 63a-5 | Pseudanabaena | 97 | FJ026734 | Florida Keys, sediment | This work |
| 73-2 | Phormidium | 97 | EU196366 | Gulf of Mexico, water column | [32] |
| 63-1 | Pseudanabaena | 98 | EU110976 | Florida Keys, sediment | [32] |
| 72-1 | Pseudanabaena | 98 | EU196365 | Florida Keys, mat on Montastraea cavernosa | [32] |
| 63a-1 | Synechococcus | 98 | EU743972 | Florida Keys, water column | This work |
| 63a-3 | Synechococcus | 98 | EU743971 | Florida Keys, water column | This work |
Identified by using classical taxonomic criteria because the BLAST search did not provide the identification of the closest relative
Not sequenced
All of the cultures were unialgal since all attempts to obtain axenic cultures were unsuccessful. It is notoriously difficult to obtain axenic cultures of gliding filamentous cyanobacteria because the gliding mechanism is based on continual extrusion of polysaccharide/nutrient-rich mucus along the length of the filament. Heterotrophic bacteria grow within this mucus and also within cyanobacterial sheaths. The bacteria present in the cultures were all derived from the BBD community. We believe that the use of non-axenic cultures in our experiments more closely mimics the conditions within BBD. The potential effect of their presence is discussed below.
Taxonomic Identification of Cyanobacterial Isolates
Identification of the isolates was based on PCR amplification and sequencing of the16S rRNA gene. Isolation of the total genomic DNA, 16S rRNA gene amplification, and its sequencing were performed as described elsewhere [31]. The cyanobacteria specific primers CYA359F and CYA781R(b) [33] were used for PCR amplification and the sequencing of the cyanobacterial 16S rRNA gene. All sequences were manually edited using FinchTV version 1.4.0 (Geospiza, Seattle, WA, USA) and assembled using ContigExpress (Invitrogen, Carlsbad, CA, USA). Sequences were analyzed using the BLAST system (http://www.ncbi.nlm.nih.gov/BLAST/) to identify their closest relatives [2]. The sequences obtained in this study have been deposited in the GenBank database under the following accession numbers: EU743966, EU743968, EU743969, FJ232377, FJ232376, FJ026734, EU743972, EU743971.
Measurement of Toxin Production
Toxin production by individual cyanobacterial isolates was determined in cell extracts (below) by using enzyme linked immunosorbent assay (ELISA) [9] and the protein phosphatase inhibition assay (PP2A) [3]. Quantification of microcystins by high-performance liquid chromatography [26] is known to be less sensitive and was not used in this work. Microcystin-DM ELISA kits were purchased from Abraxis (Warminster, PA, USA), and protein phosphatase 2A was purchased from Upstate Cell Signaling Solutions-Millipore (Billerica, MA, USA). The ELISA kit is the congener independent assay for microcystins and nodularins [18]; therefore, it does not distinguish between these two toxins or between microcystin variants. The toxin concentration was calculated as micrograms of microcystin-LR equivalent in 1 g of dry cyanobacterial biomass.
To produce the biomass of the test strains required for toxin analysis, cultures were grown in 4-L flasks in marine BG11 medium, with aeration and continuous illumination with cool-white fluorescent light at 30 μE m−2 s−1. The cultures were grown for 4 weeks after which the biomass was harvested by centrifugation and freeze-dried. Cell extracts were prepared by extracting 1 g of dry biomass in 100 mL of 50% methanol. The cell suspensions were sonicated for 30 s and then left for overnight extraction at 4°C. After centrifugation, the supernatant was filtered through a membrane filter (0.45-μm pore size) and used for ELISA and PP2A assays. In both of these assays, standards and samples were run in 96-well plates in triplicate. The presented data are the means of three separate experiments.
Effect of Environmental Factors on Toxin Production
The effect of different environmental factors on production of toxins was tested using two of the BBD isolates, Geitlerinema sp. strain W-1 and Leptolyngbya sp. strain FLK BBD1. Inocula were prepared by growing each isolate in triplicate in 3-L flasks (9 L total) of marine BG11 medium with aeration under cool-white fluorescent light at 27°C. After 4 weeks of cultivation, the biomass was harvested as described above and resuspended in 1 L of marine BG11 medium and homogenized by a hand-held homogenizer for 30 s. Homogenization was required to enable uniform distribution of the biomass during the inoculation since these strains produce “clumpy” growth. The inocula provided a starting biomass of 1 mg mL−1 of dry weight. After inoculation, the cultures were grown under the following environmental conditions: (a) photo-autotrophy at 27°C (control), (b) photoautotrophy at 30°C, (c) mixotrophy (light with glucose or fructose), (d) heterotrophy (dark with glucose or fructose), (e) pH 7, (f) pH 9. The sugars were filter-sterilized and added to BG11 medium at a final concentration of 10 mM. If not stated otherwise, cultures were maintained at a temperature of 27± 0.3°C in a temperature-controlled culture room. The cultures grown at the elevated temperature of 30 (±0.3)°C consisted of flasks being partially submerged in a temperature-controlled aquarium in which water was mixed by a constant stream of air to provide a homogeneous distribution of heat, verified using a calibrated laboratory thermometer. A constant illumination was provided with fluorescent tubes (Philips, F34T12/CW/RS/EW) with an intensity of 30 μE m−2 s−1. In order to verify a uniform intensity, light was measured with a light meter (Fisher Scientific, Pittsburgh, PA, USA), and the distance from the light source was adjusted for each flask. For cultures grown in the dark, flasks were wrapped in a double layer of aluminum foil. The pH of all cultures was maintained at pH 8.0 except for those grown at pH 7.0. Every second day, the pH was checked and if needed, it was adjusted by using 10% HCl. All cultures were hand-shaken twice a day.
After 1 week, the cultures were homogenized with a cell homogenizer. Biomass yield (dry weight) was determined gravimetrically by filtering 2 to 5 mL of culture through pre-weighed GF/C filters previously dried at 60°C to constant weight. In addition, biomass yield was determined as chlorophyll a concentration. One milliliter of homogenized biomass was centrifuged, and the pellet was extracted with 100% methanol at 4°C for 24 h. Chlorophyll concentration was determined spectrophotometrically according to the method of Dere et al. [15]. In order to determine whether bacterial biomass of non-axenic cultures contributed to and affected the biomass yield data, the chlorophyll a results were compared with those obtained gravimetrically. By using Duncan’s multiple comparison test (data not shown), it was revealed that there was no significant difference between these two sets of data (P<0.05) and regression analysis showed high correlation (r=0.92). Based on this, we concluded that bacteria in the non-axenic cultures of cyanobacteria did not significantly contribute to the dry weight values; therefore, only gravimetrical data were used as reliable indicators of culture biomass yield.
The remaining biomass was centrifuged, freeze-dried, and extracted for toxin analysis. The extracts for ELISA were prepared as described above. This experiment was repeated three times, and the presented data are the means (n=3) from one representative experiment.
Statistical Analysis
Statistical analyses were performed using the software Stat100 (Biosoft, Cambridge, UK) and SPSS. The significance between different treatments (growth in different environmental conditions) was tested by one-way ANOVA.
Results
Organisms
Eighteen cyanobacterial strains were investigated in this study, of which nine strains were isolated from BBD and nine from other marine (non-BBD) sources (Table 1). The BBD strains originated from different geographical regions and from different coral host species. The non-BBD strains were isolated from sediment, microbial mats, and from the water column. Nine of the strains were the subject of previous research in related studies of BBD (see Table 1), while nine strains are new isolates. The taxonomic identification of most of the strains was carried out by sequencing of the 16S rRNA gene followed by a BLAST search for the identification of the closest relatives. Strain Alg was not sequenced. The sequence obtained from strain HS26 was related (97% similarity) to an uncultured cyanobacterium (accession number AM177427, clone Ct-3-39), and strain 10 was similarly (97%) related to an unidentified cyanobacterium (accession number EF372582, clone number not available) in the GenBank database. These three strains were identified by using classical methods based on morphology [25].
Toxin Production
According to both ELISA and PP2A data, all strains except for two had measurable levels of toxicity (Table 2). In each assay, the two (which were different between the two assays) that did not produce toxins were among the non-BBD strains. Based on the ELISA results (see “Discussion” section), the toxin content per cyanobacterial biomass varied between 0.01 and 0.27 μg g−1 and was found to be highest in Synechococcus sp. strain 63a-1, a non-BBD isolate from the water column (Tables 1, 2).
Table 2.
Quantification of mean microcystin-LR equivalents in cyanobacterial culture extracts using ELISA and PP2A assays
| ELISA | PP2A | ||
|---|---|---|---|
|
| |||
| μg MC g−1 | |||
| BBD strains | |||
| BBD 1991 | Geitlerinema | 0.02±0.0018 | 0.01±0.002 |
| HS 217 | Geitlerinema | 0.04±0.0015 | 0.03±0.005 |
| HS 223 | Geitlerinema | 0.03±0.0 | 0.01±0.002 |
| W-1 | Geitlerinema | 0.10±0.01 | 0.01±0.015 |
| FLK BBD1 | Leptolyngbya | 0.04±0.007 | 0.01±0.02 |
| Phil 2b-2 | Leptolyngbya | 0.01±0.002 | 0.01±0.0 |
| 102a-1 | Leptolyngbya | 0.02±0.0 | 0.03±0.005 |
| 102d-1 | Leptolyngbya | 0.08±0.002 | 0.01±0.015 |
| 96-4 | Spirulina | 0.12±0.02 | 0.03±0.003 |
| Non-BBD strains | |||
| 10 | Geitlerinema | 0.03±0.0 | 0.04±0.004 |
| Alg | Leptolyngbya | 0 | 0.02±0.003 |
| HS 26 | Leptolyngbya | 0 | 0.02±0.003 |
| 63a-5 | Pseudanabaena | 0.02±0.003 | 0.03±0.005 |
| 73-2 | Phormidium | 0.026±0.003 | 0.03±0.005 |
| 63-1 | Pseudanabaena | 0.023±0.003 | 0.03±0.005 |
| 72-1 | Pseudanabaena | 0.043±0.005 | 0.00 |
| 63a-1 | Synechococcus | 0.27±0.03 | 0.03±0.005 |
| 63a-3 | Synechococcus | 0.08±0.002 | 0.00 |
Environmental Factors
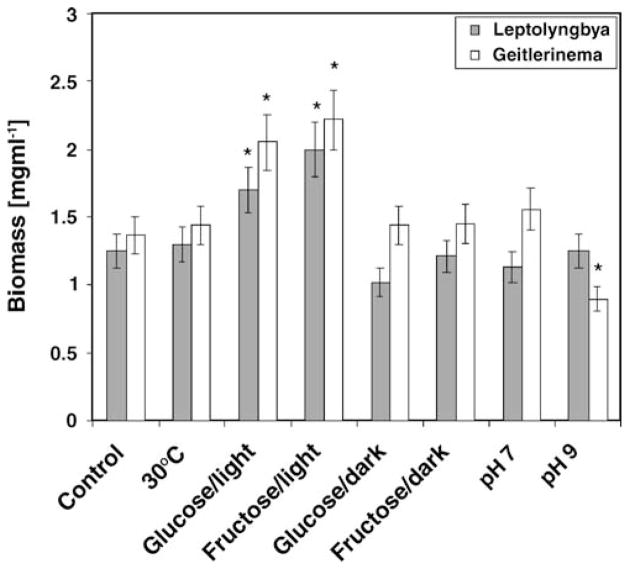
The effect of environmental factors on toxin production and culture growth was tested in two BBD strains, Geitlerinema W-1 and Leptolyngbya FLKBBD1. These results are presented in Fig. 1 (biomass yield) and Fig. 2 (toxin production). Biomass yield of both strains grown under the different environmental conditions showed similar patterns (Fig. 1). Statistical significance was tested against the control (photoautotrophic conditions at 27°C) using Duncan’s multiple comparison test. For both strains, a significant increase of biomass yield was obtained in the presence of fructose and glucose under light conditions (P<0.02). Significantly reduced biomass yield was seen for the Geitlerinema strain at pH 9. The other environmental conditions did not significantly affect the growth yield.
Figure 1.
Biomass yield of Leptolyngbya sp. strain FLK BBD1 and Geitlerinema sp. strain W-1 when grown under different environmental conditions. The control culture was grown photoautotrophically at 27°C and pH8.0. (Asterisks indicate statistically significant differences compared to control)
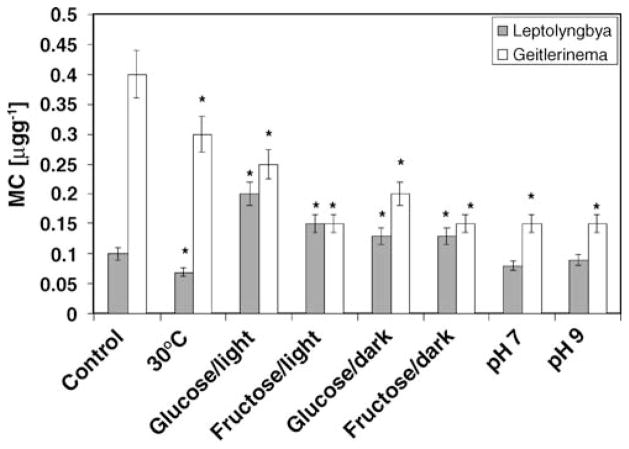
Figure 2.
Toxin production (microcystin-LR equivalent) per unit biomass of Leptolyngbya sp. strain FLK BBD1 and Geitlerinema sp. strain W-1 when grown under different environmental conditions. The control culture was grown photoautotrophically at 27°C and pH 8.0. (Asterisks indicate statistically significant differences compared to control)
The highest toxin production was exhibited by Geitlerinema sp. strain W-1 when the culture was grown under photoautotrophic conditions at 27°C, with the second and third highest production under photoautotrophic growth at 30°C and mixotrophic growth with glucose, respectively. Elevated toxin production was also observed in cultures containing glucose in darkness. For this isolate, the toxin level/biomass values varied between different environmental conditions by a factor of 2.6. In the case of Leptolyngbya FLKBBD1, the highest level of toxin production occurred under mixotrophic conditions with glucose. The lowest level of toxin produced was recorded when the culture was grown photoautotrophically at 30°C.
Discussion
Much work has been carried out on cyanotoxins in freshwater systems. This work includes identification of toxin-producing strains and the relationship between toxin production and environmental factors [13, 54, 61]. Much of this research has been driven by the fact that cyanotoxins are recognized as hazardous to human health combined with observations of an increase in the occurrence of toxin-producing cyanobacterial blooms in freshwater sources used for human consumption [54]. In contrast, much less is known about toxic cyanobacteria in marine environments [46, 52].
We have recently conducted experiments in which we exposed fragments of apparently healthy coral to purified microcystin-LR at concentrations of 1, 50, and 100 μg L−1 [44]. We found that exposure to microcystin affected the structural integrity of the coral tissue (epidermis and gastrodermis) and resulted in extrusion of zooxanthellae from their normal location in the gastrodermis [44]. We also found that exposure to the lowest concentration of microcystin (1 μg L−1) promoted bacterial growth in the coral tissue [44]. Thus, microcystin appears to be both directly toxic to corals and to indirectly affect them by stimulating growth of at least some species of bacteria.
In the present study, 16 of 18 marine cyanobacterial strains tested were positive for production of cyanotoxins (Table 2). Nine of these strains were isolated from BBD, of which 100% were toxic. This increases the number of known BBD cyanotoxin-producering genera from two (Geitlerinema and Leptolyngbya) to three (the addition of Spirulina). These results also increase the number of marine strains that test positive for toxin. These include both pelagic (Synechococcus) and benthic (Geitlerinema, Phormidium, Pseudoanabaena) genera.
By using two different methods for toxin detection (ELISA and PP2A), we were able to determine that the toxins detected belong to the microcystin/nodularin group and that toxicity was more or less equally present among BBD and non-BBD strains. All tested strains had detectable levels of toxin using at least one of the applied methods. However, the concentrations of toxin obtained by the two different methods (ELISA and PP2A) varied considerably. This discrepancy in estimating microcystin concentration by different methods is known and well documented [56] and can be explained by the fact that cell extracts such as those used in our experiments may contain different levels of non-specific protein phosphatase inhibitors [30]. Furthermore, in some cases, the cyanobacterial extracts themselves contain phosphatase activity that masks the presence of toxins [53]. Because the ELISA technique is much more specific and is less likely to produce false-positive reactions, we used ELISA data to compare toxin production among the different strains.
The amount of cyanotoxin (microcystin/nodularin) produced per biomass dry weight varied considerably among the strains tested. In order to identify the strains with “high” and “low” content of cyanotoxin, we calculated the median value by using the ELISA data. The median was 0.035 μg microcystin equivalent per gram of dry weight. Five strains of the BBD isolates and three of the non-BBD strains produced cyanotoxin at levels higher than the median value (Table 2). The highest level of cyanotoxin production was found in non-BBD Synechococcus sp. strain 63a-1. While the data comparing levels of toxicity of the individual strains should be taken with caution since it is known that cyanobacteria grown in laboratory condition tend to lose the capability to produce cyanotoxins [34], it is interesting to note that Geitlerinema strain 1991 has been maintained in our laboratory as a unilalgal culture for 17 years. This strain produced a mean microcystin-LR equivalent of 0.02±0.002 μg MC g−1, which, although lower than that of the three much more recent BBD Geitlerinema isolates, was in the range of two of the BBD Leptolyngbya isolates (Table 2). The chemical identification of the cyanotoxin (s), which appear to be novel, are currently underway in our laboratory.
Our results on the potential widespread occurrence of microcystin/nodularin toxicity among marine cyanobacteria are in accordance with the results of Carmichael and Li [10] who showed that 85% of water samples from the Salton Sea contained detectable levels of microcystin. Marine cyanobacteria in which toxicity had been reported to date include members of the genera Oscillatoria, Synechococcus, and Synechocystis [10, 29] as well as one isolate each of Leptolyngbya and Geitlerinema isolated from BBD [43]. Here, we report for the first time measurable levels of toxicity detected in marine strains belonging to three additional genera, Phormidium, Pseudanabaena, and Spirulina.
Environmental Factors
Research using freshwater strains of toxic cyanobacteria has shown that cyanotoxin, in particular microcystin, production and level of toxicity are controlled by nutrient and light levels as well as temperature [23, 27, 52, 57, 58, 61, 63]. These results have shown that microcystin production appears to be tied to energy production in that both relatively high light and replete or enhanced nutrients produced the highest levels of toxin. On the other hand, elevated temperatures were found to decrease the production of microcystin [61]. Watanabe and Oishi [61] and Van der Westhuizen and Eloff [57] both showed that lower temperatures result in higher toxin production in Microcystis aeruginosa. Similarly, Sivonen and Jones [54] showed that microcystin and nodularin levels in Anabaena, Microcystis, and Nodularia were the highest between 18°C and 25°C as opposed to higher temperatures.
Much less work has been done on the relationship between cyanotoxin production and the environment for marine cyanobacteria [46]. In general, based on the positive correlation between microcystin and energy production, one would expect that marine cyanobacteria, including BBD cyanobacteria, would also produce more microcystin with elevated nutrients and light. Field studies have demonstrated that increased nutrient levels can increase both BBD virulence and prevalence [24, 47, 60], which would agree with an increase in microcystin synthesis in response to nutrients. The effect of elevated temperature, which decreases microcystin/nodularin production in freshwater strains, might be expected to be different for BBD isolates. Field studies of the prevalence and incidence of BBD on reefs of the wider Caribbean have shown that the disease is seasonal, usually occurring during the warmer months of the year when temperatures are above 27.5°C (reviewed in Richardson [40]). Therefore, elevated temperature may induce synthesis of more cyanotoxin by BBD cyanobacteria, a pattern that would be the opposite of that found in work with freshwater strains.
In addition to the roles of nutrients, light, and temperature on cyanotoxin production, other environmental factors must be considered in studies of microcystin synthesis in BBD. BBD cyanobacteria are exposed to widely varying environmental conditions within the microbial mat that constitutes the band. It has been shown using oxygen- and sulfide-sensitive microelectrodes that the band fluctuates between conditions of anoxia/sulfide and super-saturated O2, with corresponding changes in pH, with the presence or absence of photosynthesis as is typical of other illuminated cyanobacterial mats [7]. The band itself contains high levels of organic compounds and nutrients due to the lysing coral tissue, making the mat environment very different from surrounding reef waters, other benthic environments on the reef, and the pelagic zone of freshwater lakes where most of the work on environmental effects on cyanotoxin production have been carried out.
In this work, we tested several environmental conditions for their potential effect on cyanotoxin production using two of the BBD cyanobacterial strains (Geitlerinema sp. strain W-1 and Leptolyngbya sp. strain FLKBBD1). We found that the environmental conditions investigated had different effects on cyanotoxin production (Fig. 2) in these two strains even though the overall pattern of biomass yield with the varying environmental conditions was the same in both organisms (Fig. 1). Temperature had a significant effect on toxin production in both strains, but no effect on biomass yield. Higher cyanotoxin production was detected at the lower (27°C) rather than at the higher temperature (30°C) for both isolates. Therefore, despite the fact that these temperatures were selected based on observations that BBD activity normally increases at temperatures in the range of 27.5°C to 30°C on Caribbean reefs, this temperature threshold does not appear to be related to any potential role of cyanobacteria in seasonal BBD incidence patterns in terms of toxin production [42]. Our results are, therefore, in accordance with the findings of others working with freshwater strains, as discussed above, and it appears that lower temperatures promote toxin production [54, 57, 61]
Supplementation of the mineral media used in our study with sugars also had an effect on toxin production. Toxin production was higher under both mixo- (organic carbon and light) and heterotrophic (organic carbon and dark) conditions for the Leptolyngbya isolate for both glucose and fructose when compared to the photoautotrophic control. In contrast, the Geitlerinema isolate produced lower amounts of toxin in the presence of both sugars (in the light and dark). Despite the differences in the effect of sugars on toxin production, both glucose and fructose stimulated significantly higher growth yields under mixotrophic conditions for both isolates. Toxin production was relatively lower for both strains grown photoautotrophically at pH 7 and 9 when compared to the control (pH 8). While the Leptolyngbya isolate showed no effect of pH on growth yield, the Geitlerinema isolate yielded less biomass at pH 9.
The role of cyanotoxins in BBD etiology appears to be complex, targeting the coral animal, zooxanthellae, and coral-associated bacteria [44]. While every BBD cyanobacterial isolate tested to date has been positive for toxin production, we have only investigated two strains of BBD cyanobacteria of two genera (Geitlerinema and Leptolyngbya) in terms of the effect of environment on toxin production. As discussed previously, microscopic and molecular studies indicate that there are multiple BBD cyanobacterial species. It may be that different BBD cyanobacteria produce cyanotoxin at different points in the pathobiology of BBD, with some strains producing toxin at the early part of the disease season (lower temperatures) and others producing toxin when the disease takes hold and tissue lysis begins (stimulation by organic carbon). It has been shown that for the cyanobacterium Planktothrix sp. FP1, paralytic shellfish toxin synthesis can be increased up to 61% by addition of allantoic acid or by bacterial cell extract [35]. We have shown that by changing environmental conditions, toxin production by BBD cyanobacteria can be increased up to 2.6 times (>100%), demonstrating that cyanotoxin production within BBD can indeed be affected by physicochemical factors.
We found [44] that, in general, purified microcystin stimulated growth of bacteria isolated from BBD and inhibited growth of bacteria isolated from the surface mucus layer of apparently healthy corals. Therefore, microcystin, besides affecting the coral tissue, may have a role in structuring the BBD microbial community. The presence of bacteria in our BBD cyanobacterial cultures may have had an effect on our results. It may be that BBD bacteria stimulate microcystin production by BBD cyanobacteria just as BBD cyanobacterial microcystin production stimulates growth of some BBD bacteria. At the same time, the potential role of bacteria in degradation of microcystin should not be excluded.
In conclusion, we have shown that cyanotoxin production among marine cyanobacteria is more widespread than previously demonstrated. We found that all BBD cyanobacteria tested were toxic and that seven of nine non-BBD strains were also toxic. These cultures represent seven cyanobacterial genera, including three (Phormidium, Pseudanabaena, and Spirulina) previously not known to be toxic in marine environments. We have shown that temperature affects microcystin/nodularin production by BBD cyanobacteria in the same manner as exhibited by freshwater strains, and that toxin production by BBD cyanobacteria is also affected by sugars and pH. Since all strains of cyanobacteria isolated from BBD to date have been shown to produce cyanotoxins and since the environment of BBD is dynamic in terms of organic carbon, pH, temperature, nutrients, and light, we predict that the contribution of cyanotoxins to BBD pathobiology will prove to be highly complex.
Acknowledgments
We thank Elizabeth Remily, Longin Kaczmarsky, and Joshua Voss for field assistance and Kathleen Rein for providing purified microcystin-LR. The comments of three anonymous reviewers improved this manuscript. This research was supported by NIH (NIH/ NIGMS SO6GM8205) and FIU. Sample collection in the Florida Keys National Marine Sanctuary was conducted under permit numbers FKNMS-2003-011 and FKNMS-2005-010. This is contribution 166 of the Tropical Biology Program at Florida International University.
References
- 1.Al-Moghrabi M. Unusual black band disease (BBD) outbreak in the northern tip of the gulf of Aquaba (Jordan) Coral Reefs. 2001;19:330–331. [Google Scholar]
- 2.Altschul SF, Gish W, Miller W, Meyers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.An J, Carmichael WW. Use of colorimetric protein phosphatase assay and enzyme linked immunoassay for the study of microcystins and nodularins. Toxicon. 1994;12:1495–1507. doi: 10.1016/0041-0101(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 4.Antonius A. New observations on coral destruction in reefs. 10th Mtg Assoc Isl Mar Lab Caribb; 1973. p. 3. [Google Scholar]
- 5.Barneah O, Ben-Dov E, Kramarsky-Winter E, Kushmaro A. Characterization of black band disease in Red Sea stony corals. Environ Microbiol. 2007;9:1995–2006. doi: 10.1111/j.1462-2920.2007.01315.x. [DOI] [PubMed] [Google Scholar]
- 6.Boyett HV, Bourne G, Willis BL. Elevated temperature and light enhance progression and spread of black band disease on staghorn corals of the Great Barrier Reef. Mar Biol. 2007;151:1711–1720. [Google Scholar]
- 7.Carlton RG, Richardson LL. Oxygen and sulfide dynamics in a horizontally migrating cyanobacterial mat: black band disease of corals. FEMS Microbiol Ecol. 1995;18:155–162. [Google Scholar]
- 8.Carmichael WW, Beasley V, Bunner D, Eloff J, Falconer I, Gorham P, Harada K, Yu M, Krishnamurthy T, Moore RE, Rinehart K, Runnegar M, Skulberg O, Watanabe W. Naming of cyclic heptapeptide toxins of cyanobacteria (blue-green algae). Letter to the Editor. Toxicon. 1988;26:971–973. doi: 10.1016/0041-0101(88)90195-x. [DOI] [PubMed] [Google Scholar]
- 9.Carmichael WW, An J-S. Using an enzyme immunosorbent assay (ELISA) and a protein phosphatase inhibition assay (PP1A) for the detection of microcystins and nodularins. Nat Toxins. 1999;7:377–385. doi: 10.1002/1522-7189(199911/12)7:6<377::aid-nt80>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Carmichael WW, Li RH. Cyanobacteria toxins in the Salton Sea. Saline Systems. 2006;2:5–18. doi: 10.1186/1746-1448-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castenholz RW. Culturing methods for cyanobacteria. Methods Enzymol. 1988;167:68–93. [Google Scholar]
- 12.Cohen Y, Jørgensen BB, Revsbech NP, Poplawski R. Adaptation to hydrogen sulfide of oxygenic and anoxygenic photosynthesis among cyanobacteria. Appl Environ Microbiol. 1986;51:398–407. doi: 10.1128/aem.51.2.398-407.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Codd GA, Bell SG, Kaya K, Ward CJ, Beattie K, Metcalf JS. Cyanobacterial toxins, exposure route and human health. Eur J Phycol. 1999;34:405–415. [Google Scholar]
- 14.Cooney RP, Pantos O, Le Tissier MDA, Barer MR, O’Donell A, Bythell JC. Characterization of the bacterial consortium associated with black band disease in coral using molecular microbiological techniques. Environ Microbiol. 2002;4:401–413. doi: 10.1046/j.1462-2920.2002.00308.x. [DOI] [PubMed] [Google Scholar]
- 15.Dere S, Gunes T, Sivaci R. Spectrophotometric determination of chlorophyll-a, b and total carotenoid contents of some algae species using different solvents. Tr J Botany. 1998;22:13–17. [Google Scholar]
- 16.Dinsdale E. Abundance of black band disease on corals from one location on the Great Barrier Reef: a comparison with abundance in the Caribbean region. Proc 9th Intl Coral Reef Symp. 2002;2:1239–1244. [Google Scholar]
- 17.Ducklow HW, Mitchell R. Observations on natural and artificial diseased tropical corals: A scanning electron microscope study. Microb Ecol. 1979;5:215–223. doi: 10.1007/BF02013528. [DOI] [PubMed] [Google Scholar]
- 18.Fisher WJ, Garthwaite I, Miles CO, Ross KM, Aggen JB, Chamberlin AR, Towers NA, Dietrich DR. Congener-independent immunoassay of microcystins and nodularins. Environ Sci Technol. 2001;35:4849–4858. doi: 10.1021/es011182f. [DOI] [PubMed] [Google Scholar]
- 19.Frias-Lopez J, Zerkle AL, Bonheyo GT, Fouke BW. Partitioning of bacterial communities between seawater and healthy, black band diseased, and dead coral surfaces. Appl Environ Microbiol. 2002;68:2214–2228. doi: 10.1128/AEM.68.5.2214-2228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frias-Lopez J, Bonheyo GT, Jin S, Fouke BW. Cyanobacteria associated with coral with coral black band disease in Caribbean and Indo-Pacific reefs. Appl Environ Microbiol. 2003;69:2409–2413. doi: 10.1128/AEM.69.4.2409-2413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrett P, Ducklow H. Coral diseases in Bermuda. Nature. 1975;253:349–350. [Google Scholar]
- 22.Hooser SB. Fulminant hepatocyte apoptosis in vivo following microcystin LR administration to rats. Toxicol Path. 2000;28:762–733. doi: 10.1177/019262330002800513. [DOI] [PubMed] [Google Scholar]
- 23.Kaebernick M, Neilan B. Ecological and molecular investigation of cyanotoxin production. FEMS Microbiol Ecol. 2001;35:1–9. doi: 10.1111/j.1574-6941.2001.tb00782.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaczmarsky LT, Draud M, Williams EH. Is there a relationship between proximity to sewage effluent and the prevalence of coral disease? Carib J Sci. 2005;41:124–137. [Google Scholar]
- 25.Komarek J, Anagnostidis K. Modern approach to the classification system of cyanophytes 2-Chroococcales. Arch Hydrobiol/Suppl 80 Algol Studies. 1986;43:157–226. [Google Scholar]
- 26.Lawton LA, Edwards C, Codd GA. Extraction and high-performance liquid chromatographic method for the determination of microcystins in raw and treated waters. Analyst. 1994;119:1525–1530. doi: 10.1039/an9941901525. [DOI] [PubMed] [Google Scholar]
- 27.Lee SJ, Jang MH, Kim HS, Yoon BD, Oh HM. Variation of microcystin content of Microcystis aeruginosa relative to medium N:P ratio and growth stage. Appl Microbiol. 2000;89:323–329. doi: 10.1046/j.1365-2672.2000.01112.x. [DOI] [PubMed] [Google Scholar]
- 28.MacKintosh C, Beattie KA, Klumpp S, Cohen P, Codd G. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatase 1 and 2A from mammals and higher plants. FEBS Lett. 1990;264:187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- 29.Martins R, Pereira P, Welker M, Fastner J, Vasconcelos VM. Toxicity of culturable cyanobacteria strains isolated from the Portuguese coast. Toxicon. 2005;46:454–464. doi: 10.1016/j.toxicon.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Metcalf JS, Bell SG, Codd GA. Colorimetric immunoprotein phosphatase inhibition assay for specific detection of microcystins and nodularins of Cyanobacteria. Appl Environ Microbiol. 2001;67:904–909. doi: 10.1128/AEM.67.2.904-909.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers JL, Sekar R, Richardson LL. Molecular Detection and ecological significance of the cyanobacterial genera Geitlerinema and Leptolyngbya in black band disease of corals. Appl Environ Microbiol. 2007;73:5173–5182. doi: 10.1128/AEM.00900-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myers JL, Richardson LL. Adaptation of cyanobacteria to the sulfide-rich microenvironment of black band disease of coral. FEMS Microbiol Ecol. 2009;67:242–251. doi: 10.1111/j.1574-6941.2008.00619.x. [DOI] [PubMed] [Google Scholar]
- 33.Nübel UF, Garcia-Pichel F, Muyzer G. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl Environ Microbiol. 1997;63:3327–3332. doi: 10.1128/aem.63.8.3327-3332.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prati M, Molteni M, Pomati F, Rossetti C, Bernardini G. Biological effect of the Planktothrix sp. FP1 cyanobacterial extract. Toxicon. 2002;40:267–272. doi: 10.1016/s0041-0101(01)00212-4. [DOI] [PubMed] [Google Scholar]
- 35.Pomati F, Manarolla G, Rossi O, Vigetti D, Rossetti C. The purine degradation pathway possible role in paralytic shellfish toxin metabolism in the cyanobacterium Planktothrix sp. FP1. Environ Internat. 2001;27:463–470. doi: 10.1016/s0160-4120(01)00101-5. [DOI] [PubMed] [Google Scholar]
- 36.Ragoonath DN. MS thesis. Florida International University; Miami, Fl: 2005. Heterotrophic capabilities and the molecular identification of a cyanobacterium found in black band disease of coral reefs. [Google Scholar]
- 37.Ramos AG, Martel A, Codd GA, Soler E, Coca J, Redondo A, Morrison LF, Metcalf JS, Ojeda A, Suarez S, Petit M. Bloom of the marine diazotrophic cyanobacterium Trichodesmium erythraeum in the northwest African upwelling. Mar Ecol Prog Ser. 2005;301:303–305. [Google Scholar]
- 38.Richardson LL. Horizontal and vertical migration patterns of Phormidium corallyticum and Beggiatoa spp. associated with black band disease of corals. Microb Ecol. 1996;32:323–335. doi: 10.1007/BF00183066. [DOI] [PubMed] [Google Scholar]
- 39.Richardson LL, Kuta KG, Schnell S, Carlton RG. Ecology of the black band disease microbial consortium. Proc 8th Intl Coral Reef Symp. 1997;1:597–600. [Google Scholar]
- 40.Richardson LL. Coral diseases: what is really known? TREE. 1998;13:438–443. doi: 10.1016/s0169-5347(98)01460-8. [DOI] [PubMed] [Google Scholar]
- 41.Richardson LL, Kuta KG. Ecological physiology of the black band disease cyanobacterium Phormidium corallyticum. FEMS Microbiol Ecol. 2003;43:287–298. doi: 10.1016/S0168-6496(03)00025-4. [DOI] [PubMed] [Google Scholar]
- 42.Richardson LL. Black band disease. In: Rosenberg E, Loya Y, editors. Coral Health and Disease. Springer-Verlag; Berlin: 2004. pp. 325–336. [Google Scholar]
- 43.Richardson LL, Sekar R, Myers J, Gantar M, Voss J, Kaczmarsky L, Remily E, Boyer G, Zimba P. The presence of the cyanobacterial toxin microcystin in black band disease of corals. FEMS Microbiol Lett. 2007;272:182–187. doi: 10.1111/j.1574-6968.2007.00751.x. [DOI] [PubMed] [Google Scholar]
- 44.Richardson LL, Miller AW, Broderick E, Kaczmarsky L, Gantar M, Stanić D, Sekar R. Sulfide, microcystin, and the etiology of black band disease. Dis Aq Org. 2009 doi: 10.3354/dao02083. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- 46.Roelfsema CM, Phinn SR, Dennison WC, Dekker AG, Brando VE. Monitoring toxic cyanobacteria Lyngbya majuscula (Gomont) in Moreton Bay, Australia by integrating satellite image data and field mapping. Harmful Algae. 2006;5:45–56. [Google Scholar]
- 47.Rodriguez S, Croquer A. Dynamics of black band disease in a Diploria strigosa population subjected to annual upwelling on the northeastern coast of Venezuela. Coral Reefs. 2008;27:381–388. [Google Scholar]
- 48.Rützler K, Santavy D. The black band disease of Atlantic reef corals: I Description of a cyanophyte pathogen. PSZNI: Mar Ecol. 1983;4:301–319. [Google Scholar]
- 49.Rützler K, Santavy DL, Antonius A. The black band disease of Atlantic reef corals: III Distribution, ecology and development. PSZNI: Mar Ecol. 1983;4:329–358. [Google Scholar]
- 50.Sekar R, Mills DK, Remily ER, Voss JD, Richardson LL. Microbial communities in the surface mucopolysaccharide layer and the black band microbial mat of black band diseased Siderastrea siderea. Appl Environ Microbiol. 2006;72:5963–5973. doi: 10.1128/AEM.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sekar R, Kaczmarsky LT, Richardson LL. Microbial community composition of black band disease on the coral host Siderastrea siderea from three regions of the wider Caribbean. Mar Ecol Prog Ser. 2008;362:85–98. [Google Scholar]
- 52.Sellner KG. Physiology, ecology, and toxic properties of marine cyanobacteria blooms. Limnol Oceanogr. 1997;42:1089–1104. [Google Scholar]
- 53.Sim ATR, Mudge LM. Protein phosphatase activity in cyanobacteria-consequences for microcystin toxicity analysis. Toxicon. 1993;31:1179–1186. doi: 10.1016/0041-0101(93)90133-4. [DOI] [PubMed] [Google Scholar]
- 54.Sivonen K, Jones G. Cyanobacterial toxins. In: Chorus I, Bartram J, editors. Toxic Cyanobacteria in Water. A Guide to their Public Health Consequences, Monitoring and Management. E. and FN Spoon; London: 1999. pp. 41–111. [Google Scholar]
- 55.Sutherland KP, Porter JW, Torres C. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar Ecol Prog Ser. 2004;266:273–302. [Google Scholar]
- 56.Tillmanns AR, Pick FR, Aranda-Rodriguez R. Sampling and analysis of microcystins: Implications for the development of standard methods. Environ Toxicol. 2007;22:132–143. doi: 10.1002/tox.20250. [DOI] [PubMed] [Google Scholar]
- 57.Van der Westhuizen AK, Eloff JN. Effects of temperature and light on toxicity and growth of the blue-green alga Microcystis aeruginosa [UV-006] Planta. 1985;163:55–59. doi: 10.1007/BF00395897. [DOI] [PubMed] [Google Scholar]
- 58.Vezie C, Rapala J, Vaitomaa J, Seitsonen J, Sivonen K. Effect of nitrogen and phosphorus on growth of toxic and nontoxic Microcystis strains and on intracellular microcystin. Microb Ecol. 2002;43:443–454. doi: 10.1007/s00248-001-0041-9. [DOI] [PubMed] [Google Scholar]
- 59.Viehman S, Mills DK, Meichel GW, Richardson LL. Culture and identification of Desulfovibrio spp. from corals infected by black band disease on Dominican and Florida Keys reefs. Dis Aquat Org. 2006;69:119–1276. doi: 10.3354/dao069119. [DOI] [PubMed] [Google Scholar]
- 60.Voss J, Richardson LL. Nutrient enrichment enhances black band disease progression in corals. Coral Reefs. 2006;25:569–576. [Google Scholar]
- 61.Watanabe MF, Oishi S. Effects of environmental factors on toxicity of a cyanobacterium (Microcystsic aeruginosa) under culture conditions. Appl Env Microbiol. 1985;49:1342–1344. doi: 10.1128/aem.49.5.1342-1344.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weil E. Coral reef diseases in the Wider Caribbean. In: Rosenberg E, Loya Y, editors. Coral Health and Disease. Springer-Verlag; Berlin: 2004. pp. 35–68. [Google Scholar]
- 63.Wiedner C, Visser PM, Fastner J, Metcalf JS, Codd GA, Mur LR. Effects of light on the microcystin content of Microcystis strain PCC 7806. Appl Environ Microbiol. 2003;69:1475–1481. doi: 10.1128/AEM.69.3.1475-1481.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]