Abstract
Acid-secreting intercalated cells respond to changes in systemic pH through regulation of apical H+ transporters. Little is known about the mechanism by which these cells sense changes in extracellular pH (pHo). Pyk2 is a nonreceptor tyrosine kinase activated by autophosphorylation at Tyr402 by cell-specific stimuli, including decreased pH, and is involved in the regulation of MAPK signaling pathways and transporter activity. We examined whether the Pyk2 and MAPK signaling pathway mediates the response of transport proteins to decreased pH in outer medullary collecting duct cells. Immunoblot analysis of phosphorylated Pyk2 (Tyr402), ERK1/2 (Thr202/Tyr204), and p38 (Thr180/Tyr182) was used to assay protein activation. To examine specificity of kinase activation and its effects, we used Pyk2 small interfering RNA to knockdown Pyk2 expression levels, the Src kinase inhibitor 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]-pyrimidine (PP 1) to inhibit Pyk2 phosphorylation, and the MEK inhibitor U0126 to inhibit ERK1/2 phosphorylation. The pH-sensitive fluorescent probe 2′-7′-bis(carboxyethyl)-5(6)-carboxyfluorescein-acetoxymethyl ester (BCECF-AM) was used to assay H+ transporter activity. The activity of H+ transporters was measured as the rate of intracellular pH (pHi) recovery after an NH4Cl prepulse. We show that Pyk2 is endogenously expressed and activated by acid pH in mouse-derived outer medullary collecting duct (mOMCD1) cells. Incubation of mOMCD1 cells in acid media [extracellular pH (pHo) 6.7] increased the phosphorylation of Pyk2, ERK1/2, and p38. Reduction in pHi induced by an NH4Cl prepulse also increased the phosphorylation of Pyk2, ERK1/2, and p38. Consistent with our previous studies, we found that mOMCD1 cells exhibit H+-ATPase and H+,K+-ATPase activity. Pyk2 inhibition by Pyk2 siRNA and PP 1 prevented Pyk2 phosphorylation as well as H+-ATPase-mediated recovery in mOMCD1 cells. In addition, ERK1/2 inhibition by U0126 prevented acid-induced ERK1/2 phosphorylation and H+-ATPase-mediated pHi recovery but not phosphorylation of p38. We conclude that Pyk2 and ERK1/2 are required for increasing H+-ATPase, but not H+,K+-ATPase, activity at decreased pHi in mOMCD1 cells.
Keywords: intracellular pH, kinase, regulation of H+ transport, acidosis, outer medullary collecting duct, Pyk2
the kidney plays a major role in maintaining acid-base homeostasis. Metabolism of dietary protein from a typical Western diet in a 70-kg subject produces ∼70 mmol of nonvolatile acid per day, which is released into the extracellular space (15). Epithelial cells of the kidney collecting duct “sense” small decreases in systemic pH and respond by increasing H+ secretion and HCO3− absorption to defend against metabolic acidosis (MA; Refs. 6, 15, 19). The underlying condition or disease causing MA can vary, but typically MA accompanies and accelerates the progression of chronic kidney disease (55). The kidney collecting duct consists of three segments: cortical, outer medullary (OMCD), and inner medullary collecting duct, and is responsible for final regulation of net acid excretion (9, 15, 58) and maintenance of acid-base homeostasis. The OMCD exhibits the highest rate of acid secretion, which is mediated specifically by type A acid-secreting intercalated cells (A-IC; Refs. 15, 46). A-ICs respond to metabolic acidosis by increasing apical proton secretion via H+-ATPase (14, 56, 57), via the gastric and colonic H+,K+-ATPases (9, 11, 31, 61), and by increasing basolateral bicarbonate absorption via the bicarbonate/chloride exchanger anion exchanger 1 (AE1; Refs. 1, 7). Distal renal tubular acidosis (RTA type 1) is a specific example of abnormal net H+ transport and is characterized clinically, in the complete form, by an inability of the kidney to excrete net acid appropriately, resulting in positive net acid balance and decreased systemic pH. One example of the inherited forms of distal RTA has been linked to mutations in the apical H+-ATPase in the collecting duct (18, 48).
In view of the role of the collecting duct in final adjustments of the overall acid-base status and fine tuning of kidney net acid secretion, it seems reasonable to assume that A-IC cells “sense” and respond to increases in acid load by increasing acid secretion. However, the precise mechanism involving pH sensor and afferent signaling pathways that mediate upregulation of acid secretion via the H+-ATPase and/or H+,K+-ATPase have not been completely elucidated.
One of the putative pH sensors in the kidney, Pyk2, is a 116-kDa nonreceptor tyrosine kinase that is expressed in the central nervous system (35), cartilage (30), vascular smooth muscle (41), and kidney (28, 49). Pyk2 activation by autophosphorylation at Tyr402 occurs in response to a variety of extracellular cell-specific stimuli, such as neuronal membrane depolarization (47), the inflammatory cytokine TNFα in hematopoietic cells (12), cartilage-destroying fibronectin fragment in chondrocytes (30), and decreased pH in proximal renal tubule cells (28, 40). Pyk2-mediated signaling pathways have a wide range of physiological effects, depending on cell type, including regulation of ion transport (27, 28, 40), cell movement via focal adhesion spreading (44), cell proliferation (50), and vasoconstriction (32). Activated Pyk2 leads to phosphorylation of mitogen-activated protein kinases (MAPK) including ERK1/2 (21, 35, 41), p38 (33, 38, 49), and c-Jun NH2-terminal kinase (JNK; Refs. 20, 63), which mediate the physiological effects of Pyk2.
Pyk2 has been shown to regulate the coupling of receptor proteins to MAPK signaling pathways, such as integrins (5, 30), G-protein-coupled receptors (13, 35), vascular endothelial growth factor receptor (33), and epidermal growth factor receptor (45, 51). It has been reported previously that Pyk2 is involved in signaling pathways initiated by endothelin (20) and angiotensin (52). Both hormones are involved in the regulation of H+-ATPase-mediated acid secretion in the collecting duct, for example (39, 43, 59).
In the proximal tubule, ERK1/2 signaling pathways regulate acid stimulation of the sodium-proton exchanger NHE3 and sodium-coupled bicarbonate/citrate cotransporter NaDC-1 (28, 40, 53). In the collecting duct, ERK has been shown to activate H+,K+-ATPase activity in a cAMP-dependent manner (25) and also activates the Na+,K+-ATPase (34) but inhibits the ROMK channel (60). In bicarbonate-secreting (type B) intercalated cells (B-ICs) of the cortical collecting duct, ERK1/2 activation of H+,K+-ATPase activity has been shown to occur via a PKA-mediated pathway involving Ras and Raf-1 (25). In A-ICs, ERK1/2 is involved in calcitonin-induced activation of H+,K+-ATPase through a cAMP-dependent, PKA-independent pathway involving Epac1, Rap1, and B-Raf (26).
The purpose of this study was to determine whether the acid-activated Pyk2 signaling pathway underlies the upregulation of H+ transporter activity in mouse-derived OMCD1 (mOMCD1) cells. Our results indicate that Pyk2 is expressed in mOMCD1 cells. We demonstrate further that the adaptation of mOMCD1 cells to a reduction in pH may be facilitated by a Pyk2-mediated ERK1/2 signaling pathway that regulates the apical H+-ATPase but not the H+,K+-ATPase.
MATERIALS AND METHODS
Cell Culture
The mOMCD1 cell line is an immortalized cell line cultured from a microdissected tubule isolated from the inner stripe of the OMCD of a mouse transgenic for the early region of SV40. This cell line was previously characterized by our laboratory (17) and others (31). In the present study, mOMCD1 cells were grown at 37°C in a 5% CO2 humidified incubator in DMEM/F-12 (1:1) media supplemented with 100 U/l penicillin, 100 μg/ml streptomycin, and 10% FBS. Cells were confluent and incubated in serum-free media for 24 h before starting an experiment.
Incubation of cells in acid media (extracellular pH 6.7).
Confluent, quiescent mOMCD1 cells were incubated for 2 h in antibiotic-free and serum-free DMEM/F-12 media, buffered using 35 mM HEPES and calibrated to pHo 7.4 using NaOH. The ionic strength was corrected by addition of NaCl. With the exception of the cells used for the zero time-point, each dish was incubated in acid media (pHo 6.7) for one of the following time periods: 1, 3, 5, 10, or 15 min, and then lysed. Cells, growing in 3.5-cm Petri dishes, were lysed using 200-μl 2× Laemmli sample buffer (23) containing 10% β-mercaptoethanol, scraped, and sonicated.
Intracellular acidification using an NH4Cl prepulse.
We employed the NH4Cl prepulse, a widely accepted technique, to study the effect of decreased intracellular pH (pHi) on H+ transport (17, 29, 42). The solutions used to manipulate pHi (for the NH4Cl prepulse) are shown in Table 1. Renal epithelial cells recover from an acid load by increasing the rate of proton transport and acid extrusion. The rate of pHi recovery (dpH/dt) varies depending on the presence and activity of specific transporters (6, 17). To measure changes in pHi, confluent monolayers of mOMCD1 cells grown on coverslips were incubated for 10 min in physiological saline solution (PSS) containing 10 μM BCECF-AM, followed by incubation in PSS (see Table 1) for 10 min at 37°C. Coverslips were then transferred into a quartz cuvette and placed into the chamber of a fluorospectrophotometer [Deltascan Photon Technology International, South Brunswick, NJ] at a 45° angle to the light beam of the high-speed multiwavelength illuminator. The coverslip was then perfused with PSS, and an intracellular acid load was induced using an NH4Cl prepulse in Na+, K+, HCO3−-free solutions, as previously described by our laboratory and others (17, 22).
Table 1.
Composition of solutions used in the NH4Cl prepulse
| CaCl2 | MgSO4 | Glucose | HEPES | NaCl | KCl | NH4Cl | NMDG | |
|---|---|---|---|---|---|---|---|---|
| PSS | 1.8 | 0.8 | 5.5 | 10 | 135 | 5 | 0 | 0 |
| NH4Cl | 1.8 | 0.8 | 5.5 | 10 | 0 | 0 | 20 | 120 |
| 0K+/0Na+ | 1.8 | 0.8 | 5.5 | 10 | 0 | 0 | 0 | 140 |
| 5K+/0Na+ | 1.8 | 0.8 | 5.5 | 10 | 0 | 5 | 0 | 135 |
Final composition of each solution is shown in mM. All solutions were adjusted to pHo 7.4 and maintained at 37°C. PSS, physiological salt solution; NMDG, N-methyl-d-glucamine chloride.
The solutions used for NH4Cl prepulse are shown in Table 1. They were sequentially and continuously perfused through the cuvette and changed at the following time-points: PSS for 5 min, NH4Cl solution for 5 min, 0K+/0Na+ or 5K+/0Na+ solution for 10 min, followed by PSS for 10 min. BCECF was alternately excited at 488 nm (pH-sensitive wavelength) and 440 nm (pH-insensitive wavelength, the isosbestic point for BCECF). Fluorescence intensity was measured at 535 nm. Felix software (Photon Technology International, NJ) was used for data acquisition and analysis. At the end of each experiment, the ratio of 488/440 was converted to pHi using a fluorescence-pHi ratio calibration curve obtained with pH 7.5, 7.0, and 6.5 solutions containing 10 μM nigericin and high K+ solutions, as previously described (17). pHi recovery was measured as the rate of pHi change, expressed as ΔpHi/min, and interpreted as reflecting apical proton secretion.
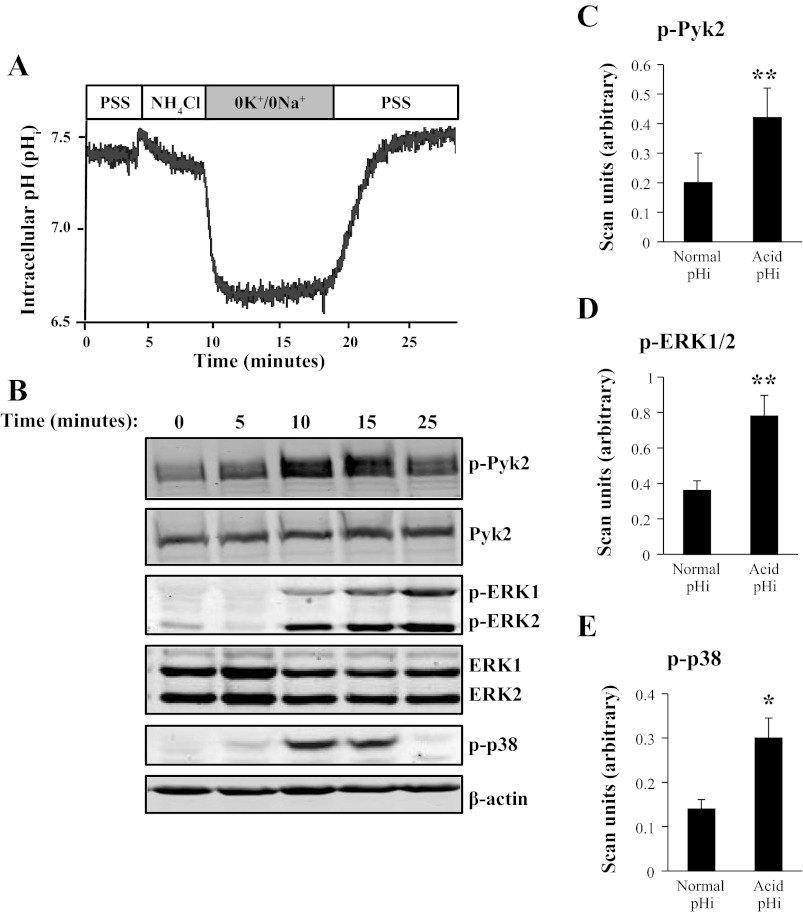
For parallel immunoblots of the phosphorylation of Pyk2 and MAPKs (ERK1/2 and p38) during an NH4Cl prepulse, 3.5-cm dishes of confluent mOMCD1 cells were lysed at the following time points during an NH4Cl prepulse: 2-min incubation in PSS, after 5 min in the NH4Cl solution, 1-min incubation in the 0K+/0Na+ solution, 5-min incubation in the 0K+/Na+ solution, and after 5 min of the final incubation in PSS solution. For immunoblot analysis of cells at “normal pHi,” cells were incubated in PSS for 2 min and lysed. For immunoblot analysis of cells at “acid pHi,” cells were lysed after 5 min in PSS, followed by 5 min in NH4Cl solution and then 1 min in 0K+/0Na+ solution (Table 1). This point in the NH4Cl prepulse corresponds to the minimum pHi achieved during the prepulse (see representative tracing in Fig. 2A at 10 min).
Fig. 2.
An NH4Cl prepulse to decrease pHi enhances Pyk2 and MAPK phosphorylation. A representative tracing in A illustrates changes in pHi after an NH4Cl prepulse in BCECF-AM loaded mOMCD1 cells. Tracing aligns with immunoblots shown in B, which demonstrate changes in phosphorylation of Pyk2, ERK1/2, and p38. Data were normalized using β-actin as a loading standard. Bar graphs in C (p-Pyk2; n = 10), D [p-ERK1/2 (p-ERK1 + p-ERK2); n = 10], and E (p-p38; n = 5) represent mean band intensity and compare the first lane of the immunoblot (normal pHi 7.4) to the fourth lane (acid pHi ∼6.6). PSS, physiological saline solution. *P < 0.05, **P < 0.01 vs. untreated cells. These same data were normalized as well for total kinase expression (Table 2), and the results are not statistically different.
Small Interfering Pyk2 Small Interfering RNA
Following the approach described by Nicodemo et al. (35) and Preisig (40), 80% confluent mOMCD1 cells, in 3.5-cm Petri dishes, were transiently transfected with 25 nM (final concentration) ON-TARGETplus nontargeting pool (control) small interfering (si)RNA (Dharmacon, Lafayette, CO. no. D-001810–10) or ON-TARGETplus SMARTpool mouse PTK2B siRNA (Dharmacon no. L-040719–00). Transfection was performed in antibiotic-free and serum-free media using 8 μM (final concentration) Dharmafect transfection reagent (Dharmacon no. T-2001), as described by the manufacturer. After a 3-h incubation at 37°C, 1 ml media containing serum was added to each dish. Twenty-four hours after transfection, fresh serum-free media was added to cells. Forty-eight hours after transfection, cells were stimulated with or without an NH4Cl prepulse through incubation in 0K+/0Na+ solution for 1 min (normal or acid pHi). Immunoblot was used to confirm knockdown of Pyk2 and analyze the effects of Pyk2 siRNA on the phosphorylation of Pyk2 and MAPKs. Equal loading of the gel was verified by β-actin quantification as described previously by our laboratory (10, 11); additionally, the quantification of phospho-kinases was normalized for total kinase expression.
Pyk2 and ERK1/2 Inhibition
4-Amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]-pyrimidine (PP 1) is known to inhibit Src family kinases, including Pyk2 (21, 49, 62). U0126 is a highly selective inhibitor of the MEK1/2 kinases that phosphorylate ERK1/2 (4, 20). We tested the effect of these inhibitors on the phosphorylation of Pyk2 and ERK1/2 on pHi recovery in mOMCD1 cells. Cells were preincubated in serum-free media containing PP 1 (50 μM) for 30 min at 37°C before stimulation or in serum-free media containing U0126 (10 μM) for 30 min 2 h before stimulation. The potential effect of DMSO was verified by addition of DMSO to control groups. Inhibition of Pyk2 and ERK1/2 phosphorylation by PP 1 and U0126, respectively, was confirmed by an immunoblot. Equal loading was verified as described above.
Inhibition of H+-ATPase
Confluent mOMCD1 cells were pretreated with bafilomycin A1 (10 nM), a specific inhibitor of the H+-ATPase (17, 36, 37, 54), for 30 min at 37°C before stimulation using an NH4Cl prepulse. The same concentration of bafilomycin A1 was maintained during the 0K+/0Na+ or 5K+/0Na+ treatment during pHi recovery.
Immunoblots
We followed the approach described previously by our laboratory (8, 10). Briefly, 50 μl of each sample were separated on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. Equal loading of the gel was verified by staining the membranes in Ponceau S solution and later by immunoblot of β-actin. In our assay, the membranes were blocked for 1 h, shaking in 5% nonfat dry milk in PBS-Tween [10 mM sodium phosphate (pH 7.5), 150 mM NaCl, and 0.05% Tween-20]. Membranes were incubated in primary antibodies (1:1,000 in PBS-Tween) shaking for 2 h, rinsed in PBS-Tween (3 × 15 min), and incubated in secondary antibodies conjugated to infrared fluorophores. Membranes were rinsed in PBS-Tween (3 × 15 min) and scanned using the Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE). Bands were quantified using Odyssey V3.0 software. For total PYK2 and ERK, the phosphoblots were stripped and reprobed.
Antibodies
Monoclonal anti-p-Pyk2 (the antibody specifically detects phosphorylation of Pyk2 at Tyr 402; Santa Cruz Biotechnology no. sc-101790); polyclonal anti-Pyk2 (Santa Cruz Biotechnology no. sc-1515); monoclonal anti-ERK (Santa Cruz Biotechnology no. sc-135900); monoclonal anti-p-ERK1/2 (Thr202/Tyr204) (Santa Cruz Biotechnology no. sc-7378); polyclonal anti-p-p38 (Thr180/Tyr182)-R (Santa Cruz Biotechnology no. sc-17852-R); monoclonal anti-β-actin (Sigma-Aldrich no. A5316); Alexa Fluor 680 goat anti-mouse (Invitrogen no. A-21058); and Alexa Fluor 680 goat anti-rabbit (Invitrogen no. A-21109).
Statistical Analysis
Significance of data was determined using t-tests of the mean value of multiple independent experiments indicated, n-values shown in results. Two-tailed two-sample t-tests assuming equal variance were used to analyze the significance of our data (in our data, *P < 0.05, **P < 0.01). Error bars represent means values ± SE. Statistical analyses were done using PSI-Plot Version 7.5 (Poly Software International, Pearl River, NY).
RESULTS
Acute Decrease in pHo Induces a Decrease in pHi and an Increase in Pyk2 and MAPK Phosphorylation
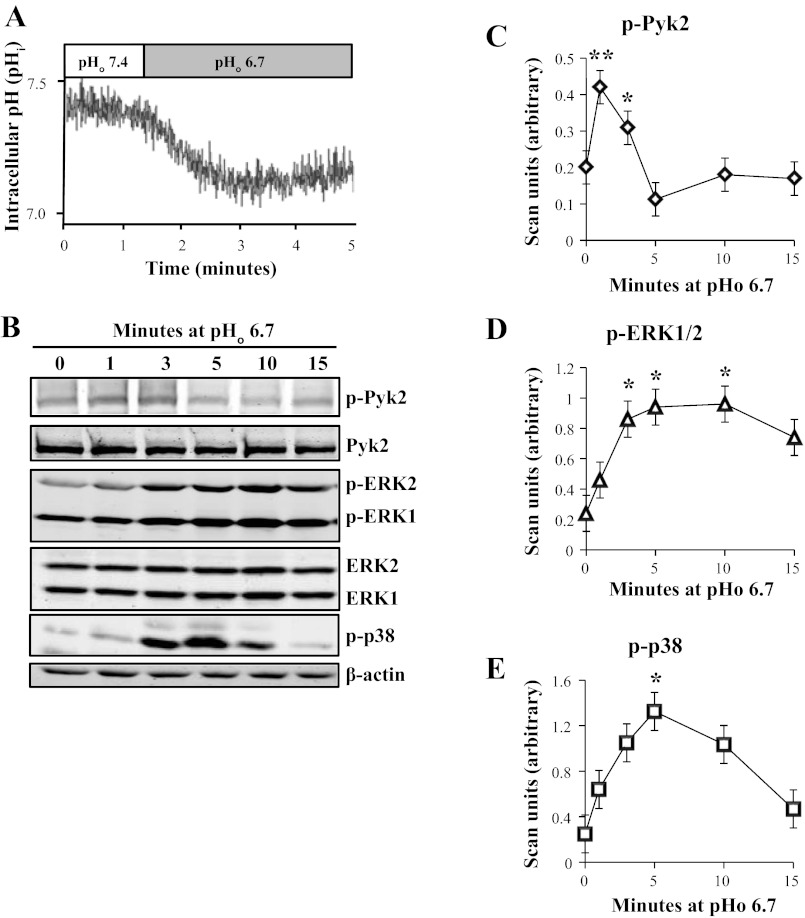
Incubation of mOMCD1 cells at decreased pHo 6.7 decreased pHi from 7.4 to 7.1 ± 0.1, as illustrated in a representative tracing in Fig. 1A (n = 4). We next tested whether acid pH elicits phosphorylation of Pyk2 and MAPKs. Each 3.5-cm Petri dish of confluent and quiescent mOMCD1 cells was lysed after incubation at media pHo 6.7 for the time indicated above each lane of the representative immunoblot, from 0 to 15 min (Fig. 1B). The data were normalized using β-actin. We observed a rapid increase in Pyk2 phosphorylation (210% of control) that peaked after a 1-min incubation in acid media and lasted through a 3-min incubation (130% of control; P < 0.05; n = 7) as illustrated in the line graph in Fig. 1C. Total Pyk2 did not change in response to decreased pH (Fig. 1B). ERK1/2 phosphorylation increased after a 3-min incubation in acid media (420%), peaked at 5 min (480%), and lasted through 10 min (400%; P < 0.05, n = 9), as shown in a representative immunoblot in Fig. 1B and summarized in a line graph in Fig. 1D. ERK1/2 expression did not change in response to decreased pH (Fig. 1B). Finally, phosphorylation of p38 also increased after a 5-min incubation in acid media (650%; P < 0.05; n = 4; Fig. 1, B and E). The antibody used to detect phosphorylated Pyk2 is specific for phosphorylated Tyr402, which is the widely accepted autophosphorylation site on Pyk2. Increased autophosphorylation at Tyr402 is generally interpreted as Pyk2 activation (24, 28, 40).
Fig. 1.
An acute decrease in extracellular pH (pHo) reduces intracellular pH (pHi) and increases Pyk2 and MAPK phosphorylation. Representative tracing in A shows that decreasing extracellular pH to 6.7 elicits a parallel decrease in intracellular pH in 2′-7′-bis(carboxyethyl)-5(6)-carboxyfluorescein-acetoxymethyl ester (BCECF-AM)-loaded mouse-derived outer medullary collecting duct (mOMCD1) cells. B: representative immunoblots of confluent mOMCD1 cells, which were lysed after incubation in acid media (pHo 6.7) for the time indicated above each lane. Data were normalized using β-actin. Line graphs show mean band intensities illustrating phosphorylation of Pyk2 (p-Pyk2; C; n = 7), ERK1/2 (p-ERK1 + p-ERK2; D; n = 9), and p38 (p-p38; E; n = 4) as a function of time (*P < 0.05, **P < 0.01 vs. untreated cells). In this and Figs. 2–6, molecular mass of Pyk2 and p-Pyk2 was ∼116 kDa, ERK1 and p-ERK1 was ∼44 kDa, ERK2 and p-ERK2 was ∼42 kDa, p-p38 was ∼38 kDa, and β-actin was ∼45 kDa.
Acute Decrease in pHi Using an NH4Cl Prepulse Increases Pyk2 and MAPK Phosphorylation
The second, more direct method to manipulate pHi is the NH4Cl prepulse. Figure 2A illustrates a representative tracing of changes in pHi during a NH4Cl prepulse obtained using BCECF-AM loaded mOMCD1 cells. Since the NH4Cl prepulse produced a sustained decrease in pHi and allowed measurement of dpH/dt, providing a functional “readout” of the various conditions examined, we used the NH4Cl prepulse in the remainder of the experiments.
The tracing in Fig. 2A aligns with representative immunoblots of p-Pyk2, p-ERK1/2, and p-p38 shown in Fig. 2B. Data were normalized using β-actin. Bar graphs in Fig. 2, C–E, summarize mean band intensities and compare the first lane (normal pHi) to the third lane (acid pHi) of the immunoblot (Fig. 2B). These graphs demonstrate that acid pHi elicits phosphorylation of Pyk2 (Fig. 2C), ERK1/2 (Fig. 2D), and p38 (Fig. 2E). Pyk2 phosphorylation increased 333% of control at acid pHi (P < 0.01; n = 10), ERK1/2 increased 220% of control (P < 0.01; n = 16), and p38 increased 230% of control (P < 0.05; n = 5). There was no significant change in JNK phosphorylation in mOMCD1 cells in response to acid pH (data not shown). Taken together, these data suggest the possibility that acid pHi induces increased phosphorylation of Pyk2, ERK1/2, and p38 and ultimately may lead to an increase in proton secretion. The quantification of phosphokinases p-Pyk2 or p-ERK1/2 was normalized by either total β-actin expression or alternatively for total kinase expression. Similar results were obtained with either approach. The data corrected for β-actin expression are shown in Fig. 2, and correction for total Pyk2 or total ERK1/2 expression is shown in Table 2, respectively.
Table 2.
Summary of data displayed in Figs. 2–5 showing normalization of phosphorylation band intensity (Pyk2 and ERK1/2) by total kinase expression
| p-Pyk2/Pyk2 |
p-ERK1,2/ERK1,2 |
|||
|---|---|---|---|---|
| Condition | Normal pH | Acid pH | Normal pH | Acid pH |
| Control cells | 0.433 ± 0.076 | 1.810 ± 0.086* | 0.397 ± 0.021 | 0.920 ± 0.093* |
| Pyk2 siRNA | 0.487 ± 0.037 | 0.532 ± 0.018 | 0.337 ± 0.058 | 0.542 ± 0.0759 |
| PP 1 | 0.444 ± 0.012 | 0.482 ± 0.081 | 0.413 ± 0.043 | 0.473 ± 0.114 |
| U0126 | 0.482 ± 0.082 | 1.709 ± 0.282* | 0.304 ± 0.045 | 0.258 ± 0.041 |
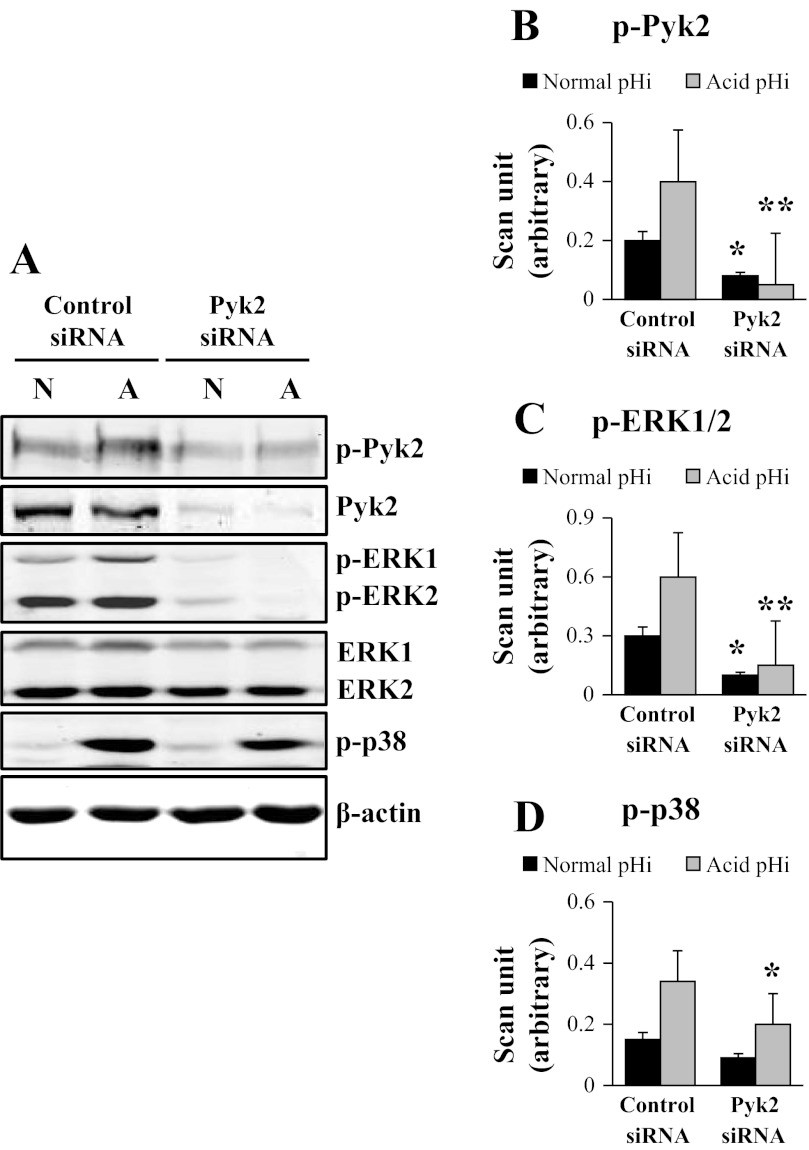
Pyk2 siRNA Blocks acid pHi-Induced ERK1/2 Phosphorylation
mOMCD1 cells were transfected with control siRNA or Pyk2 siRNA. After 48 h, the cells were lysed at normal or acid pHi. pHi was decreased using an NH4Cl prepulse and protein phosphorylation and expression were analyzed by immunoblot. Knockdown of Pyk2 was confirmed by assessing the level of total Pyk2 expression in cells transfected with Pyk2 siRNA. Pyk2 siRNA decreased expression of total Pyk2 84% (P < 0.05; n = 4), as illustrated in the representative immunoblot in Fig. 3A. Compared with the cells transfected with the control siRNA, a decrease in the level of total Pyk2 resulted in attenuation of Pyk2 phophorylation at normal pHi (P < 0.05; n = 4; Fig. 3B). Compared with the cells transfected with the control siRNA and incubated at acid pHi, Pyk2 phosphorylation was significantly reduced in cells transfected with Pyk2 siRNA and incubated at acid pHi (88% inhibition; P < 0.01; n = 4), as shown in Fig. 3A and in the bar graph summarizing mean band intensities in Fig. 3B. We next examined the effect of Pyk2 knockdown on MAPK phosphorylation at acid pHi. The representative blots are shown in Fig. 3A. A decrease in Pyk2 levels caused by Pyk2 siRNA significantly attenuated ERK1/2 phosphorylation at normal pHi (60% inhibition) as well as at acid pHi (75% inhibition; P < 0.05; n = 4; Fig. 3A, summarized in Fig. 3C) without affecting total ERK1/2 expression (Fig. 3A). Pyk2 knockdown had a marginal effect on the acid-induced phosphorylation of p38 (n = 4; Fig. 3A, summarized Fig. 3D). Taken together, these data indicate that Pyk2 is required for ERK1/2 phosphorylation by acid pHi in mOMCD1 cells. Table 2 displays the same data for which p-Pyk2 and p-ERK1/2 expression was corrected by total Pyk2 and total ERK1/2 kinase expression, respectively.
Fig. 3.
Pyk2 small interfering (si)RNA blocks acid pHi induced ERK1/2 phosphorylation. Representative immunoblot in A and the corresponding bar graphs show the effects of control siRNA and Pyk2 siRNA on acid-induced phosphorylation of Pyk2 (B), ERK1/2 (p-ERK1+ p-ERK2; C), and p38 (D). Cells were lysed at normal pHi (N) or acid pHi (A). The acid pHi was induced by an NH4Cl prepulse. Data were normalized using β-actin and the graphs illustrate the mean band intensity. In the statistical analysis of the data displayed in panels B-D, bar 1 from the left was compared with bar 3, and bar 2 with bar 4. These comparisons emphasize the effect of acute intracellular acidosis induced by an NH4Cl prepulse. Similar comparisons were done in Figs. 4 and 5. In Figs. 3–5, as in the experiments described above, *P < 0.05 and **P < 0.01. These same data were normalized as well for total kinase expression (Table 2), and the results are not statistically different.
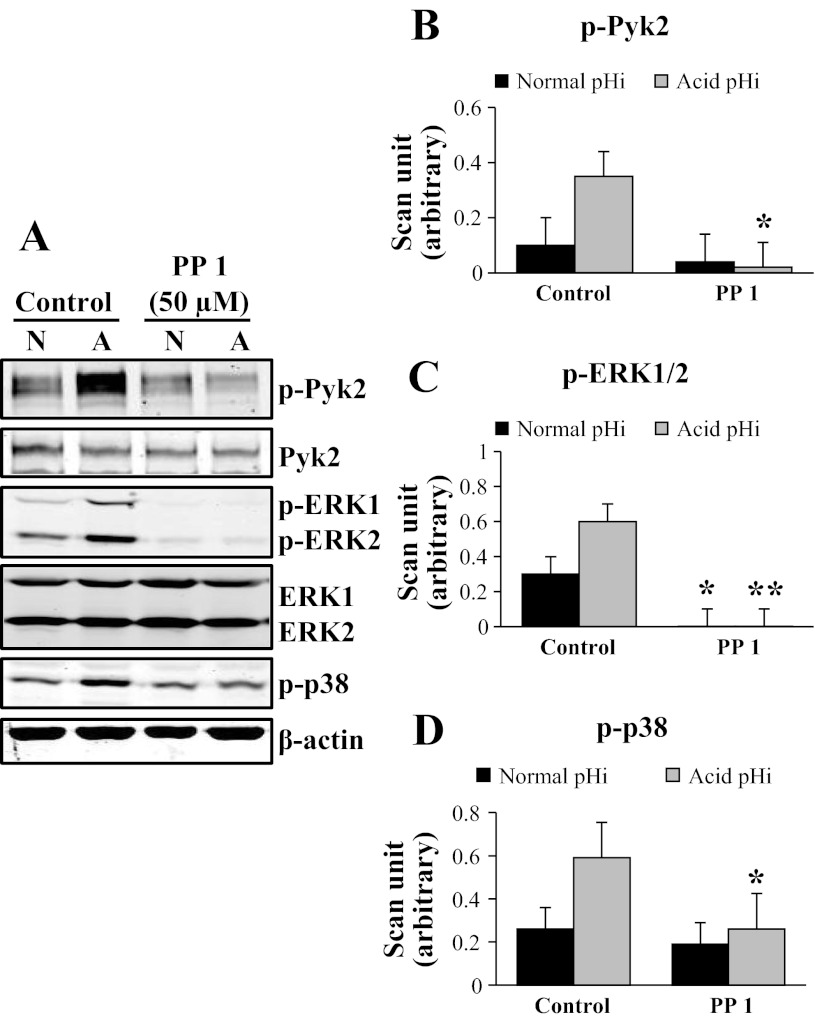
PP 1 Inhibits Acid pHi-Induced Pyk2 and ERK1/2 Phosphorylation
In the next set of experiments, we used the Src kinase inhibitor PP 1 (50 μM) to inhibit Pyk2 and determine its effect on MAPK phosphorylation by acid pHi in mOMCD1 cells. A representative immunoblot of the effects of PP 1 pretreatment on Pyk2, ERK1/2, and p38 phosphorylation at acid pHi is shown in Fig. 4A. Compared with the control cells, PP 1 pretreatment significantly reduced phosphorylation of Pyk2 at acid pHi (91% inhibition; P < 0.05; n = 3; Fig. 4B). PP 1 pretreatment, attenuated ERK1/2 phosphorylation at both normal pHi (99% inhibition; P < 0.05; n = 3) as well as at acid pHi (99% inhibition; P < 0.01; n = 3; Fig. 4C). PP 1 pretreatment attenuated p38 phosphorylation in acid pHi by 56% (P < 0.05; n = 3; Fig. 4D). In summary, inhibition of Pyk2 phosphorylation with PP 1 abolished Pyk2 and ERK1/2 phosphorylation at acid pHi, as well as p38 phosphorylation, although to a lesser degree. Table 2 displays the same data for which p-Pyk2 and p-ERK1/2 expression were normalized by total Pyk2 and total ERK1/2 kinase expression, respectively.
Fig. 4.
4-Amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]-pyrimidine (PP 1) inhibits acid pHi-induced Pyk2 and ERK1/2 phosphorylation. Representative immunoblot in A and the corresponding bar graphs show the effects of PP 1 on acid-induced phosphorylation of Pyk2 (B), ERK1/2 (p-ERK1+ p-ERK2; C), and p38 (D). Cells were pretreated with 0.25% DMSO (control) or PP 1 (50 μM) for 30 min before acid stimulation. Cells were lysed at normal pHi (N) or acid pHi (A). Acid pHi was induced by an NH4Cl prepulse. Data were normalized using β-actin and the graphs illustrate the mean band intensity. *P < 0.05, **P < 0.01, vs. control cells; n = 3. B-D were compared as described in Fig. 3. These same data were normalized as well for total kinase expression (Table 2), and the results are not statistically different.
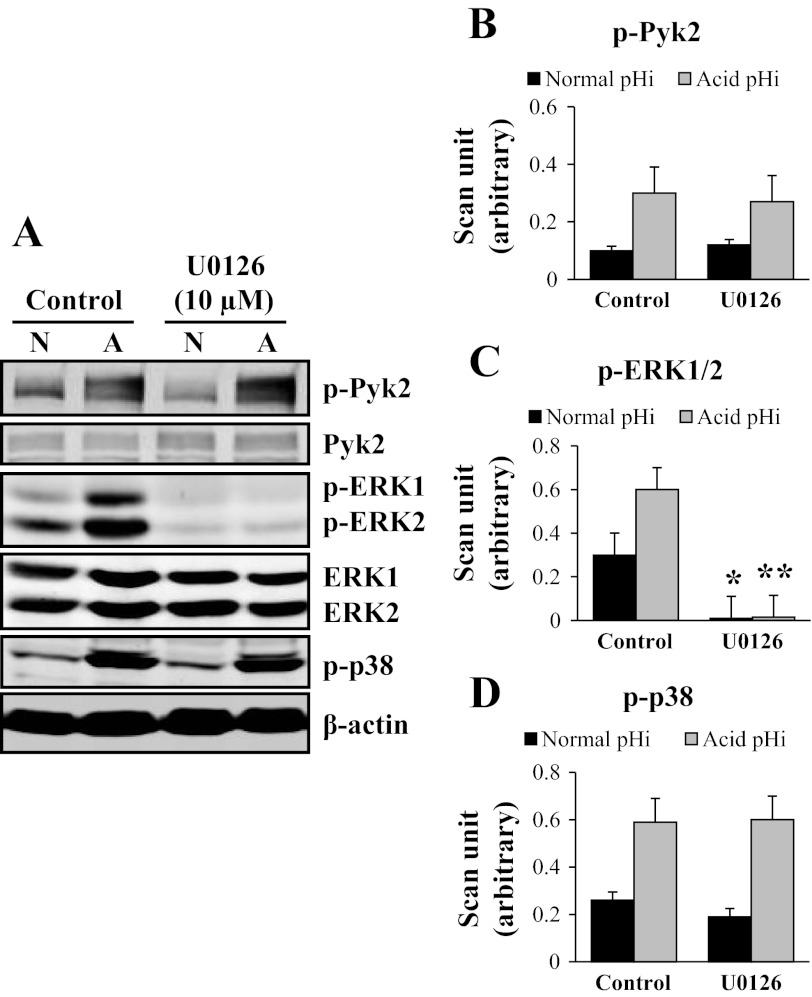
U0126 Blocks Acid pHi-Induced ERK1/2 Phosphorylation
In the next set of experiments, we used U0126, an inhibitor of MEK1/2 that blocks ERK1/2 phosphorylation. A representative immunoblot of the effects of U0126 on Pyk2, ERK1/2, and p38 phosphorylation at acid pHi in mOMCD1 cells is shown in Fig. 5A. Compared with control cells, U0126 pretreatment did not affect phosphorylation of Pyk2, as expected, and marginally decreased acid induced phosphorylation of p38 at acid pHi (Fig. 5A, B, and D). As expected, U0126 significantly inhibited ERK1/2 phosphorylation at both normal pHi (98% inhibition; P < 0.05; n = 3) and acid pHi (99% inhibition; P < 0.01; n = 3), as shown in Fig. 5, A and C. In summary, inhibition of ERK1/2 with U0126 abolished most of ERK1/2 phosphorylation, without affecting Pyk2 or p38 phosphorylation. Table 2 displays the same data for which p-Pyk2 and p-ERK1/2 expression were normalized by total Pyk2 and total ERK1/2 kinase expression, respectively.
Fig. 5.
U0126 blocks acid pHi induction of ERK1/2 phosphorylation. Representative immunoblot in A and the corresponding bar graphs show the effects of U0126 on acid-induced phosphorylation of Pyk2 (B), ERK1/2 (p-ERK1+ p-ERK2; C), and p38 (D). Cells were pretreated with 0.05% DMSO (control) or U0126 (10 μM) for 30 min before stimulation. Cells were lysed at normal pHi (N) or acid pHi (A). Acid pHi was induced by an NH4Cl prepulse. Data were normalized using β-actin and the graphs illustrate the mean band intensity. *P < 0.05, **P < 0.01, vs. control cells; n = 3. B-D were compared as described in Fig. 3. These same data were normalized as well for total kinase expression (Table 2), and the results are not statistically different.
Inhibition of Pyk2 and ERK1/2 Blocks H+-ATPase-Mediated pHi Recovery
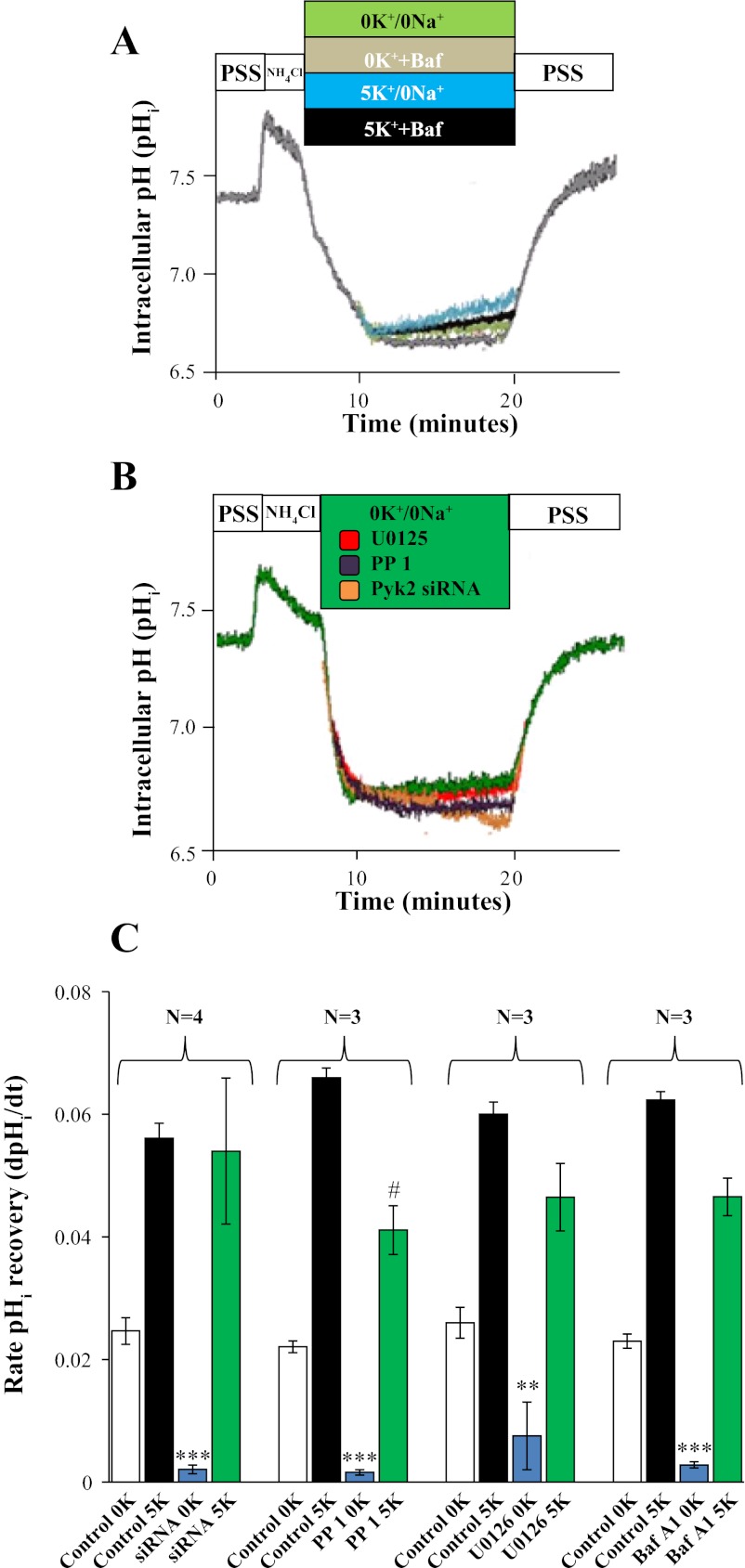
To correlate the phosphorylation profile of Pyk2 and ERK1/2 with the rate of ATPase-mediated pHi recovery, we used the pH-sensitive probe BCECF-AM in mOMCD1 cells. The average rate of K+- and Na+-independent recovery after an NH4Cl prepulse, indicating H+-ATPase-mediated proton secretion, was 0.022 ± 0.002 pH U/min, (n = 4). A representative tracing is shown in Fig. 6A, and the average rate is summarized in Fig. 6C. To confirm that the observed proton secretion was mediated by H+-ATPase, we used bafilomycin A1 (10 nM) as a specific H+-ATPase inhibitor (17, 36, 37, 54). Incubation of mOMCD1 cells with bafilomycin A1 resulted in a 90% inhibition of the rate of Na+- and K+-independent pHi recovery (0.002 ± 0.001 pH U/min; n = 3; P < 0.05 vs. 0.020 ± 0.002 in Fig. 6). Compared with Na+ and K+-independent pHi recovery, representing H+-ATPase-mediated pHi recovery, the rate of pHi recovery increased in the presence of extracellular K+ (5 mM) to an average rate of 0.072 ± 0.01 (n = 4) pH U/min (representative of H+,K+-ATPase plus H+-ATPase activity) vs. 0.022 ± 0.002 pH U/min (representing the H+-ATPase; n = 4, P < 0.001; Fig. 6, A and C). The activity of H+,K+-ATPase was calculated as 1) the difference in the average rates of pHi recovery before and after K+ addition (0.052 ± 0.0003 pH U/min; n = 4; Fig. 6, A and C); and 2) as the average rate of pHi recovery in the presence of 5 mM K+ and bafilomycin A1, which inhibited H+-ATPase activity (0.045 ± 0.01 pH U/min; n = 3; Fig. 6, A and C). Taken together, these data demonstrate H+-ATPase and H+,K+-ATPase mediate pHi recovery in mOMCD1 cells in response to an acute decrease in pHi.
Fig. 6.
Inhibition of Pyk2 and ERK1/2 blocks H+-ATPase-mediated pHi recovery. pHi was measured in BCECF-AM loaded mOMCD1 cells during an NH4Cl prepulse. A: overlapping representative tracings of pHi in the presence or absence of extracellular K+ (5 mM) and in the presence or absence of bafilomycin A1 (10 nM). Na+-independent, K+-independent pHi recovery (0K+/0Na+) is shown in green and the effect of 10 nM bafilomycin A1 on Na+-independent, K+-independent pHi recovery (0K+ + Baf) in gray. Na+-independent, K+-dependent pHi recovery (5K+/0Na+) is shown in blue and the effect of 10 nM bafilomycin A1 on K+-dependent pHi recovery (5K+ + Baf) is shown in black. B: representative tracings of the effect of Pyk2 and ERK1/2 inhibitors on Na+-independent, K+-independent pHi recovery (0K+/0Na+). Tracings in B show control cells in green, cells pretreated with U0126 in red and PP 1 in purple, and cells expressing Pyk2 siRNA in orange. Bar graph in C summarizes the average rates of pHi recovery under a variety of inhibitory conditions as depicted along the x-axis. In each group, bar 1 from left should be compared with bar 3 (indicates H+-ATPase response to the inhibitor) and bar 2 to bar 4 (indicates H+,K+-ATPase response to the inhibitor). Otherwise, the effect of 5K+ is evident when comparing bar 1 to 2 and 3 to 4. #P < 0.05 compared with control (5K+/0Na+ in the experiment designed to test the effect of PP 1) and **P < 0.01, ***P < 0.001, compares the H+-ATPase activity in each specific group.
To ascertain the specificity of Pyk2 effects on the rate of ATPase-mediated pHi recovery, we used BCECF-AM to measure proton secretion in mOMCD1 cells transfected with control or Pyk2 siRNA. H+-ATPase-mediated proton secretion measured as the average rate of K+- and Na+-independent recovery after an NH4Cl prepulse in cells transfected with Pyk2 siRNA was 0.0017 ± 0.0005 pH U/min, which represents 92% inhibition compared with 0.02 ± 0.002 pH U/min in the cells transfected with the control siRNA (n = 4; P > 0.05; Fig. 6, B and C). The rate of pHi recovery in cells transfected with Pyk2 siRNA increased in the presence of extracellular K+ (5 mM) to an average rate of 0.05 ± 0.01 pH U/min, representative of H+,K+-ATPase activity. Compared with the cells transfected with control siRNA (0.072 ± 0.001 pH U/min), this represents 31% inhibition but was not statistically significant (P > 0.05; n = 4), (Fig. 6, B and C). Cells transfected with control siRNA exhibited rates of pHi recovery similar to the nontransfected, control mOMCD1 cells (data not shown). These data demonstrate involvement of Pyk2 in the stimulation of H+-ATPase-mediated pHi recovery.
To further correlate the phosphorylation of Pyk2 and ERK1/2 with the rate of ATPase-mediated pHi recovery, we inhibited the respective kinases with PP 1 and U0126. Inhibition of Pyk2 and ERK1/2 substantially decreased the activity of H+-ATPase, measured as K+- and Na+-independent pHi recovery. The activity of H+-ATPase was nearly abolished in cells treated with PP 1 (from 0.020 ± 0.002 pH U/min in control cells pretreated with DMSO to 0.0020 ± 0.0002 pH U/min in cells treated with PP 1; n = 3; P < 0.01, 99% inhibition; Fig. 6, B and C). The ERK1/2 inhibitor U0126 inhibited 61% of H+-ATPase activity (from 0.025 ± 0.005 pH U/min in the control cells pretreated with DMSO to 0.0078 ± 0.003 pH U/min in cells treated with U0126; n = 3; P < 0.05; Fig. 6, B and C). Inhibition of Pyk2 with 50 μM PP 1, had a significant, but much less dramatic effect on H+,K+-ATPase. The activity of H+,K+-ATPase, measured as Na+-independent, K+-dependent pHi recovery, decreased from 0.065 ± 0.004 pH U/min in the control cells to 0.04 ± 0.008 pH U/min in cells treated with PP 1 (n = 3, P < 0.05), a decrease of 33%, (Fig. 6C). Inhibition of ERK1/2 with U0126, however, did not affect H+,K+-ATPase activity, (pHi recovery declined from 0.060 ± 0.003 pH U/min, in control cells, to 0.047 ± 0.01 pH U/min in cells treated with U0126; n = 3; P > 0.05 or 22% inhibition; Fig. 6C). Taken together, these data demonstrate that the Pyk2/Src complex and phosphorylated ERK1/2 mediate acid activation of the H+-ATPase.
DISCUSSION
Acid-secreting type A-IC cells of the OMCD are known to respond to changes in systemic pH through parallel regulation of luminal proton secretion and basolateral bicarbonate absorption (15, 46, 54), but the afferent pathways that sense acid pH and augment H+ transport are not completely understood. In our study, we report that Pyk2, a putative pH sensor in the kidney (28, 40) is expressed in mOMCD1 cells and responds rapidly to a reduction in pHi by enhanced phosphorylation of Pyk2 at Tyr402 (Figs. 1, B and C, and 2, B and 2C), ERK1/2 at Thr180/Tyr182 (Figs. 1, B and D, and 2, B and D) and p38 at Thr202/Tyr204 (Figs. 1, B and E, and 2, B and E). This increase in phosphorylation is observed independently of the method used to normalize the phosphorylation data (correcting for expression of either β-actin or total kinase). This finding supports the possibility that phosphorylation of these kinases mediates an increase in proton secretion via the bafilomycin A1-sensitive H+-ATPase (Fig. 6).
To confirm the specificity and requirement of Pyk2 and ERK1/2 phosphorylation for the activation of the H+-ATPase and H+,K+-ATPase by a reduction in pHi, we examined the effects of molecular inhibition of Pyk2 mRNA stability (siRNA), chemical inhibition of Pyk2 phosphorylation (PP 1), and chemical inhibition of ERK1/2 phosphorylation (U0126). By knockdown of Pyk2 expression using Pyk2 siRNA or by inhibition of Pyk2 phosphorylation with PP 1 we blocked acid induced Pyk2 phosphorylation (Figs. 3, A and B, and 4, A and B) and acid induced ERK1/2 phosphorylation (Figs. 3, A and C, and 4, A and C). Each of these maneuvers inhibited stimulation of acid secretion by the H+-ATPase similarly (Fig. 6). The inhibition of p38 phosphorylation elicited by a reduction in pH was less evident (Figs. 3, A and D, and 4, A and D), suggesting that p38 is not downstream of Pyk2/ERK1/2. Taken together, our results indicate that the signaling pathway is initiated by the activation of Pyk2, followed by the activation of ERK1/2, and stimulation of the H+-ATPase. This interpretation is in agreement with the observations of Li et al. (28) and Preisig (40) who demonstrated Pyk2-mediated acid-activation of NHE3 in opossum kidney proximal tubule cells. In our studies using mOMCD1 cells, and in the studies by Li et al. (28) using opossum kidney proximal cells, Pyk2 phosphorylation increased within 2 min after reduction in pHi and was required for MAPK-mediated acid secretion (Fig. 6, B and C); however, the time frame to detect an increase in proton excretion was very different. In studies of Li's et al., the experiments were performed after 6 h of acidosis induced by reduction of pHo and were consistent with an increase in NHE3 mRNA, as demonstrated by Amemiya et al. (3). Our experiments were performed a few minutes after the NH4Cl prepulse, a time frame that suggests that mRNA synthesis was not involved.
Our studies demonstrate that Pyk2/ERK1/2 signaling plays an important role in acute stimulation of H+-ATPase-mediated acid secretion, providing further information on the mode of activation of the H+-ATPase in the OMCD. Alzamora et al. (2) and Gong et al. (16) have shown that pH-sensitive activation of H+-ATPase in the collecting duct involves phosphorylation of the pump subunits and is mediated by cAMP and PKA. Winter et al. (62) demonstrated that aldosterone regulates H+-ATPase by the PKA/cAMP pathway and by the PKC/Ca2+-mediated ERK signaling pathway that facilitates the nongenomic, more rapid effects of aldosterone on H+-ATPase activity. Our results appear to be consistent with the later possibility; however, the assumption that acid and aldosterone regulate apical H+-ATPase activity via a common pathway cannot be supported at this time. Further studies will be needed to determine the phosphorylation sites and ATPase subunits involved in the Pyk2-mediated upregulation of proton secretion by the H+-ATPase in OMCD in response to a decrease in pHi.
In summary, we have shown for the first time that Pyk2 and ERK1/2 are activated by an acute reduction in pHi in the OMCD in vitro (mOMCD1 cells). We corroborated that pHi recovery after an NH4Cl prepulse involves the regulation of both the H+-ATPase and the H+,K+-ATPase in these cells. Moreover, our studies demonstrate that Pyk2-mediated MAPK signaling pathways stimulate H+-ATPase activity in mOMCD1 cells in response to a reduction in pHi (Fig. 6, B and C). That there was an inhibitory effect of Pyk2 siRNA, PP 1, and U0126 on ERK1/2 phosphorylation and K+-independent pHi recovery demonstrates that the H+-ATPase is regulated by Pyk2 and ERK1/2. These results also suggest that acid activation of the H+,K+-ATPase may be regulated by a different signaling cascade than the H+-ATPase and will require further investigation. In conclusion, our studies demonstrate that Pyk2 may function as a pH sensor in mOMCD1 cells to increase the activity of the H+-ATPase, most likely via an ERK1/2-mediated signaling pathway.
GRANTS
This work is supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases DK-030603 (awarded to T. D. DuBose, Jr.) and by the generous support of research development funds from the Department of Internal Medicine, Wake Forest University School of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.D.F., J.C., and T.D.D. conception and design of research; K.D.F. performed experiments; K.D.F., J.C., S.P., and T.D.D. analyzed data; K.D.F., J.C., S.P., and T.D.D. interpreted results of experiments; K.D.F. prepared figures; K.D.F. drafted manuscript; K.D.F., J.C., S.P., and T.D.D. edited and revised manuscript; K.D.F., J.C., and T.D.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Richard F. Loeser and Raymond B. Penn for critical reading of the manuscript and numerous helpful suggestions and comments during the preparation of this manuscript and Dr. Alain Doucet for comments on ERK1/2 pathway.
REFERENCES
- 1. Alper SL. Genetic diseases of acid-base transporters. Annu Rev Physiol 64: 899–923, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Alzamora R, Thali RF, Gong F, Smolak C, Li H, Baty CJ, Bertrand CA, Auchli Y, Brunisholz RA, Neumann D, Hallows KR, Pastor-Soler NM. PKA regulates vacuolar H+-ATPase localization and activity via direct phosphorylation of the A subunit in kidney cells. J Biol Chem 285: 24676–24685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amemiya M, Yamaji Y, Cano A, Moe OW, Alpern RJ. Acid incubation increases NHE-3 mRNA abundance in OKP cells. Am J Physiol Cell Physiol 269: C126–C133, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J 371: 199–204, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blaukat A, Ivankovic-Dikic I, Gronroos E, Dolfi F, Tokiwa G, Vuori K, Dikic I. Adaptor proteins Grb2 and Crk couple Pyk2 with activation of specific mitogen-activated protein kinase cascades. J Biol Chem 274: 14893–14901, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Boron WF. Regulation of intracellular pH. Adv Physiol Educ 28: 160–179, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Bruce LJ, Cope DL, Jones GK, Schofield AE, Burley M, Povey S, Unwin RJ, Wrong O, Tanner MJ. Familial distal renal tubular acidosis is associated with mutations in the red cell anion exchanger (Band 3, AE1) gene. J Clin Invest 100: 1693–1707, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Codina J, Delmas-Mata JT, DuBose TD., Jr Expression of HKα2 protein is increased selectively in renal medulla by chronic hypokalemia. Am J Physiol Renal Physiol 275: F433–F440, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Codina J, DuBose TD., Jr Molecular regulation and physiology of the H+,K+-ATPases in kidney. Semin Nephrol 26: 345–351, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Codina J, Liu J, Bleyer AJ, Penn RB, DuBose TD., Jr Phosphorylation of S955 at the protein kinase A consensus promotes maturation of the alpha subunit of the colonic H+,K+-ATPase. J Am Soc Nephrol 17: 1833–1840, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Codina J, Opyd TS, Powell ZB, Furdui CM, Petrovic S, Penn RB, DuBose TD., Jr pH-dependent regulation of the α-subunit of H+,K+-ATPase (HKα2). Am J Physiol Renal Physiol 301: F536–F543, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dikic I, Dikic I, Schlessinger J. Identification of a new Pyk2 isoform implicated in chemokine and antigen receptor signaling. J Biol Chem 273: 14301–14308, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature 383: 547–550, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Finberg KE, Wagner CA, Bailey MA, Paunescu TG, Breton S, Brown D, Giebisch G, Geibel JP, Lifton RP. The B1-subunit of the H(+) ATPase is required for maximal urinary acidification. Proc Natl Acad Sci USA 102: 13616–21, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finkel KW, DuBose TD., Jr Metabolic acidosis. In: Acid-Base and Electrolyte Disorders: A Companion to Brenner and Rector's The Kidney, edited by DuBose TD, Hamm LL. Philadelphia, PA: Saunders, 2002, p. 55–66 [Google Scholar]
- 16. Gong F, Alzamora R, Smolak C, Li H, Naveed S, Neumann D, Hallows KR, Pastor-Soler NM. Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. Am J Physiol Renal Physiol 298: F1162–F1169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guntupalli J, Onuigbo M, Wall S, Alpern RJ, DuBose TD., Jr Adaptation to low-K+ media increases H+-K+-ATPase but not H+-ATPase-mediated pHi recovery in OMCD1 cells. Am J Physiol Cell Physiol 273: C558–C571, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, Rodriguez-Soriano J, Santos F, Cremers CW, di Pietro A, Hoffbrand BI, Winiarski J, Bakkaloglu A, Ozen S, Dusunsel R, Goodyer P, Hulton SA, Wu DK, Skvorak AB, Morton CC, Cunningham MJ, Jha V, Lifton RP. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet 21: 84–90, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Kleinman JG. Proton ATPases and urinary acidification. J Am Soc Nephrol 5: S6–11, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Kodama H, Fukuda K, Takahashi E, Tahara S, Tomita Y, Ieda M, Kimura K, Owada KM, Vuori K, Ogawa S. Selective involvement of p130Cas/Crk/Pyk2/c-Src in endothelin-1-induced JNK activation. Hypertension 41: 1372–1379, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Kodama H, Fukuda K, Takahashi T, Sano M, Kato T, Tahara S, Hakuno D, Sato T, Manabe T, Konishi F, Ogawa S. Role of EGF receptor and Pyk2 in endothelin-1-induced ERK activation in rat cardiomyocytes. J Mol Cell Cardiol 34: 139–150, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Kuwahara M, Fu WJ, Marumo F. Functional activity of H-K-ATPase in individual cells of OMCD: localization and effect of K+ depletion. Am J Physiol Renal Fluid Electrolyte Physiol 270: F116–F122, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970 [DOI] [PubMed] [Google Scholar]
- 24. Lakkakorpi PT, Bett AJ, Lipfert L, Rodan GA, Duong LT. PYK2 autophosphorylation, but not kinase activity, is necessary for adhesion-induced association with c-Src, osteoclast spreading, and bone resorption. J Biol Chem 278: 11502–11512, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Laroche-Joubert N, Marsy S, Luriau S, Imbert-Teboul M, Doucet A. Mechanism of activation of ERK and H-K-ATPase by isoproterenol in rat cortical collecting duct. Am J Physiol Renal Physiol 284: F948–F954, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Laroche-Joubert N, Marsy S, Michelet S, Imbert-Teboul M, Doucet A. Protein kinase A-independent activation of ERK and H,K-ATPase by cAMP in native kidney cells: role of Epac I. J Biol Chem 277: 18598–18604, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature 376: 737–745, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Li S, Sato S, Yang X, Preisig PA, Alpern RJ. Pyk2 activation is integral to acid stimulation of sodium/hydrogen exchanger 3. J Clin Invest 114: 1782–9, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu W, Pastor-Soler NM, Schreck C, Zavilowitz B, Kleyman TR, Satlin LM. Luminal flow modulates H+-ATPase activity in the cortical collecting duct (CCD). Am J Physiol Renal Physiol 302: F205–F215, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loeser RF, Forsyth CB, Samarel AM, Im HJ. Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase C-dependent pathway. J Biol Chem 278: 24577–24585, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lynch IJ, Greenlee MM, Gumz ML, Rudin A, Xia SL, Wingo CS. Heterogeneity of H-K-ATPase-mediated acid secretion along the mouse collecting duct. Am J Physiol Renal Physiol 298: F408–F415, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matsui A, Okigaki M, Amano K, Adachi Y, Jin D, Takai S, Yamashita T, Kawashima S, Kurihara T, Miyazaki M, Tateishi K, Matsunaga S, Katsume A, Honshou S, Takahashi T, Matoba S, Kusaba T, Tatsumi T, Matsubara H. Central role of calcium-dependent tyrosine kinase PYK2 in endothelial nitric oxide synthase-mediated angiogenic response and vascular function. Circulation 116: 1041–1051, 2007 [DOI] [PubMed] [Google Scholar]
- 33. McMullen M, Keller R, Sussman M, Pumiglia K. Vascular endothelial growth factor-mediated activation of p38 is dependent upon Src and RAFTK/Pyk2. Oncogene 23: 1275–1282, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Michlig S, Mercier A, Doucet A, Schild L, Horisberger JD, Rossier BC, Firsov D. ERK1/2 controls Na,K-ATPase activity and transepithelial sodium transport in the principal cell of the cortical collecting duct of the mouse kidney. J Biol Chem 279: 51002–51012, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Nicodemo AA, Pampillo M, Ferreira LT, Dale LB, Cregan T, Ribeiro FM, Ferguson SS. Pyk2 uncouples metabotropic glutamate receptor G protein signaling but facilitates ERK1/2 activation. Mol Brain 3: 4, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ohkuma S, Shimizu S, Noto M, Sai Y, Kinoshita K, Tamura H. Inhibition of cell growth by bafilomycin A1, a selective inhibitor of vacuolar H(+)-ATPase. In Vitro Cell Dev Biol Anim 29A: 862–866, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Ono S, Guntupalli J, DuBose TD., Jr Role of H+-K+-ATPase in pHi regulation in inner medullary collecting duct cells in culture. Am J Physiol Renal Fluid Electrolyte Physiol 270: F852–F861, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Pandey P, Avraham S, Kumar S, Nakazawa A, Place A, Ghanem L, Rana A, Kumar V, Majumder PK, Avraham H, Davis RJ, Kharbanda S. Activation of p38 mitogen-activated protein kinase by PYK2/related adhesion focal tyrosine kinase-dependent mechanism. J Biol Chem 274: 10140–10144, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Pech V, Zheng W, Pham TD, Verlander JW, Wall SM. Angiotensin II activates H+-ATPase in type A intercalated cells. J Am Soc Nephrol 19: 84–91, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Preisig PA. The acid-activated signaling pathway: starting with Pyk2 and ending with increased NHE3 activity. Kidney Int 72: 1324–1329, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Rocic P, Govindarajan G, Sabri A, Lucchesi PA. A role for PYK2 in regulation of ERK1/2 MAP kinases and PI 3-kinase by ANG II in vascular smooth muscle. Am J Physiol Cell Physiol 280: C90–C99, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Roos A, Boron WF. Intracellular pH Physiol Rev 61: 296–434, 1981 [DOI] [PubMed] [Google Scholar]
- 43. Rothenberger F, Velic A, Stehberger PA, Kovacikova J, Wagner CA. Angiotensin II stimulates vacuolar H+-ATPase activity in renal acid-secretory intercalated cells from the outer medullary collecting duct. J Am Soc Nephrol 18: 2085–2093, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Rufanova VA, Alexanian A, Wakatsuki T, Lerner A, Sorokin A. Pyk2 mediates endothelin-1 signaling via p130Cas/BCAR3 cascade and regulates human glomerular mesangial cell adhesion and spreading. J Cell Physiol 219: 45–56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schauwienold D, Sastre AP, Genzel N, Schaefer M, Reusch HP. The transactivated epidermal growth factor receptor recruits Pyk2 to regulate Src kinase activity. J Biol Chem 283: 27748–27756, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Schuster VL. Function and regulation of collecting duct intercalated cells. Annu Rev Physiol 55: 267–288, 1993 [DOI] [PubMed] [Google Scholar]
- 47. Siciliano JC, Toutant M, Derkinderen P, Sasaki T, Girault JA. Differential regulation of proline-rich tyrosine kinase 2 cell adhesion kinase beta (PYK2/CAK beta) and pp125(FAK) by glutamate and depolarization in rat hippocampus. J Biol Chem 271: 28942–28946, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Smith AN, Skaug J, Choate KA, Nayir A, Bakkaloglu A, Ozen S, Hulton SA, Sanjad SA, Al-Sabban EA, Lifton RP, Scherer SW, Karet FE. Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat Genet 26: 71–75, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Sorokin A, Kozlowski P, Graves L, Philip A. Protein-tyrosine kinase Pyk2 mediates endothelin-induced p38 MAPK activation in glomerular mesangial cells. J Biol Chem 276: 21521–21528, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Sun CK, Man K, Ng KT, Ho JW, Lim ZX, Cheng Q, Lo CM, Poon RT, Fan ST. Proline-rich tyrosine kinase 2 (Pyk2) promotes proliferation and invasiveness of hepatocellular carcinoma cells through c-Src/ERK activation. Carcinogenesis 29: 2096–2105, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Tahara S, Fukuda K, Kodama H, Kato T, Miyoshi S, Ogawa S. Potassium channel blocker activates extracellular signal-regulated kinases through Pyk2 and epidermal growth factor receptor in rat cardiomyocytes. J Am Coll Cardiol 38: 1554–1563, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Tang H, Zhao ZZJ, Landon EJ, Inagami T. Regulation of calcium-sensitive tyrosine kinase Pyk2 by angiotensin II in endothelial cells - Roles of Yes tyrosine kinase and tyrosine phosphatase SHP-2. J Biol Chem 275: 8389–8396, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Tsuganezawa H, Sato S, Yamaji Y, Preisig PA, Moe OW, Alpern RJ. Role of c-SRC and ERK in acid-induced activation of NHE3. Kidney Int 62: 41–50, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Tsuruoka S, Schwartz GJ. Metabolic acidosis stimulates H+ secretion in the rabbit outer medullary collecting duct (inner stripe) of the kidney. J Clin Invest 99: 1420–1431, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Turner JM, Bauer C, Abramowitz MK, Melamed ML, Hostetter TH. Treatment of chronic kidney disease. Kidney Int 81: 351–362, 2012 [DOI] [PubMed] [Google Scholar]
- 56. Valles P, Lapointe MS, Wysocki J, Batlle D. Kidney vacuolar H+ -ATPase: physiology and regulation. Semin Nephrol 26: 361–374, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar-ATPase. Physiol Rev 84: 1263–1314, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Wagner CA, Geibel JP. Acid-base transport in the collecting duct. J Nephrol 15, Suppl 5: S112–S127, 2002 [PubMed] [Google Scholar]
- 59. Wall SM, Fischer MP, Glapion DM, De La Calzada M. ANG II reduces net acid secretion in rat outer medullary collecting duct. Am J Physiol Renal Physiol 285: F930–F937, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Wang WH. Regulation of ROMK (Kir1.1) channels: new mechanisms and aspects. Am J Physiol Renal Physiol 290: F14–F19, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wingo CS. Active proton secretion and potassium absorption in the rabbit outer medullary collecting duct. Functional evidence for proton-potassium-activated adenosine triphosphatase. J Clin Invest 84: 361–365, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Winter C, Kampik NB, Vedovelli L, Rothenberger F, Paunescu TG, Stehberger PA, Brown D, John H, Wagner CA. Aldosterone stimulates vacuolar H(+)-ATPase activity in renal acid-secretory intercalated cells mainly via a protein kinase C-dependent pathway. Am J Physiol Cell Physiol 301: C1251–C1261, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yu H, Li X, Marchetto GS, Dy R, Hunter D, Calvo B, Dawson TL, Wilm M, Anderegg RJ, Graves LM, Earp HS. Activation of a novel calcium-dependent protein-tyrosine kinase. Correlation with c-Jun N-terminal kinase but not mitogen-activated protein kinase activation. J Biol Chem 271: 29993–29998, 1996 [DOI] [PubMed] [Google Scholar]