Abstract
Aberrant melanocortin signaling has been implicated in the pathogenesis of wasting in chronic kidney disease (CKD). Previously, we demonstrated that agouti-related peptide (AgRP), a melenocortin-4 receptor antagonist, reduced CKD-associated cachexia in CKD mice. Our previous studies with AgRP utilized dual energy X-ray (DXA) densitometry to assess the body composition in mice (Cheung W, Kuo HJ, Markison S, Chen C, Foster AC, Marks DL, Mak RH. J Am Soc Nephrol 18: 2517–2524, 2007; Cheung W, Yu PX, Little BM, Cone RD, Marks DL, Mak RH. J Clin Invest 115: 1659–1665, 2005). DXA is unable to differentiate water content in mice, and fluid retention in CKD may lead to an overestimate of lean mass. In this study, we employed quantitative magnetic resonance technique to evaluate body composition change following central administration of AgRP in a CKD mouse model. AgRP treatment improved energy expenditure, total body mass, fat mass, and lean body mass in CKD mouse. We also investigated the effect of CKD-associated cachexia on the signaling pathways leading to wasting in skeletal muscle, as well as whether these changes can be ameliorated by central administration of AgRP. AgRP treatment caused an overall decrease in proinflammatory cytokines, which may be one important mechanism of its effects. Muscle wasting in CKD may be due to the activation of proteolytic pathways as well as inhibition of myogenesis and muscle regeneration processes. Our results suggest that these aberrant pathological pathways leading to muscle wasting in CKD mice were ameliorated by central administration of AgRP.
Keywords: chronic kidney disease, muscle wasting, melanocortin signaling, proinflammatory cytokines, muscle regeneration
cachexia is prevalent among patients with chronic kidney disease (CKD) and is associated with anorexia and progressive loss of body fat and lean mass (26, 27). Although calorie deficiency from anorexia is a common feature, cachexia is not prevented by increased caloric intake. Existing pharmaceutical therapy has been relatively ineffective (24). Thus the development of new therapy for this disorder is urgently needed. One novel approach to treat this disorder is to target the regulation of energy homeostasis. The pivotal role played by the central melanocortin system in regulating energy homeostasis has made this an attractive target for novel cachexia therapy. The mixed melanocortin receptor antagonist agouti-related peptide (AgRP) is an endogenous peptide that induces hyperphagia (23). Previously, we showed that AgRP attenuated cachexia in CKD mice (8, 10). Although promising, our previous studies (38, 39) with AgRP have one major caveat, as we used dual energy X-ray absorptiometry (DXA) to assess the body composition in mice. DXA cannot measure body water. This is particularly pertinent to CKD. The presence of fluid overload in CKD often leads to an overestimate of lean mass by DXA. The superior precision offered by the quantitative magnetic resonance (QMR) approach over DXA technique to body composition analysis has been demonstrated (21, 43, 44). QMR measurements utilize inherent differences in the nuclear magnetic resonance properties of hydrogen atoms and hydrogen density in fluids and tissues to derive estimates of fat mass, lean mass, total body water, and free water (body fluids not bound in tissues; Ref. 33).
Insulin-like growth factor-I (IGF-I) and myostatin are important regulators of muscle mass (25, 28). Proinflammatory cytokines, such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α, induce protein catabolism, enhance lipolysis, suppress appetite, and increase energy expenditure in patients with CKD (2, 9, 46). Aberrant expression of forkhead family transcription factor 1 (FoxO1), the ubiquitin ligases muscle atrophy F-box (Atrogin-1), and muscle ring finger 1 (MuRF-1) as well as myogenic differentiation gene (MyoD) have been implicated in the pathogenesis of muscle atrophy (15, 16, 32, 37, 49). Skeletal muscle regeneration following injury involves proliferation and differentiation of satellite cells leading to the formation of new myofibers (7). Recent studies (41, 48, 53) suggest that chemokine CXCL-16 and monocyte chemoattractant protein-1 (MCP-1) are critical for the regeneration process occurring in the injured muscle tissue.
In the present study, we employed QMR technique to measure body composition change following central administration of AgRP in a surgically induced CKD mouse model. We further investigated the effect of CKD-associated cachexia on the signaling pathways leading to wasting in skeletal muscle as well as whether these pathological pathways can be ameliorated by central administration of AgRP.
MATERIALS AND METHODS
Animals.
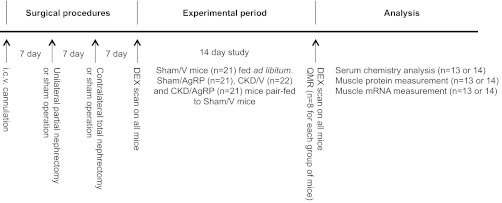
Male C57BL/6J mice (8-wk of age, Jackson Laboratory) were maintained on a normal 12:12-h light-dark cycle. Scheme of experimental procedure is outlined in Fig. 1. Experiments were conducted in accordance with the National Institutes Health's Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committees of the University of California, San Diego.
Fig. 1.
Scheme representation of the experimental procedures. Surgical implantation of intracerebroventricular (icv) cannulation was performed in all mice. Subsequent 2-stage nephrectomy or sham operation was performed. All mice underwent body composition analysis twice, that is, at the start and at the end of 14-day observation by dual energy X-ray (DXA). At the end of study, following DXA measurement in conscious live mice, eight mice from each study group were killed and shipped for postmortem quantitative magnetic resonance (QMR) analysis. The remaining mice in each group were killed for serum chemistry analysis. Gastrocnemius muscle samples were extracted for protein and mRNA analysis. Four groups of mice were included: sham-operated, normal saline treated (Sham/V); sham operated, AgRP treated (Sham/AgRP); CKD, normal saline treated (CKD/V); and CKD, AgRP treated (CKD/AgRP).
Mouse chronic kidney disease surgeries.
CKD in mice was induced by standard subtotal two-stage nephrectomy operation as described previously (10). Sham operation was performed in a control mouse.
Mouse intracerebroventricular AgRP administration.
Cannulation implantation was performed in all mice (10). Mice were divided into four groups: sham-operated, normal saline treated (Sham/V); sham operated, AgRP treated (Sham/AgRP); CKD, normal saline treated (CKD/V); and CKD, AgRP treated (CKD/AgRP). Normal saline or 2 nmol of AgRP (82–131 amino acid fragment; Phoenix Pharmaceuticals) were infused into the lateral ventricle of the mice, using a 10-μl syringe (Hamilton). For this study, mice were infused at days 0, 3, 6, 9, and 12 relative to the initial injection.
Mouse diet manipulation.
CKD/V mice were fed ad libitum with powdered mouse diet 5015 containing 17% crude protein (LabDiet) whereas Sham/V, Sham/AgRP, and CKD/AgRP mice were pair fed with CKD/V mice.
Indirect calorimetry.
Oxygen consumption (V̇o2) was determined by indirect calorimetry (Oxymax; Columbus Instruments; Ref. 10). Mice were housed in separated chambers at 24 ± 1°C. Mice were first acclimatized to the chambers for 2 days before the study was conducted. Measurements were recorded for 4–6 h during the light cycle (0900–1700). Samples were recorded every 3 min with the room air reference taken every 30 min and the airflow to chambers 500 ml/min. Basal oxygen consumption (ml·kg−1·h−1) was determined as the average of the three lowest measurements obtained during the day of observation.
Body composition analysis by DXA.
In vivo body composition in anesthetized mice was determined twice, i.e., before the initiation and at the end of study by PiXimus mouse densitometer (MEC Lunar; Ref. 10). All animals were fasted for 12 h before DXA analysis to minimize the effect of indigested food on the DXA analysis. Food efficiency was calculated by dividing the total weight gained (in grams) by the total amount of food consumed (in grams).
Body composition analysis by QMR.
At the end of study, following DXA measurements in conscious live mice, eight mice from each group were killed by CO2 asphyxia, enclosed individually in airtight plastic bags, snapped frozen in liquid nitrogen, and shipped in dry ice for post mortem QMR analysis (EchoMRI-100; Echo Medical Systems). Intact carcasses were warmed to 37°C in a hybridization oven but were not removed from plastic bags to limit water loss. Total fat mass, lean mass, and water content of mice were analyzed. The percentage of fat mass, lean mass, and water content in mice was calculated by dividing the total fat mass (gram), lean mass (gram), and water content (gram) by the body weightt of individual mouse (gram).
Serum chemistry analysis.
The remaining mice in each group were killed for serum chemistry analysis. Blood urea nitrogen (BUN) and blood bicarbonate levels were assayed by standard laboratory methods. Serum creatinine levels were analyzed using QuantiChrom creatinine assay kit (BioAssay Systems).
Muscle protein measurement.
Gastrocnemius muscle from experimental mice was dissected for protein analysis. Muscle protein was extracted, and protein concentration was determined by Pierce BCA protein assay kit (Thermo Scientific, product no. 23227). Muscle tissue lysate protein levels of IL-1α, IL-1β, IL-6, IL-10, IFN-γ, TNF-α, CXCL-16, and MCP-1 were quantified with Mouse Quantibody Custom Array (RayBiotech).
RNA isolation and analysis.
Total RNA from gastrocnemius muscles were extracted and reversely transcribed with standard procedures. Appropriate primers and probes for mouse IGF-I, myostatin, MyoD, FoxO-1, Atrogin-1, MuRF-1, IL-6, TNF-α, MCP-1, CXCL-16, and endogenous control GAPDH were obtained. Identities for mouse Taqman Gene expression Assays-On-Demand (Applied Biosystems) are listed in Table 1. The condition for PCR amplification for targeted genes was described previously (10). GAPDH was used as internal control. Relative quantification of target gene was related to the PCR signal of the specific transcript of Sham/V mice. Final results were expressed in arbitrary units, with one unit being the mean mRNA level determined in the Sham/V mice.
Table 1.
RT-PCR primer information
| mRNA | Taqman Gene Expression Assays-On-Demand Identity |
|---|---|
| IGF-I | Mm00439560_m1 |
| Myostatin | Mm00440328_m1 |
| MyoD | Mm00440387_m1 |
| FoxO1 | Mm00490672_m1 |
| Atrogin-1 | Mm00499518_m1 |
| MuRF-1 | Mm01185221_m1 |
| IL-6 | Mm00446190_m1 |
| TNF-α | Mm00443258_m1 |
| MCP-1 | Mm00441242_m1 |
| CXCL-16 | Mm00469712_m1 |
| GAPDH | 4352339E |
IGF-I, insulin-like growth factor-I; MCP-1, monocyte chemoattractant protein-1.
Statistical analysis.
Values are presented as means ± SE, and results were analyzed using Student's t-test when two experimental groups were compared or using ANOVA when data from ≥3 groups were studied. For ANOVA analyses, pair-wise comparisons were made by the Student-Newman-Keuls test. Data sets were analyzed for statistical significance using SPSS 16.0 software package (SPSS).
RESULTS
Mice develop CKD-associated cachexia following two-stage nephrectomy.
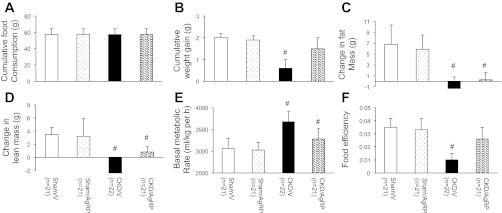
Male c57BL/6J mice were used for this study. Subtotal nephrectomized mice (CKD) treated with vehicle (V) were uremic but not acidotic. CKD/V mice had higher levels of BUN and creatinine (65.9 ± 2.3 and 0.6 ± 0.0 mg/dl) than sham mice treated with vehicle (Sham/V; 30.7 ± 2.7 and 0.2 ± 0.0 mg/dl; P < 0.001; Table 2). Blood bicarbonate levels were not different between CKD/V (27.5 ± 3.2 mmol/l) and Sham/V mice (26.9 ± 5.9 mmol/l; Table 2). CKD/V mice were fed ad libitum (57.3 ± 7.2 g), whereas Sham/V mice were pair-fed to CKD/V mice (Fig. 2A) for the study period of 14 days. Sham/V gained significantly more weight than CKD/V mice (gain of 2.0 ± 0.2 vs. gain of 0.6 ± 0.4 g; P < 0.001; Fig. 2B).
Table 2.
Serum chemistry of experimental mice
| Sham/V (n = 13) | Sham/AgRP (n = 13) | CKD/V (n = 14) | CKD/AgRP(n = 13) | |
|---|---|---|---|---|
| Initial weight, g | 19.4 ± 0.3 | 20.3 ± 2.3 | 19.4 ± 1.3 | 19.7 ± 1.0 |
| Serum chemistry | ||||
| BUN, mg/dl | 30.7 ± 2.7 | 32.1 ± 6.8 | 65.9 ± 2.3* | 63.2 ± 11.6* |
| Creatinine, mg/dl | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.6 ± 0.0* | 0.6 ± 0.0* |
| Biocarbonate, mmol/l | 26.9 ± 5.9 | 28.7 ± 3.9 | 27.5 ± 3.2 | 28.5 ± 4.6 |
Data are expressed as means ± SE. Mice were kiled at the end of 14 days.
AgRP, agouti-related peptide; BUN, blood urea nitrogen; CKD, 2-stage subtotal 5/6 nephrectomy; V, normal saline as vehicle. Four groups of mice were included: sham operated, normal saline treated (Sham/V); sham operated, AgRP treated (Sham/AgRP); CKD, normal saline treated (CKD/V); and CKD, AgRP treated (CKD/AgRP). Sham/AgRP, CKD/V, and CKD/AgRP mice were compared with Sham/V mice.
P < 0.001.
Fig. 2.
Chronic kidney disease (CKD) and sham mice were administered with agouti-related peptide (AgRP) or vehicle for 14 days. At the end of study, cumulative food consumption (A) and cumulative weight gain (B) were recorded. Live mice were scanned by DXA to determine the change in fat mass and lean mass. Mice were scanned 1 day before the starting of the experiment, followed 14 days later by a second DXA scan. Final results of change in fat (C) and lean mass (D) relative to baseline are shown. Basal metabolic rate (E) and food efficiency (F) were measured in experimental animals at the end of the study. Basal metabolic rate (ml·kg−1·h−1) is calculated as the average three lowest readings obtained during the recording period. Food efficiency was calculated by dividing the total weight gained (in grams) by the total amount of food consumed (in grams) over the 14-day treatment period. Four groups of mice were included: Sham/V, Sham/AgRP, CKD/V, and CKD/AgRP. Sham/AgRP, CKD/V, and CKD/AgRP mice were compared with Sham/V mice. Data are means ± SE. #P < 0.001.
Body composition of mice was first analyzed before and after the treatment by DXA. Significant accumulation of fat and lean mass occurred in Sham/V mice but not in CKD/V mice. The percent increase in fat mass per animal over the course of the 14 days was significantly higher in Sham/V mice relative to CKD/V mice (gain of 6.8 ± 3.5 vs. loss of 1.3 ± 2.1%; P < 0.001; Fig. 2C). Similarly, the percent increase in lean mass was significantly higher in Sham/V mice compared with that in CKD/V mice (gain of 3.5 ± 1.1 vs. loss of 2.4 ± 2.4%; P < 0.001; Fig. 2D).
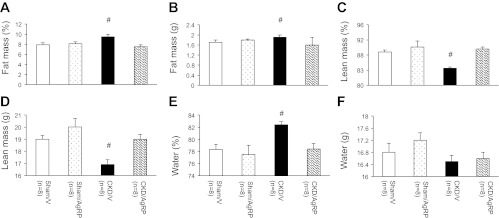
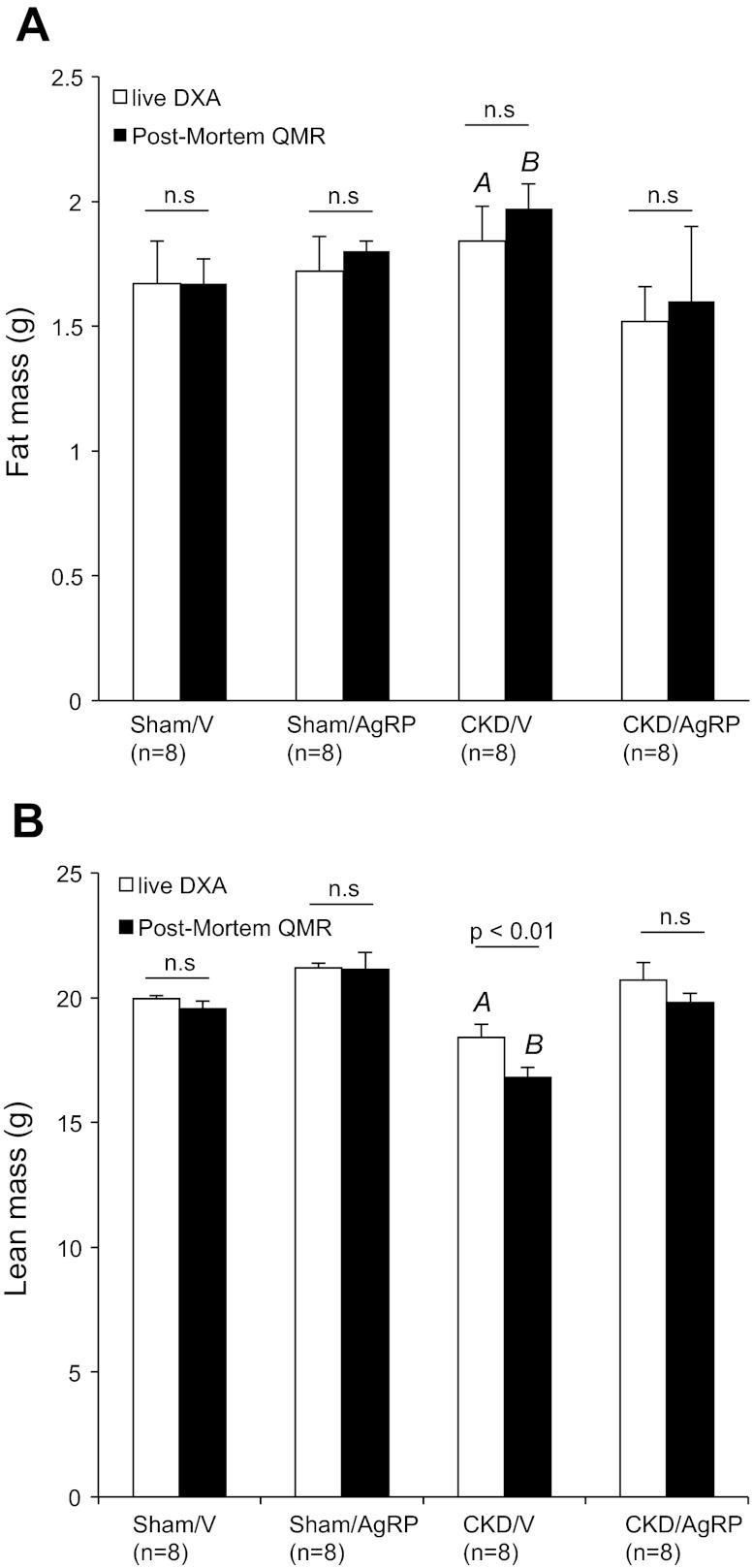
These results were confirmed by QMR analysis. At the end of 14 days, QMR measurements were performed on eight mice in all study groups. The percentage of fat mass and total fat mass was significantly increased in CKD/V mice (9.5 ± 0.5% and 1.9 ± 0.1 g) relative to Sham/V mice (7.9 ± 0.4% and 1.7 ± 0.1 g; P < 0.001; Fig. 3, A and B). Conversely, the percentage of lean mass and total lean mass was significantly lower in CKD/V mice (84.5 ± 0.3% and 16.9 ± 0.4 g) compared with Sham/V mice (88.8 ± 0.5% and 19.0 ± 0.3 g; P < 0.001; Fig. 3, C and D). The percentage of water content in CKD/V mice was significantly higher than Sham/V mice (82.4 ± 0.5 vs. 78.3 ± 0.9%; P < 0.001; Fig. 3E). Total water content was not different in CKD/V mice compared with Sham/V mice (16.5 ± 0.2 vs. 16.8 ± 0.3 g; Fig. 3F). We compared measurements of body composition by DXA vs. QMR for the same Sham/V and CKD/V mice. Fat mass determined by DXA vs. post mortem by QMR was not different within each study group (Fig. 4A). Fat mass was significantly increased in CKD/V mice relative to Sham/V mice either by DXA (1.8 ± 0.1 vs. 1.7 ± 0.2 g; P < 0.001; Fig. 4A) or QMR analysis (1.9 ± 0.1 vs. 1.7 ± 0.1 g; P < 0.001; Figs. 3B and 4A). Lean mass determined by DXA vs. QMR was not different for Sham/V mice (Fig. 4B). However, measurement of lean mass by DXA in CKD/V mice was greater than values estimated by QMR (18.2 ± 0.5 vs. 16.9 ± 0.4 g; P < 0.01; Fig. 4B).
Fig. 3.
Body composition analysis of postmortem mice by QMR. At the end of study, 8 mice from all groups were scanned by QMR to determine the percentage of fat mass (A), total fat mass (B), percentage of lean mass (C), total lean mass (D), percentage of water content (E), and total water mass (F). Final results are means ± SE. Sham/AgRP, CKD/V, and CKD/AgRP mice were compared with Sham/V mice. #P < 0.001.
Fig. 4.
Comparison of fat and lean mass in live mice assessment by DXA vs. postmortem analysis by QMR. At the end of 2-wk study, 8 conscious live mice from each group were anesthetized and scanned at room temperature for DXA analysis. The same mice were also used for QMR study. Values of fat mass (A) and lean mass (B) of mice are listed. Values of fat and lean mass were estimated by DXA. Sham/AgRP, CKD/V, and CKD/AgRP mice were compared with Sham/V mice. AP < 0.001. Similarly, fat and lean mass was also estimated by QMR. Sham/AgRP, CKD/V, and CKD/AgRP mice were compared with Sham/V mice. BP < 0.001. In addition, values of fat and lean mass measured by DXA were compared with values derived from QMR analysis within the same group of mice.
Basal metabolic rate was significantly higher in CKD/V mice compared with Sham/V mice (3,678.2 ± 243.7 vs. 3,061.4 ± 231.3 ml·kg−1·h−1; P < 0.001; Fig. 2E). On the contrary, food efficiency was significantly lower in CKD/V mice (0.010 ± 0.005) compared with Sham/V mice (0.035 ± 0.007; P < 0.001; Fig. 2F).
AgRP administration attenuates CKD-associated cachexia.
Cachexia in CKD mice was attenuated by intracranial administration of AgRP. CKD mice treated with AgRP (CKD/AgRP) mice were still uremic but not acidotic. CKD/AgRP mice had higher BUN and creatinine levels (63.2 ± 11.6 and 0.6 ± 0.0 mg/dl) than did Sham/V and AgRP-treated sham (Sham/AgRP) mice (32.1 ± 6.8 and 0.2 ± 0.0 mg/dl; P < 0.001; Table 2). Blood bicarbonate levels in CKD/AgRP mice (28.5 ± 4.6 mmol/l) were comparable to those in Sham/V and Sham/AgRP mice (28.7 ± 3.9 mmol/l). AgRP treatment ameliorated CKD-associated cachexia. Our previous results (8) showed that administration of AgRP significantly increased the food consumption of CKD mice. To ensure that the observed effects on body mass regulation was not due to difference in nutritional intake, CKD/AgRP and Sham/AgRP mice were pair fed to CKD/V mice (Fig. 2A). Weight gain in CKD/AgRP mice was normalized (gain of 1.5 ± 0.5 g, Fig. 2B). CKD/AgRP mice gained fat mass and lean mass (gain of 0.3 ± 1.3 and 0.8 ± 0.8%), although still less than Sham/V or Sham/AgRP mice (gain of 5.9 ± 2.6 and 3.2 ± 2.7%; P < 0.001; Fig. 2, C and D).
At the end of study, we used QMR to analyze body composition in CKD/AgRP and Sham/AgRP mice. The percentage of fat mass and total fat mass was not different between CKD/AgRP mice (7.5 ± 0.4% and 1.6 ± 0.3 g), Sham/V mice and Sham/AgRP mice (8.1 ± 0.4% and 1.8 ± 0.0 g; Fig. 3, A and B). The percentage of lean mass and total lean mass was significantly improved in CKD/AgRP mice (89.6 ± 0.5% and 19.0 ± 0.4 g) than CKD/V mice (P < 0.001; Fig. 3, C and D). The percent of lean mass and total lean mass was normalized in CKD/AgRP mice compared with Sham/AgRP (90.1 ± 1.6% and 20.0 ± 0.7 g) mice or Sham/V mice (Fig. 3, C and D). The percentage of water content in CKD/AgRP mice was significantly lower than CKD/V mice (78.4 ± 0.9%; P < 0.001) and was not different than that in Sham/AgRP (77.5 ± 1.5%) mice or Sham/V mice (Fig. 3E). Total water content was not different between CKD/AgRP (16.6 ± 0.2 g) and Sham/AgRP mice (17.2 ± 0.25 g) compared with CKD/V or Sham/V mice (Fig. 3F). Body composition of eight Sham/AgRP and eight CKD/AgRP mice was further analysis. Fat mass and lean mass determined by DXA vs. QMR was not different within each study group (Fig. 4, A and B).
Blockade of the melanocortin receptor by AgRP has been associated with a decrease in basal oxygen consumption (8, 10, 11). Basal metabolic rate was lower in CKD/AgRP mice (3,278.1 ± 235.8 ml·kg−1·h−1) than CKD/V mice and not different with Sham/V or Sham/AgRP mice (3,028 ± 177.9 ml·kg−1·h−1; P < 0.001; Fig. 2E). In addition, food efficiency improved in CKD/AgRP mice (0.026 ± 0.009) relative to CKD/V mice (P < 0.001; Fig. 2F) and was not different than that in Sham/V or Sham/AgRP mice (0.033 ± 0.009; Fig. 2F).
Signaling pathways associated with skeletal muscle wasting in CKD mice.
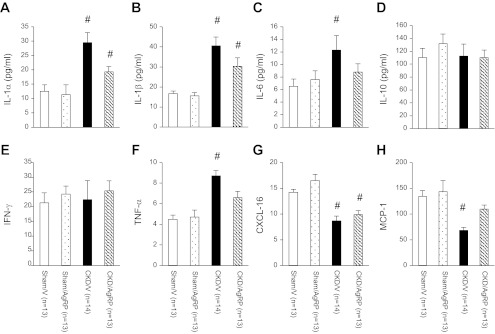
We investigated the impact of AgRP administration on muscle mass regulatory pathways. Gastrocnemius muscle from experimental mice was dissected, and muscle lysate protein was extracted. Muscle lysate protein levels of IL-1α, IL-1β, IL-6, IL-10, IFN-γ, TNF-α, CXCL-16, and MCP-1 were quantified. There was no difference in skeletal muscle protein profile between Sham/AgRP and Sham/V mice (Fig. 5). However, we detected significant differences in skeletal muscle protein profile in CKD/V relative to Sham/V mice. CKD/V mice exhibited a significant increase in skeletal muscle protein levels of IL-1α, IL-1β, IL-6, and TNF-α while CXCL-16 and MCP-1 protein concentration was lower relative to Sham/V mice. There was no difference in IL-10 and IFN-γ protein levels in CKD/V mice vs. Sham/V mice. AgRP normalized protein levels of IL-6, TNF-α, and MCP-1 in CKD/AgRP mice compared with Sham/V mice. Protein levels of IL-1α and IL-1β were significantly lower in CKD/AgRP mice compared with CKD/V mice although still higher than those in Sham/V mice (Fig. 5, A and B). In addition, protein levels of CXCL-16 were still decreased in CKD/AgRP mice compared with Sham/V mice (Fig. 5G).
Fig. 5.
Protein profiling in gastrocnemius muscle. IL-1α (A), IL-1β (B), IL-6 (C), IL-10 (D), IFN-γ (E), TNF-α (F), CXCL-16 (G), and monocyte chemoattractant protein-1 (MCP-1; H) protein concentrations in gastrocnemius muscle tissue lysate. Sham/AgRP, CKD/V, and CKD/AgRP mice were compared with Sham/V mice. Results are means ± SE. #P < 0.01.
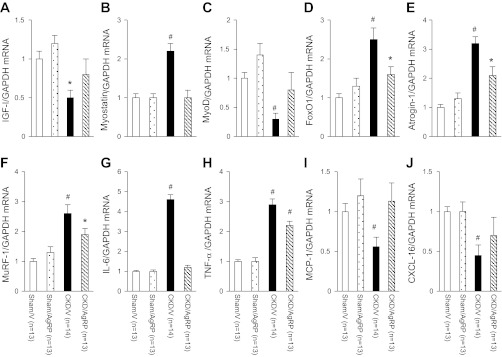
Total RNA from gastrocnemius muscles were extracted and reversely transcribed. Gene expression of IGF-I, myostatin, MyoD, FoxO1, Atrogin-1, MuRF-1, IL-6, TNF-α, MCP-1, and CXCL-16 were measured. Similarly to protein data, there was no difference in gene expression of any targeted molecule between Sham/AgRP and Sham/V mice (Fig. 6). Myostatin, FoxO1, Atrogin-1, MuRF-1, IL-6, and TNF-α mRNA level was significantly increased in gastrocnemius muscles of CKD/V mice relative to Sham/V mice while IGF-I, MyoD, MCP-1, and CXCL-16 mRNA content was lower in gastrocnemius muscle of CKD/V mice than Sham/V mice (Fig. 6). AgRP corrected aberrant gene expression of IGF-I, myostatin, MyoD, IL-6, MCP-1, and CXCL-16 in CKD/AgRP mice compared with Sham/V mice. Gene expression of FoxO1, Atrogin-1, and MuRF-1 in CKD/AgRP mice was still lower than that in Sham/V mice (Fig. 6, D–F). In addition, TNF-α mRNA level was significantly lower in CKD/AgRP mice compared with CKD/V mice, although still higher than that in Sham/V mice (Fig. 6I).
Fig. 6.
Gene expression in gastrocnemius muscle. Comparative 2−ΔΔCt method was used to determine the relative quantification of IGF-I (A), myostatin (B), MyoD (C), FoxO1 (D), Atrogin-1 (E), MuRF-1 (F), IL-6 (G), TNF-α (H), MCP-1 (I), and CXCL-16 (J) mRNA content. To normalize each sample for RNA content, the control gene GAPDH was used. Relative quantification of target gene was related to the PCR signal of the specific transcript of Sham/V mice. Final results were expressed in arbitrary units, with 1 unit being the mean mRNA level determined in the Sham/V control mice. Data are means ± SE. #P < 0.01. *P < 0.05.
DISCUSSION
Elevated circulating levels of cytokines such as leptin may be an important cause of CKD-associated cachexia via signaling through the hypothalamic melanocortin system (10). In this study, we investigated the effect of AgRP administration on body composition in CKD mice using QMR technique. We used QMR to measure fat mass, lean mass, and water content in mice. We examined the effect of AgRP on expression of key molecules implicated in muscle wasting. Our previous studies (10) indicate that AgRP administration stimulated nutritional intake in CKD mice. Thus to examine the salutary effects of AgRP beyond its nutritional effects, we have employed pair-feeding experimental strategies. All mice had intracranial cannulation to avoid unintended effects of the surgical procedures. Despite the same nutritional intake as CKD/V mice (Fig. 2A), a significant weight gain was observed in CKD/AgRP mice (Fig. 2B). The most significant aspect of this study is the maintenance of fat and lean mass in CKD mice treated with AgRP compared with sham mice (Fig. 2, C and D). Our results were in agreement with other published data (42). Small et al. (42) studied energy metabolism in AgRP-treated ad libitum rats vs. pair-fed saline control group. AgRP administration significantly increased body weight in ad libitum fed animals compared with saline-treated controls. A significant increase in the epididymal fat pad weight and interscapular brown adipose tissue weight was observed in the ad libitum fed group, suggesting that AgRP caused metabolic changes independent of increased food intake (42).
QMR provides detailed measurement of body composition in CKD mice in response to dietary and pharmacological modulation. Measurements of whole body composition in eight mice in all study groups were made using DXA compared with postmortem QMR. Precision measurement of lean mass in mice was found to be better for QMR than for DXA. QMR and DXA did not differ in the total amount of fat mass within the same group of mice, but values of lean mass derived from DXA analysis for CKD/V mice were greater than values estimated by QMR (Fig. 4B). This is not surprising as DXA is unable to differentiate water content in mice and fluid retention in CKD may lead to an overestimate of lean mass. Progressive loss of renal function may lead to water retention. The percentage of water content was significantly higher in CKD/V mice relative to Sham/V mice (Fig. 3, E and F). AgRP administration resulted in significant improvement in fat mass (Figs. 3, A and B, and 4A), lean mass (Figs. 3, C and D, and 4B), and normalization of water content (Fig. 3E) in CKD/AgRP mice.
AgRP treatment may modulate intestinal absorption of nutrients in CKD mice. Dietary fat is a major source of energy. Fat is delivered to tissues by apolipoprotein B-containing lipoproteins synthesized in the liver and intestine with the help of an intracellular chaperone, microsomal triglyceride transfer protein (MTP). Regulation of MTP expression is a major determinant of hepatic and intestinal fat mobilization (18). Recent evidence suggests that, indeed, MTP expression is differentially expressed in the intestine and liver. Leptin regulates energy metabolism. Leptin reduces body weight by inhibiting AgRP neurons and release of pro-opiomelanocortin (POMC), a polypeptide precursor that is cleaved to generate α- and γ-melanocortin-stimulating hormone (MSH). Interaction of MSH with melanocortin 4 receptor (MC4R) in the hypothalamus decreases food intake and increases energy expenditure. In contrast, the MC4R antagonist AgRP increases food intake and decreases energy expenditure (9, 27). Enterocytes express leptin receptor, POMC, AgRP, and MC4R. Leptin might regulate intestinal MTP expression through the melanocortin pathway. Recent data suggest that leptin-melanocortin pathway may modulate intestinal MTP metabolism and intestinal lipid absorption (19).
How could AgRP ameliorate muscle wasting in a catabolic condition such as CKD? One possibility is that AgRP attenuated muscle wasting by correcting the imbalance between IGF-I and myostatin. IGF-I mRNA level was 50% downregulated in skeletal muscle of CKD mice while expression of myostatin mRNA was 120% upregulated in skeletal muscle of CKD mice. AgRP treatment normalized skeletal muscle IGF-I and myostatin mRNA contents in CKD mice (Fig. 6, A and B). Decreased expression of IGF-I accelerates muscle proteolysis via signaling through caspase-3 and ubiquitin-proteasome system (3). Myostatin influences myogenesis by negatively regulating myoblast proliferation (30). Myostatin induces muscle wasting via signaling through AKT-FoxO1 pathway (13). Elevated expression of myostatin has been implicated in several cachexia-associated diseases such as AIDS, sarcopenia, glucocorticoid-induced muscle dystrophy, and CKD (13). Systemic administration of myostatin protein induced muscle wasting (31). Alternatively, a lack of myostatin results in increased muscle growth and repair (1). Myostatin blockade in mdx mice, a model of Duchenne muscular dystrophy, results in an increase in muscle mass and muscle force production (45). Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in CKD mice (52).
Our data showed that AgRP caused an overall decrease in proinflammatory cytokines in CKD mice (Figs. 5 and 6), which may be the mechanism for reversal of cachexia and improvement in fat mass and lean mass in CKD mice. Indeed, proinflammatory cytokines such as IL-1, IL-6, and TNF-α have been associated with anorexia and cachexia in CKD (2, 9, 46, 54). Skeletal muscle protein levels of IL-1α, IL-1β, IL-6, TNF-α, and mRNA level of IL-6 and TNF-α were significantly increased in CKD mice (Figs. 5 and 6). AgRP normalized skeletal muscle protein and mRNA levels of IL-6 in CKD/AgRP mice (Figs. 5C and 6G). AgRP normalized skeletal muscle TNF-α protein level in CKD/AgRP mice (Fig. 5F). Muscle TNF-α mRNA level was significantly lower in CKD/AgRP mice compared with CKD/V mice although still higher than that in Sham/V mice (Fig. 6H). Cytokines influence physiologic functions of skeletal muscle cells, including anabolic and catabolic processes and programmed cell death (54). Proinflammatory cytokines may exert their biological effects in skeletal muscle in a paracrine/autocrine manner (54). IL-6 mediates its effects on target cells through a complex receptor system (sIL-6R or gp80) and a signal-transducing glycoprotein (gp130; Ref. 34). Skeletal muscle cells are capable of producing IL-6 and TNF-α in response to various stimuli. Muscle-derived IL-6 functions as an exocrine hormone and exerts its effect on the liver and adipose tissue (14, 35). IL-6 interferes IGF-I signaling pathway (34). IL-6 interacts with JAK/STAT signaling pathways, leading to changes in SOCS expression, and this process may lead to a decrease in signaling associated with the growth hormone and/or IGF-I receptors (17). Our result is relevant because elevated IL-6 level impairs IGF-I signaling and inhibition of this pathway will decrease muscle protein synthesis and increase protein degradation in CKD mice. Previous studies (51) indicated that IL-6 stimulated SOCS-3 activity, leading to degradation of IRS-1 and suppression of p-Akt and p-FoxO, and promoted muscle proteolysis. IL-6 elicited skeletal muscle atrophy in healthy rats (17). Raj et al. (36) quantified muscle IL-6 efflux in patients with end-stage renal disease. Their study (36) suggests that intradialytic IL-6 activation is a major protein catabolic signaling in renal patients. The same group measured IL-6 protein content of skeletal muscle biopsies in end-stage renal disease patients. Muscle protein catabolism was positively associated with skeletal muscle IL-6 content (4). Local infusion of IL-6 disproportionately affected myofibrillar protein compartment, suggesting that IL-6 treatment would have a meaningful skeletal muscle functional impact (17). TNF-α contributes to muscle wasting by inducing reactive oxygen species and by activating the proinflammatory NF-κB system and via this inducing the ubiquitin-proteasome system (20). Increased oxidative stress also leads to upregulation of autophage related genes via p38MAPK atrophic muscles (29). There is precedence for a link between inflammatory cytokines and myostatin expression. Results of a recent study (52) suggest that TNF-α triggered myostatin production in muscle, which in turn, stimulated IL-6 expression and its release in CKD mice.
Conversely, proinflammatory cytokines are known to inhibit the release of AgRP from hypothalamus (9, 24, 27). This will be a maladaptive response in CKD-associated cachexia. Increasing the hypothalamic level of AgRP in our study may help to overcome this aberrant pathophysiologic phenomenon. Furthermore, inflammation is associated with increased fat mass (obesity) and decreased muscle mass (2, 27). It is an interesting finding that AgRP increased fat mass and yet decreased inflammation in our study. Although AgRP caused an increase in fat mass (Figs. 2, C and D, 3, A and B, and 4A) as well as a decrease in inflammatory cytokines in CKD mice, the CKD/AgRP mice are hardly obese compared with control mice (Fig. 2B). They just have a lesser degree of cachexia, which is characterized by decreased fat mass relative to control mice.
AgRP may improve muscle mass in CKD mice by suppressing the expression of Atrogin-1 and MuRF-1 through regulation of FoxO1. FoxO1 expression is significantly induced during atropic conditions including fasting, cancer, diabetes mellitus, and renal failure (15, 16, 40). Upregulation of Atrogin-1 and MuRF-1 has been observed in various models of skeletal muscle atrophy. In this study, we reconfirmed the upregulation of skeletal muscle FoxO1, Atrogin-1, and MuRF-1 mRNA expression in CKD mice. We found that skeletal muscle mRNA content of FoxO1, Atrogin-1, and MuRF-1 was 150, 220, and 160% increased in CKD mice relative to control mice. Skeletal muscle mRNA content of FoxO1, Atrogin-1, and MuRF-1 was still 60, 110, and 90% elevated in CKD/AgRP mice relative to control mice, although significantly lower than that in CKD/V mice (Fig. 6, E and F). Inhibition of Atrogin-1 attenuated muscle loss in fasting mice as did MuRF-1 inhibition in cellular models (12). Suppression of FoxO1, Atrogin-1, and MuRF-1 expression will lead to reduced proteolysis (47). In mice with a muscle-specific FoxO1 deletion, the absence of FoxO1 attenuated Atrogin-1 and MuRF-1 expression in CKD mice. Subsequent in vitro and in vivo studies (50) indicated that FoxO1 is an important mediator of CKD-induced muscle wasting.
Another important aspect of muscle wasting in CKD is the impaired muscle regeneration. Skeletal muscle regeneration following injury involves proliferation and differentiation of satellite cells and leads to the formation of new myofibers (5, 6, 32). MyoD influences muscle differentiation and myogenesis (6, 22). Downregulation of MyoD leads to muscle wasting. Loss of myoD function leads to failure to maintain myonuclear density, a mechanism potentially linked to satellite cells differentiation defects (5). We examined MyoD gene expression in response to AgRP treatment in CKD mice. Indeed, skeletal muscle MyoD mRNA was found to be dramatically downregulated by 70% in CKD mice and expression of skeletal muscle MyoD was attenuated in CKD/AgRP mice (Fig. 6C). Chemokines, signaling through the CCR2 receptor, involve in skeletal muscle regeneration. CXCL-16 and MCP-1 are crucial for muscle regeneration in injured muscle. Data from the laboratory of Zhang et al. (53) indicates that CXCL-16 promotes neutrophil and macrophage infiltration into injured muscle and induces muscle regeneration. Muscle regeneration was severely impaired in CXCL-16-deficient mice compared with that in wild-type mice. There was a significant decreased of MyoD and myogenin expression in regenerating muscle in CXCL-16 deficient mice, indicating impaired satellite cell proliferation and differentiation. Reports of Warren et al. (48) and Shireman et al. (41) suggest that MCP-1 and its receptor, CCR2, were also critical for the regeneration processes in injured muscle. Targeted deletion of CCR2 receptor or blocking the action of MCP-1 significantly delayed the muscle regeneration in injured tissue. At 14 days after a second nephrectomy, skeletal muscle protein and mRNA levels of CXCL-16 and MCP-1 were significantly decreased in CKD/V mice relative to control mice. AgRP treatment normalized mRNA levels of CXCL-16 and protein and mRNA content of MCP-1 in CKD/AgRP mice (Figs. 5, G and H, and 6, I and J). Our results are consistent with the notion that CXCL-16 and MCP-1 are integral to muscle regeneration processes. Lower expression of CXCL-16 and MCP-1 in muscle may lead to decreased macrophage infiltration and eventually impairs muscle regeneration in CKD mice.
In conclusion, we demonstrated that AgRP treatment improved energy expenditure, total body mass, fat mass, and lean body mass in a CKD mouse model. AgRP treatment caused an overall decrease in proinflammatory cytokines, which may be an important mechanism of its effects and further highlights potential benefits for its therapeutic usage. Muscle wasting in CKD may be due to the activation of proteolytic pathways and inhibition of myogenesis and muscle regeneration processes. The improvement in lean mass accrual in CKD mice treated with AgRP was likely mediated by modulation of pathological signaling pathways associated with skeletal muscle wasting.
GRANTS
This work was supported by an Amgen Young Investigator Grant of the National Kidney Foundation (to W. W. Cheung) and National Institute of Diabetes and Digestive and Kidney Diseases Grants K24-DK-59524 and U01-DK-3-012 and Satellite Healthcare (to R. H. Mak). Body compositions of mice were measured by the Cincinnati Mouse Metabolic Phenotyping Center (DK-059630) using a quantitative magnetic resonance instrument, EchoMRI.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.W.C. and R.H.M. conception and design of research; W.W.C. performed experiments; W.W.C. and R.H.K.M. analyzed data; W.W.C. and R.H.M. interpreted results of experiments; W.W.C. prepared figures; W.W.C. drafted manuscript; W.W.C. and R.H.M. edited and revised manuscript; W.W.C. and R.H.M. approved final version of manuscript.
REFERENCES
- 1.Amthor H, Macharia R, Navarrete R, Schuelke M, Brown SC, Otto A, Voit T, Muntoni F, Vrbóva G, Partridge T, Zammit P, Bunger L, Patel K. Lack of myostatin results in excessive muscle growth but impaired force generation. Proc Natl Acad Sci USA 104: 1835–1840, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avesani CM, Carrero JJ, Axelsson J, Qureshi AR, Lindholm B, Stenvinkel P. Inflammation and wasting in chronic kidney disease: partners in crime. Kidney Int 70: S8–S13, 2006 [Google Scholar]
- 3.Bailey JL, Zheng B, Hu Z, Price SR, Mitch WE. Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy. J Am Soc Nephrol 17: 1388–1394, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Boivin MA, Battah SI, Dominic EA, Kalantar-Zadeh K, Ferrando A, Tzamaloukas AH, Dwivedi R, Ma TA, Moseley P, Raj DS. Activation of caspase-3 in the skeletal muscle during haemodialysis. Eur J Clin Invest 40: 903–910, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brack AS, Bildsoe H, Hughes SM. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J Cell Sci 118: 4813–4821, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Braun T, Gautel M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat Rev Mol Cell Biol 12: 349–361, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 84: 209–238, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Cheung W, Kuo HJ, Markison S, Chen C, Foster AC, Marks DL, Mak RH. Peripheral administration of the melanocortin-4 receptor antagonist NBI-12i ameliorates uremia-associated cachexia in mice. J Am Soc Nephrol 18: 2517–2524, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Cheung W, Paik KH, Mak RH. Inflammation and cachexia in chronic kidney disease. Pediatr Nephrol 25: 711–724, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Cheung W, Yu PX, Little BM, Cone RD, Marks DL, Mak RH. Role of leptin and melanocortin signaling in uremia-associated cachexia. J Clin Invest 115: 1659–1665, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung W, Rosengren S, Boyle DL, Mak RH. Modulation of melanocortin signaling ameliorates uremic cachexia. Kidney Int 74: 180–186, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Cong H, Sun L, Liu C, Tien P. Inhibition of atrogin-1/MAFbx expression by adenovirus-delivered small hairpin RNAs attenuates muscle atrophy in fasting mice. Hum Gene Ther 22: 313–324, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Elliott B, Renshaw D, Getting S, Mackenzie R. The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol (Oxf) 205: 324–340, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Febbraio MA, Steensberg A, Keller C, Starkie RL, Nielsen HB, Krustrup P, Ott P, Secher NH, Pedersen BK. Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. J Physiol 549: 607–612, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foletta VC, White LJ, Larsen AE, Léger B, Russell AP. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflügers Arch 461: 325–335, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Glass D, Roubenoff R. Recent advances in the biology and therapy of muscle wasting. Ann NY Acad Sci 1211: 25–36, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol 98: 911–917, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res 44: 22–32, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Iqbal J, Li X, Chang BH, Chan L, Schwartz GJ, Chua SC, Jr, Hussain MM. An intrinsic gut leptin-melanocortin pathway modulates intestinal microsomal triglyceride transfer protein and lipid absorption. J Lipid Res 51: 1929–1942, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 287: C834–C843, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Künnecke B, Verry P, Bénardeau A, von Kienlin M. Quantitative body composition analysis in awake mice and rats by magnetic resonance relaxometry. Obes Res 12: 1604–1615, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Lagirand-Cantaloube J, Cornille K, Csibi A, Batonnet-Pichon S, Leibovitch MP, Leibovitch SA. Inhibition of atrogin-1/MAFbx mediated MyoD proteolysis prevents skeletal muscle atrophy in vivo. PLos One 4: e4973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo N, Marcelin G, Liu SM, Schwartz G, Chua S., Jr Neuropeptide Y and Agouti-related peptide mediate complementary functions of hyperphagia and reduced energy expenditure in leptin receptor deficiency. Endocrinology 152: 883–889, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mak RH, Cheung W. Therapeutic strategy for cachexia in chronic kidney disease. Curr Opin Nephrol Hypertens 16: 542–546, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Mak RH, Cheung W, Robert CTJR. The growth hormone-insulin like growth factor-I axis in chronic kidney disease. Growth Horm IGF Res 18: 17–25, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mak RH, Cheung W, Zhan Shen Q JY, Foster BJ. Cachexia and protein-energy wasting in children with chronic kidney disease. Pediatr Nephrol 27: 173–181, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mak RH, Ikizler AT, Kovesdy CP, Raj DS, Stenvinkel P, Kalantar-Zadeh K. Wasting in chronic kidney disease. J Cachexia Sarcopenia Muscle 2: 9–25, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mak RH, Rotwein P. Myostatin and insulin-like growth factors in uremic sarcopenia: the yin and yang in muscle mass regulation. Kidney Int 70: 410–412, 2006 [DOI] [PubMed] [Google Scholar]
- 29.McClung JM, Judge AR, Powers SK, Yan Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol 298: C542–C549, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 162: 1135–1147, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McFarlane C, Plummer E, Thomas M, Hennebry A, Ashby M, Ling N, Smith H, Sharma M, Kambadur R. Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol 209: 501–514, 2006 [DOI] [PubMed] [Google Scholar]
- 32.McKinnell IW, Rudnicki MA. Molecular mechanisms of muscle atrophy. Cell 119: 907–910, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Nixon JP, Zhang M, Wang C, Kuskowski MA, Novak CM, Levine JA, Billington CJ, Kotz CM. Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity 18; 1652–1659, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedersen Edward F BK. Adolph distinguished lecture: muscle as an endocrine organ: IL-6 and other myokines. J Appl Physiol 107:1006–1014, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Pedersen BK, Steensberg A, Keller P, Keller C, Fischer C, Hiscock N, van Hall G, Plomgaard P, Febbraio MA. Muscle-derived interleukin-6: lipolytic, anti-inflammatory and immune regulatory effects. Pflügers Arch 446: 9–16, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Raj DS, Moseley P, Dominic EA, Onime A, Tzamaloukas AH, Boyd A, Shah VO, Glew R, Wolfe R, Ferrando A. Interleukin-6 modulates hepatic and muscle protein synthesis during hemodialysis. Kidney Int 73: 1054–1061, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Rajan VR, Mitch WE. Muscle wasting in chronic kidney disease: the role of the ubiquitin proteasome system and its clinical impact. Pediatr Nephrol 23: 527–535, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rashid R, Neill E, Maxwell H, Ahmed SF. Growth and body composition in children with chronic kidney disease. Br J Nutr 97: 232–238, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Rashid R, Neill E, Smith W, King D, Beattie TJ, Murphy A, Ramage IJ, Maxwell H, Ahmed SF. Body composition and nutritional intake in children with chronic kidney disease. Pediatr Nephrol 21: 1730–1738, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shireman PK, Contreras-Shannon V, Ochoa O, Karia BP, Michalek JE, McManus LM. MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leukoc Biol 81: 775–785, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Small CJ, Kim MS, Stanley SA, Mitchell JR, Murphy K, Morgan DG, Ghatei MA, Bloom SR. Effects of chronic central nervous system administration of agouti-related protein in pair-fed animals. Diabetes 50: 248–254, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem 377: 990–1002, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes Res 12: 150–160, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Tsuchida K. Myostatin inhibition by a follistatin-derived peptide ameliorates the pathophysiology of muscular dystrophy model mice. Acta Myol 27: 14–18, 2008 [PMC free article] [PubMed] [Google Scholar]
- 46.Utaka S, Avesani CM, Draibe SA, Kamimura MA, Andreoni S, Cuppari L. Inflammation is associated with increased energy expenditure in patients with chronic kidney disease. Am J Clin Nutr 82: 801–805, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 147: 4160–4168, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Warren GL, Hulderman T, Mishra D, Gao X, Millecchia L, O'Farrell L, Kuziel WA, Simeonova PP. Chemokine receptor CCR2 involvement in skeletal muscle regeneration. FASEB J 19: 413–415, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr 91: 1128S–1132S, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Xu J, Li R, Workeneh B, Dong Y, Wang X, Hu Z. Transcription factor FoxO1, the dominant mediator of muscle wasting in chronic kidney disease, is inhibited by microRNA-486. Kidney Int 2012. April 4 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L, Du J, Hu Z, Han G, Delafontaine P, Garcia G, Mitch WE. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J Am Soc Nephrol 20: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Rajan V, Lin E, Hu Z, Han HQ, Zhou X, Song Y, Min H, Wang X, Du J, Mitch WE. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J 25: 1653–1663, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, Ran L, Garcia GE, Wang XH, Han S, Du J, Mitch WE. Chemokine CXCL16 regulates neutrophil and macrophage infiltration into injured muscle, promoting muscle regeneration. Am J Pathol 175: 2518–2257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zoico E, Roubenoff R. The role of cytokines in regulating protein metabolism and muscle function. Nutr Rev 60: 39–51, 2002 [DOI] [PubMed] [Google Scholar]