Abstract
This paper highlights the role of institutional resources and policies, whose origins lie in political processes, in shaping the genetic etiology of body mass among a national sample of adolescents. Using data from Waves I and II of the National Longitudinal Study of Adolescent Health, we decompose the variance of body mass into environmental and genetic components. We then examine the extent to which the genetic influences on body mass are different across the 134 schools in the study. Taking advantage of school differences in both health-related policies and social norms regarding body size, we examine how institutional resources and policies alter the relative impact of genetic influences on body mass. For the entire sample, we estimate a heritability of .82, with the remaining .18 due to unique environmental factors. However, we also show variation about this estimate and provide evidence suggesting that social norms and institutional policies often mask genetic vulnerabilities to increased weight. Empirically, we demonstrate that more-restrictive school policies and policies designed to curb weight gain are also associated with decreases the proportion of variance in body mass that is due to additive genetic influences.
Keywords: policies, gene-environment interactions, BMI, obesity, schools
1. Introduction
According to recent estimates, more than one in three adolescents are overweight or at risk of being overweight (Ogden et al., 2006), and prevalence rates of obesity are 10 times higher in the United States than in other developed nations (Lissau et al., 2004). Given the links of obesity to chronic health problems such as type-2 diabetes and hypertension, understanding the social, behavioral, and biological mechanisms responsible for differences in the risk of obesity is a critical public health issue. ‘Obesity politics’ (Kersch, 2009) has taken center stage in policy debates regarding the most effective means to improve the health of the public but the bulk of policy research on obesity has focused on individuals and their corresponding behavioral risk factors such as diet and exercise. Recent research, however, has begun to examine characteristics of the built, social, and institutional environment that may facilitate or hinder proper energy balance behaviors (Brescoll, Kersh, & Brownell, 2008; Gordon-Larsen et al., 2006). By contributing to the knowledge and understanding of political and institutional processes at play, political science has the potential to be centrally located in debates regarding the causes and consequences of obesity and can help to develop weight management initiatives that begin with institutions such as schools and work places.
The heritability (the proportion of variance that is due to genetic factors) of body mass index has ranged from as low as .3 to as high as .9 (Cornes et al., 2007; Franz et al., 2007; Maes et al., 1997; Ordonana et al., 2007; Schousboe et al., 2003; Silventoinen et al., 2007; Wardle et al., 2008; Haworth et. al 2008; Haberstick et al. 2010). Despite this large range, to date no research has examined the effectiveness of institutional norms and policies as a function of genetic vulnerability to weight gain. That is, some policies may be effective at reducing the intake of fatty or high-caloric food and increasing the prevalence of regular exercise for the overall population, but these policies may be relatively ineffective for those who are more likely to gain weight because of very small differences across their genome. As such, the interplay of genetics and environment (GxE) paradigm is critical for generating effective policies aimed at reducing the prevalence of obesity by identifying and creating environmental sources which also reduce the genetic influences on weight gain and energy balance behaviors (Faith and Kral 2006).
This is particularly relevant to current state of the ‘obesity epidemic’ (Mokdad et al. 2000) and the related ‘obesity politics’ (Kersch, 2009). Health policy makers have been engaged in heated debates about the implementation of punitive sanctions for those who are obese. For example, Arizona has proposed introducing a $50 fee for engaging in unhealthy behaviors and they single out obese individuals (Williams 2011). This emphasis on choices frames the increased prevalence of obesity as an individual-level phenomena and the corresponding policies are aimed at influencing individual’s behaviors rather than examining the environment in which these behaviors exist. Equally important, these policies assume that increasing levels of control will lead to decreases in obesity levels but some research suggests that this is not the case, especially if obese individuals feel further marginalized and stigmatized by these policies (Story, Nanney, and Schwartz, 2009).
In this paper, we review the general GxE framework and illustrate how twin and sibling models can be used to further our understanding of an important political and public policy issue like obesity. To demonstrate the usefulness of this perspective, we take advantage of the policy differences that exist across the schools that are included in the National Longitudinal Study of Adolescent Health (Add Health). We conclude by discussing the implications of these findings for policy-related work in general and for the genetics of obesity in particular.
2. Gene-environment interactions: the social scientific perspective
Gene-environment interaction studies, characterized as the interplay between heritable traits and environmental factors, have been a focus of many decades of research attempting to unravel the overly simplified ‘Nature vs. Nurture’ debate (Freese and Shostak, 2009). While scientists may share enthusiasm for GxE research, there is very little agreement in the broad research community about what actually constitutes the environment. With ‘obesity politics’ and public policy institutes such as The Rudd Center for Food Policy and Obesity, social scientists in general and political scientists specifically, can provide important insights about the contours of the political, social, and institutional contexts in which individuals interact with one another. Apart from their families, schools are the most important social contexts for most adolescents and schools provide clearly defined environments for specific health related policies. In this section of the paper, we describe the current perspectives which frame the social and institutional environments, with an eye towards policy suggestions that are sensitive to the current social and political climate.
As noted by Kersch (2009), the dominant paradigm of current research on obesity has focused on individual behaviors and activities. This criticism is particularly important in the gene-environment interaction literature because the environment continues to be operationalized and measured at the individual level. Different social policies and social norms are useful ways to characterize the broad social environment and research has shown predictable changes in genetic factors as a function of these different environments. For example, research on the genetic and environmental inputs into smoking has examined social contexts before and after the implementation of anti-smoking legislation (Boardman et al. 2011; Boardman et al., 2010; Kohler et al., 1999), cultural contexts within schools (Boardman et al., 2008; Rowe et al., 1999), and tax-rate differences across states (Boardman, 2009). However, no existing work has extended this line of inquiry to the study of body mass or obesity.
Given the classical and modern focus of political science on how institutions and social norms shape behaviors (Hall and Taylor 1996; Gerber, Green and Larimer, 2008), the study of how institutions and social norms influence the expression of heritability of body mass certainly overlaps with heritability research areas in political science. Adding a GxE framework to policy research, though methodologies such as observing how the heritability of obesity may be altered (1) by student norms/stigmatization of weight or (2) school resources for weight management, may also enhance health policy research which are extensions of these established research areas.
3. Gene-environment interactions and body mass: conceptual models
Within the GxE paradigm, four perspectives have emerged to explain how social and genetic forces interact to affect an individual’s risk of obesity. These models include social trigger, social control, social push, and social distinction and they are depicted graphically in Figure 1. According to the social trigger model, genetic factors related to obesity should only emerge in the most risky social environments because these environments trigger genetic tendencies for behaviors and lifestyles that are linked to increases in physical weight. For example, Loos and Bouchard (2003) point to differences in energy balance behaviors across different social contexts as the reason for the observed differences in heritability estimates across studies. According to their model, individuals may have latent genetic tendencies to gain weight but this trait only manifests in ‘obesogenic’ environments characterized by high caloric intake and no physical activity. In contrast, the social control model anticipates that the social environment is more likely to suppress genetic influences than it is to trigger them. Informal social norms and formal institutional rules (e.g., laws) are sources of control that place real limits on the types of behaviors in which individuals can engage. As an example, Boardman (2009) shows that the heritability of regular smoking is significantly reduced for residents of states with higher taxes on cigarettes and states with the most limitations on the sale of cigarettes compared to residents of states without these controls. The trigger and control models are similar to each other because they both assume that the environment causes genes to function differently. They are different from each other because they anticipate a different threshold at which environmental influences are expected. As indicated in Figure 1, the social trigger model emphasizes the relevance of genetic risk in the most risky environments; body mass may not be a heritable trait in non-risk environments. In contrast, the social control model anticipates a main genetic influence in most environments except in those with the highest level of social control (e.g., lowest level of social risk).
Figure 1.
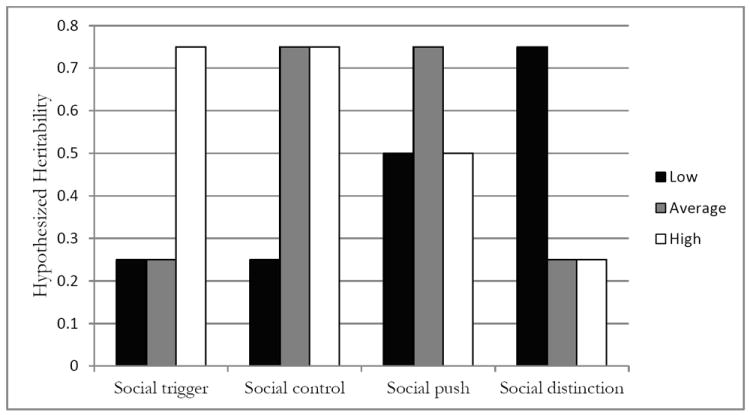
Gene-environment interaction models: hypothesized influence of school factors on the heritability of body mass.
Note: Figures represent hypothesized models of gene-environment interaction typology. Darker bars represent areas with the lowest level of social risk compared to average (grey) and high (white) social risk categories.
In contrast to these causal models, the social push and social distinction models propose non-causal mechanisms for gene-environment interactions. In other words, the environment is not hypothesized to cause changes in genetic associations, rather the environment may mask genetic associations which are only evident when considered across the spectrum of different environments. The social push model emphasizes the difference between typical and extreme social environments and anticipates that genetic factors will emerge as more salient within typical but not extreme environments. Within the extreme ends of the distribution, the social factors are causing behaviors and there is very little room for genotype to differentiate individuals from one another. However, in the typical range, when social forces on either end are minimized, genes become relatively important because the environment allows for ‘biology to shine through’ (Raine, 2002: 314). Like the social push model, the social distinction model emphasizes the ‘noisiness’ of certain social environments. However, in contrast, the social distinction model suggests that it is in the least risky environments where genetic influences will have the greatest effect on BMI. If one examines cases (obese) and controls (non-obese) in environments characterized by a low prevalence of obesity, where there is cheap access to healthy foods, and few limits on a healthy lifestyle, one is more likely to identify genes linked to BMI than if one examines cases and controls within ‘obesogenic’ environments (Loos and Bouchard 2003).
4. Schools and the genetics of obesity
In this paper, we build on this body of work by examining differences in the genetics of body mass as a function of the institutional and normative characteristics of more than 130 schools in the Add Health study. Using the GxE paradigm discussed above, we demonstrate how social norms and institutional factors may impact the heritability of BMI. In doing so, we pull examples from recent research. Given recent findings that overweight children face stigmatization and marginalization by peers of ‘normal’ weight (Puhl, Luedicke, and Heuer, 2011) we examine how school-level mean BMI (e.g., ‘norms’ for BMI) and variation around the mean BMI (e.g., ‘enforcement of BMI’) among students who report being of normal weight may alter heritability of BMI. For example, if the social trigger model is the most relevant, then the heritability of BMI should be the highest within schools that have a higher value of “normal weight” compared to schools with lower values of “normal weight” (e.g., stronger norms about physical size). Alternatively, if the heritability of BMI is the highest in schools with average weight expectations for “normal weight” compared to either extreme (e.g., the “normal weight” is either very low or very high) then the social push model would receive support.
Additionally, we examine how school resources, punitive policies, and issues of social disorganization may lead to differences in the heritability of BMI. Our examination of school resources reflects research suggesting school resources for health and fitness reduce obesity (Story, Nanney, and Schwartz, 2009). Given recent political and discussions about potential dangers of punitive treatment of obesity in laws and school policy (Kersch, 2010; Story, Nanney, and Schwartz, 2009) that mirrors the associations within families (Odoms-Young and Fitzgibbon, 2009) it is important to examine the extent to which heritability is influenced by the degree to which schools discipline their students. Specifically, this body of work suggests that increasingly strict environments may actually increase the risk of weight gain among those who may be predisposed to increases in body weight. Finally, given social disorganization in a child’s environment is associated with obesity (Burdette, Wadden, and Whitaker, 2006), we also examine how measures of social disorganization may be associated with differences in heritability of BMI. In this manner, we illustrate how institutional characteristics (e.g., the extent to which schools experience social problems), health resources, and punitive policies may impact the heritability of weight and weight gain.
5. Data
This study uses data from the National Longitudinal Study of Adolescent Health (Add Heath) (Udry, 1998). Add Health was designed to examine health and health-related behaviors among a nationally representative sample of adolescents in seventh through twelfth grade. In 1994, 90,118 adolescents from 134 schools completed questionnaires about their daily activities, health-related behaviors, and basic social and demographic characteristics. Following the in-school survey, 20,747 respondents were re-interviewed in their homes (Wave I) between April and December of 1995, again one year later (Wave II), and again between 2001-2001 (Wave III). In 1995, more-detailed school characteristics were collected through a second round of interviews with school administrators.
Two aspects of this study are particularly useful for our purposes. First, the Add Health study over-sampled twin pairs identified in the in-school survey (Harris et al. 2006). During Wave III of the study, respondents who indicated that they had a full sibling or a twin during Wave I were asked to provide saliva specimens to be genotyped. Of these 3,139 individuals, 83 percent (n = 2,612) agreed to take part in the study. Researchers then used 11 genetic markers to confirm the reported zygosity of the twin and sibling pairs. As a result of this test, 34 pairs were reassigned zygosity status. Based on this criterion of zygosity, we use 234 monozygotic (MZ, or identical) twin pairs, 370 dizygotic (DZ, or fraternal) twin pairs, 864 full sibling pairs, 238 half-sibling pairs, and 96 cousin pairs, for a total of 1,802 unique pairs. The pair design makes it possible to decompose phenotypic variation into environmental and genetic components, and the multilevel design allows for these estimates to vary across social environments such as schools (Boardman et al., 2008). Second, because nearly all students and administrators in the schools surveyed completed interviews, it is possible to measure aspects of schools that are otherwise difficult to assess. Our analysis uses data from all 134 schools, with an average of 15.52 pairs per school (SD = 24.18). Descriptive statistics and pairwise correlations for BMI are provided in Table 1.
Table 1.
Descriptive Statistics by Sibling Type
| MZ Twin Pairs | DZ Twin Pairs | Full Sibling Pairs | Half-sibling Pairs | Cousin Pairs | Total | |
|---|---|---|---|---|---|---|
| Age (Wave I) | 16.22 (1.62) | 15.98 (1.65) | 16.41 (1.63) | 16.06 (1.84) | 16.44 (1.71) | 16.25 (1.68) |
| Black | 0.21 (0.50) | 0.24 (0.50) | 0.14 (0.50) | 0.35 (0.50) | 0.54 (0.50) | 0.21 (0.41) |
| Hispanic | 0.15 (0.36) | 0.13 (0.33) | 0.16 (0.37) | 0.15 (0.36) | 0.18 (0.39) | 0.15 (0.36) |
| Other race | 0.11 (0.32) | 0.06 (0.24) | 0.15 (0.36) | 0.04 (0.20) | 0.18 (0.37) | 0.11 (0.32) |
| White | 0.53 (0.50) | 0.57 (0.49) | 0.56 (0.50) | 0.46 (0.50) | 0.12 (0.33) | 0.52 (0.50) |
| Male | 0.52 (0.50) | 0.52 (0.50) | 0.51 (0.50) | 0.50 (0.50) | 0.52 (0.50) | 0.52 (0.50) |
| Mean BMI (Wave I) | 21.99 (4.08) | 21.84 (3.82) | 22.44 (4.42) | 22.34 (4.12) | 22.83 (4.14) | 22.25 (4.21) |
| Correlation (BMI) | .82 | .39 | .40 | .10 | .16 | |
| Number of Individuals | 468 | 740 | 1,557 | 403 | 139 | 3,307 |
| Number of Sibling Pairs | 234 | 370 | 864 | 238 | 96 | 1,802 |
Note: all data come from Wave I of the National Longitudinal Study of Adolescent Health (Add Health). Cell entries represent descriptive statistics (means and standard deviations in parentheses) for the pairs sample by sibling type. Correlations within each pair type are provided at the bottom of the table.
In our analysis, we examine the degree to which school norms within schools and institutional characteristics of schools influence the genetic, shared environmental, and unique individual components of body mass. To accomplish this task, we selected biologically related siblings who have non-missing data from Wave I interviews, attended schools where school administrators had completed surveys about school characteristics, and attended schools in which at least 20 students were Wave I respondents so that BMI means and standard deviations could be reliably estimated for those reporting ‘normal BMI’.
6. Measures
6.1 Dependent variable
We use the body mass index (BMI) to assess the weight of our respondents. This measure is simply respondents weight (in kg.) divided by their height (in meters) squared. BMI at Wave I is based on self-reports of respondent height and weight. Although later waves of Add Health collected height and weight data through both self-reports and interviewer measurements, we use Wave I self-reported BMI because the Wave II sample did not include high school seniors who graduated during Waves I and II and the Wave II measures occur 12 months after our school-level indicators are assessed. It is important to note that is Wave I self-reported BMI and Wave II objective BMI are highly correlated with measured BMI (r = .93) and this correlation does not vary as a function of zygosity. Similarly, the pairwise correlations for measured (rMZ = .77; rDZ = .40) and self-reported (rMZ = .71; rDZ = .36) BMI provide roughly equivalent heritability estimates for BMI (h2 ~ .74 to .70).
6.2 School-level variables
We use five indicators of the school environment that are theoretically linked to the heritability of BMI through social or institutional mechanisms. Means, standard deviations, and correlations for these five variables are presented in Table 2. We discuss these variables below.
Table 2.
Correlations and Descriptive Statistics for School-Level Information Factors
| Weight-Loss Resources | Punishment Severity | Mean BMI | Variance BMI | Social Disorganization | |
|---|---|---|---|---|---|
| Number of Weight-Loss Resources | 1.00 | ||||
| School Punishment Severity | 0.18* | 1.00 | |||
| Mean BMI | −0.05 | 0.02 | 1.00 | ||
| Variance BMI | −0.19* | 0.03 | 0.45*** | 1.00 | |
| Social Disorganization | 0.22** | 0.22* | 0.13+ | 0.04 | 1.00 |
| Mean | 0.57 | 21.89 | 21.09 | 2.65 | 20.29 |
| Standard Deviation | 0.65 | 3.83 | 0.83 | 0.60 | 3.85 |
Note:
p< .001,
p< .01,
p< .05,
p < .10.
All data come from the In school, the Wave I school administrator, and the Wave II school administrator surveys of the National Longitudinal Study of Adolescent Health (Add Health). Cell entries represent correlations of the variables within schools.
Social disorganization is assessed using data from the Wave I school administrator questionnaire. This scale represents the school administrator’s views of severity of a number common problems found in the school’s student population. The items used in the scale are the severity of the following issues: smoking or tobacco use, drug use, alcohol use, gang violence, teenage pregnancy, sexual harassment, vandalism, eating disorders, racial conflict, and stress or pressure. Administrator responses to these items were coded as 0 for ‘no problem’, 1 for a ‘minor problem’, and 2 for a ‘major problem’. These items were summed to provide a measure of social disorganization for the school (α = .85).
We estimate the degree to which schools are more or less punitive using a school punishment severity score. This measure relies on Wave I data from school administrators, who were asked about school punitive policies for a range of 12 first-time occurrences for misbehavior or misconduct. These items consist of policies for the following infractions: cheating, fighting with another student, injuring another student, possessing alcohol, possessing an illegal drug, possessing a weapon, drinking alcohol at school, using an illegal drug at school, smoking at school, verbally abusing a teacher, physically injuring a teacher, and stealing school property. To differentiate levels of policies, we code scores for each item as 0 for ‘minor actions’, 1 for ‘in school suspension’, 2 for ‘out of school suspension’, and 3 for ‘expulsion’. A school’s punishment severity score is measured as the sum of these 12 items (α = .76).
School weight-loss resources is assessed using data from Wave II, in which school administrators were asked if they had an in-school recreation center and weight counseling. To examine the extent to which these in-school weight-control (e.g., institutional weight) resources are differentially associated with heritability of BMI, we estimate genetic, common environmental, and unique environmental components of BMI for (1) schools that had neither weight counseling nor a recreational center, (2) schools that had either weight counseling or a recreational center, and (3) schools that had both weight-loss counseling and recreational facilities. In supplemental analyses that are available upon request, we also examined for variation in schools having a recreation center or weight-loss counseling and found no significant variation from the results reported below.
We assess school body-size norms using the average weight among those who claimed to be normal weight. During Wave I in-home interviews, respondents were asked, How do you think of yourself in terms of weight? Response options were ‘very underweight’, ‘slightly underweight’, ‘about the right weight’, ‘slightly overweight’, or ‘very overweight’. To obtain a measure of socially acceptable BMI, we estimate the average BMI for the subgroup of individuals at a given school who viewed themselves as being ‘about the right weight’ (i.e., as being neither overweight nor underweight). For schools with a sufficiently large sub-population, we then estimate heritability and environmental components for schools with the lowest, middle, and highest mean normal BMIs.
We assess the BMI norm enforcement using variation in BMI among those who consider themselves to be normal weight. That is, while the mean ‘normal’ BMI may capture the socially acceptable BMI within a school, limited variation in the spread of BMI about the mean in that school may indicate that the school body-size norm is strictly enforced. Tremendous variation in weight for those who claim to be normal weight would indicate less consensus and marginalization for those with ‘non-normal’ weight, providing indirect evidence for loosely proscribed social norms regarding body size. In estimating the heritability and environmental components for schools with lowest, middle, and highest variation in perceived normal BMI, we attempt to capture how adherence to an ideal normal weight may alter the scope of environmental and genetic influences of BMI.
Because we present our multivariate biometrical models (described below) by school type for the respondents in the sample, we examine correlations of the school-level factors that are used in our analyses. These results and descriptive statistics for the school-level measures are presented in Table 2. The number of school weight-loss resources is only weakly correlated with the level of the BMI norm within schools (r = −.05) but is moderately correlated with norm enforcement of BMI (r = −.19). That is, there is significantly less variability in physical weight among those who perceive themselves to be normal weight in schools that have both weight-loss resources. Schools with both weight-loss resources are also more likely to have more-strict punishments (r = .18) but more signs of social disorganization (r = .22). Like the number of school weight-loss resources, social disorganization and school punishment severity are only weakly correlated with average BMI (r = .13 and r = .02, respectively). This is important because it suggests that the school effects that we describe in Section 8 are likely to be operating independently of one another.
7. Methods
Using statistical procedures outlined by Guo and Wang (2002) and McArdle and Prescott (2005), we use PROC MIXED in SAS to estimate genetic and environmental components of BMI. These techniques are extensions of basic multilevel modeling techniques commonly used in the social sciences, adapted to provide estimates of the variance components arising from heritability and the environment. While the Add Health data provide a wide array of contextual variables, the school-based design reduces shared environmental and genetic variance relative to twin and family-based designs commonly used in behavioral genetics. In addition, by analyzing individually structured data (rather than pairwise data), we can estimate variance components while controlling for individual factors that are related to BMI and differentially distributed across sibling types. These methods provide estimates for the proportion of variance in BMI that is due to additive genetic (A), shared environmental (C), and unique environmental (E) sources. As shared environmental variance component is often zero, we use fit statistics to determine if ACE variance or A-E variance models are the optimal fit. We present models that have optimal fit in the output provided below. Importantly, our models will be estimated for different school types that we identify as the most or least risky with respect to social and institutional factors unique to each school. By comparing the proportion of variance in BMI that is due to environmental differences across schools, we can test the various GxE models described above. These methods are described in detail in Appendix 1.
Due to GxE interactions potentially having non-linear effects when models such as social push or a ‘threshhold’ trigger effect are present, we estimate variance models separately for the third lowest, middle, and high categories of each school environmental variable. For each environmental variable, we display the estimated heritability and confidence intervals for these three categories in Figure 2.
Figure 2.
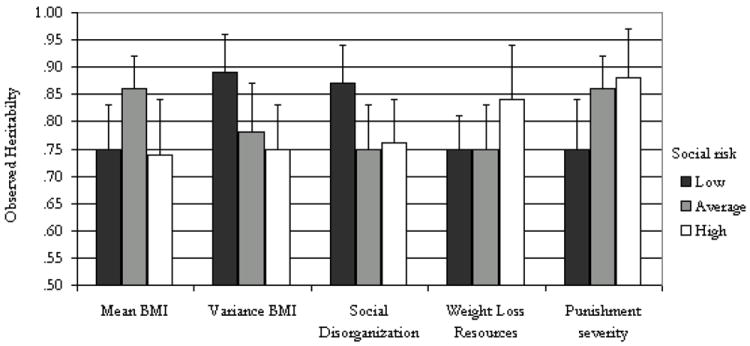
Observed Heritability of Body Mass by School Characteristics
Note: Estimates derived from the error variance terms presented in Table 3. Standard errors for these estimates are used to calculate the confidence intervals (the standard errors are not provided in the table and are available upon request). The risk categories have been changed to reflect increasing social risk to allow a better comparison to the conceptual model in Figure 1.
8. Results
The correlations presented in Table 1 provide the first evidence that genetic factors contribute substantially to BMI. Among the 234 MZ twins, the pairwise correlation for BMI is .82. As expected, this measure is considerably smaller among DZ twins (r = .39) and full siblings (r = .40) and is virtually identical for these two types of siblings, who are genetically similar in that they share an average of one-half of their genes by descent. Taking twice the difference of the MZ and DZ correlations provides a rough heritability estimate of .86 for BMI. This estimate is in line with estimates found in other work using these data (Haberstick et al., 2010).
Table 3 presents the multilevel parameter estimates and the corresponding variance components for the ACE estimates from the models described earlier. The results suggest that the AE model is the best-fitting model in 11 of the 15 models. Specifically, dropping the C estimate significantly changes the model fit for 4 of the 15 models due to A-E models providing best-fit statistics; as shown in the model, however, this simply implies that the contribution of C to the overall distribution is negligible, and the parameter estimates themselves are not statistically different from zero. Nevertheless, we include these estimates because they slightly improve model fit. For the full sample, we estimate that additive genetic factors account for 82 percent [h2 = 10.44 / (10.44 + 3.44) = .82] of the variance of BMI. This heritability estimate is differs slightly from the simple comparison of MZ and DZ pairs because the model includes controls for age, gender, and race and because we also include full-sibling, half-sibling, and cousin pairs in the analysis.
Table 3.
Multilevel Parameter Estimates and ACE Variance Components for BMI by School Type
| A | C | E | −2LL | N | h2 | c2 | e2 | |
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Full Sample | 14.12 | 3.12 | 18,317 | 3,308 | 0.82 | 0.18 | ||
| Mean Normal BMI | ||||||||
| Low | 10.44 | 3.44 | 5,865 | 1,098 | 0.75 | 0.25 | ||
| Medium | 15.57 | 0.98 | 1.65 | 6,033 | 1,091 | 0.86 | 0.05 | 0.09 |
| High | 13.95 | 4.78 | 6,320 | 1,116 | 0.74 | 0.26 | ||
| Variance Normal BMI | ||||||||
| Low | 13.25 | 1.61 | 5,826 | 1,094 | 0.89 | 0.11 | ||
| Medium | 12.05 | 3.31 | 5,005 | 920 | 0.78 | 0.22 | ||
| High | 15.06 | 4.9 | 7,392 | 1291 | 0.75 | 0.25 | ||
| School weight loss resources | ||||||||
| None | 15.36 | 3.02 | 10,591 | 1,894 | 0.84 | 0.16 | ||
| One | 11.60 | 0.30 | 3.52 | 5,874 | 1,078 | 0.75 | 0.02 | 0.23 |
| Both | 12.87 | 1.62 | 2.58 | 1,826.40 | 334 | 0.75 | 0.09 | 0.15 |
| Social Disorganization | ||||||||
| Low | 14.26 | 2.16 | 6,264 | 1,151 | 0.87 | 0.13 | ||
| Medium | 14.02 | 1.69 | 3.07 | 5,641 | 1,004 | 0.75 | 0.09 | 0.16 |
| High | 13.38 | 4.29 | 4,799 | 859 | 0.76 | 0.24 | ||
| School Punishment Severity | ||||||||
| Low | 14.39 | 1.92 | 4,458 | 819 | 0.88 | 0.12 | ||
| Medium | 15.24 | 2.49 | 5,855 | 1,057 | 0.86 | 0.14 | ||
| High | 12.90 | 4.34 | 7,833 | 1,406 | 0.75 | 0.25 | ||
Note: all data come from the National Longitudinal Study of Adolescent Health (Add Health). Cell entries represent parameter estimates from a mixed quantitative genetic model described in the paper. The proportion of variance in body mass index that is due to genetic (h2), shared environmental (c2), and non-shared environmental (c2) factors are derived from the ACE estimates. All models adjust for individual-level age, race/ethnicity, and gender.
The standardized heritability estimates and 95% confidence intervals from these models are summarized in Figure 2. As in previous work on the school moderation of genetic influences (Boardman et al., 2008; Rowe et al., 1999), these results indicate that the estimate of .82 is an average and that it varies predictably across school types. For example, the genetic influences on BMI are the highest for students who attend schools with a medium level of normal BMI, but genetic factors are considerably less influential for students in schools with higher or lower levels of normal BMI. This functional form is anticipated by the social push perspective. This non-causal model suggests that an examination of genetic influences on BMI is highest in schools which have close to the mean school BMI of the entire sample, suggesting that heritability of BMI is highest in schools which reflect the norms of the larger population of schools in the sample.
A similar non-causal model also seems to account for the differences by norm enforcement of BMI (e.g., variance in normal BMI) and for social disorganization. In both cases, the heritability for BMI is significantly higher in schools characterized as the least socially risky. Schools with the least variation in BMI among those who report to be ‘normal weight’ provide evidence for the enforcement of norms about body weight. If the structure of this association was causal in nature then the environment would be characterized as one in which social enforcement of a normal BMI may stigmatize those with high weight gain, possibly creating a hostile structure for non-heritable weight changes to emerge. However, we observe the lowest heritability of BMI in schools with the least social enforcement of BMI norms may not be as stigmatized, possibly creating a social structure where weight gain may be more easily influenced by environmental factors. This finding is in line with the social push model, but we refer to this functional form as social distinction because genetic factors emerge as the strongest primarily in the most-supportive and the most-controlled environments (rather than the in average environments). This non-causal social distinction model is also shown for social disorganization: the heritability of BMI is the highest in schools in which the principal reports the lowest number of problems such as fights, graffiti, and drug use. Given the negative relationship between social disorganization and BMI, these results suggest that negative environmental influences may influence weight changes, reducing the effect of heritability on BMI. In sum, these results suggest that meso-level social-normative factors may be best characterized as non-causal GxE associations as related to BMI.
However, when the environment is considered from an institutional (i.e., top-down) perspective, rather than from a normative or behavioral perspective, the models take on a causal orientation. For example, as the severity of punishment increases, the heritability of BMI decreases Schools with low and average levels of punishment have heritability estimates of .88 and .86, respectively. As indicated by the confidence intervals in Figure 2, the estimated heritability is not significantly different in these two contexts. However, the schools with the highest level of punishment, the heritability drops to .75 and this value is significantly different from the other types of schools. Given the functional form of this association and the nature of the measure, these results provide support for the social control model. In other words, the more controlled social environments, especially those with the highest levels of disciplinary may create environments that hold obesity rates below their ‘natural’ levels. Similarly, the availability of weight-loss resources seems to moderate the heritability of BMI that is in line with the social trigger model. For example, schools with no resources in place to help with healthy behaviors around weight management have the highest heritability (h2=.84), compared to schools with one or both resources in place (h2=.75). This nine point differences represents a twelve percent increase in the genetic influence on BMI for schools without any resources in place and is in line with the ‘weak’ social trigger model because the trigger is not required to observe genetic risks, it simply exacerbates genetic tendencies for elevated BMI.
9. Discussion
The goal of this paper was to demonstrate the relevance of the GxE perspective for understanding how social norms about body size and health policies may moderate the heritability of body weight. While obesity has traditionally been a studied within public health and epidemiology (Ogden, 2006), the increased prevalence of obesity in the United States and the related burdens on the public health infrastructure have made ‘obesity politics’ an increasingly prominent political issue that is an area of study within political science. (Brescoll, Kersh, & Brownell, 2008; Kersch 2009). For political scientists and policy makers, understanding the biological and social etiologies of this phenomenon cross a number of research boundaries. Importantly, by using the GxE framework, our findings illustrate the importance of considering contextual environmental factors.
Our findings distinguish between causal and non-causal GxE models and these models appear to align with different environmental stimuli. Specifically, when environmental factors are characterized as the behaviors and norms of the students within the schools, the GxE associations for BMI are best described by non-causal models (i.e., social distinction and social push). However, when the environment is considered from the ‘top-down’ institutional perspective the causal GxE models (e.g., trigger and control) were the most relevant. As noted by Reiss and Leve (2007: 1006–1007), a large number of studies report a triggering mechanism such that ‘[an] association between allele and behavior is observed under adverse environmental circumstances but not under favorable circumstances’. Here, adversity is the lack of health-related resources within schools that may help students to engage in healthy behaviors.
By using schools as the backdrop for our study, we focus on the embodiment of complex and multilevel social arrangements typical of institutional-level analysis (Krieger, 2001). Critical aspects of the structured social spaces in which people interact are consistently linked to the prevalence of obesity (Popkin et al., 2005). Thus, policies that are aimed at reducing the prevalence of obesity in the population should take social spaces such as schools as an important component of formulating policy. As institutions, schools have a great deal of influence over the caloric intake and expenditure of their students. As shown here, schools differ with respect to their ability to provide weight counseling or cost-free use of recreational services which may affect the ability of their students in making healthy choices regarding nutrition and exercise.
Finally, our findings make an important contribution to political science in general because they challenge researchers to be explicitly state assumptions about the homogeneity of populations. That is, our results provide additional evidence for the notion that social policies may influence the prevalence of a particular health outcome or health behavior in the population but without explicit efforts to compare the genetic and social contributions to the prevalence before and after the policy, it is difficult to know if the same policy will continue to exhibit the same level of effectiveness over time. That is, imagine a world in which individuals engage in behaviors for either genetic or social reasons. Then imagine that a policy is developed, such as anti-smoking and ‘just say no’ campaigns, that are designed to change the norms regarding this behavior and ultimately reduce the likelihood that people will engage in this behavior. This policy is likely to be effective for those who are using drugs or smoking cigarettes for social reasons (e.g., to be cool, to distinguish one’s self, to indicate belonging to a particular group). However, for those who smoke or abuse other substances because of physiological reasons related to addiction, these policies are likely to be ineffective. As such, over time, the social-genetic composition of those who remain in or enter this group (e.g., smokers) is likely to tilt toward the genetic side. This has been shown for smoking (Boardman et al. 2011) and this research suggests that policies that might have effectively reduced smoking in the past (e.g., primarily social-behavioral models) might but not be effective anymore because compared to previous time periods, a larger proportion of smokers in the current time do so for biological rather than social reasons. As such, policies should emphasize basic physiological processes such as nicotine replacement for long term chronic smokers in this most recent cohort of smokers.
This is relevant to obesity research because it suggests that researchers should make efforts to better characterize the social-genetic composition of the obese population and to understand the social forces that enable or control genetic tendencies for weight gain for particular populations at particular points in time. As with smoking, specific policies may reduce the prevalence of obesity in the population, but they may not effectively reduce the risk of obesity among genetically vulnerable individuals. Our findings point to the important role of schools as a vehicle to enact salutary policies that better enable students to make healthy choices. This general perspective also suggests that a proper understanding of the genetic etiology of obesity should consider the social and political norms in which the study is designed because it is possible that genetic effects are masked by elevated levels of background noise.
10. Conclusion
Both behavioral genetics and political science stand to gain from increased collaborative work. For behavioral genetics, incorporating political processes, social norms, and institutions into current research can help to more fully explain how genes and environment interact to predict outcomes such as obesity. For political scientists, the incorporation of behavior genetics models can provide new insights into social and institutional factors, while also helping to better implement social policies. Our results suggest this is true for obesity and likely holds for other topical issues. Future research in political science can also expand upon current research. For example, an important next step is to examine the behaviors that link the environment to observed body size because these behaviors are also shown to be highly heritable. For example, regular breakfast consumption (h2 = .60) (Keski-Rahkonen et al., 2004), sedentary behavior and fast food consumption (h2 ~ .24−.34) (Nelson et al., 2006), meal frequency and size (h2 =.46−.56) (deCastro, 1993), and total food intake (h2 = .33) (Faith et al., 1999; Hur et al., 1998) have all been shown to be moderately to highly heritable. Therefore, rather than focusing on the phenotype (e.g., obesity), future work should detail the endophenotypic pathways through which genetic and social factors simultaneously operate. Similarly, efforts need to be made to identify specific genetic loci that are responsible for the large heritability estimates that we present here. This is important because, with some exceptions (Yang et al. 2010), results from genome wide association studies have produced heritability estimates that are significantly smaller than comparable estimates derived from twin and sibling studies. Our results suggest that some of this ‘missing heritability’ (Manolio, 2009) may be due to ways in which social and institutional factors interact with genes to influence weight and weight gain. As our results generally suggest, examination of social and institutional factors through the lens of political science will likely help to account for an important portion of this missing variation.
Acknowledgments
This research uses data from Add Health, a program project designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris, and funded by a Grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Persons interested in obtaining data files from Add Health should contact Add Health, Carolina Population Center, 123 W. Franklin Street, Chapel Hill, NC 27516-2524 (addhealth@unc.edu). No direct support was received from Grant P01-HD31921 for this analysis. This paper is part of a larger study funded by the National Institute of Child Health and Human Development (R01HD060726). Research funds were also provided by the NIH/NICHD funded CU Population Center (R24HD066613).
Appendix – Description of multilevel ACE model
Typically, twin and sibling studies rely on structural equation techniques that use highly specific models in which the unit of analysis is the twin (or sibling) pair (Neale et al., 2003). The multilevel approach has recently emerged as a more flexible and (at times) more efficient method of decomposing phenotypic variance into environmental and genetic components in which the level of analysis is the individual and individuals are nested within pairs. McArdle and Prescott (2005) provide a detailed description of the model and examples of SAS codes for various biometrical models. These models provide not only estimates of the variance components but also standard errors for genetic, common environmental, and unique environmental components of variance.
Equation 1 specifies a multilevel model in which BMI of the ith adolescent nested within the jth family is described by an intercept (μ); a set of individual risk factors including age, race, and sex (βXij); and three error terms capturing additive genetic (aij), shared environmental (cj), and non-shared environmental variance (eij).
| (1) |
As shown in Equation 2, total phenotypic variance is then characterized as the sum of the three sources of residual variance, which are independently and normally distributed.
| (2) |
Therefore, a heritability estimate is derived as follows:
| (3) |
In total, this specification of A, C, and E as components of Equation 1 is conceptually similar to a traditional random-effects model. The distinction of the biometrical models is that the covariance of the random effects is conditional on the zygosity. As such, a series of covariance matrices are specified using the same genetic theory that underlies structural equation approaches (Neale et al. 2003). The matrices in Equations 4-6 are used to describe this method. If there are two unrelated twin pairs j = 1, 2 with twin i = 1, 2 in each pair, where the first pair are MZ twins and the second are DZ twins, then considering that MZ twins share 100% of their genes and DZ pairs share (on average) one-half their genes, the additive genetic covariance matrix is
| (4) |
Similarly the shared environmental covariance matrix is given by
| (5) |
Finally, the non-shared covariance matrix is given by
| (6) |
Therefore, if the phenotypes for the individuals i in twin pair j is given as Y = (y11, y21, y12, y22), then the phenotypic covariance within and between pairs is given as
| (7) |
This model is quite flexible because additional covariates can be estimated at the individual level. For example, to address potential BMI variation arising from basic demographic characteristics, we include controls for the respondent’s gender, racial phenotype, and age. Gender is coded as whether the respondent reported being female at Wave I. Racial phenotype is coded as mutually exclusive categories from respondent’s Wave I self-reports of being white, black, Hispanic, or a different race/ethnicity (i.e., Native American, Asian, or ‘other’ racial category). Age was also assessed at Wave I interviews. The mixed model described above also allow for the use of complex sample designs, generalized linear modeling allows for the analysis of variables of any measurement, and they can be extended to longitudinal growth curves (McCardle, 2006). Additional updates have been made to extend these models to other statistical software packages, including the GLLAMM procedure in STATA (Rabe-Hesketh et al., 2008), and they are quite flexible because they allows for the inclusion of different types of pairs, including full siblings, half-siblings, and cousins. Similar to the covariance matrices presented in Equations 4-6, these additional pairs take on different values for alleles shared by descent according to genetic theory.
Footnotes
Although the MZ correlation is slightly more than twice the DZ correlation (indicating a small possibility of dominance) previous research (Haberstick et al., 2010) suggests that the inclusion of the dominance parameter estimate does not improve model fit. Thus, we only describe ACE models in our multivariate approach.
Figure 2 arranges the values in terms of social risk. As such, the lowest level of punishment severity is characterized as a social risk because we equate this with less social control. Thus, the low and high values from Table 3 appear to be ‘reversed’ in the Figure 2. This was done so that the values are conceptually similar to the hypothesized models presented in Figure 1.
References
- Boardman JD. State-level moderation of genetic tendencies to smoke. American Journal of Public Health. 2009;99:480–486. doi: 10.2105/AJPH.2008.134932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JD, Saint Onge JM, Haberstick BC, Timberlake DS, Hewitt JK. Do schools moderate the genetic determinants of smoking? Behavior Genetics. 2008;38:234–246. doi: 10.1007/s10519-008-9197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JD, Blalock CL, Pampel FC, Hatemi PK, Heath AC, Eaves LJ. Population composition, public policy, and the genetics of smoking. Demography. 2011;48(4):1517–1533. doi: 10.1007/s13524-011-0057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JD, Blalock CL, Pampel FC. Trends in the genetic influences on smoking. Journal of Health and Social Behavior. 2010;51:108–123. doi: 10.1177/0022146509361195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette HL, Wadden TA, Whitaker RC. Neighborhood Safety, Collective Efficacy, and Obesity in Women with Young Children. Obesity. 2006;14:518–525. doi: 10.1038/oby.2006.67. [DOI] [PubMed] [Google Scholar]
- Brescoll VL, Kersh R, Brownell KD. Assessing the feasibility and impact of federal childhood obesity policies. Annals of the American Academy of Political and Social Science. 2008;6l5(l):178–194. [Google Scholar]
- Cornes BK, Zhu G, Martin NG. Sex differences in genetic variation in weight: A longitudinal study of body mass index in adolescent twins. Behavior Genetics. 2007;37:648–660. doi: 10.1007/s10519-007-9165-0. [DOI] [PubMed] [Google Scholar]
- de Castro JM. Independence of genetic influences on body size, daily intake, and meal patterns of humans. Physiology & Behavior. 1993;54:633–639. doi: 10.1016/0031-9384(93)90070-v. [DOI] [PubMed] [Google Scholar]
- Faith MS, Kral TVE. Institute of Medicine (U.S.) Committee on Assessing Interactions Among Social Behavioral and Genetic Factors in Health. Social environmental and genetic influences on obesity and obesity promoting behaviors: fostering research integration. In: Hernandez LM, Blazer DG, editors. Genes, Behavior, and the Social Environment: Moving Beyond the Nature/Nurture Debate. Washington, DC: National Academies Press; 2006. pp. 236–280. [Google Scholar]
- Faith MS, Rha SS, Neale MC, Allison DB. Evidence for genetic influences on human energy intake: results from a twin study using measured observations. Behavior Genetics. 1999;29:145–154. doi: 10.1023/a:1021683716700. [DOI] [PubMed] [Google Scholar]
- Franz CE, Grant MD, Jacobson KC, Kremen WS, Eisen SA, Xian H, Romeis J, Thompson-Brenner H, Lyons MJ. Genetics of body mass stability and risk for chronic disease: A 28-year longitudinal study. Twin Research and Human Genetics. 2007;10:537–545. doi: 10.1375/twin.10.4.537. [DOI] [PubMed] [Google Scholar]
- Freese J, Shostak S. Genetics and Social Inquiry. Annual Review of Sociology. 2009;35:107–128. [Google Scholar]
- Gerber AS, Green DP, Larimer CW. Social pressure and voter turnout: Evidence from a large-scale field experiment. American Political Science Review. 2008;102(1):33–48. [Google Scholar]
- Gordon-Larsen P, Nelson MC, Page P, Popkin BM. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics. 2006;117:417–424. doi: 10.1542/peds.2005-0058. [DOI] [PubMed] [Google Scholar]
- Guo G, Wang J. The mixed or multilevel model for behavior genetic analysis. Behavior Genetics. 2002;32:37–49. doi: 10.1023/a:1014455812027. [DOI] [PubMed] [Google Scholar]
- Haberstick BC, Lessem JM, McQueen M, Boardman JD, Hopfer CJ, Smolen A, Hewitt JK. Stable genes and changing environments: Body mass index across adolescence and young adulthood. Behavior Genetics. 2010;40:495–504. doi: 10.1007/s10519-009-9327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PA, Taylor RCR. Political Science and the Three New Institutionalisms. Political Studies. 1996;44:936–957. [Google Scholar]
- Harris KM, Halpern CT, Smolen A, Haberstick BC. The National Longitudinal Study of Adolescent Health (Add health) twin data. Twin Research and Human Genetics. 2006;9:988–997. doi: 10.1375/183242706779462787. [DOI] [PubMed] [Google Scholar]
- Haworth CMA, Plomin R, Carnell S, Wardle J. Obesity in children: genetic and environmental overlap with normal range BMI. Obesity. 2008;16:1585–1590. doi: 10.1038/oby.2008.240. [DOI] [PubMed] [Google Scholar]
- Hur YM, Bouchard TJ, Jr, Eckert E. Genetic and environmental influences on self-reported diet: A reared-apart twin study. Physiology & Behavior. 1998;64:629–636. doi: 10.1016/s0031-9384(98)00101-2. [DOI] [PubMed] [Google Scholar]
- Kersch R. The politics of obesity: a current assessment and look ahead. Milbank Quarterly. 2010;87:295–316. doi: 10.1111/j.1468-0009.2009.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Rahkonen A, Viken RJ, Kaprio J, Rissanen A, Rose RJ. Genetic and environmental factors in breakfast eating patterns. Behavior Genetics. 2004;34:503–514. doi: 10.1023/B:BEGE.0000038488.22974.b1. [DOI] [PubMed] [Google Scholar]
- Kohler H-P, Rodgers JL, Christensen K. Is fertility behavior in our genes? Findings from a Danish twin study. Population and Development Review. 1999;25:253–288. [Google Scholar]
- Krieger N. Theories for social epidemiology in the 21st century: An ecosocial perspective. International Journal of Epidemiology. 2001;30:668–677. doi: 10.1093/ije/30.4.668. [DOI] [PubMed] [Google Scholar]
- Lissau I, Overpeck MD, Ruan WJ, Due P, Holstein BE, Hediger ML. Body mass index and overweight in adolescents in 13 European countries, Israel, and the United States. Archives of Pediatrics & Adolescent Medicine. 2004;158:27–33. doi: 10.1001/archpedi.158.1.27. [DOI] [PubMed] [Google Scholar]
- Loos RJ, Bouchard C. Obesity—Is it a genetic disorder? Journal of Internal Medicine. 2003;254:401–425. doi: 10.1046/j.1365-2796.2003.01242.x. [DOI] [PubMed] [Google Scholar]
- Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behavior Genetics. 1997;27:325–352. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- Manolio TA. Cohort studies and the genetics of complex disease. Nature Genetics. 2009;41:5–6. doi: 10.1038/ng0109-5. [DOI] [PubMed] [Google Scholar]
- McArdle JJ. Latent curve analyses of longitudinal twin data using a mixed-effects biometric approach. Twin Research and Human Genetics. 2006;9:343–359. doi: 10.1375/183242706777591263. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Prescott CA. Mixed-effects variance components models for biometric family analyses. Behavior Genetics. 2005;35:631–652. doi: 10.1007/s10519-005-2868-1. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. Richmond, VA: Virginia Institute for Psychiatric and Behavioral Genetics Virginia Commonwealth University, Department of Psychiatry; 2003. http://www.vipbg.vcu.edu/~vipbg/software/mxmanual.pdf. [Google Scholar]
- Nelson MC, Gordon-Larsen P, Song Y, Popkin BM. Built and social environments associations with adolescent overweight and activity. American Journal of Preventative Medicine. 2006;31:109–117. doi: 10.1016/j.amepre.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. Journal of the American Medical Association. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Odoms-Young AM, Fitzgibbon M. Familial and environmental factors that contribute to pediatric overweight in African American populations: Implications for prevention and treatment. Progress in Pediatric cardiology. 2008;25:147–151. [Google Scholar]
- Ordonana JR, Rebollo-Mesa I, Gonzalez-Javier R, Perez-Riquelme F, Martinez-Selva JM, Willemsen G, Boomsma DI. Heritability of body mass index: A comparison between the Netherlands and Spain. Twin Research and Human Genetics. 2007;10:749–756. doi: 10.1375/twin.10.5.749. [DOI] [PubMed] [Google Scholar]
- Popkin BM, Duffey K, Gordon-Larsen P. Environmental influences on food choice, physical activity and energy balance. Physiology & Behavior. 2005;86:603–613. doi: 10.1016/j.physbeh.2005.08.051. [DOI] [PubMed] [Google Scholar]
- Puhl RM, Luedicke J, Heuer C. Weight-based victimization toward overweight adolescents: observations and reactionsof peers. Journal of School Health. 2011;81:696–703. doi: 10.1111/j.1746-1561.2011.00646.x. [DOI] [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondral A, Gjessing HK. Biometrical modeling of twin and family data using standard mixed model software. Biometrics. 2008;64(1):280–288. doi: 10.1111/j.1541-0420.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: a review. Journal of Abnormal Child Psychology. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Reiss D, Leve LD. Genetic expression outside the skin: Clues to mechanisms of Genotype × Environment interaction. Development and Psychopathology. 2007;19:1005–1027. doi: 10.1017/S0954579407000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DC, Almeida DM, Jacobson KC. School context and genetic influences on aggression in adolescence. Psychological Science. 1999;10:277–280. [Google Scholar]
- Schousboe K, Willemsen G, Kyvik KO, Mortensen J, Boomsma DI, Cornes BK, Davis CJ, Fagnani C, Hjelmborg J, Kaprio J, de Lange M, Luciano M, Martin NG, Pedersen N, Pietiläinen KH, Rissanen A, Saarni S, Sørensen TIA, van Baal GCM, Harris JR. Sex differences in heritability of BMI: A comparative study of results from twin studies in eight countries. Twin Research. 2003;6:409–421. doi: 10.1375/136905203770326411. [DOI] [PubMed] [Google Scholar]
- Silventoinen K, Pietiläinen KH, Tynelius P, Sørensen TIA, Kaprio J, Rasmussen F. Genetic and environmental factors in relative weight from birth to age 18: The Swedish Young Male Twins Study. International Journal of Obesity. 2007;31:615–621. doi: 10.1038/sj.ijo.0803577. [DOI] [PubMed] [Google Scholar]
- Story M, Nanney MS, Schwartz MB. Schools and obesity prevention: creating school environments and policies designed to promote healthy eating and physical activity. Milbank Quarterly. 2009;87(1):71–100. doi: 10.1111/j.1468-0009.2009.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udry JR. The National Longitudinal Study of Adolescent Health (Add Health), Waves I & II, 1994–1996 [machine-readable data file and documentation] Chapel Hill, NC: Carolina Population Center, University of North Carolina at Chapel Hill; 1998. [Google Scholar]
- Wardle J, Carnell S, Haworth CMA, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. American Journal of Clinical Nutrition. 2008;87:398–404. doi: 10.1093/ajcn/87.2.398. [DOI] [PubMed] [Google Scholar]
- Williams T. Under an Arizona plan, smokers and the obese would pay Medicaid fee. New York Times. 2011 http://www.nytimes.com/2011/05/31/us/31questions.html?_r=1.
- Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, Madden PA, Heath AC, Martin NG, Montgomery GW, Goddard ME, Visscher PM. Common SNPs explain a large proportion of the heritability for human height. Nature Genetics. 2010;42:565–569. doi: 10.1038/ng.608. [DOI] [PMC free article] [PubMed] [Google Scholar]


