Abstract
Phosphorylase kinase (PhK), a 1.3 MDa enzyme complex that regulates glycogenolysis, is composed of four copies each of four distinct subunits (α, β, γ, and δ). The catalytic protein kinase subunit within this complex is γ, and its activity is regulated by the three remaining subunits, which are targeted by allosteric activators from neuronal, metabolic, and hormonal signaling pathways. The regulation of activity of the PhK complex from skeletal muscle has been studied extensively; however, considerably less is known about the interactions among its subunits, particularly within the non-activated versus activated forms of the complex. Here, nanoelectrospray mass spectrometry and partial denaturation were used to disrupt PhK, and subunit dissociation patterns of non-activated and phospho-activated (autophosphorylation) conformers were compared. In so doing, we have established a network of subunit contacts that complements and extends prior evidence of subunit interactions obtained from chemical crosslinking, and these subunit interactions have been modeled for both conformers within the context of a known three-dimensional structure of PhK solved by cryoelectron microscopy. Our analyses show that the network of contacts among subunits differs significantly between the nonactivated and phospho-activated conformers of PhK, with the latter revealing new interprotomeric contact patterns for the β subunit, the predominant subunit responsible for PhK's activation by phosphorylation. Partial disruption of the phosphorylated conformer yields several novel subcomplexes containing multiple β subunits, arguing for their self-association within the activated complex. Evidence for the theoretical αβγδ protomeric subcomplex, which has been sought but not previously observed, was also derived from the phospho-activated complex. In addition to changes in subunit interaction patterns upon phospho-activation, mass spectrometry revealed a large change in the overall stability of the complex, with the phospho-activated conformer being more labile, in concordance with previous hypotheses on the mechanism of allosteric activation of PhK through perturbation of its inhibitory quaternary structure.
In the cascade activation of glycogenolysis in skeletal muscle, phosphorylase kinase (PhK),1 upon becoming activated through phosphorylation, subsequently phosphorylates glycogen phosphorylase in a Ca2+-dependent reaction. This phosphorylation of glycogen phosphorylase activates its phosphorolysis of glycogen, leading to energy production (1). The 1.3 MDa (αβγδ)4 PhK complex was the first protein kinase to be characterized and is among the largest and most complex enzymes known (2). As such, the intact complex has proved to be refractory to high resolution x-ray crystallographic or NMR techniques; however, low resolution structures of the nonactivated and Ca2+-saturated conformers of PhK have been deduced through modeling (3) and solved by means of three-dimensional electron microscopic (EM) reconstruction (4–7), and they show that the complex is a bilobal structure with interconnecting bridges. Approximate locations of small regions of each subunit in the complex are known (8–10) and show that the subunits pack head-to-head as apparent αβγδ protomers that form two octameric (αβγδ)2 lobes associating in D2 symmetry (11), although direct evidence that the αβγδ protomers are discrete, functional subcomplexes has been lacking until now.
Approximately 90% of the mass of the PhK complex is involved in its regulation. Its kinase activity is carried out by the catalytic core of the γ subunit (44.7 kDa), with the kcat being enhanced up to 100-fold by multiple metabolic, hormonal, and neural stimuli that are integrated through allosteric sites on PhK's three regulatory subunits, α, β, and δ (12). The small δ subunit (16.7 kDa), which is tightly bound integral calmodulin (13), binds to at least the C-terminal regulatory domain of the γ subunit (γCRD) (14, 15), thereby mediating activation of the catalytic subunit by the obligate activator Ca2+ (16). The α and β subunits, as deduced from DNA sequencing, are polypeptides of 1237 and 1092 amino acids, respectively, with calculated masses prior to post-translational modifications of 138.4 and 125.2 kDa (17, 18). Both subunits can be phosphorylated by numerous protein kinases, including cAMP-dependent protein kinase and PhK itself (2). The α and β subunits are also homologous (38% identity and 61% similarity); however, each subunit has unique phosphorylatable regions that contain nearly all the phosphorylation sites found in these subunits (17, 18).
The regulation of PhK activity by both Ca2+ (19–23) and phosphorylation has been studied extensively (reviewed in Ref. 24); however, only the structural effects induced by Ca2+ are well characterized (25), primarily through comparison of the non-activated and Ca2+-activated conformers using three-dimensional EM reconstructions (4), small angle x-ray scattering modeling (3), and biophysical (26–28) and chemical crosslinking methods (29–32). In contrast to the Ca2+-activated versus non-activated conformers, there are no reported structures of phosphorylated PhK to compare against the non-activated form. A very small amount of structural information for phospho-activated PhK derived from chemical crosslinking raises the possibility of phosphorylation-dependent communication between the β and γ subunits: Arg-18 in the N-terminal phosphorylatable region of β was found to be relatively near the γCRD (33). Several lines of evidence suggest that transduction of the activating phosphorylation signal in PhK occurs concomitantly with conformational changes in β (33) that are detected via various methods (10, 34), including chemical crosslinking (35). For example, crosslinking of only the phosphorylated conformer by the short-span crosslinker 1,5-difluoro-2,4-dinitrobenzene results in the formation of β homodimers (35). Correspondingly, more recent two-hybrid screens of the full length β subunit against itself yielded positive binding interactions only for point mutants in which the N-terminal phosphorylatable serine residues were mutated to phosphomimetic glutamates (33). It should be noted, however, that both chemical crosslinking and two-hybrid screening have potential drawbacks in the study of subunit interactions within a multisubunit complex. In the case of the latter, it is difficult when observing homodimeric two-hybrid interactions to determine whether they correspond to naturally occurring interactions between two like subunits within a complex or between two interacting regions within a single subunit of that complex. Studying subunit interactions in a complex through chemical crosslinking comes with its own inherent limitations. For example, an initial mono-derivatization can potentially cause a conformational change in one subunit that might affect the subsequent crosslinking reaction. This is particularly the case if the crosslinker contains a functionality, such as an aromatic group, that can unexpectedly direct it to a specific locus on the protein complex (36, 37). In addition, the spacer arms on many crosslinkers are sufficiently long to confound interpretation as to whether two subunits within a complex are actually in contact. Similarly, it should be proved that any observed crosslinked conjugate is formed from subunits within a complex, as opposed to between complexes (38, 39), a control that is often not run. Thus, it is prudent to analyze subunit interactions within a complex using a variety of approaches.
To corroborate, complement, and expand the previous two-hybrid screening and chemical crosslinking studies of PhK's subunit interactions and to investigate changes in the pattern of subunit interactions induced by phosphorylation, we carried out comparative MS analyses of both intact and partially denatured forms of nonactivated and phospho-activated PhK using mass spectrometers modified specifically to enhance the transmission of large noncovalently bound protein complexes (40–42). The array of subunit interactions detected for the nonactivated PhK complex largely replicated those reported in the crosslinking literature for this conformer, both corroborating those earlier studies and validating the use of these MS approaches to study subunit interactions within the PhK complex. Additionally, several novel subcomplexes of PhK were revealed, most notably an αβγδ protomer, which corroborates the observed packing of this subcomplex in the D2 symmetrical (αβγδ)4 native complex (9, 11). Moreover, we show herein that the array of subunit interactions detected for phospho-activated PhK differs significantly from that observed for the nonactivated conformer, with only the former showing extensive self-interactions between and among the regulatory β subunits. As is discussed, this suggests that activation through phosphorylation is associated with increased interprotomeric interactions in the bridged core of the PhK complex (33, 35).
EXPERIMENTAL PROCEDURES
Proteins
Nonactivated PhK was purified from the psoas muscle of female New Zealand White rabbits as described elsewhere (43). The enzyme was stored at −80 °C in 50 mm HEPES buffer (pH 6.8, 10% w/v sucrose, and 0.2 mm EDTA). Phospho-activated PhK was prepared via autophosphorylation essentially as described (43), resulting in a 40-fold activation of phosphorylase conversion at pH 6.8. Nonactivated PhK was incubated at 30 °C for 20 min in 50 mm HEPES (pH 8.2) containing ATP (1.2 mm), Ca2+ (0.2 mm), Mg(CH3CO2)2 (10 mm), and EGTA (0.1 mm). The reaction was quenched by the addition of excess EDTA (25 mm final concentration). Phospho-PhK was then purified via size exclusion chromatography over Sepharose 6B, concentrated to 1.45 mg/ml by ultrafiltration using an Amicon Ultra-2 (3 kDa) concentration device, and stored in 50 mm β-glycerophosphate buffer (pH 6.8, 10% w/v sucrose, and 2 mm EDTA). The extent of the incorporation of phosphate into the α and β subunits of PhK was determined using 32P-labeled ATP as described elsewhere (33).
Sample Preparation for MS
Nonactivated and phospho-activated PhK (0.13 and 0.08 nmole, respectively) were buffer-exchanged into 50 mm ammonium acetate (pH 6.8) using a Vivaspin (5 kDa) column (Vivascience, Hannover, Germany) and concentrated to ∼30 μl. To generate subcomplexes of the enzyme via partial denaturation (44), the pH levels of the solutions were adjusted by means of dilution with either ammonium hydroxide (10% to 20%: v/v) or acetic acid (10% to 20%: v/v). The final pH values measured were 9.9 (10% ammonium hydroxide), 10.2 (20% ammonium hydroxide), 3.0 (10% acetic acid), and 2.7 (20% acetic acid). Unless otherwise stated, partial denaturants were typically incubated with protein samples at room temperature for 5 min prior to MS analyses.
Nanoelectrospray MS
Nondenaturing nanoelectrospray (nES) mass spectra were acquired on a Q-ToF 2 mass spectrometer (Micromass/Waters, Milford, MA), modified for high mass detection (42), or on a Qstar XL (MDS Sciex, Applied Biosystems, Carlsbad, CA) using a previously described protocol optimized for the transmission of noncovalent protein complexes (41). Analysis of PhK on the Qstar XL instrument was carried out in positive ion mode using the following general parameters: ion spray voltage, 1.4 kV; declustering potential, 100 V and 15 V; focusing potential, 100 V; quadrupole voltage, 150 V; collision gas, 12; ion release delay, 6; and ion release width, 5. Experiments were performed with a collision cell pressure of ∼11.8 mbar. The following experimental parameters were applied in positive ion mode for the analysis of intact phospho-PhK on the Q-time-of-flight 2 instrument: capillary voltage, 1.7 kV; sample cone, 194 V; extraction cone, 5 V; collision energy, 200 V; collision cell pressure, 20 mbar; hexapole ion guide pressure, 42 mbar; analyzer pressure, 1.5 × 10−4 mbar; backing pressure, 1.0 mbar; and ToF pressure, 2.1 × 10−6 mbar.
LC/MS Analyses of PhK Conformers
Capillary high pressure LC/MS analyses of individual PhK and phospho-PhK subcomplexes were carried out using an LC-Packings Ultimate System (Dionex, Sunnyvale, CA) equipped with a capillary UV detector set at 214 and 280 nm. Samples were prepared in a 1:1 (v/v) solution of 0.1% TFA, and 1 μl of sample was applied to a capillary polystyrene-divinylbenzene reverse-phase monolithic column (200 μm inner diameter × 5 cm; Dionex) equilibrated at 90% solvent A (0.05% TFA) and 10% solvent B (0.04% TFA, 90% acetonitrile). A linear gradient of solvent B (10% to 70%) was developed over 25 min using a flow rate of 3 μl/min. The column effluent was analyzed via nES MS using a Q-star Pulsar (ABI/Sciex, Sunnyvale, CA) mass spectrometer.
Data Analyses
Spectra were acquired and processed using MassLynx v4.1 (Waters, Manchester, UK) and Analyst QS (MDS Sciex, Applied Biosystems). Errors are reported as ±1 standard deviation. All spectra were calibrated externally using a 100 mg/ml solution of cesium iodide. All spectra are shown with minimal smoothing and without background subtraction. Subcomplex compositions were determined using the iterative search algorithm SUMMIT (45).
RESULTS
MS Analyses of the Intact 1.3 MDa Complex of Nonactivated PhK
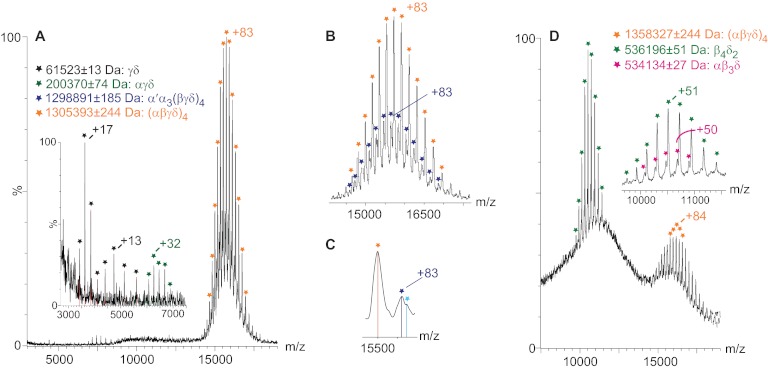
To compare subunit interactions in the two PhK conformers, we first established conditions for measuring the intact (αβγδ)4 complexes via MS from nondenaturing solutions (41, 44). nES MS of native, nonactivated PhK revealed a predominant and well-resolved charge state series centered on a +83 charge state, with an experimental mass (massEXP) of 1,305,393 ± 244 Da, which corresponds to the partially solvated (αβγδ)4 hexadecameric form of the complex (Fig. 1A). The measured mass exceeds the theoretical mass (massTHEO = 1,298,994 Da) of PhK by 6388 Da; this mass difference likely reflects buffer and water molecules associated with the protein complex (46), a phenomenon of the soft ionization process required to observe biological complexes in the gas phase (47). In addition to the intact (αβγδ)4 complex, αγδ (massTHEO = 199,664 Da) and γδ (massTHEO = 61,339 Da) subcomplexes were detected at lower m/z values by measurements for two well-resolved charge state series that corresponded, respectively, to masses of 200,370 ± 74 and 61,573 ± 13 Da. Corroborating these results, both subcomplexes have been observed previously after dissociation of the PhK complex with high concentrations of LiBr (48, 49).
Fig. 1.
Top-down MS of nonactivated and phospho-activated PhK. A, mass spectrum of intact, nonactivated (αβγδ)4 PhK in 50 mm ammonium acetate (orange), with two less intense but well-resolved series corresponding to the αγδ trimer (green) and the γδ dimer (black). B, magnification of the charge state series (orange) corresponding to the intact complex, but showing an additional, less intense partially overlapping charges state series (dark blue) that is consistent in terms of mass with the intact complex, in which one α subunit is replaced by a lower mass splice variant, termed α′ (52). C, discrete shoulders (light blue) shown in a magnified view of two peaks at 15,500 m/z from the intact PhK charge series suggesting the possible substitution of more than one molecule of α′ for α in the hexadecameric complex. D, nanoelectrospray mass spectrum of intact phospho-activated (αβγδ)4 PhK (orange), with two partially resolved charge series (magnified in inset) corresponding to β4δ2 (green) and αβ3δ subcomplexes (pink).
Close inspection of the charge series for the intact complex (Fig. 1B, orange) revealed an additional charge series (Fig. 1B, dark blue) corresponding to an intact hexadecameric complex in which one α subunit is replaced by a lower mass splice variant of this subunit (α′), an isoform found predominately in slow twitch oxidative skeletal muscle (50, 51). Alternative splicing of the α subunit results in an internal deletion and loss of residues 654–712 (52), with a corresponding mass loss of 6414 Da. The experimental mass difference of 6370 Da (0.69% error) measured between α′α3(βγδ)4 (massTHEO = 1,292,580 Da) and the (αβγδ)4 complex is in close agreement. Multiple substitutions of α′ for α in other complexes are suggested by discrete shoulders observed in the second charge series (Fig. 1C); however, these were not resolved sufficiently for accurate mass calculations.
MS Analyses of the Intact 1.3 MDa Complex of Phospho-activated PhK
Analysis via nondenaturing nES MS of the phospho-PhK conformer revealed three significant charge state series (Fig. 1D). The first, ranging approximately between 15,000 and 17,000 m/z values and centering on the +83 charge state, yielded a measured mass of 1,358,327 ± 211 Da, which corresponds to the intact, partially solvated, phosphorylated (αβγδ)4 complex. The phosphorylation of the complex resulted in the incorporation of 3.9 and 2.2 mol -PO3 per α and β subunit, respectively (see Experimental Procedures), for a total of 24.4 mol -PO3 per (αβγδ)4 complex. The 1927 Da additional mass from the -PO3 groups raises the massTHEO of the complex to 1,300,921 Da, with the mass difference between the calculated and experimental values again attributed to retention of small molecules. In comparison with native PhK, the broader peaks observed for the phospho-PhK spectrum are consistent with the increased level of heterogeneity associated with phosphorylation of the complex (reviewed in Ref. 24). The second charge series observed for the phospho-conformer was centered on a +51 charge state at ∼11,000 Da (Fig. 1D), with a measured mass of 536,196 ± 51 Da (Fig. 1D inset, green stars). After evaluating all possible combinations of subunits, we found that the subcomplex most closely corresponding to this series in terms of mass is a β4δ2 hexamer (massTHEO = 533,728 Da). In addition, a less intense third series in the same m/z range corresponded by mass (massEXP = 534,134 ± 27 Da) to an αβ3δ subcomplex having a theoretical mass of 530,272 Da (Fig. 1D inset, pink). As is discussed further on, both of these novel subcomplexes containing three and four β subunits indicate that the β subunits must occupy the core of the bridged bilobal dimer of (αβγδ)2 octameric lobes that describe PhK (5, 9, 11). Moreover, the detection of subcomplexes comprising primarily the β subunits in the mass spectrum of only phospho-PhK is consistent with previous reports demonstrating homodimeric crosslinking of β only with phosphorylated forms of PhK (35, 53).
When the mass spectra of non-activated and phospho-activated PhK were compared, we found that the highest intensity charge state peaks from the latter corresponded to its subcomplexes, whereas with the nonactivated conformer, the intact complex accounted for the most intense charge state peaks. This difference implies that the phospho-activated complex is more labile than its nonactivated counterpart. Similarly, the activated conformer of PhK produced by Ca2+-saturation is increasingly labile to thermal perturbation relative to the native, nonactivated conformer (27). The above two results are consistent with the oft postulated view that the activity of the native, nonactivated conformer of PhK is under extreme quaternary constraint and that perturbation of its quaternary structure leads to activation or, more specifically, deinhibition (33, 54). It should be noted, however, that the subunits in all forms of the complex remain tightly associated under standard solution conditions (i.e., buffer and low salt) and do not readily dissociate (see Ref. 55 and references therein).
Subcomplexes Formed from the Native, Nonactivated (αβγδ)4 PhK Complex
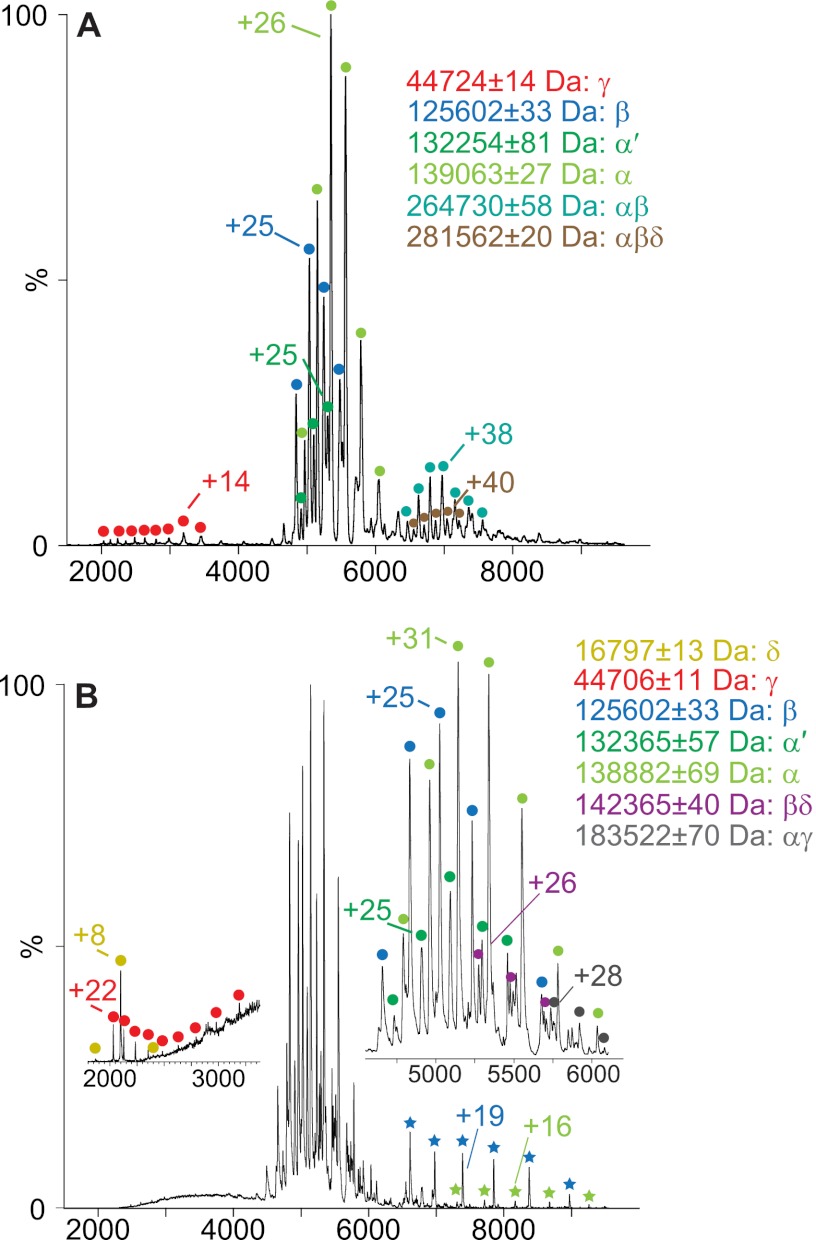
Manipulating the pH enabled us to disassemble the native, nonactivated PhK hexadecamer into subcomplexes (Table I) and individual subunits (Table II). Disruption occurred more readily at low pH values. The addition of acetic acid (final concentration 10% v/v) to PhK in 50 mm ammonium acetate (pH 6.8) yielded individual α (139,063 ± 27 Da), β (125,602 ± 33 Da), and γ (44,724 ± 14 Da) subunits, as well as two subcomplexes corresponding to an αβ dimer (264,730 ± 58 Da) and an αβδ trimer (281,562 ± 199 Da) (Fig. 2A). This association of the three regulatory subunits (α, β, and δ) with one another in the absence of the catalytic γ subunit has not previously been observed in, or suggested by, other approaches and was unexpected, given that each of these three regulatory subunits is known to directly interact with the γ subunit (33). A low intensity charge series centered on a +38 charge state (massEXP = 132,254 ± 58 Da) between 6000 and 8000 m/z corresponds well to a splice variant of α (52) termed α′ (massTHEO = 131,893 Da), which is present in minor quantities in preparations of PhK from rabbit psoas muscle (50).
Table I. Calculated and observed masses of intact complexes of PhK and subcomplexes generated from the (αβγδ)4 complex by either solution disruption with partial denaturants or CID.
| Complexes and subcomplexes | Theoretical mass (Da) | Experimental mass (Da) |
|---|---|---|
| (αβγδ)4 | 1,298,994 | 1,305,393 ± 24 |
| α′α3(βγδ)4 | 1,292,580 | 1,298,891 ± 19 |
| β4δ2 | 533,730 | 536,196 ± 51 |
| αβ3δ | 530,275 | 534,134 ± 27 |
| αββ | 388,494 | 390,819 ± 30 |
| αβγδ | 324,749 | 326,892 ± 87 |
| αβδ | 280,106 | 281,562 ± 20 |
| αβ | 263,410 | 264,730 ± 58 |
| ββ | 250,169 | 251,368 ± 59 |
| αγδa | 199,664 | 201,148 ± 81 |
| αγ | 182,968 | 183,522 ± 70 |
| βγ | 169,727 | 170,409 ± 69 |
| αδ | 155,021 | 155,698 ± 32 |
| βδ | 141,780 | 142,365 ± 40 |
| γγ | 89,286 | 89,569 ± 64 |
| γδ | 61,339 | 61,523 ± 13 |
Table II. Calculated and observed masses of the subunits of PhK.
masses for the large α and β regulatory subunits were determined via CID, and masses for γ and δ were determined via LC/MS.
| Subunits | Theoretical mass (Da) | Experimental mass (Da) |
|
|---|---|---|---|
| Nonactivated PhK | Phospho-activated PhK | ||
| α | 138,325 | 138,792 ± 40 | 139,358 ± 47 |
| β | 125,084 | 125,602 ± 33 | 125,660 ± 30 |
| γ | 44,643 | 44,667 ± 0.8 | 44,669 ± 3.0 |
| δ | 16,696 | 16,787 ± 0.3 | 16,784 ± 7.2 |
Fig. 2.
Nanoelectrospray mass spectra of partially denatured, nonactivated PhK. A, spectrum of PhK acquired in 10% (v/v) acetic acid. B, spectrum acquired for PhK under solution conditions identical to those described under A, but with activating MS conditions. Inset represents a magnified view of the major charge series peak. Green and blue stars in the full spectrum indicate ions corresponding to low-charge “stripped” α and β subunits, respectively. Low m/z (200–3000) inset shows charge series corresponding to the small γ and δ subunits.
Further analyses of subunit contacts in the non-activated conformer were carried out by altering either parameters governing the MS activation (Fig. 2B) or the acidic solution disruption conditions (supplemental Fig. S1). Regarding the former, we further analyzed subunit contacts in the complex by using more energetic gas phase ion activation conditions to promote dissociation of the complex under the same slightly acidic solution conditions as described above. Gas phase activation in an argon-filled collision cell results in the ejection of a coulombically unfolded, highly charged monomer (ejected species) from a dimer or higher order oligomer, resulting in a relatively low-charged “stripped” oligomer missing one subunit (56). For the most part, the dissociation of oligomers via this process is asymmetric (40). As shown in Fig. 2B, such activation was evidenced by detectable levels of stripped α and β subunits (green and blue stars, respectively), each revealed by a greater extent of desolvation and a lower charge state (+16 and +19, respectively) than observed for α (+31) and β (+25) under less activating conditions (Fig 2B, inset). The small δ subunit was also observed at low m/z values (Fig. 2B, low m/z inset). Additionally, two new species corresponding to αγ (massEXP = 183,522 ± 70 Da) and βδ (massEXP = 142,365 ± 40 Da) dimers were detected. In contrast to MS activation of the complex, increasing the final concentration of acetic acid to 20% (v/v) while retaining the original lower energy MS activation parameters yielded a distinct charge series that corresponded to a βγ subcomplex (massEXP = 170,409 ± 69 Da) (Table I and supplemental Fig. S1). In summary, of the six heterodimeric subcomplexes that are theoretically possible upon disruption of the (αβγδ)4 complex, the only one not observed with disassembly of the non-activated PhK conformer was the αδ dimer.
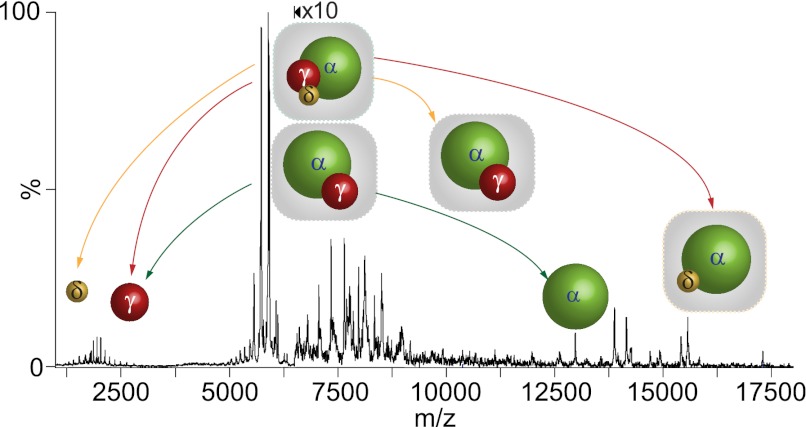
To probe further for potential direct interactions between the α and δ subunits within the PhK complex, we selected, using tandem MS, an activated charge state series corresponding to the αγδ trimer, which happened to overlap the spectrum for the αγ dimer. As described above for activation of PhK, CID of the αγδ and αγ heteromers in the collision cell of the tandem MS provides an alternative mechanism to probe for subunit interactions in these subcomplexes by following the production of ejected and stripped species from each subcomplex (40). The pathways of dissociation for both subcomplexes are shown schematically from a tandem MS spectrum of the corresponding charge series isolated at 5196 m/z from a 10% v/v ammonium hydroxide solution of PhK (Fig. 3), with full details described under supplemental Fig. S2. Activation of αγδ by CID revealed two pathways resulting from the ejection of either the catalytic γ subunit (44,702 ± 17 Da) or the δ subunit (16,869 ± 23 Da) to form, respectively, the corresponding stripped αδ (155,698 ± 32 Da) or αγ (183,580 ± 41 Da) subcomplexes (Table I). The results of CID analyses show that the α subunit interacts directly with both the catalytic γ subunit and the δ subunit. Consequently, all six theoretically possible heterodimers are observed, indicating that each subunit interacts with the remaining three, forming an extensive communication network in the nonactivated form of PhK.
Fig. 3.
Nanoelectrospray tandem mass spectrum of PhK in 10% v/v ammonium hydroxide obtained from mass selection of αγδ and αγ subcomplexes at 5916 m/z. Because of the proximity of their subcomplex mass series, both the αγδ trimer and the αγ dimer were isolated for analysis by CID. The two CID pathways detected for αγδ are shown by red and yellow arrows depicting, respectively, asymmetric ejection of γ and δ to form stripped αδ and αγ subcomplexes. The CID pathway of dissociation for αγ is depicted by green arrows.
Subcomplexes Formed from the Phospho-activated PhK Complex
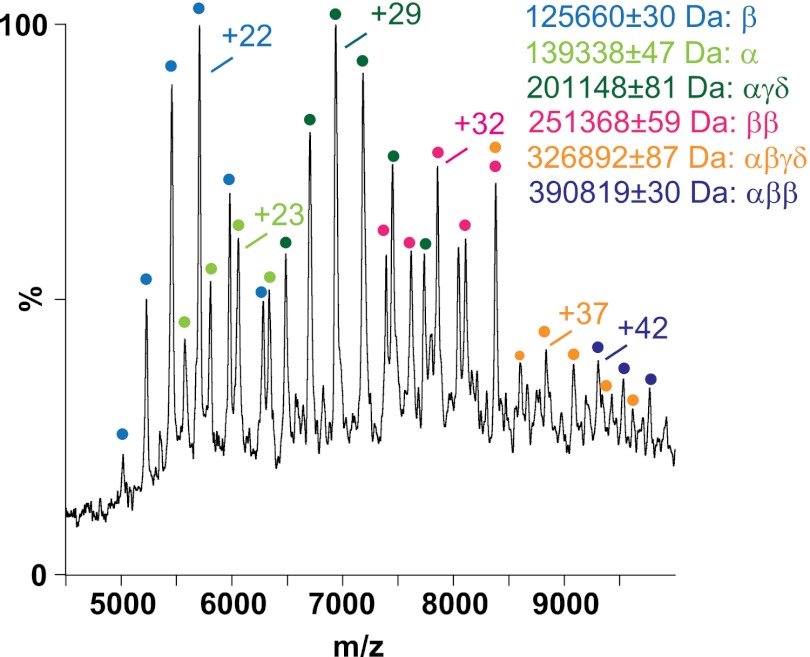
Unlike in the situation with nonactivated PhK, the detection of subcomplexes was readily achieved with its phospho-activated counterpart, with the use of only minimal amounts of either acidic or basic denaturants (not exceeding 10% total volume). This ease of partial dissociation provides further evidence that the phospho-activated complex is more labile than the nonactivated conformer. The subcomplexes and individual subunits observed with the addition of acetic acid (supplemental Fig. S3A) or ammonium hydroxide (Fig. 4 and supplemental Fig. S3B) to phospho-activated PhK were in many cases identical to those observed upon disruption of the native, nonactivated conformer. Several new species were observed, however, most notably an intact αβγδ protomer (326,892 ± 87 Da) (Fig. 4, orange dots). The observation of this subcomplex represents the first direct evidence of this tetramer within the quaternary structure of PhK. Two additional species, an αβ2 trimer (390,819 ± 30 Da) and a β2 dimer (251,368 ± 59 Da), are consistent with the αβ3δ and β4δ2 subcomplexes observed in MS of the intact phospho-activated PhK complex (Fig. 1D). It is important to note that no subcomplexes containing more than one β subunit were obtained from the nonactivated conformer of PhK.
Fig. 4.
Nanoelectrospray mass spectrum of phospho-activated PhK. Both subunits and subcomplexes were observed in the presence of 10% (v/v) ammonium hydroxide.
DISCUSSION
MS methods designed for optimal transmission and analyses of large macromolecular assemblies have previously proved successful in determining the stoichiometry and subunit architecture of large multisubunit complexes in the MDa mass range (56). These MS methods complement structural information for such complexes obtained from chemical crosslinking (45). An advantage of these MS methods over crosslinking is that large subcomplexes can usually be unambiguously identified via MS (46), whereas crosslinking of large protein complexes to capture oligomeric conjugates contained within is generally less selective, resulting in the formation of a continuum of crosslinked subunits that is sometimes too complex to adequately fractionate and analyze components (39). Consequently, crosslinking is usually most accurate when identifying small product conjugates, such as dimers (39). Our MS results with the nonactivated conformer of PhK show that all but one (αα) of the dimeric subunit–subunit interactions identified in a large body of crosslinking literature were observed in either solution disruption or CID experiments (Table I). These similar findings both corroborate the pertinent previous crosslinking results and validate the MS approaches used herein to analyze subunit interactions within the PhK complex; however, one superiority of the top-down MS approaches over crosslinking is evident when comparing the nonactivated to the activated conformers of PhK. Although both approaches readily show differences in subunit interaction maps, the difference in stability of the two complexes is shown only by MS.
The observation of the α′α3(βγδ)4 subcomplex has implications regarding hetero-oligomeric interactions and tissue-dependent isoform expression. PhK containing the α subunit is found in fast twitch skeletal muscle, whereas the alternatively spliced α′ species, in which one exon is deleted (52), is found in PhK from slow twitch oxidative or cardiac muscle (50). That both subunit types can be found within the same subcomplex (Fig. 1B) indicates a lack of selectivity for PhK's quaternary structure by tolerating such heterogeneity. It would also seem to indicate that both subunits are simultaneously expressed in certain muscle cells, unless in a solution of purified PhK there can be exchange of α and α′ subunits between (αβγδ)4 and (α′βγδ)4 complexes. There is clearly a small number of red fiber types in our source of “white” muscle (i.e., the presence of α′); however, there is no published evidence of subunit exchange involving the α subunit. In fact, even when the α subunit is extensively proteolyzed, its fragments remain tightly bound as part of the PhK complex (57). Consequently, it seems likely that both α and α′ are synthesized in some cells and that both can simultaneously occur within the same hexadecameric PhK complex.
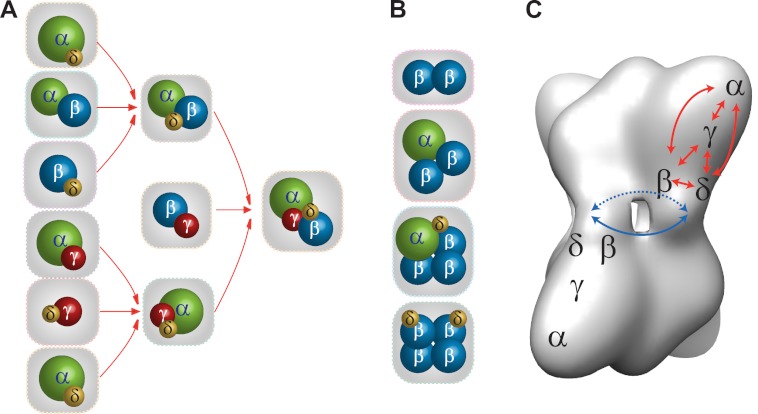
Several large hetero-oligomeric subcomplexes containing single copies of each subunit, including an αγδ trimer and an αβγδ tetramer, were observed in mass spectra of the intact and solution disrupted forms of the PhK complex; these indicate extensive subunit communication networks, as well as distinct substructures within PhK's quaternary structure (33). Based on the stoichiometry and positions of the subunits (8–10), the PhK complex has been suggested to comprise four αβγδ protomers that pack as head-to-head pairs to form two long (176 Å) (αβγδ)2 octameric lobes that perpendicularly associate with D2 symmetry through four central interconnecting bridges (11). Our results herein provide the first direct evidence for such an αβγδ protomer, suggesting that it is a structural and functional moiety in the complex (11). Given the large axial ratio (length/width > 2.3) of the lobes (4) and the peripheral locations (8, 9) and relatively small masses of the γ and δ subunits, we presume that most of the heterodimers identified through chemical crosslinking and our MS analyses reflect subunit contacts within individual αβγδ protomers. If this is true, then the six observed heterodimers (αβ, αγ, αδ, βγ, βδ, γδ), the maximal possible theoretical number, would suggest a localized, but extensive, set of protein–protein interactions in which each subunit interacts with the remaining three within its protomer. The various subcomplexes observed in this study are depicted in Fig. 5, in which the extensive interactions among all the subunits are shown in the context of a three-dimensional cryoEM reconstruction of the non-activated PhK complex (Fig. 5C). The trimeric αγδ subcomplex detected in spectra of intact native PhK and solution disrupted forms of the kinase is an expected dissociation product, as this stable subcomplex has been observed previously (48).
Fig. 5.
Comparison of subunit interaction networks determined for nonactivated and phospho-activated PhK. A, connectivity among the subunits within the αβγδ protomer deduced by detection of heteromeric complexes. B, subcomplexes detected only in phospho-activated PhK that show interprotomeric interactions mediated by the β subunits in the (αβγδ)4 complex. C, as determined previously by EM methods (8–10), the approximate placement of the four subunits is shown for two αβγδ protomers that are located on opposite (αβγδ)2 lobes of the cryoEM density map of PhK (5). The red and blue arrows indicate, respectively, subunit interaction networks determined by top-down MS among α, β, γ, and δ subunits in a protomer and the β subunits from each of four protomers in the bridge region of PhK.
Within the (αβγδ)4 complex, there is considerable evidence of a Ca2+-dependent communication network involving the α, γ, and δ (calmodulin) subunits. For instance, binding of Ca2+ to δ causes a mass redistribution on the (αβγδ)2 lobe tips (4) where the carboxy-terminal region of α has been localized (9), and it also alters proteolysis of α near its carboxy-terminus (32). Moreover, the catalytic activities of (αβγδ)4 and αγδ are stimulated in parallel by Ca2+ (49, 58). There is strong indirect evidence that the effects on α of the binding of Ca2+ to δ are mediated by γ, specifically by the γCRD, and adjacent regions of its primary structure have been shown to interact with α and δ within PhK. The question of whether a direct δ–α interaction also occurs that could potentially participate in this δ ↔ γ ↔ α communication network has not been addressed, because previous evidence for α–δ interactions has been indirect and weak. For example, crosslinking of PhK by several reagents results in the formation of conjugates that correspond by apparent mass to αδ dimers but crossreact only with an antibody against the α subunit (30, 37), presumably because the epitope on the δ subunit is masked by crosslinking. CID of the αγδ complex has shown unequivocally for the first time a direct interaction between the α and δ subunits, and together with the other evidence above, this demonstrates that all three subunits interact with one another in the αγδ subcomplex, and presumably within the αβγδ protomeric structures, of PhK.
One of the primary reasons for performing this work was to determine whether changes in subunit interactions could be observed upon activation of PhK by phosphorylation. Using electron microscopy (4) and small-angle x-ray scattering (3), it has been established that activation of the nonphosphorylated enzyme by the binding of Ca2+ causes structural changes in both its lobes and bridges, but it has not been known whether related structural changes occur upon activation of PhK by phosphorylation. Because any relatively small subcomplex containing more than two of a given subunit (e.g., αβ3δ and β4δ2) must, by definition, contain the bridged core connecting PhK's two (αβγδ)2 lobes, the β subunits must compose that bridged core.
Thus, based on the results herein, we can now conclude that the phospho-activated PhK complex, similar to the Ca2+-activated form, also demonstrates significant structural changes in the region of its bridges. β-Dimers and higher order oligomers containing two or more copies of this subunit were detected only in spectra of phospho-PhK (either intact or solution disrupted), revealing significant differences in the subunit interaction patterns between the nonactivated and phospho-activated conformers (Fig. 5). The self-interactions of β indicated by the detection of β2, αβ2, αβ3δ, and β4δ2 subcomplexes only in phospho-activated PhK are consistent with our previous yeast two-hybrid screens showing that self-interaction of the β subunit is greatly enhanced by mutation of its known N-terminal phosphorylatable regulatory serines to phosphomimetic glutamates (33). The β subunit is predominant in the regulation of PhK by phosphorylation (33), in that its phosphorylation parallels activation of the catalytic kinase γ subunit in the complex (59, 60). The phosphorylation of PhK has also been reported to reduce its stringency for activation by Ca2+ (20), and a linkage between β and the Ca2+-binding δ subunit is seen in the βδ and β4δ2 subcomplexes observed in spectra of intact and solution disrupted forms of phospho-PhK. Moreover, detection of the αβγδ protomer in the phospho-activated conformer of PhK suggests that β is simultaneously able to maintain interprotomeric contacts with adjacent β subunits and intraprotomeric contacts with the α, γ, and δ subunits. Our models of nonactivated and phospho-activated PhK depicted in Fig. 5 show for the first time in a global context the architecture of subunit interactions in each conformer and the changes induced in the subunit interaction pattern upon phosphorylation.
Supplementary Material
Footnotes
* Supported by NIH grant DK32953 (to GMC) and the Royal Society and the Engineering and Physical Science Research Council (to CVR).
 This article contains supplemental Figs. S1 to S3.
This article contains supplemental Figs. S1 to S3.
1 The abbreviations used are:
- EM
- electron microscopy
- γCRD
- γ subunit C-terminal regulatory domain
- nES
- nanoelectrospray
- PhK
- phosphorylase kinase.
REFERENCES
- 1. Krebs E. G., Fischer E. H. (1956) The phosphorylase b to a converting enzyme of rabbit skeletal muscle. Biochim. Biophys. Acta 20, 150–157 [DOI] [PubMed] [Google Scholar]
- 2. Brushia R. J., Walsh D. A. (1999) Phosphorylase kinase: the complexity of its regulation is reflected in the complexity of its structure. Front. Biosci. 4, D618–D641 [DOI] [PubMed] [Google Scholar]
- 3. Priddy T. S., MacDonald B. A., Heller W. T., Nadeau O. W., Trewhella J., Carlson G. M. (2005) Ca2+-induced structural changes in phosphorylase kinase detected by small-angle X-ray scattering. Protein Sci. 14, 1039–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nadeau O. W., Carlson G. M., Gogol E. P. (2002) A Ca(2+)-dependent global conformational change in the 3D structure of phosphorylase kinase obtained from electron microscopy. Structure 10, 23–32 [DOI] [PubMed] [Google Scholar]
- 5. Nadeau O. W., Gogol E. P., Carlson G. M. (2005) Cryoelectron microscopy reveals new features in the three-dimensional structure of phosphorylase kinase. Protein Sci. 14, 914–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Venien-Bryan C., Jonic S., Skamnaki V., Brown N., Bischler N., Oikonomakos N. G., Boisset N., Johnson L. N. (2009) The structure of phosphorylase kinase holoenzyme at 9.9 angstroms resolution and location of the catalytic subunit and the substrate glycogen phosphorylase. Structure 17, 117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Venien-Bryan C., Lowe E. M., Boisset N., Traxler K. W., Johnson L. N., Carlson G. M. (2002) Three-dimensional structure of phosphorylase kinase at 22 A resolution and its complex with glycogen phosphorylase b. Structure 10, 33–41 [DOI] [PubMed] [Google Scholar]
- 8. Traxler K. W., Norcum M. T., Hainfeld J. F., Carlson G. M. (2001) Direct visualization of the calmodulin subunit of phosphorylase kinase via electron microscopy following subunit exchange. J. Struct. Biol. 135, 231–238 [DOI] [PubMed] [Google Scholar]
- 9. Wilkinson D. A., Marion T. N., Tillman D. M., Norcum M. T., Hainfeld J. F., Seyer J. M., Carlson G. M. (1994) An epitope proximal to the carboxyl terminus of the alpha-subunit is located near the lobe tips of the phosphorylase kinase hexadecamer. J. Mol. Biol. 235, 974–982 [DOI] [PubMed] [Google Scholar]
- 10. Wilkinson D. A., Norcum M. T., Fizgerald T. J., Marion T. N., Tillman D. M., Carlson G. M. (1997) Proximal regions of the catalytic gamma and regulatory beta subunits on the interior lobe face of phosphorylase kinase are structurally coupled to each other and with enzyme activation. J. Mol. Biol. 265, 319–329 [DOI] [PubMed] [Google Scholar]
- 11. Norcum M. T., Wilkinson D. A., Carlson M. C., Hainfeld J. F., Carlson G. M. (1994) Structure of phosphorylase kinase. A three-dimensional model derived from stained and unstained electron micrographs. J. Mol. Biol. 241, 94–102 [DOI] [PubMed] [Google Scholar]
- 12. Heilmeyer L. M., Jr. (1991) Molecular basis of signal integration in phosphorylase kinase. Biochim. Biophys. Acta 1094, 168–174 [DOI] [PubMed] [Google Scholar]
- 13. Cohen P., Burchell A., Foulkes J. G., Cohen P. T. (1978) Identification of the Ca2+-dependent modulator protein as the fourth subunit of rabbit skeletal muscle phosphorylase kinase. FEBS Lett. 92, 287–293 [DOI] [PubMed] [Google Scholar]
- 14. Dasgupta M., Honeycutt T., Blumenthal D. K. (1989) The gamma-subunit of skeletal muscle phosphorylase kinase contains two noncontiguous domains that act in concert to bind calmodulin. J. Biol. Chem. 264, 17156–17163 [PubMed] [Google Scholar]
- 15. Jeyasingham M. D., Artigues A., Nadeau O. W., Carlson G. M. (2008) Structural evidence for co-evolution of the regulation of contraction and energy production in skeletal muscle. J. Mol. Biol. 377, 623–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meyer W. L., Fischer E. H., Krebs E. G. (1964) Activation of skeletal muscle phosphorylase b kinase by Ca. Biochemistry 3, 1033–1039 [DOI] [PubMed] [Google Scholar]
- 17. Kilimann M. W., Zander N. F., Kuhn C. C., Crabb J. W., Meyer H. E., Heilmeyer L. M., Jr. (1988) The alpha and beta subunits of phosphorylase kinase are homologous: cDNA cloning and primary structure of the beta subunit. Proc. Natl. Acad. Sci. U.S.A. 85, 9381–9385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wullrich A., Hamacher C., Schneider A., Kilimann M. W. (1993) The multiphosphorylation domain of the phosphorylase kinase alpha M and alpha L subunits is a hotspot of differential mRNA processing and of molecular evolution. J. Biol. Chem. 268, 23208–23214 [PubMed] [Google Scholar]
- 19. Burger D., Cox J. A., Fischer E. H., Stein E. A. (1982) The activation of rabbit skeletal muscle phosphorylase kinase requires the binding of 3 Ca2+ per delta subunit. Biochem. Biophys. Res. Commun. 105, 632–638 [DOI] [PubMed] [Google Scholar]
- 20. Cohen P. (1980) The role of calcium ions, calmodulin and troponin in the regulation of phosphorylase kinase from rabbit skeletal muscle. Eur. J. Biochem. 111, 563–574 [DOI] [PubMed] [Google Scholar]
- 21. Heilmeyer L. M., Jr., Meyer F., Haschke R. H., Fischer E. H. (1970) Control of phosphorylase activity in a muscle glycogen particle. II. Activation by calcium. J. Biol. Chem. 245, 6649–6656 [PubMed] [Google Scholar]
- 22. Kilimann M. W., Heilmeyer L. M., Jr. (1982) Multiple activities on phosphorylase kinase. 1. Characterization of three partial activities by their response to calcium ion, magnesium ion, pH, and ammonium chloride and effect of activation by phosphorylation and proteolysis. Biochemistry 21, 1727–1734 [DOI] [PubMed] [Google Scholar]
- 23. Brostrom C. O., Hunkeler F. L., Krebs E. G. (1971) The regulation of skeletal muscle phosphorylase kinase by Ca2+. J. Biol. Chem. 246, 1961–1967 [PubMed] [Google Scholar]
- 24. Pickett-Gies C. A., Walsh D. A. (1986) Phosphorylase kinase. In The Enzymes (Boyer P. D., Krebs E. G., eds) 3rd Ed., pp. 395–459, Academic Press, Orlando, FL [Google Scholar]
- 25. Nadeau O. W., Carlson G. M. (2012) A review of methods used for identifying structural changes in a large protein complex. Methods Mol. Biol. 796, 117–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu W., Priddy T. S., Carlson G. M. (2008) Physicochemical changes in phosphorylase kinase associated with its activation. Protein Sci. 108, 2111–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Priddy T. S., Middaugh C. R., Carlson G. M. (2007) Electrostatic changes in phosphorylase kinase induced by its obligatory allosteric activator Ca2+. Protein Sci. 16, 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Priddy T. S., Price E. S., Johnson C. K., Carlson G. M. (2007) Single molecule analyses of the conformational substates of calmodulin bound to the phosphorylase kinase complex. Protein Sci. 16, 1017–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ayers N. A., Nadeau O. W., Read M. W., Ray P., Carlson G. M. (1998) Effector-sensitive cross-linking of phosphorylase b kinase by the novel cross-linker 4-phenyl-1,2,4-triazoline-3,5-dione. Biochem. J. 331, 137–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nadeau O. W., Sacks D. B., Carlson G. M. (1997) The structural effects of endogenous and exogenous Ca2+/calmodulin on phosphorylase kinase. J. Biol. Chem. 272, 26202–26209 [DOI] [PubMed] [Google Scholar]
- 31. Nadeau O. W., Traxler K. W., Fee L. R., Baldwin B. A., Carlson G. M. (1999) Activators of phosphorylase kinase alter the cross-linking of its catalytic subunit to the C-terminal one-sixth of its regulatory alpha subunit. Biochemistry 38, 2551–2559 [DOI] [PubMed] [Google Scholar]
- 32. Rice N. A., Nadeau O. W., Yang Q., Carlson G. M. (2002) The calmodulin-binding domain of the catalytic gamma subunit of phosphorylase kinase interacts with its inhibitory alpha subunit: evidence for a Ca2+ sensitive network of quaternary interactions. J. Biol. Chem. 277, 14681–14687 [DOI] [PubMed] [Google Scholar]
- 33. Nadeau O. W., Anderson D. W., Yang Q., Artigues A., Paschall J. E., Wyckoff G. J., McClintock J. L., Carlson G. M. (2007) Evidence for the location of the allosteric activation switch in the multisubunit phosphorylase kinase complex from mass spectrometric identification of chemically crosslinked peptides. J. Mol. Biol. 365, 1429–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trempe M. R., Carlson G. M. (1987) Phosphorylase kinase conformers. Detection by proteases. J. Biol. Chem. 262, 4333–4340 [PubMed] [Google Scholar]
- 35. Fitzgerald T. J., Carlson G. M. (1984) Activated states of phosphorylase kinase as detected by the chemical cross-linker 1,5-difluoro-2,4-dinitrobenzene. J. Biol. Chem. 259, 3266–3274 [PubMed] [Google Scholar]
- 36. Carlson G. M. (1984) Precautions when determining kinetically the order of inactivation of enzymes by functionally irreversible inhibitors. Biochim. Biophys. Acta 789, 347–350 [DOI] [PubMed] [Google Scholar]
- 37. Nadeau O. W., Sacks D. B., Carlson G. M. (1997) Differential affinity cross-linking of phosphorylase kinase conformers by the geometric isomers of phenylenedimaleimide. J. Biol. Chem. 272, 26196–26201 [DOI] [PubMed] [Google Scholar]
- 38. Nadeau O. W., Carlson G. M. (1994) Zero length conformation-dependent cross-linking of phosphorylase kinase subunits by transglutaminase. J. Biol. Chem. 269, 29670–29676 [PubMed] [Google Scholar]
- 39. Nadeau O. W., Carlson G. M. (2005) Protein interactions captured by chemical cross-linking. In Protein-Protein Interactions: A Molecular Cloning Manual (Golemis E., Adams P. D., eds) 2nd Ed., pp. 105–127, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 40. Benesch J. L., Ruotolo B. T., Simmons D. A., Robinson C. V. (2007) Protein complexes in the gas phase: technology for structural genomics and proteomics. Chem. Rev. 107, 3544–3567 [DOI] [PubMed] [Google Scholar]
- 41. Hernandez H., Robinson C. V. (2007) Determining the stoichiometry and interactions of macromolecular assemblies from mass spectrometry. Nat. Protoc. 2, 715–726 [DOI] [PubMed] [Google Scholar]
- 42. Sobott F., Hernandez H., McCammon M. G., Tito M. A., Robinson C. V. (2002) A tandem mass spectrometer for improved transmission and analysis of large macromolecular assemblies. Anal. Chem. 74, 1402–1407 [DOI] [PubMed] [Google Scholar]
- 43. King M. M., Carlson G. M. (1981) Synergistic activation by Ca2+ and Mg2+ as the primary cause for hysteresis in the phosphorylase kinase reactions. J. Biol. Chem. 256, 11058–11064 [PubMed] [Google Scholar]
- 44. Hernandez H., Dziembowski A., Taverner T., Seraphin B., Robinson C. V. (2006) Subunit architecture of multimeric complexes isolated directly from cells. EMBO Rep. 7, 605–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taverner T., Hernandez H., Sharon M., Ruotolo B. T., Matak-Vinkovic D., Devos D., Russell R. B., Robinson C. V. (2008) Subunit architecture of intact protein complexes from mass spectrometry and homology modeling. Acc. Chem. Res. 41, 617–627 [DOI] [PubMed] [Google Scholar]
- 46. McKay A. R., Ruotolo B. T., Ilag L. L., Robinson C. V. (2006) Mass measurements of increased accuracy resolve heterogeneous populations of intact ribosomes. J. Am. Chem. Soc. 128, 11433–11442 [DOI] [PubMed] [Google Scholar]
- 47. Aebersold R., Mann M. (2003) Mass spectrometry-based proteomics. Nature 422, 198–207 [DOI] [PubMed] [Google Scholar]
- 48. Chan K. F., Graves D. J. (1982) Isolation and physicochemical properties of active complexes of rabbit muscle phosphorylase kinase. J. Biol. Chem. 257, 5939–5947 [PubMed] [Google Scholar]
- 49. Chan K. F., Graves D. J. (1982) Rabbit skeletal muscle phosphorylase kinase. Catalytic and regulatory properties of the active alpha gamma delta and gamma delta complexes. J. Biol. Chem. 257, 5948–5955 [PubMed] [Google Scholar]
- 50. Burchell A., Cohen T. W., Cohen P. (1976) Distribution of isoenzymes of the glycogenolytic cascade in different types of muscle fibre. FEBS Lett. 67, 17–22 [DOI] [PubMed] [Google Scholar]
- 51. Jennissen H. P., Heilmeyer L. M., Jr. (1974) Multiple forms of phosphorylase kinase in red and white skeletal muscle. FEBS Lett. 42, 77–80 [DOI] [PubMed] [Google Scholar]
- 52. Harmann B., Zander N. F., Kilimann M. W. (1991) Isoform diversity of phosphorylase kinase alpha and beta subunits generated by alternative RNA splicing. J. Biol. Chem. 266, 15631–15637 [PubMed] [Google Scholar]
- 53. Cheng A., Fitzgerald T. J., Carlson G. M. (1985) Adenosine 5′-diphosphate as an allosteric effector of phosphorylase kinase from rabbit skeletal muscle. J. Biol. Chem. 260, 2535–2542 [PubMed] [Google Scholar]
- 54. Paudel H. K., Carlson G. M. (1987) Inhibition of the catalytic subunit of phosphorylase kinase by its alpha/beta subunits. J. Biol. Chem. 262, 11912–11915 [PubMed] [Google Scholar]
- 55. Paudel H. K., Carlson G. M. (1990) The quaternary structure of phosphorylase kinase as influenced by low concentrations of urea. Evidence suggesting a structural role for calmodulin. Biochem. J. 268, 393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Benesch J. L., Robinson C. V. (2006) Mass spectrometry of macromolecular assemblies: preservation and dissociation. Curr. Opin. Struct. Biol. 16, 245–251 [DOI] [PubMed] [Google Scholar]
- 57. Trempe M. R., Carlson G. M., Hainfeld J. F., Furcinitti P. S., Wall J. S. (1986) Analyses of phosphorylase kinase by transmission and scanning transmission electron microscopy. J. Biol. Chem. 261, 2882–2889 [PubMed] [Google Scholar]
- 58. Boulatnikov I. G., Peters J. L., Nadeau O. W., Sage J. M., Daniels P. J., Kumar P., Walsh D. A., Carlson G. M. (2009) Expressed phosphorylase b kinase and its alphagammadelta subcomplex as regulatory models for the rabbit skeletal muscle holoenzyme. Biochemistry (Mosc.) 48, 10183–10191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. King M. M., Fitzgerald T. J., Carlson G. M. (1983) Characterization of initial autophosphorylation events in rabbit skeletal muscle phosphorylase kinase. J. Biol. Chem. 258, 9925–9930 [PubMed] [Google Scholar]
- 60. Ramachandran C., Goris J., Waelkens E., Merlevede W., Walsh D. A. (1987) The interrelationship between cAMP-dependent alpha and beta subunit phosphorylation in the regulation of phosphorylase kinase activity. Studies using subunit specific phosphatases. J. Biol. Chem. 262, 3210–3218 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.