Abstract
Black band disease (BBD) consists of a cyanobacterial dominated, sulfide-rich microbial mat that migrates across coral colonies degrading coral tissue. The mat contains diverse bacteria that include photoautotrophs (cyanobacteria), sulfate-reducers, sulfide-oxidizers, and organoheterotrophs. It is known that BBD sulfate-reducers contribute to BBD pathobiology by production of sulfide, which causes coral tissue lysis and death, and that the cyanotoxin microcystin is produced by BBD cyanobacteria. In this study we used a model system of coral fragments to investigate the roles of sulfide and microcystin in BBD by exposure to the metabolic inhibitors Na molybdate and DCMU (3-(3’,4’-dichlorophenyl)-1,1-dimethylurea), which inhibit sulfate reduction and oxygenic photosynthesis respectively. Exposure of BBD inocula to Na molybdate prior to inoculation prevented infection of healthy fragments but did not prevent continued band migration and coral tissue lysis by previously inoculated BBD infections. Exposure to DCMU did not inhibit either the initiation of BBD or continued migration of active BBD. Exposure of healthy coral fragments to sulfide, purified microcystin, and a combination of both revealed that both microcystin and sulfide are toxic to coral and act synergistically. Exposure of bacteria isolated from BBD and the healthy coral surface mucopolysaccharide layer (SML) revealed that relatively more BBD isolates were stimulated by microcystin compared to coral SML isolates, although effects were not uniform and the majority exhibited no effect. Our results indicate that sulfide is required for initiation of BBD, that microcystin and sulfide induce coral tissue degradation, and that bacteria from healthy coral and BBD respond differently to microcystin.
Keywords: black band disease, coral disease, polymicrobial disease, microcystin, sulfide
INTRODUCTION
Black band disease (BBD) is one of the most widespread and destructive of coral diseases. Since it’s first record in the 1970s on reefs of the wider Caribbean (Antonius 1973) it has spread to the Red Sea, the Indo-Pacific, and the Great Barrier Reef (Antonius 1985, Antonius 1988, Dinsdale 2002). Although BBD prevalence is typically low (<1 %) compared to other coral diseases, it is considered to be severe due to the fact that it targets slow growing reef-framework scleractinian coral species that cannot outgrow the migrating, tissue-lysing band (Richardson 2004).
Studies of the epizootiology of BBD have focused on its effects on coral reef host populations (Edmunds 1991, Kuta & Richardson 1996, Bruckner & Bruckner 1997, Dinsdale 2002) as well as the relationship between disease dynamics and environmental factors such as nutrients and temperature (Kuta & Richardson 2002, Kaczmarsky 2004, Rodriguez & Croquer 2007). Additionally a large number of studies have been carried out to describe BBD-associated microorganisms using both microscopic (Garrett & Ducklow 1975, Ducklow & Mitchell 1979, Rützler & Santavy 1983, Rützler et al. 1983) and molecular (Cooney et al. 2002, Frias-Lopez et al. 2004, Sekar et al. 2006, 2008) approaches. To date the cumulative results of these studies suggest that BBD is a polymicrobial disease and that disease etiology is based on diverse members of four BBD physiological functional groups - photoautotrophs (cyanobacteria), sulfate-reducers, sulfide-oxidizers, and organoheterotrophs (Richardson 2004). Studies of BBD using molecular community profiling techniques suggest that the specific members of each of these four groups are highly variable across geographic region and host coral species (Cooney et al. 2002, Frias-Lopez et al. 2004, Myers et al. 2007, Sekar et al. 2008).
Relatively little work has been conducted documenting the specific toxins or toxicants involved in scleractinian coral disease pathobiology. To date it is known that a toxic peptide is involved in bacterial bleaching of Vibrio shiloi (Banin et al. 2001) and an extracellular factor causes tissue lysis associated with “white plague-like” disease of corals (Barash et al. 2005). We have been working on identifying the toxins and toxicants associated with BBD. It is well known that three of the four major BBD physiological groups are associated with production of harmful compounds. The sulfide produced by BBD (and other) sulfate reducers is toxic to eukaryotes in general (Vismann 1991) and has been shown to cause tissue lysis and coral death in corals exposed to concentrations measured in intact BBD infections using sulfide-sensitive microelectrodes (Carlton & Richardson 1995, Richardson et al. 1997). We have recently determined that the cyanotoxin microcystin is present in BBD and produced in cultures of cyanobacteria isolated from BBD (Richardson et al. 2007, Gantar and Richardson, unpublished data). Microcystin, a potent toxin that inhibits cellular processes such as protein phosphatase (Sim & Mudge 1993) and can induce apoptosis (Hooser 2000), is potentially active in coral tissue death. While we have not documented or measured toxins produced by BBD organoheterotrophic bacteria, we have repeatedly detected sequences most closely homologous to bacteria associated with toxic dinoflagellates in our studies of BBD bacterial communities using cloning and sequencing of the 16S rRNA gene (Sekar et al. 2006, 2008).
In this report we present results of experiments in which we used metabolic inhibitors to disrupt the major energy yielding metabolic pathways associated with two of the four major BBD bacterial physiological groups, oxygenic photosynthesis of BBD cyanobacteria and sulfate reduction of BBD sulfate reducers, to assess the contribution of these metabolic processes to BBD infection in corals. We additionally performed experiments in which healthy coral fragments were exposed to sulfide, microcystin, and a combination of the two, with the effects assessed using Scanning Electron Microscopy (SEM) to investigate the effects of these two BBD associated toxic compounds in BBD pathobiology. Finally, we assessed the effect of microcystin exposure on growth of bacteria isolated from BBD and the surface mucopolysaccharide layer (SML) of healthy coral colonies to determine a potential role of these compounds in the BBD and coral bacterial communities.
MATERIALS AND METHODS
Collection of coral fragments and black band inocula
Coral fragments were collected on reefs of Lee Stocking Island, Bahamas, for use in metabolic inhibition experiments, and from reefs of the Florida Keys for microcystin and sulfide exposure experiments, while SCUBA diving. All fragments were from the reef-framework species Montastraea annularis, and consisted of ‘skirt fragments’. This term refers to the edges of colonies (the ‘skirt’) which were chipped off using a chisel and hammer. Skirt fragments were approximately 3–5 cm by 3–4 cm. Fragments were placed immediately after collection into plastic bags (underwater) with ample seawater to allow the fragments to be suspended. For transport to the laboratory the bags were floated in a cooler with ambient temperature seawater.
Fresh BBD for inocula was collected from infected colonies of three coral species (Montastraea annularis, Siderastrea siderea, and Colpophyllia natans) on reefs of Lee Stocking Island using sterile 10 or 60 ml syringes by aspirating the band from the coral surface. After collection, syringes were maintained at ambient temperature (floated in freshly collected seawater in a cooler) until return to the laboratory. Once at the laboratory, BBD samples were placed in 125 ml Erlenmeyer flasks with approximately 100 ml of fresh seawater and placed in experimental flumes (see below). BBD samples were maintained overnight prior to infection the next morning. Experimental infections were conducted over two time periods in 2004 (August 8 to 12) and 2005 (July 11 to 15) to ensure that each experiment, which contained varying replicates (minimum of three per treatment) was repeated three times. In each experiment M. annularis fragments were infected.
Metabolic inhibition experiments
Metabolic inhibition experiments were conducted in the wet laboratory facilities at the Perry Institute for Marine Sciences, Lee Stocking Island, Bahamas. This facility contains flumes supplied with sand-filtered (2X) seawater. The flumes are housed in a greenhouse-type room with double-layer screening that acts as a neutral density filter.
Experimental chambers, consisting of 750 ml plastic containers, were placed in the flumes. For acclimation (pre-experiment) and control coral fragments, seawater continually flowed into each experimental chamber via a PVC pipe system. Each chamber had an outflow and the chambers themselves sat in approximately 6 cm of water in the flumes to maintain temperature at ambient (reef) levels.
Experimental coral fragments were placed, after acclimation and recovery from sampling, in one liter glass beakers placed in the experimental chambers. The beakers were raised above the chamber floor such that the overflow level of the chamber was slightly below the rim of the beaker. The inhibitors Na molybdate and DCMU (3-(3’,4’-dichlorophenyl)-1,1-dimethylurea) were added to the beakers, which contained experimental coral fragments. Temperature and light were the same as the control fragments in the chambers. Because there was no flow through of water in the experimental beakers (to prevent flushing of the inhibitors) each beaker was aerated near the surface using tubing attached to an air pump. The tubing was placed such that air bubbles would swirl around the circumference of the beaker with no direct bubbling onto the experimental corals.
To inoculate coral fragments a 3–4 mm diameter piece of BBD mat was collected using a Pasteur pipette. The clump was carefully placed near the edge of each experimental fragment on the surface of healthy coral tissue, and monitored visually. During the first 20 to 30 min, the aerator tubing was removed from the experimental beakers to prevent the inoculum clump from being dislodged from the coral surface by water movement. During this time period the coral would at times push the clump off of the coral surface, presumably via mucus extrusion. When this happened, the clump was retrieved using a Pasteur pipette and redeposited onto the coral fragment. In a few cases, the coral repeatedly removed the BBD inoculum; in these cases, the tip of a Pasteur pipette was positioned to rest on the clump to hold it onto the colony. In all cases it was visually apparent when the clump had firmly attached to the coral surface (the first step of infection).
For inhibition experiments the inhibitor Na molybdate, which inhibits sulfate reduction, was prepared using a stock solution (0.2 M) made in sterile seawater to a final concentration of 2 mM. To inhibit oxygenic photosynthesis DCMU was prepared in seawater to a final concentration of 5 µM from a 10−4 M stock solution (made in sterile seawater).
Two sets of inhibition experiments were conducted. In the first, the BBD inoculum was exposed overnight to the inhibitors prior to inoculation of healthy coral fragments. In these experiments inhibitors were added to the experimental beakers immediately after inoculation. In the second set of experiments, fragments were inoculated with BBD and the inhibitors added to experimental beakers only after the band had formed and was actively migrating across the coral. Control beakers had only seawater. For each experiment, migration and tissue lysis by BBD were observed and recorded, and documented by photographing the coral fragments.
Microcystin and sulfide exposure experiments
To assess the effects of microcystin and sulfide on corals, fragments of Montastraea annularis collected from the Florida Keys were exposed (after acclimation in laboratory aquaria) to these substances under controlled conditions in the laboratory. Exposure experiments were performed at Florida International University using plexiglass chambers constructed such that the interior could be stirred with a magnetic stirrer without disrupting the coral fragment (see below). Each chamber (volume = 150 ml) was first filled with 125 ml of ASN III (a mineral seawater medium). For microcystin exposure experiments microcystin was added to this media from a stock solution (100 mg l−1) of purified microcystin-LR (obtained from K. Rein, FIU) to final concentrations of 1 µg l−1, 50 µg l−1, or 100 µg l−1. For sulfide exposure experiments, chambers containing ASN III (with microcystin when appropriate) were first bubbled (in a hood) with 100% reagent grade N2 gas for 20 minutes and capped with a rubber stopper. To add sulfide the stopper was carefully lifted, and the sulfide was added using a syringe under a stream of N2 gas. Sulfide was added from a stock solution of 0.1 M Na2S·9H2O to a final concentration of 0.5 mM.
In these experiments a small coral fragment (approximately 1 cm2) was placed on a stand in the center of the chamber and held in place with a small piece of modeling clay. The stand was elevated to allow the presence of a stirbar at the base of the chamber. In each experiment one fragment was maintained as a control (no microcystin or sulfide were added). Experimental conditions (with duplicate fragments for each condition in each experiment) were as follows: microcystin (MC-LR) at 1 µg l−1, 50 µg l−1, and 100 µg l−1 (no sulfide); 50 µg l−1 microcystin plus 0.5 mM sulfide; 100 µg l−1 microcystin plus 0.5 mM sulfide; and 0.5 mM sulfide (no microcystin). In all experiments using sulfide, the coral fragment was mounted on the stand in the chamber under a stream of N2 gas and immediately capped with a rubber stopper prior to introduction of sulfide, which was then introduced as described above.
Experimental chambers were maintained at 30 °C in a 15 l aquarium with each chamber positioned above a magnetic stirrer. Light was maintained at 246 +/− 9.6 µE m2 sec−1. Experiments (each conducted in duplicate except for the control) were repeated for a total of 3 times. Incubations (exposure to microcystin and/or sulfide) ranged from 18 to 22.5 hours before harvesting for SEM, at which time fragments were removed from the chambers and photographed on a Leica Mz6 dissecting scope with a Leica DC 500 digital camera system.
Scanning electron microscopy
In preparation for viewing with SEM, harvested fragments were fixed in a solution of 2% gluteraldehyde in 0.05 M sodium cacodylate buffered sea-water fixative and maintained at 4 °C until processing. To process samples, fragments were placed in 0.05 M sodium cacodylate buffer made with filtered seawater (three changes at 10 min each), and post-fixed in 1% osmium tetroxide for 45 min (added directly to the buffer). Fragments were partially decalcified in a solution of 22% formic acid buffered with 10% sodium citrate, after which they went through another series of three changes of cacodylate buffer (10 min each). Samples were then dehydrated through a series of graded ethanols by placing them in three washes of each concentration of 20%, 40%, 60%, 70%, 90%, and 100% ethanol (10 min for each wash). This was followed by further dehydration (three changes at 10 min each) using Hexamethyldisilazane (HMDS) with out-gassing overnight. Fragments were affixed to an aluminum stub using carbon adhesive tape, and were coated in a thin layer of palladium using a sputter coater (Cressington 108 Sputtercoater, Cressington Scientific). Fragments were viewed using a Philips XL30 ESEM-FEG located at the University of Miami Center for Advanced Microscopy.
The effect of microcystin on bacterial growth
Bacteria used in this study were isolated from BBD on infected colonies, and the SML of apparently healthy colonies, of Siderastrea siderea on reefs of the Florida Keys (SML) and Lee Stocking Island, Bahamas (BBD). Additionally, cultures of the known coral pathogens Aurantimonas coralicida, Vibrio shiloi, and Serratia marcescens were tested.
To obtain isolates the black band mat or the SML were sampled using sterile needleless 10 ml syringes. Samples were placed in cryovials, maintained at ambient (seawater) temperature, and upon return to the lab plated onto Difco marine agar 2216. After incubation at room temperature colonies with different morphologies were picked and replated to purity. Cultures were maintained at room temperature on marine agar slants. For this study, 10 isolates from BBD, 12 from the SML of apparently healthy corals, and three known coral pathogens were used. BBD isolates were cloned and sequenced, with sequences BLAST searched in GenBank for identification, as described in Sekar et al. 2006.
Prior to the microcystin exposure experiments, each isolate was first grown in marine broth for two days. For each experiment three different concentrations (1, 100 and 500 µg l−1) of microcystin-LR were used for each isolate with assays conducted in plastic 96 well micro plates in triplicate. For each treatment 20 µl of microcystin LR (dissolved in methanol at the appropriate concentration) were added to experimental wells and placed under a sterile hood to evaporate the solvent. The control wells did not contain microcystin. After evaporation 190 µl of marine broth (Diffco) were added to all wells. To start the experiment 10µl of each bacterial culture was added to all control and experimental wells (each in triplicate), and incubated at 26° C overnight for 12h. Control and experimental wells were inoculated from the same culture at the same time. Optical densities of the plates were read on a microplate reader (BioTek, model Synergy2) at 405 nm after the 12 h incubation period. The effect of the different concentrations of microcystin on bacterial growth was calculated by averaging the final concentration (optical density) of bacteria in both the triplicate control and experimental wells. The control and experimental wells were then statistically compared by a two-sample t-Test assuming equal variances with an alpha value of 0.05.
RESULTS
Metabolic inhibition experiments
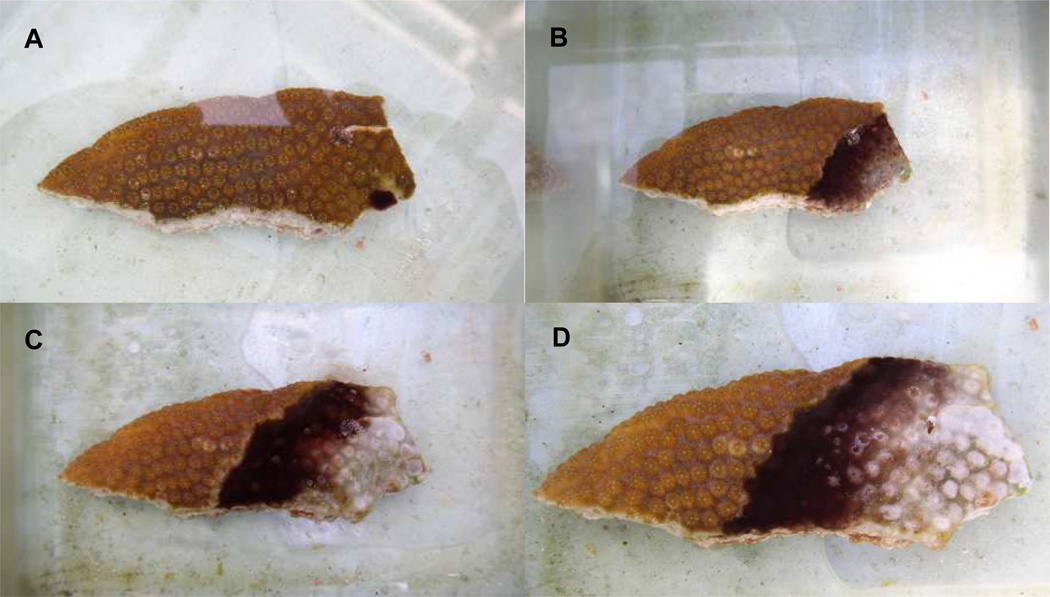
The model used in this study is shown in Figure 1. Inoculation (Figure 1a) was always performed near the edge of a healed fragment. The coral was not wounded prior to infection. Such inoculation resulted in formation of a band within one day, which then actively migrated across the fragment, lysing coral tissue similar to naturally occurring BBD infections observed in the field (Figures 1B–D).
Figure 1.
Black band infection model. Coral fragments are from the host Montastraea annularis. A. t = 0 (immediately after inoculation). B. t = 3 days. C. t = 7 days. D. t = 8 days.
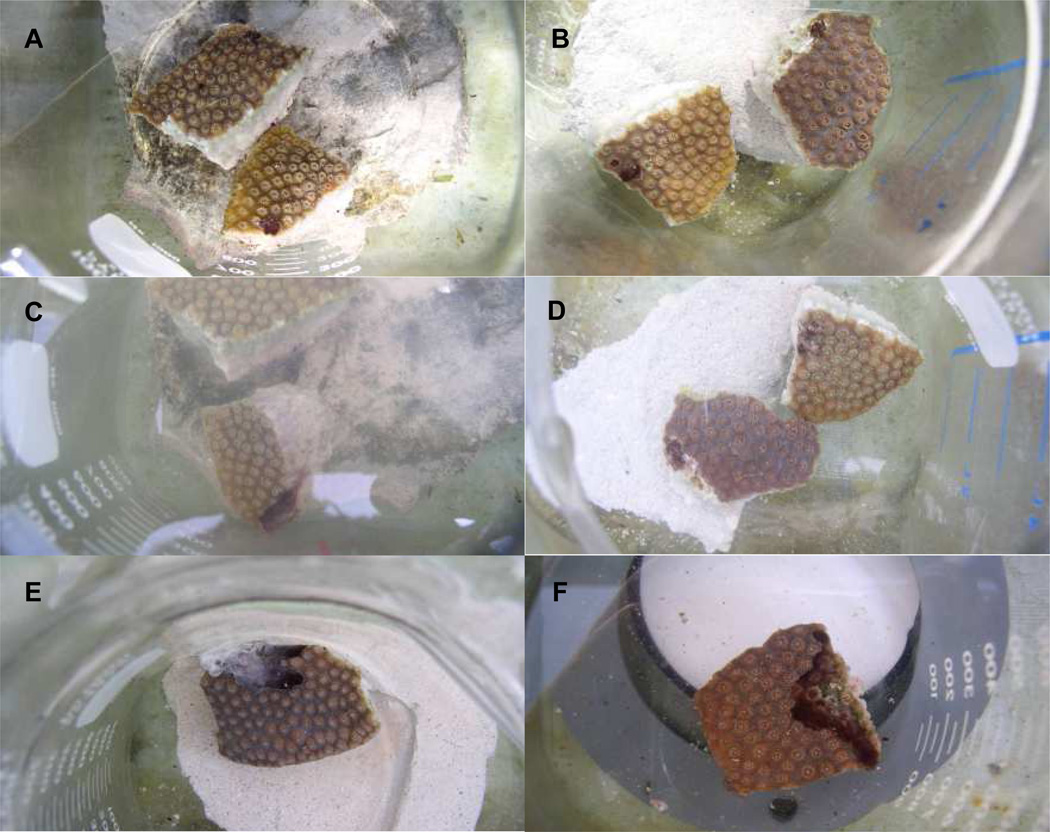
To assess the effect of the inhibitors DCMU and Na molybdate on BBD infection BBD inocula were exposed to each inhibitor overnight with infection occurring the following morning. Figure 2A–D show the results of one representative experiment. In all cases (n = 3 experiments, minimum of 3 replicates per experiment) DCMU failed to prevent infection. As seen in Figure 2C a band formed similar to the control and progressed across the coral fragment, lysing tissue. In contrast, exposure to Na molybdate prevented BBD infection in all cases attempted (Figure 2D). These results indicate that sulfate reduction is required to initiate BBD from a freshly collected BBD microbial mat. These results also indicate that oxygenic photosynthesis is not required for infection.
Figure 2.
Effect of the inhibitors DCMU and Na molybdate on black band infection (A–D) and on an actively progressing disease band (E,F). Coral fragments are from the host Montastraea annularis. In panels A and B the fragments were inoculated with BBD exposed (prior to inoculation) to DCMU (A) and Na molybdate (B), both at t = 0. Panels C and D are the same fragments at t = 31 hours. Panels E and F show fragments that had been successfully inoculated with BBD (bands had formed and were actively migrating) and then exposed to DCMU (E) and Na molybdate (F). Both panels are at t = 3 days. The white material on the surface of the mat in panel E is the population of the sulfide-oxidizer Beggiatoa, which has risen to the mat surface following the oxygen/sulfide inteface.
The second set of experiments involved exposure of coral fragments with actively migrating BBD to the two inhibitors (DCMU and Na molybdate). As seen in Figures 1 E (DCMU) and F (Na molybdate), the migrating bands continued to migrate in the presence of both inhibitors. These results show that disrupting sulfate reduction or oxygenic photosyntheseis had no effect on active BBD disease. Therefore, as opposed to BBD infection, sulfate reduction was not required to sustain existing BBD.
In all cases of each of the above experiments the control fragments infected with BBD (no inhibitors) produced a band which migrated across the coral fragment, and the control fragments with no BBD and no inhibitors remained healthy (not shown).
Effects of microcystin and sulfide exposure on coral tissue
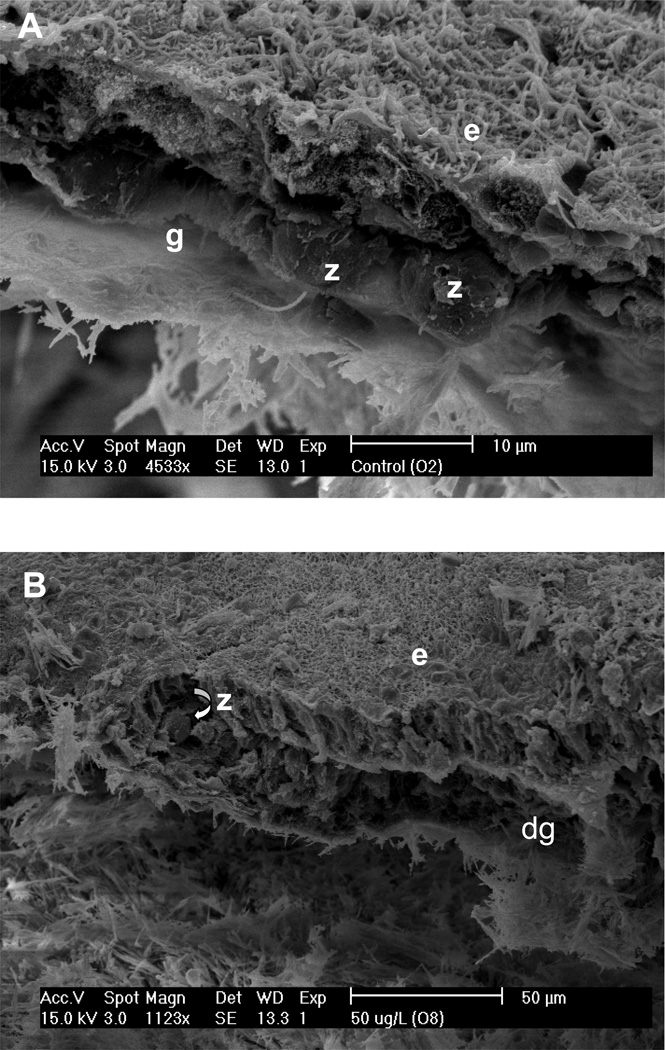
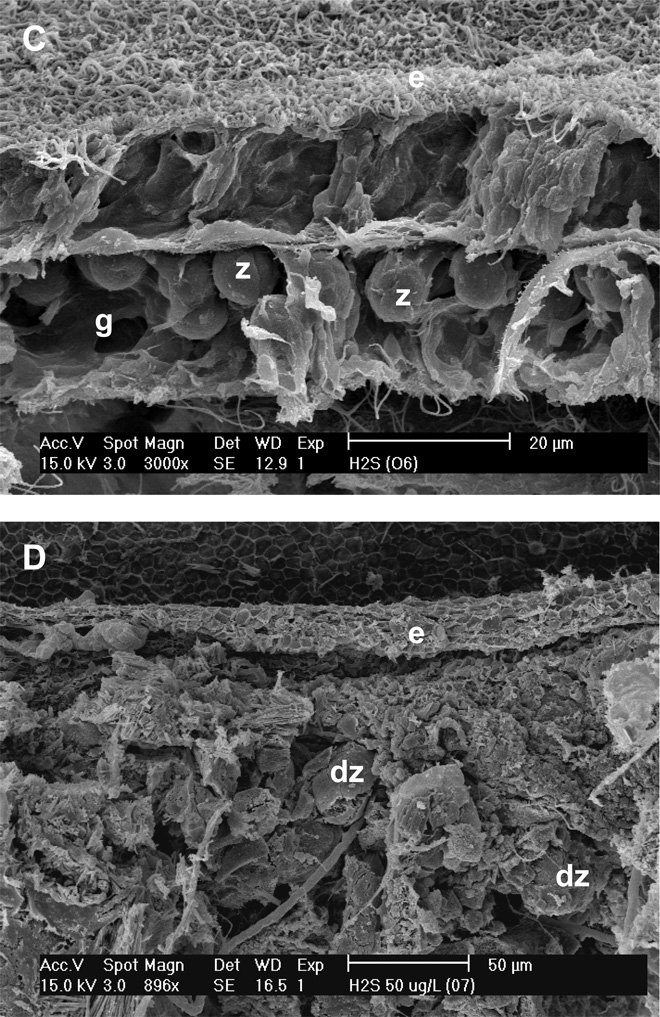
Table 1 summarizes the effects of the exposure studies on the coral tissue, zooxanthellae, and bacterial community of experimental coral fragments. When examined visually, fragments exposed to increasing microcystin concentrations (1, 50 and 100 µg l−1) exhibited corresponding decreases in coral surface topography. This effect was exacerbated with exposure to sulfide (with and without microcystin). When the fragments were examined using SEM pronounced effects were apparent. Control fragments (an example is shown in Figure 3A) contained apparently healthy, intact zooxanthellae which were located in their normal position in the gastrodermis. The epidermis displayed normal columnar cells, with little damage, and normal mucocytes. In contrast, fragments that were exposed to microcystin exhibited a loss of structural integrity in both the epidermis and gastrodermis (Figures 3B). These fragments also exhibited extrusion of zooxanthellae from the gastrodermis, or formation of clusters of zooxanthellae, but the zooxanthellae generally appeared to be healthy. Columnar cells in the epidermis of microcystin-exposed coral tissues were vacuolated, and there was an absence of mucocytes. In many cases, the epidermis was drastically thinned or absent.
Table 1.
Effect of exposure of apparently healthy coral fragments to purified microcystin LR (MC-LR) and sulfide. Exposure ranged from 18 to 22.5 hours. Representative scanning electron micrographs are shown in Figure 3.
| Exposure to MC-LR and/or Sulfide |
Bacterial Growth |
Coral Tissue |
Zooxanthellae |
|---|---|---|---|
| Control (seawater) | Very low | Healthy and intact epidermis and gastrodermis | Healthy, in gastrodermis |
| 1 µg l−1 MC-LR | High | Epidermis thinned or absent, gastrodermis degraded | Healthy, in clusters within gastrodermis or on surface |
| 50 µg l−1 MC-LR | Low | Epidermis vacuolated, degraded gastrodermis | Healthy, in clusters within gastrodermis |
| 100 µg l−1 MC-LR | Low | Epidermis thinned or absent, degraded gastrodermis | Some degradation, in clusters within gastrodermis or on surface |
| 50 µg l−1 MC-LR 0.5 mM sulfide | Low | Epidermis thinned or vacuolated, degraded gastrodermis | Some degradation, extruded from gastrodermis |
| 100 µg l−1 MC-LR 0.5 mM sulfide | Low | Epidermis and gastrodermis thinned or absent | Large clusters on surface, some degradation |
| 0.5 mM sulfide | Cyano-bacterial filaments present | Epidermis vacuolated or thinned, gastrodermis degraded | Appear healthy, in clusters within gastrodermis |
Figure 3.
Scanning electron micrographs of Montastraea annularis fragments exposed to 50 µg l−1 microcystin LR (B), 0.5 mM sulfide (C), and 50 µg l−1 microcystin LR plus 0.5 mM sulfide (D). Panel A is a control. Note different scales on panels. e = epidermis; g = gastrodermis; z = zooxanthella; d = degraded.
Coral fragments exposed to sulfide exhibited effects similar to those exposed to microcystin, with a loss of organized structure in the tissue layers and zooxanthellae that appeared either to be floating freely in the gastrodermis or to be present as clusters extruded from the gastrodermis (Figure 3C). Again, the zooxanthellae appeared normal. With sulfide exposure cyanobacteria were present at the base of the coral tissue (not shown).
The effects of exposure to microcystin or sulfide were exacerbated when the two substances were combined (Figure 3D). In this case there was vacuolation of the epidermis, severe loss of structure of the columnar cells of the epidermis (when present), and little to no gastrodermis. With combined exposure the zooxanthellae exhibited some degradation.
Effect of microcystin exposure on bacterial growth
In the experiments described above, it was noted that exposure of coral fragments to 1µg l−1 microcystin was accompanied by an increase in the number of bacteria observable in the coral tissue and on the surface of the zooxanthellae. At the increased microcystin concentrations bacteria were still present, but at much lower densities; however they were more prevalent than in the control (data not shown). Based on these observations, the effect of exposure of the growth of bacteria isolated from BBD and SML was examined. These results are summarized in Table 2.
Table 2.
Effect on bacterial growth of isolates from BBD and SML to purified microcystin (MC-LR) at 1, 100, and 500 µg l−1. Growth was compared to that of controls (no microcystin) for each isolate for each experiment (N = 3 experiments, each with triplicate incubations).
| Bacterial isolate Source Strain designation |
Identification (closest GenBank sequence, % similarity) |
Response/[MC]a | P value b |
|---|---|---|---|
| BBD | |||
| BBD-216-1a | Vibrio harveyi (AY750576, 99%) | - | |
| BBD-216-4a | Methylarcula sp. (AJ534208, 96%) | S/100 µg l−1 | p < 0.02 |
| BBD-216-3d | Bacillus megaterium (AJ717381, 99%) | - | |
| BBD-216-4f | Marinobacter sp. (AY196982, 99%) | - | |
| BBD-216-4g | M. aquaeolei (AJ000726, 99%) | - | |
| BBD-216-4i | Idiomarina sp. (AB167036, 98%) | S/ 1 µg l−1 | p < 0.01 |
| BBD-217-1c | Photobacterium eurosenbergii (AJ842346, 99%) | S/500 µg l−1 | p < 0.05 |
| BBD-217-1a | Bacillus cereus (AY305275, 99%) | - | |
| BBD-217-2d | Vibrio harveyi (AY750576, 99%) | I/100 µg l−1 | p < 0.05 |
| BBD-217-2g | Alteromonas sp. (AY626838, 99%) | - | |
| SML | |||
| H-1-2 | not identified | I/ 1 µg l−1 | p < 0.0005 |
| H-1-3 | “ | I/500 µg l−1 | p < 0.05 |
| H-1-5 | “ | - | |
| H-1-7 | “ | I/ 1 µg l−1 | p = 0.005 |
| H-1-8a | “ | - | |
| H-1-8b | “ | I/ 1 µg l−1 | p = 0.001 |
| S/500 µg l−1 | p < 0.005 | ||
| H-1-9 | “ | - | |
| H-1-10 | “ | - | |
| H-1-11 | “ | - | |
| H-1-12 | “ | - | |
| H-1-13 | “ | - | |
| H-1-16 | “ | I/ 1 µg l−1 | p < 0.005 |
| S/100 µg l−1 | p = 0.0002 | ||
| Known coral pathogens | |||
| Aurantimonas coralicida | na | - | |
| Serratia marcescens | na | S/500 µg l−1 | p < 0.05 |
| Vibrio shiloi | na | S/ 1 µg l−1 | p < 0.01 |
Response designated as S (stimulation), I (inhibition) - (no effect)
two sample t-Test, assuming equal variances
na = not applicable
Of the 22 bacterial isolates from BBD (10) or coral SML (12), nine exhibited a response (change in growth relative to the control) when exposed to microcystin LR for 12 hours (Table 2). Three of the BBD isolates (strains BBD-2164a, BBD-2164i, and BBD-2171c) were stimulated by microcystin and one was inhibited (BBD-2172d). The isolates whose growth was stimulated exhibited this effect when exposed to microcystin concentrations of 1, 100 and 500 µg l−1, but exhibited the effect only at one of the three concentrations to which they were exposed (see Table 2). The one BBD isolate that exhibited growth inhibition did so at a concentration of 100 µg l−1 of microcystin; the other two concentrations had no effect. In contrast three of the SML isolates (strains H-1-2, H-1-3, and H-1-7) exhibited growth inhibition at 1 or 500 µg l−1, with no effect at other concentrations (see Table 2). Two of the SML isolates (H-1-8b and H-1-16) exhibited inhibition at the lowest microcystin concentration (1 µg l−1) and stimulation at the higher concentrations. Of the known coral pathogens, growth of both Vibrio shiloi and Serratia marcescens were stimulated while that of Aurantimonas coralicida was not affected.
DISCUSSION
Effects of disruption of oxygenic photosynthesis and sulfate reduction in black band disease
The metabolic inhibition experiments targeted two of the major metabolic pathways functioning within the BBD microbial consortium, oxygenic photosynthesis and dissimilatory sulfate reduction, carried out by BBD cyanobacteria and sulfate-reducing bacteria respectively. Both pathways are tied to production of toxic substances, but in different ways. In the case of microcystin production the connection is indirect in that it is known that microcystin synthesis is positively correlated with cyanobacterial energy production (Kaebernick & Neilan 2001, Kaebernick et al. 2002, Codd et al. 2005), which can involve different energy-yielding metabolic pathways in addition to oxygenic photosynthesis (Stal 1995). In contrast, production of sulfide by sulfate reducers is the result of sulfide being a specific byproduct of the (energy yielding) dissimilatory sulfate reduction pathway. Therefore whereas inhibition of sulfate reduction by Na molybdate will completey block sulfide production, inhibition of oxygenic photosynthesis by DCMU would not block microcystin formation if other energy yielding pathways are operating.
Disruption of the two pathways using the specific inhibitors DCMU and Na molybdate had very different effects. Exposure of BBD inocula to Na molybdate had a pronounced and striking effect in that it always prevented BBD infection. These results indicate that sulfide is required for the initiation of this disease. In contrast, the disruption of oxygenic photosynthesis had no effect on BBD infection. Neither inhibitor prevented continued BBD tissue degradation when a band had formed and was actively migrating and lysing coral tissue. Together these results indicate that although sulfide is required for BBD infection it is not required for continued tissue lysis. Therefore, while sulfide appears to be required for BBD pathogenesis, there must be another toxin(s) or toxicant(s) present for sustained tissue lysis associated with BBD pathobiology.
Although not definitive, these results indicate that microcystin is potentially a second operative toxin. Specifically, the result that disruption of oxygenic photosynthesis did not stop tissue lysis (or infection) is inconclusive in assigning a definitive and necessary role of microcystin since microcystin synthesis could be powered by other metabolic pathways. In other words, disruption of one of the major BBD energy yielding metabolic pathways (oxygenic photosynthesis) may not be important in BBD disease etiology due to the fact that cyanobacteria are metabolically very flexible (Stal 1995). It is well known that microcystin synthesis is tied to energy production and is positively correlated with increasing nutrients and light levels, the latter of which is directly related to oxygenic photosynthesis (Van der Westhuizen & Eloff 1985, Lee et al. 2000, Vezie et al. 2002). However, BBD cyanobacteria may be contributing to toxin production based on alternate energy yielding pathways that could support microcystin synthesis. In our experiments the BBD cyanobacteria in our model system, similar to natural BBD infections, were exposed to organic carbon (and high nutrients) in both the light and dark due to the fact that the BBD mat overlays lysing coral tissue. This organic carbon is an energy source in that all cyanobacteria are capable of aerobic respiration or organic carbon as an energy yielding pathway (Stal 1995). Additionally, one cultured BBD cyanobacterium, a member of the genus Geitlerinema, has recently been shown to exhibit enhanced survival under both aerobic and anaerobic conditions in darkness when supplied with exogenous organic carbon (sugars and amino acids) (Richardson & Ragoonath 2008). A second BBD isolate, a member of the genus Leptolyngbya, showed increased microcystin synthesis in the presence of fructose and glucose in the light and dark when compared to photoautotrophic controls (Gantar and Richardson, unpublished data). Therefore microcystin production could very well still occur when oxygenic photosynthesis is shut down.
Effect of sulfide and microcystin on coral tissue
Exposure of healthy coral fragments to microcystin resulted in degradation of the coral tissue layers at all concentrations tested (Table 1). At 1 µg l−1, there was also an increase in the number of bacterial cells associated with coral tissue and zooxanthellae. It is possible that the increased bacterial growth is a result of stimulation by microcystin (discussed below) which may have contributed to coral tissue degradation. At 50 µg l−1 microcystin bacterial growth was much lower. Under this experimental conditions the columnar cells in the epidermis were vaculated, and zooxanthellae were extruded from the gastrodermis, suggesting that microcystin directly degrades coral tissue and may inhibit bacteria. These effects were exacerbated with exposure to 100µg l−1 microcystin, in which the epidermis appeared to be significantly thinned, or absent, leaving behind clusters of zooxanthellae.
Exposure to sulfide caused effects similar to exposure to the higher concentrations of microcystin, with severely vacuolated columnar cells and degraded gastrodermis. Zooxanthellae again appeared to clump into clusters within the gastrodermis, but appeared healthy. With exposure to sulfide it was interesting to note the appearance of filamentous cyanobacteria below the calicodermis.
The above effects were exacerbated when coral fragments were exposed to combinations of the two substances, both in terms of the degree of the deleterious effect and length of time before the effect was observed. In these experiments exposure to both substances led to complete degradation of the epidermal and gastrodermal layers and these effects were apparent sooner than when the substances were used alone. The zooxanthellae in fragments exposed to a combination of sulfide and microcystin showed some signs of degradation, but not to the extent of the coral animal, suggesting that zooxanthellae may not suffer the same effects of BBD infection as the coral animal (lysis and death). These results are supported by the fact that examination of freshly collected BBD field samples using light microscopy often reveals intact zooxanthellae in the BBD microbial mat, presumably released from the physical symbiosis when the coral tissue is lysed.
Our finding that both sulfide and microcystin have toxic effects on coral tissue and zooxanthellae is not surprising considering the fact that the two substances act differently on target cells. Sulfide is toxic in that it poisons both respiratory and photosynthetic electron transport (Vismann 1991). Microcystin is toxic because it inhibits protein synthesis, protein and nuclear protein phosphatases, disrupts membrane integrity and conduction, and can induce apoptosis (MacKintosh et al. 1990, Sim & Mudge 1993, Hooser 2000). Thus these two substances may target different members of the coral holobiont.
There may be an additional way in which the two compounds interact. The requirement for sulfide production for the initiation of BBD when an infective piece of BBD mat is placed on the surface of healthy coral may be because sulfide exposure, which is known to cause coral tissue lysis (Richardson et al. 1997), is necessary to allow microcystin to penetrate coral cells and tissues. Microcystin is, structurally, a cyclic heptapeptide and, being hydrophilic, would not easily penetrate intact coral tissue (Codd et al. 2005). This could be the basis for the finding of other investigators (Aeby & Santavy 2006) that BBD infection requires that the coral surface be wounded for infection to occur.
Effect of microcystin on bacterial growth
Based on the observations of a pronounced increase in the number of bacteria present in the gastrodermis of corals exposed to microcystin at 1 µg l−1, and relatively more bacteria at higher concentrations, we assessed the effect of exposure of bacterial cultures to this compound. While only a limited number of cultures were tested, we did find a pattern in which BBD bacterial isolates appeared to be, in general, stimulated by microcystin (3 of 10 isolates stimulated, one isolate inhibited) while SML bacteria were more often inhibited (3 of 12 isolates). Two of the SML isolates were inhibited at the lowest concentration of microcystin but stimulated at the highest concentration. Thus there may be a role of microcystin besides its toxicity in the pathobiology of BBD. It is known that some bacteria can grow on microcystin as a carbon and energy source (Park et al. 2001, Eleuterio & Batista 2005). The known production of microcystin by BBD cyanobacteria (Richardson et al. 2007) may, in addition to contributing directly to coral tissue lysis and death, have an etiological role in selecting for BBD microorganisms and against potentially beneficial members of the coral SML. These aspects of BBD are completely unexplored.
Additional potential toxins in BBD
The experiments described in this study do not rule out the presence of additional toxins operating in BBD. To date only three of potentially many cyanotoxins have been investigated in BBD. While we did find microcystin in BBD two other cyanobacterial toxins, saxitoxin and anatoxin-a (both neurotoxins) were not detected in BBD field samples or cultures of BBD cyanobacteria using analytical techniques (Richardson et al. 2007). Many more cyanotoxins have not, to our knowledge, been the subject of study in BBD research. As mentioned previously, sequences homologous to bacteria associated with toxin-producing dinoflagellates are common in BBD (discussed in detail in (Sekar et al. 2008). Again, to our knowledge no one has investigated the potential role of these toxins in BBD. In summary, our work has shown that at least two toxins, sulfide and microcystin, are present in BBD and that they deleteriously affect both the coral animal and associated zooxanthellae. We have also shown that, in the case of the coral animal, these two substance act synergistically. Finally, we have presented preliminary results that indicate that these substances also have effects, both positive and negative, on coral and BBD associated bacteria and therefore may have roles in structuring the complex BBD microbial community.
Acknowledgements
We thank J.D. Voss and J. Pinzón for assistance in the field, K. Rein for providing purified microcystin, P. Blackwelder, H. Al Sayegh, and A. Renegar for assistance with SEM, and G.M. King for providing customized experimental chambers. This research was supported by NIH (NIH/NIGMS SO6GM8205), and NOAA’s Caribbean Marine Research Center (CMRC-04-PRJV-01-04C). This is contribution XXX of the Tropical Biology Program at Florida International University.
REFERENCES
- Aeby GS, Santavy DL. Factors affecting susceptibility of the coral Montastrea faveolata to black-band disease. Mar Ecol Prog Ser. 2006;318:103–110. [Google Scholar]
- Antonius A. New observations on coral destruction in reefs. 10th Mtg Assoc Isl Mar Lab Carib. 1973;10:3. [Google Scholar]
- Antonius A. Coral diseases in the Indo-Pacific: A first record. PSZNI Mar Ecol. 1985;6:197–218. [Google Scholar]
- Antonius A. Distribution and dynamics of coral diseases in the eastern Red Sea. Proc 6th Intl Coral Reef Symp. 1988;3:145–150. [Google Scholar]
- Banin E, Khare SK, Naider F, Rosenberg E. Proline-rich peptide from the coral pathogen Vibrio shiloi that inhibits photosynthesis of zooxanthellae. Appl Env Microbiol. 2001;67:1536–1541. doi: 10.1128/AEM.67.4.1536-1541.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash Y, Sulam R, Loya Y, Rosenberg E. Bacterial strain BA-3 and a filterable factor cause a white plague -like disease in corals from the Eilat coral reef. Aquat Microb Ecol. 2005;40:183–189. [Google Scholar]
- Bruckner A, Bruckner R. The persistence of black band disease in Jamaica: Impact on community structure. Proc 8th Intl Coral Reef Symp I. 1997:601–606. [Google Scholar]
- Carlton R, Richardson L. Oxygen and sulfide dynamics in a horizontally migrating cyanobacterial mat: Black band disease of corals. FEMS Microbiol Ecol. 1995;18:155–162. [Google Scholar]
- Codd GA, Lindsay J, Young FM, Morrison LF, Metcalf JS. Cyanobacterial toxins. In: Huisman J, Matthijs HCP, Visser PM, editors. Harmful cyanobacteria. Dordrecht: Springer; 2005. pp. 1–23. [Google Scholar]
- Cooney R, Pantos O, Le Tissier M, Barer M, O'Donnell A, Bythell J. Characterization of the bacterial consortium associated with black band disease in coral using molecular microbiological techniques. Env Microbiol. 2002;4:401–413. doi: 10.1046/j.1462-2920.2002.00308.x. [DOI] [PubMed] [Google Scholar]
- Dinsdale E. Abundance of black band disease on corals from one location on the Great Barrier Reef: a comparison with abundance in the Caribbean region. Proc 9th Intl Coral Reef Symp. 2002;2:1239–1244. [Google Scholar]
- Ducklow H, Mitchell R. Observations on naturally and artificially diseased tropical corals: A scanning electron microscope study. Microb Ecol. 1979;5:215–223. doi: 10.1007/BF02013528. [DOI] [PubMed] [Google Scholar]
- Edmunds PJ. Extent and effect of black band disease on Caribbean reefs. Coral Reefs. 1991;10:161–165. [Google Scholar]
- Eleuterio L, Batista JR. Intl Symp Cyano Harmful Alg Blooms (ISOC-HAB) Durham, N.C., USA: 2005. Removal of the cyanobacterial toxin microcystin-LR by biofiltration. p Abstract. [Google Scholar]
- Frias-Lopez J, Klaus JS, Bonheyo GT, Fouke BW. Bacterial community associated with black band disease in corals. Appl Env Microbiol. 2004;70:5955–5962. doi: 10.1128/AEM.70.10.5955-5962.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett P, Ducklow H. Coral disease in Bermuda. Nature. 1975;253:349–350. [Google Scholar]
- Hooser SB. Fulminant hepatocyte apoptosis in vivo following microcystin LR administration to rats. Toxicol Path. 2000;28:762–733. doi: 10.1177/019262330002800513. [DOI] [PubMed] [Google Scholar]
- Kaczmarsky L. Is sewage associated with coral disease incidence and prevalence? Carib J Sci. 2004 [Google Scholar]
- Kaebernick M, Dittmann E, Borner T, Neilan BA. Multiple alternate transcripts direct the biosynthesis of microcystin, a cyanobacterial. Appl Env Microbiol. 2002;68:449–455. doi: 10.1128/aem.68.2.449-455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaebernick M, Neilan B. Ecological and molecular investigation of cyanotoxin production. FEMS Microbiol Ecol. 2001;35:1–9. doi: 10.1111/j.1574-6941.2001.tb00782.x. [DOI] [PubMed] [Google Scholar]
- Kuta KG, Richardson L. Abundance and distribution of black band disease of corals in the northern Florida Keys. Coral Reefs. 1996;15:219–223. [Google Scholar]
- Kuta KG, Richardson LL. Ecological aspects of black band disease of corals: relationships between disease incidence and environmental factors. Coral Reefs. 2002;21:393–398. [Google Scholar]
- Lee SJ, Jang MH, Kim HS, Yoon BD, Oh HM. Variation of microcystin content of Microcystis aeruginosa relative to medium N:P ration and growth stage. Appl Env Microbiol. 2000;89:323–329. doi: 10.1046/j.1365-2672.2000.01112.x. [DOI] [PubMed] [Google Scholar]
- MacKintosh C, Beattie K, Klumpp S, Cohen P, Codd G. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990;264:187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- Myers JL, Sekar R, Richardson L. Molecular detection and ecological significance of the cyanobacteria Geitlerinema and Leptolyngbya in black band disease of corals. Appl Env Microbiol. 2007;73:5173–5182. doi: 10.1128/AEM.00900-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HD, Y S, Maruyama T, Yanagisawa E, Hiraishi A, Kato K. Degradation of the cyanobacterial microcystin by a new bacterium isolated from a hypertrophic lake. Env Toxicol. 2001;16:337–343. doi: 10.1002/tox.1041. [DOI] [PubMed] [Google Scholar]
- Richardson L, Kuta K, Schnell S, Carlton R. Ecology of the black band disease microbial consortium. Proc 8th Intl Coral Reef Symp. 1997;1:597–600. [Google Scholar]
- Richardson LL. Black band disease. In: Loya Y, Rosenberg E, editors. Coral health and disease. Springer-Verlag; 2004. pp. 325–336. [Google Scholar]
- Richardson LL, Ragoonath D. Organic carbon enhances dark survival of the cyanobacterium Geitlerinema sp. isolated from black band disease of corals. Rev Biol Trop. 2008;56(Supl 1):119–126. [Google Scholar]
- Richardson LL, Sekar R, Myers JL, Gantar M, Remily ER, Kaczmarsky LT, Voss JD, Boyer GL, Zimba PV. The presence of the cyanobacterial toxin microcystin in black band disease of corals. FEMS Microbiol Lett. 2007;272:182–187. doi: 10.1111/j.1574-6968.2007.00751.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez S, Croquer A. Dynamics of black band disease in a Diploria strigosa population subjected to annual upwelling on the northeastern coast of Venezuela. Coral Reefs. 2007;27:381–388. [Google Scholar]
- Rützler K, Santavy D. The black band disease of Atlantic reef coralsIDescription of the cyanophyte pathogen. PSZNI Mar Ecol. 1983;4:301–319. [Google Scholar]
- Rützler K, Santavy D, Antonius A. The black band disease of Atlantic reef corals. III. Distribution, ecology and development. PSZNI Mar Ecol. 1983;4:329–358. [Google Scholar]
- Sekar R, Kaczmarsky LT, Richardson LL. Microbial community composition of black band disease on the coral host Siderastrea siderea from three regions of the wider Caribbean. Mar Ecol Prog Ser. 2008;362:85–98. [Google Scholar]
- Sekar R, Mills D, Remily E, Voss J, Richardson L. Microbial communities in the surface mucopolysaccharide layer and the black band microbial mat of black band diseased Siderastrea siderea. Appl Env Microbiol. 2006;72:5963–5973. doi: 10.1128/AEM.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]