Abstract
A panel of six murine monoclonal antibodies (MAbs) recognizing inner core lipopolysaccharide (LPS) epitopes of Neisseria meningitidis was prepared and characterized in order to determine the diversity of inner core LPS glycoforms among disease and carrier isolates. Two of these MAbs, L2-16 (immunoglobulin G2b [IgG2b]) and LPT3-1 (IgG2a), together with a third, previously described MAb, L3B5 (IgG3), showed reactivity, either individually or in combination, with all except 3 of 143 disease and carriage isolates (125 of 126 strains from blood, cerebrospinal fluid, or skin biopsy samples and 15 of 17 from nasopharyngeal cultures). MAbs L3B5, L2-16, and LPT3-1 were further characterized in an indirect immunofluorescence assay. All three MAbs bound to the bacterial cell surface, findings that correlated strongly with whole-cell enzyme-linked immunosorbent assay and immunodot blots. However, in contrast to our findings with L3B5, cell surface binding of L2-16 or LPT 3-1 did not correlate with functional activity as determined by bactericidal or infant rat passive protection assays against wild-type N. meningitidis strains. These findings are provocative with respect to the requirements for protective activity of antibodies and the development of inner core LPS vaccines against invasive meningococcal disease.
Invasive meningococcal disease remains a major cause of meningitis and septicemia in children under 2 years of age. Effective polysaccharide-based conjugate vaccines are available for all the major serogroups except for serogroup B, which remains the most common cause of meningococcal meningitis and septicemia in the developed world (36). There is an urgent requirement for a serogroup B vaccine. The capsular polysaccharide of Neisseria meningitidis group B (NmB) is α-2,8-linked polysialic acid, which is also expressed on human neuronal cell adhesion and other surface-expressed molecules, and therefore poses potential problems of autoimmunity as a vaccine candidate (6). Alternative approaches include outer membrane vesicles (7, 28), a variety of outer membrane proteins identified through exploitation of whole genome sequences (26, 31, 41), signature-tagged mutagenesis (40), and an approach based on the induction of cross-reactive antigens of Neisseria lactamica (29). We have taken an approach that involves the use of inner core lipopolysaccharide (LPS) epitopes of N. meningitidis as vaccine candidates (32, 33, 34, 35). The LPS structure of Neisseria comprises a lipid A backbone attached to a core oligosaccharide unit with an inner core di-heptose-N-acetyl-glucosamine backbone, wherein the two heptose residues can provide a point of attachment for the outer core oligosaccharide residues (20).
Meningococcal LPS has been classified into 12 distinct LPS immunotypes (L1 to L12), originally defined by monoclonal antibody (MAb) reactivities (37), and further defined by subsequent structural analyses of immunotypes L1/6 (5, 43), L2 (8), L3 (30), L4/7 (23), L5 (27), and L9 (12). A key determinant of the immunotyping scheme is the location of a phosphoethanolamine (PEtn) moiety on the distal inner core heptose residue (HepII) at either position 3 or position 6 or this moiety's absence. The length and nature of oligosaccharide extension from the proximal heptose residue (HepI) and the presence or absence of an α-linked glucose unit at HepII also dictate the immunotype. Oligosaccharides extending from HepI often mimic human glycolipid structures and include sialylated lacto-N-neotetraose (L2, L3, L4, and L5), lactose (L8), and globotriose (Pk antigen) (L1). The genes encoding the glycosyltransferases are known, including lgtA, lgtB, and lgtE (13); lgtC (9); rfaC (39); lgtF and rfaK (21, 22); rfaF (14); and lgtG (2). A characteristic of meningococcal LPS is reversible, high-frequency phase variation of outer core oligosaccharide structures mediated by mutation in homopolymeric DNA tracts present in LPS biosynthetic genes (16). These homopolymeric tracts are absent in inner core LPS biosynthetic genes such as rfaC, rfaK, rfaF, and lgtF. This inner core structure (Fig. 1a) is therefore relatively conserved and an attractive candidate for incorporation into a vaccine (33). An understanding of the genes involved in the biosynthesis of N. meningitidis LPS has provided us with the genetic tools to construct genetically defined mutants, including those expressing truncated LPS glycoforms. These have been used to produce and characterize antibodies to defined inner core LPS structures. The absence of galactose residues in the conserved inner core structure of meningococcal LPS has led to the utilization of mutants defective in the enzyme UDP glucose-4-epimerase (GalE) in the construction of truncated LPS structures. This enzyme is essential for N. meningitidis to synthesize UDP-Gal for incorporation of galactose into its LPS and is encoded by the gene galE (15). Mutation of this gene results in truncation of the oligosaccharide chain at the glucose residue attached to HepI. Previously we have described an inner core LPS epitope defined by a MAb, L3B5, that is conserved in 76% of NmB strains (70% of all major N. meningitidis serogroups) and is accessible in fully encapsulated N. meningitidis (33). This epitope is accessible in N. meningitidis grown in vivo, and the MAb has functional activity in vitro (bactericidal, opsonic) and in vivo against wild-type NmB strains (32, 34, 35). L3B5 recognizes an inner core LPS epitope in immunotype L3 that has an absolute requirement for PEtn at position 3 of HepII (33) (Fig. 1c). In previous studies of L3B5 reactivity, it was clear that two genes, lpt3 and lgtG, were critical in determining the structures of the inner core epitopes. The gene lpt3 codes for an LPS PEtn transferase that adds PEtn to position 3 of HepII and competes with lgtG, which encodes the enzyme that adds glucose (Glc) to position 3 of HepII (24). Whether lgtG is switched on or off depends on the number of cytidines in the homopolymeric tract that affects whether the gene is in or out of frame and therefore affects the nature and functionality of the translated product. One mechanism resulting in N. meningitidis strains that do not react with L3B5 is when the lgtG gene has a number of cytidines permissive for transcription of lgtG. This results in the addition of glucose at position 3 of HepII instead of PEtn rendering the LPS nonreactive with L3B5. The gene responsible for the transfer of PEtn to position 6 of HepII is currently unknown.
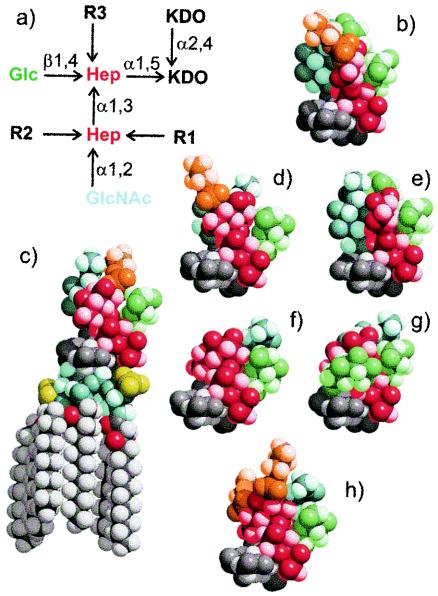
FIG. 1.
(a) Structural representation of seven inner core galE LPS structures of the N. meningitidis immunotypes. (b to h) Three-dimensional space-filling molecular models of the galE mutant inner core structures color coded as follows: PEtn (orange), Glc (green), GlcNAc (pale blue), heptose (Hep) (red), 2-keto-3-deoxyoctulosonic acid (KDO) (dark grey), and lipid A (pale grey). Molecular models were constructed using a Metropolis Monte Carlo approach and are drawn with the same orientation for the KDO residue. The lipid A moiety is shown in panel c. (b) L2 galE (R1 = PEtn-6, R2 = Glc-α1,3, R3 = H); (c) L3 galE (R1 = H, R2 = PEtn-3, R3 = H); (d) L4 galE (R1 = PEtn-6, R2 = H, R3 = H); (e) L5 galE (R2 = Glc-α1,3, R1 = H, R3 = H); (f) MC58 lpt3 galE (R1, R2, R3 = H); (g) 1000 galE (R1 = H, R2 = H, R3 = Glc-β1,2); and (h) 2220Y galE (R1 = PEtn-6, R2 = PEtn-3, R3 = H). L2-16 was raised to structures b and d, LPT3-1 was raised to structure f, and L3B5 was raised to structure c (see Materials and Methods).
The aim of this study was to develop additional MAbs to inner core LPS epitopes so as to increase our capacity to detect structural diversity. These MAbs were used to identify inner core LPS variants and to determine their diversity (number and frequency) in different N. meningitidis strains taken from patients during carriage or invasive disease. The MAbs were characterized in terms of their cross-reactivity to purified LPS, binding to whole cells by whole-cell enzyme-linked immunosorbent assay (whole-cell ELISA [WCE]), indirect immunofluorescence (IF), and functional activity in vitro (opsonophagocytosis and serum bactericidal [SB] assays). These MAbs have also resulted in the identification of novel inner core LPS glycoforms (3).
MATERIALS AND METHODS
Bacterial strains.
The N. meningitidis wild-type and mutant strains used in this paper are described in Table 1. The disease and carriage sets of isolates are described in Table 2. All N. meningitidis strains used were encapsulated (serogroup B or C). Bacterial strains were grown overnight (18 h) at 37°C on solid brain heart infusion (BHI) medium (Merck) in an atmosphere of 5% CO2.
TABLE 1.
N. meningitidis strains used in this study
| Strain | Immunotype and/or genotype | Source and/or reference |
|---|---|---|
| H44/76 | L3 | 10 |
| galE | 14 | |
| MC58 | L3 | 35 |
| galE | 14 | |
| lpt3, lpt3 galE | 23 | |
| 35E | L2 | 7 |
| galE | 28 | |
| 89I | L4 | 22 |
| galE | 28 | |
| 8047 | L3 | 30 |
| lpt3 | This study | |
| 2996 | L3 | 30 |
| lpt3 | This study | |
| M981 | L5 | 25 |
| 1000 | NT | 33 |
| galE | 3, 28 | |
| NGH38 | L2 and L5 | 33 |
| galE | 28 | |
| 2220Y | lgtA siaD | This study |
TABLE 2.
Details of N. meningitidis strain collections used in this study
| Strain collection | No. of strains | Serogroups | Country of origin (yr) | Reference and/or source |
|---|---|---|---|---|
| Global | 100 | All major groups | Global (1963-1996) | 24, 28 |
| United Kingdom | 26 | B, C, Y | United Kingdom (1996-1999) | 9; this study |
| Czech | 17 | B, C | Czech Republic (1993) | 18 |
LPS extraction and purification.
LPS samples of N. meningitidis were obtained as described previously (33). LPS was O-deacylated (odA) with anhydrous hydrazine and dephosphorylated (HF) using 48% aqueous hydrogen fluoride.
Structural analysis of purified LPS.
Mass spectrometry (MS) (negative ion electrospray-MS) and nuclear magnetic resonance techniques were employed to determine structural features of purified LPS-derived oligosaccharides as described previously (33).
Preparation of inner core LPS MAbs.
MAbs to inner core N. meningitidis LPS were developed as described for L3B5 (33). Briefly, female BALB/c mice, 6 to 8 weeks of age, were immunized intraperitoneally (i.p.) with formalin-killed galE mutant whole cells. Each mouse received 108 cells in 0.5 ml of phosphate-buffered saline (PBS). The mice received boosters on days 14 and 35, and blood samples were taken on day 45. One or two mice showing a high antibody titer to the homologous galE and wild-type LPS antigens were given two final injections on about day 56, an i.p. injection as described previously, and an intravenous injection of 2 × 107 cells in 0.1 ml of PBS. The fusion was performed 3 days following the last injections. Stimulated spleen cells were fused with SP2/O-Ag14 myeloma cells at a ratio of 10:1 in 33% polyethylene glycol 1450. Putative hybrids resulting from hypoxanthine-aminopterin-thymidine selection were screened against the purified immunizing galE mutant and/or the complete LPS of the same immunotype in an ELISA. Hybrids producing antibodies of interest were selected and cloned twice by limiting dilution to ensure stability and clonality. Immunoglobulin (Ig) subclass was determined with spent supernatant by using an enzyme immunosorbent assay mouse MAb isotyping kit (Amersham Canada, Oakville, Ontario, Canada). Clones were expanded as ascites by i.p. injection of 106 hybridoma cells in BALB/c mice 10 to 14 days following i.p. priming with 0.5 ml of 2,6,10,14-tetramethyl-pentadecane (pristane). Ascitic fluid was tapped 7 to 14 days postinjection.
ELISA.
Purified and well-characterized wild-type and mutant LPS were used in a solid-phase indirect ELISA to determine the binding specificities of the MAbs (34). Nuclear magnetic resonance, MS, and/or sodium dodecyl sulfate-polyacrylamide gel electrophoresis confirmed the structural integrity of each antigen utilized. Nunc Maxisorp EIA (96-well) plates were coated with 1.0 μg of purified LPS in 0.05 M carbonate buffer containing 0.02 M MgCl2, pH 9.8, at 37°C for 1 h. Wells were then blocked with 1% bovine serum albumin (BSA)-PBS for 3 h at room temperature, wells were washed with PBS-0.05% Tween 20 (PBS-T), culture supernatants or ascites were added, and the plates were incubated for 1 to 3 h at room temperature. Following washing with PBS-T, alkaline phosphatase-labeled goat anti-mouse IgG (Cedarlane Laboratories, Hornby, Ontario, Canada) diluted 1:3,000 in 1% BSA-PBS was added for 1 h at room temperature. The plates were then washed and developed with Phosphatase Substrate System (Kirkegaard and Perry Laboratories, Gaithersburg, Md.). After 30 to 60 min the A405-410 was determined.
Immunotyping MAbs.
To determine the immunotype of N. meningitidis strains studied, the following murine MAbs were used in dot blots: MN42F12.31 (L2 and L5), MN4A8B2 (L3, L7, and L9), MN4C1B (L4, L6, and L9), MN40G11.7 (L6), MN3A8C (L5), MN14F21-11 (L1), and MN 14-1-L10 (L10). These MAbs were obtained from the National Institute of Public Health and the Environment, Bilthoven, The Netherlands.
Purification of inner core LPS MAbs.
Murine MAbs were raised against LPS mutants of encapsulated strains as described above. Culture supernatant or ascites fluid was purified as described previously (35). This was achieved using a Sephadex G-25 precolumn (to remove phenol red from the medium) and anion exchange column chromatography (Resource 15Q anion). The concentration of pure antibody was quantified by absorbance at 280 nm and by a standard protein assay (2 mg/ml; Harlan Sera Lab Ltd., Leicester, United Kingdom). The purity of the antibody was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the antigenic specificity was determined by LPS ELISA (34).
WCE.
The WCE was performed as described by Abdillahi and Poolman (1) and Plested et al. (33). Wells of Nunc Maxisorp EIA plates were coated with 100 μl of either phenol-killed or ethanol-fixed bacteria (optical density at 620 nm [OD620] of 0.1) for 1 to 3 h at 37°C and then stored at 4°C prior to use. Plates were blocked, washed, and incubated with MAb for 1 h at room temperature. Plates were washed prior to addition of anti-mouse IgG conjugated to alkaline phosphatase for 1 h at room temperature and development with nitrophenyl phosphate substrate (1 mg/ml; Sigma). OD was measured at A405-410 using a microtiter plate reader.
IBs.
Bacterial suspensions of whole-cell lysates were prepared for each N. meningitidis strain as described previously (33) and standardized to 5 × 107 cells/ml. Two microliters of each bacterial suspension was applied to a nitrocellulose filter (Schleicher and Schuell), allowed to air dry, blocked with 1% BSA-PBS, incubated with primary MAb, washed, incubated with anti-mouse IgG alkaline phosphatase, and developed using 5-bromo-4-chloro-3-indoyl phosphate-Nitro Blue Tetrazolium substrate, as described previously for WCE (33).
IF microscopy.
Cultured 16HBE140 epithelial cells were cultured using standard methods (33) and infected with strains of N. meningitidis for 3 h at 37°C. The accessibility of inner core LPS epitopes of live whole-cell N. meningitidis to specific MAbs was determined using immunofluorescence and confocal microscopy as described previously. Briefly, glass coverslips coated with monolayers of 16HBE140 cells were infected with N. meningitidis strains for 3 h at 37°C. Coverslips were washed, blocked in 3% BSA-PBS, incubated with primary antibody (MAb), washed, and then incubated with secondary fluorescein isothiocyanate (FITC). Coverslips were then washed, incubated with a MAb, L4A3, conjugated directly to Rhodamine Red X (RRX; Molecular Probes kit) for 1 h, washed, and then mounted and viewed for immunofluorescence by using appropriate filters (Zeiss microscope with Fluorograbber and Adobe Photoshop). L4A3 recognizes a common outer membrane component conserved in all N. meningitidis strains tested (unpublished data). Coverslips were either fixed after primary antibody (MAb) incubation stage or after addition of secondary FITC. As there was no difference in immunofluorescence comparing these two different stages of fixation, in all subsequent experiments bacteria were fixed after the primary antibody incubation.
OPA.
The opsonophagocytic activity of MAbs with NmB strains was determined using the flow cytometry-based opsonophagocytosis assay (OPA) as described previously (32). Briefly, fluorescently stained (with RRX) ethanol-fixed NmB cells were opsonized with MAb and human complement in a 96-well microtiter plate (Costar) for 10 min at 37°C with shaking at 500 rpm. The human complement source used for the OPA is from a patient with hypogammaglobulemia (IgG, 0.6 g/liter; IgM, 0.2 g/liter; IgA, <0.1 g/liter). Polymorphonuclear leukocytes (PMNs) were prepared as described previously from heparinized donor blood and added to each well (1.25 × 107 cells/ml), and the plates were incubated for a further 10 min (32). The reaction was stopped by addition of 150 μl of PBS-EDTA kept on ice and then added to a tube containing 50 μl of trypan blue. Approximately 10,000 PMNs were collected using flow cytometry (FACScan and Cellquest; Becton Dickinson). The percentage phagocytosis was defined as the percentage of gated cells and refers to the percentage of PMNs that have taken up fluorescently labeled bacteria (32).
SB assay.
N. meningitidis cells were harvested from BHI agar plates following overnight growth and suspended in PBS at approximately 109 CFU/ml. The SB assay was performed as described previously (35). Briefly, bacteria were incubated with the appropriate MAb for 10 min at 37°C prior to 45 min of incubation at 37°C without agitation. The final reaction mixture contained 50 μl of N. meningitidis in buffer (PBS-0.1% glucose) or N. meningitidis with MAb, 50 μl of buffer, and 50 μl of serial dilutions of the complement source (final range from 40 to 0.04% [vol/vol]). Twenty-five microliters of reaction mixture from each well was transferred to BHI agar plates and incubated to determine the number of CFU per milliliter. A sample of bacteria was taken at time zero and plated out to enumerate the CFU per milliliter in the starting inoculum. The percentage survival was calculated by comparing the number of CFU in each sample to that of decomplemented control.
Infant rat model.
Five-day-old outbred Wistar infant rats were used to determine the ability of MAbs L2-16 and LPT3-1 to protect against challenge with infant rat-passaged N. meningitidis strains when given simultaneously with antibody, as described previously (35). Strains 35E (C:20:P1.1), 89I (C:NT:P1.16), MC58 (B:15:P1.7,16b), MC58 lpt3, MC58 lpt3 galE, 8047 (B:2b:P1.5,2), 8047 lpt3, 2996 (B:2b:P1.5-1,2-2), and 2996 lpt3 were passaged three times in the infant rat model prior to use in the passive protection model. Blood samples were taken at 6 and 18 h postchallenge to determine the number of CFU per milliliter relative to negative controls given PBS-BSA and positive controls given antibodies that were expected to protect in vivo. These included murine anticapsular serogroup C MAb from the National Institute for Biological Standards and Control (NIBSC); human serum containing anticapsular serogroup C antibodies at a concentration of 12 to 15 μg/ml; and murine anti-porin P1.1, P1.16, and P1.7 MAbs from NIBSC.
Molecular modeling.
Molecular modeling of LPS epitopes was carried out as described previously (33, 35).
RESULTS
The aim of this study was to make MAbs to N. meningitidis strains in which the LPS lacks the inner core epitopes recognized by a MAb, L3B5. Our previous studies had shown that N. meningitidis strains of the LPS immunotypes L2, L4, and L5 were nonreactive with L3B5. Thus, BALB/c mice were immunized with formalin-killed galE mutants of N. meningitidis strains 35E (L2) and 89I (L4) (Fig. 1) in which PEtn is located at position 6 of HepII. In addition, we used a double mutant (lpt3 galE) of MC58 to immunize mice and raise MAbs to an inner core structure lacking PEtn at both positions 3 and 6 of HepII.
Preparation and selection of MAbs.
Following immunization with the galE mutant of 89I (L4), nine stable hybridomas were identified from one fusion. The hybridomas were tested for reactivity by LPS ELISA with purified galE mutant LPS of immunotypes L4, L2, and L3. One of these MAbs, L4A4 (IgG2a), reacted with glycoforms of strain 35E (L2) and 89I (L4). In a second series of immunizations, mice were given a mixture of the galE mutants of strains 35E (L2) and 89I (L4), but this time the screening LPS antigens were fully extended LPS of strains L2, L4, and L5 (M981). Two fusions were carried out, the first one resulting in 10 hybridomas. Two of these were selected for more detailed characterization: L4-7 (IgG2a) reacted with fully extended L4 LPS, and L5-10 (IgG1) reacted with L5 LPS. In a second fusion, resulting in 13 hybridomas, an IgG2b MAb (L2-16) was identified that recognized the fully extended (wild-type) LPS glycoforms from both L4 and L2 immunotypes. Finally, we sought to produce MAbs that would react with inner core structures that did not require the presence of PEtn at either the 3 or 6 position of HepII. To this end, we immunized mice with the double mutant MC58 lpt3 galE strain, a strain known to be deficient in any addition of PEtn to HepII (24). Three hybridomas producing MAbs that did not require the presence of PEtn at HepII were established as a result of three fusions. The most reactive of these, LPT3-1, an IgG2a producer, was chosen for further testing.
Characterization of MAbs by LPS ELISA.
ELISA results for the newly established MAbs, together with our previously published results for L3B5 (IgG3), are summarized in Fig. 2. To determine the importance of O-acyl groups and of PEtn at HepII as determinants in the reactivity of the six MAbs, galE LPS obtained from strains of L2, L3, and L4 immunotypes was treated with hydrazine and HF to remove O-acyl and PEtn groups, respectively.
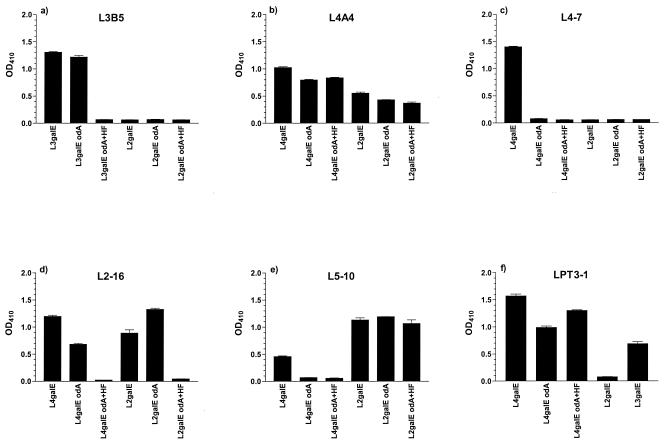
FIG. 2.
ELISA (OD410) of six inner core LPS MAbs against a series of galE, galE odA, and galE odA and HF LPS from N. meningitidis strains (see Materials and Methods). (a) L3B5 against LPS from L3 (MC58) and L2 (35E) N. meningitidis strains; (b) L4A4; (c) L4-7; (d) L2-16; and (e) L5-10 against LPS from L4 (89I) and L2 (35E) N. meningitidis strains; (f) LPT3-1 against LPS from L4 (89I), L2 (35E), and L3 (MC58) N. meningitidis strains.
The ELISA reactivity of the MAbs against odA and HF LPS indicated that L3B5 binds an inner core epitope that requires PEtn at the O-3 position of HepII regardless of the presence or absence of O-acyl groups (Fig. 2a).
L4A4 bound an inner core epitope in which PEtn is not present. This MAb recognized the epitope in both the L2 and L4 inner core environment (Fig. 2b) but not when PEtn is at the O-3 position of HepII (data not shown).
A lack of reactivity with odA L4 indicated that L4-7 requires O-acyl groups for presentation of the epitope. The binding to L4 galE was completely lost after O-deacylation of the LPS, and there was no binding to L2 galE (Fig. 2c).
L2-16 demonstrated a complete loss of binding with both L2 galE and L4 galE once the PEtn had been chemically removed, indicating a requirement for PEtn at the O-6 position of HepII (Fig. 2d). This inner core epitope is also presented in fully extended LPS (data not shown).
N. meningitidis epitopes recognized by L5-10 are presented in the L2 inner core environment but appear to require O-acyl groups for appropriate presentation when HepII does not have a Glc present at the O-3 position, as seen in L4 galE (Fig. 2e). Thus, L5-10 weakly recognized L4 galE, but this recognition was lost on O-deacylation of the LPS.
LPT3-1 binding (Fig. 2f) to L4 galE was not affected by loss of O-acyl groups or the presence or absence of PEtn at position 6 of HepII, but the results with L2 galE demonstrated that di-substitution with both PEtn at position 6 and Glc at position 3 of HepII inhibits binding. Electrospray-MS analysis of L2 galE showed two major glycoforms, one with Glc at position 3 and PEtn at position 6 of HepII (60%) and the other glycoform without PEtn at position 6 (40%) (data not shown). This mixed population of LPS containing 40% of glycoforms having no PEtn present at position 6 of HepII did not react, suggesting that Glc at position 3 of HepII alone is sufficient to preclude binding of LPT3-1. Corroborating evidence was obtained from LPS ELISA on immunotype L5 LPS which has only mono-substitution of Glc at position 3 of HepII (27) and was also nonreactive (Tables 3 and 4). PEtn at position 3 of HepII as in L3 galE is recognized. Thus, LPT3-1 can recognize epitopes in which HepII is unsubstituted or mono-substituted with either PEtn at position 6 or 3, but recognition is lost with Glc at position 3 and with di-substitution at positions 3 and 6 of HepII.
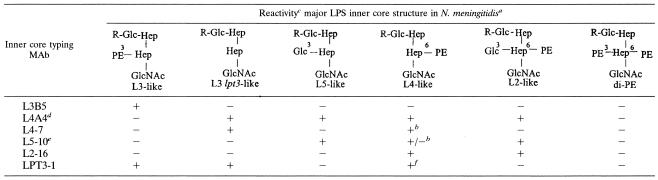
TABLE 3.
MAb reactivities to LPS inner core N. meningitidis structures
PE, PEtn in structural models.
O-Acyl groups important.
Symbols: +, reactive; −, not reactive; +/−, reactivity just above background.
R = H only.
R = Glc or R = H only.
R = H.
TABLE 4.
WCE to N. meningitidis strains using six inner core LPS MAbs
| MAb | OD410a of whole cells of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| MC58 | Mc58 galE | MC58 lpt3 | MC58 lpt3 galE | L4 | L4 galE | L2 | L2 galE | |
| L3B5 | + | + | − | − | − | − | − | − |
| L4A4 | − | − | − | +++ | − | ++ | − | ++ |
| L4-7 | − | − | − | +++ | + | +++ | − | − |
| L5-10 | − | − | − | − | − | ++ | − | +++ |
| L2-16 | − | − | − | − | − | ++ | +++ | +++ |
| LPT3-1 | + | + | ++++ | ++++ | +/− | ++++ | − | − |
Symbols: ++++, >2.0; +++, >1.0; ++, >0.5; +, >0.2; +/−, >0.1.
Novel inner core LPS glycoforms.
During this work a novel variant inner core LPS structure was discovered in strain 2220Y that has PEtn at both positions 3 and 6 of HepII (4). The strain was not recognized by any of the six MAbs (data not shown). Thus, at present there appear to be six different inner core LPS structures in NmB showing differences in substitution patterns at HepII (Table 3). These are as follows: (i) immunotype L3-like with PEtn at position 3 of HepII; (ii) L3 lpt3-like with no extension from HepII; (iii) L5-like with Glc at position 3 of HepII; (iv) L4-like with PEtn at position 6 of HepII; (v) L2-like with both Glc at position 3 and PEtn at position 6 of HepII; and (vi) the novel variant with PEtn at both position 3 and position 6 of HepII.
Reactivity of MAbs to whole cells of N. meningitidis strains.
To further characterize these inner core LPS structures, whole-cell lysates were prepared from various N. meningitidis strains and immunoblotting analysis performed as described previously (33) using the subset of inner core LPS MAbs (L3B5, L2-16, LPT3-1) and immunotyping MAbs (37). A summary of MAb cross-reactivity and immunotyping of 3 collections of N. meningitidis strains described in Table 2 is shown in Fig. 3.
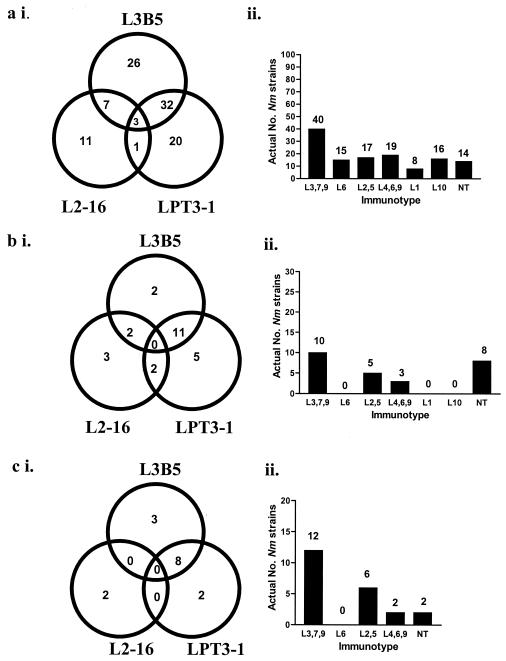
FIG. 3.
Venn diagrams showing (i) the cross-reactivity of three inner core LPS MAbs (L3B5, L2-16, and LPT3-1) and (ii) LPS immunotypes using immunotyping MAbs, in three collections of N. meningitidis strains (see Materials and Methods). (a) Global strain collection (n = 100); (b) United Kingdom strain collection (n = 26); (c) carriage isolates from Czech Republic (n = 17). The numbers in the Venn diagrams indicate the actual numbers of strains reacting to each specific MAb by immunoblot.
Global collection of hypervirulent N. meningitidis strains.
All 100 N. meningitidis hypervirulent strains of all major serogroups in the global strain collection (Table 2) were recognized using the three MAbs (L3B5, L2-16, and LPT3-1) compared to 14 that were nontypeable using conventional immunotyping MAbs (Fig. 3a, panels i and ii). Generally there was a good correlation between inner core LPS MAb reactivity and immunotype. Three out of 100 N. meningitidis strains cross-reacted with all three MAbs. One strain, 1000, did not react with MAbs L3B5 or L2-16, but did react with LPT3-1. Structural analysis of 1000 LPS revealed a novel structure for meningococcal LPS where the core oligosaccharide contained a major glycoform with an extra glucose at position 2 of HepI (3). The other three MAbs (L5-10, L4-7, and L4A4) bound to 16, 56, and 100 of the 100 N. meningitidis isolates, respectively.
United Kingdom collection of virulent N. meningitidis strains.
Of the 26 N. meningitidis virulent strains from the United Kingdom strain collection (Table 2), all except one strain (NM93) were reactive with the three MAbs and none of the strains reacted with all three MAbs (Fig. 3b, panels i and ii). Generally there was a good correlation between inner core LPS MAb reactivity with immunotype determined using conventional MAbs and 8 of the strains were nontypeable. Strain NM115 (serogroup B) was not reactive with either L3B5 or L2-16 (but did react very weakly with LPT3-1). Structural analysis of LPS from this strain revealed glycoforms in which HepI is further substituted at position 2 with glucose (3). A second strain, NM93 (serogroup Y), was not reactive with any of the three MAbs and the LPS structure is currently under investigation.
Czech collection of N. meningitidis carriage strains.
Of the 17 isolates studied in the Czech N. meningitidis strain collection (Table 2) all except two strains (425/93 and 30/93) reacted with the inner core LPS MAbs, and 2 of the 17 strains were nontypeable using immunotyping MAbs (Fig. 3c, panels i and ii). Structural analysis revealed strain 425/93 (B:NT:NST ST-144; virulent prediction) to have the same previously unidentified structure as strain NM115 (3), and strain 30/93 (PA:NT:P1.5, 10: L2,5 L3,7,9: nonvirulent prediction) is currently under investigation.
Collection of N. meningitidis LPS mutants.
Wild-type and galE mutants of L2, L3, L4, and MC58 lpt3 were tested using inner core LPS MAbs for accessibility by WCE (Tables 4 and 5). This shows that the majority of these inner core LPS MAbs have a preference for epitopes in a truncated LPS environment. With the exception of L4A4 and L5-10, the MAbs can recognize inner core LPS in wild-type strains, although it cannot be definitively ruled out that this recognition may be due to the presence of a minority of truncated glycoforms in the wild-type population.
TABLE 5.
Comparison of IF, WCE, and IB to determine accessibility of three inner core LPS MAbs (L2-16, LPT3-1, L3B5) to N. meningitidis strains and mutants
| MAb | Strain (immunotype) | Genotype | Result ofa
|
||
|---|---|---|---|---|---|
| IF | WCE (OD410) | IB | |||
| L2-16 | 35E (L2) | Wild type | + | 1.1 | +++ |
| galE | + | 0.98 | +++ | ||
| 89I (L4) | Wild type | − | 0.13 | − | |
| galE | + | 0.63 | +++ | ||
| LPT 3-1 | MC58 (L3) | Wild type | + | 0 | + |
| galE | + | 0.20 | + | ||
| lpt3 | + | 1.51 | +++ | ||
| lpt3 galE | + | 2.01 | +++ | ||
| L3B5 | MC58 (L3) | Wild type | + | 0.04 | + |
| galE | + | 0.58 | +++ | ||
Symbols: +, positive; +++, strongly positive; −, negative.
Accessibility studies using live whole cells of N. meningitidis.
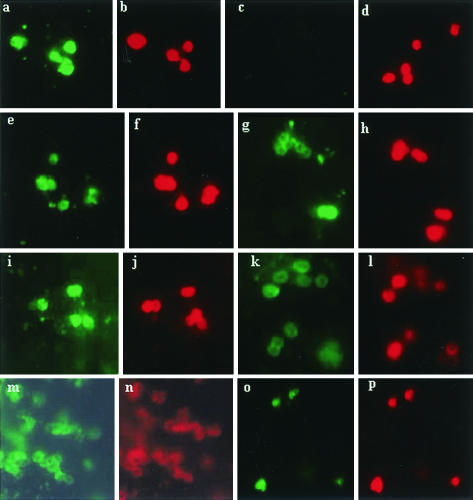
IF microscopy with live N. meningitidis adherent to monolayers of 16HBE140 epithelial cells was used to determine the binding of MAbs L2-16, LPT3-1, and L3B5 to live whole cells from N. meningitidis wild-type and galE mutants of L2 (35E), L4 (89I), MC58 lpt3 and MC58 (L3) (Fig. 4a to p). This demonstrated that L2-16 binds to L2 wild type, L2 galE, and L4 galE but not L4 wild-type whole cells (Fig. 4a, c, e, and g). LPT3-1 binds to MC58 lpt3 galE whole cells and shows very low binding to MC58 lpt3 wild-type cells (Fig. 4k and i). L3B5 prefers to bind to MC58 (L3) galE whole cells with very low binding to MC58 (L3) wild-type cells (Fig. 4m and o). A strong correlation was obtained with MAbs L3B5, L2-16, and LPT3-1 using the three different accessibility assays, IF, WCE, and IBs (Table 5).
FIG. 4.
IF of live N. meningitidis strains on monolayers of 16HBE140 cells probed with inner core LPS MAbs (see Materials and Methods), either MAbs L2-16 (a, c, e, and g) or LPT3-1 (i and k) or L3B5 (m and o) and detected using anti-mouse IgG-FITC (green), followed by direct staining using a MAb, L4A3-RRX conjugate (red), respectively (b, d, f, h, j, l, n, and p) (see Materials and Methods). Consecutive pairs of letters—e.g., a and b, c and d, and so on—indicate the same field of view observed for green or red fluorescence. L4A3 recognizes a common outer membrane component conserved in all N. meningitidis strains tested. N. meningitidis strains were as follows: 35E (L2) wild type (a and b), 89I (L4) wild-type (c and d), 35E (L2) galE mutant (e and f), 89I (L4) galE mutant (g and h), MC58 (L3) lpt3 (i and j), MC58 (L3) lpt3 galE mutant (k and l), MC58 (L3) galE (m and n), and MC58 (L3) wild type (o and p).
Functional assays in vitro and in vivo using MAbs.
The inner core LPS MAbs were studied using the following functional assays: an OPA, an SB assay, and an infant rat model of infection, as described previously (34, 35).
(i) OPA.
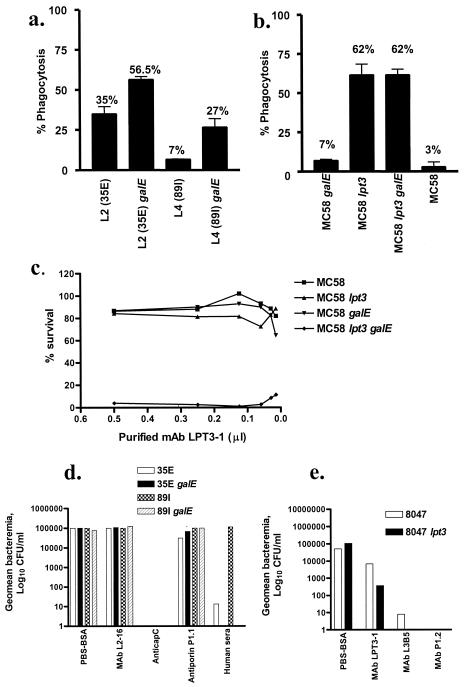
OPA was performed using ethanol-fixed fluorescently labeled whole bacteria with human donor PMNs and human complement. Purified L2-16 demonstrated opsonic activity against L2 (35E) wild type, L2 (35E) galE, and L4 (89I) galE but not L4 (89I) wild-type strain (Fig. 5a). MAbs L4-7, L5-10, and L4A4 had very low opsonic activity against L2 wild type (5.1, 4.0, and 3.4%, respectively), against L2 galE mutant (7.2, 6.0, 11.1%, respectively), and against L4 wild type (10.6, 3.2, and 3.7%, respectively) (data not shown). MAbs L4-7 and L4A4 had some opsonic activity against L4 galE mutant (15.9 and 23.5%, respectively), whereas L5-10 had none (0%). Purified LPT3-1 was opsonic against MC58 lpt3 galE and MC58 lpt3 wild type, but not against the MC58 galE mutant or MC58 wild type as expected (Fig. 5b).
FIG. 5.
Functional assays using MAbs L2-16 and LPT3-1. (a) OPA with ethanol-fixed N. meningitidis (see Materials and Methods) showing opsonic activity (percent phagocytosis) of L2-16 against 35E (L2) wild type, 35E (L2) galE, and 89I (L4) galE, but not against 89I (L4) wild type. Complement-only controls for L2 (35E), L2 (35E) galE, L4 (89I), and L4 (89I) galE mutants were 3.2, 4.5, 3.4, and 12.2%, respectively. (b) OPA with ethanol-fixed N. meningitidis showing opsonic activity (percent phagocytosis) of LPT3-1 against MC58 lpt3 and MC58 lpt3 galE but not against MC58 galE and MC58 wild type. Complement-only controls for MC58 galE, MC58 lpt3, MC58 lpt3 galE, and MC58 were 16.2, 17.8, 20.3, and 14.6%, respectively. (c) SB assay using N. meningitidis, LPT3-1 and a fixed amount of human complement (see Materials and Methods) showing a dose-response effect on bactericidal activity of LPT3-1 against MC58 lpt3 galE but not against MC58, MC58 lpt3, and MC58 galE. (d) Passive protection studies with L2-16 using a 5-day-old infant rat model (see Materials and Methods): showing the geometric mean bacteremia (log10 CFU/ml) in blood samples taken 18 h after simultaneous treatment with L2-16 (20 μg/rat) and challenge with N. meningitidis strains 35E (L2), 35E (L2) galE, 89I (L4), or 89I (L4) galE. Positive control MAbs include anticapsular serogroup C mouse MAb (NIBSC) (undiluted), anti-porin P1.1 mouse MAb (NIBSC) (1:1,000 dilution in PBS-BSA), and human serum (individual with high-titer anticapsular C antibody; equivalent, 0.2 μg/rat). Negative controls were given PBS-BSA. (e) Passive protection studies with LPT3-1 using 5-day-old infant rat model (see Materials and Methods) showing geometric mean bacteremia (log10 CFU/ml) in blood samples taken 18 h after simultaneous treatment with LPT3-1 (20 μg/rat) and challenge with N. meningitidis strain 8047 or 8047 lpt3. Positive controls included L3B5 (20 μg/rat) and anti-porin P1.2 (NIBSC) (1:1,000 dilution in PBS-BSA). Negative controls were given PBS-BSA.
(ii) SB assay.
SB activity was observed, using purified LPT3-1 and human complement source, against only the MC58 lpt3 galE mutant, not against the MC58 lpt3 or MC58 galE mutants or the MC58 wild-type strain (Fig. 5c). No SB activity was observed with L2-16 against wild type or galE mutants of L2 (35E) or L4 (89I) strains (data not shown).
(iii) Infant rat model.
An infant rat model was used to determine the ability of MAbs (i) L2-16 and (ii) LPT3-1 to passively protect against i.p. challenge with infant rat-passaged strains, either (i) 35E (L2) wild type, 89I (L4) wild type and their galE mutants with L2-16 or (ii) MC58, MC58 lpt3, and their galE mutants, 8047, 8047 lpt3, 2996, and lpt3 with LPT3-1. There was no protection observed with L2-16 (20 μg/rat) at 6 h or 18 h postchallenge following simultaneous injection of bacteria and MAb with any of the N. meningitidis strains tested (Fig. 5d). Complete protection was achieved with anticapsular serogroup C MAb (undiluted) against all N. meningitidis serogroup C strains tested and human sera protected against 35E but not 89I (Fig. 5d). With LPT3-1 (20 μg/rat), although there was a slight reduction in bacteremia at 6 and 18 h against the double mutant MC58 lpt3 galE, there was not a consistently high level of bacteremia in the control PBS-BSA group with this strain (data not shown). Therefore, this experiment was repeated with strains 8047, 8047 lpt3, 2996, and 2996 lpt3 previously shown to produce high bacteremia in PBS-BSA controls. These results demonstrated a 2-log reduction in bacteremia at 18 h with LPT3-1 (20 μg/rat) compared to PBS-BSA controls against 8047 lpt3 mutant (Fig. 5e). This was consistent with the bactericidal activity observed against MC58 lpt3 galE (Fig. 5c). L3B5 (20 μg/rat) and anti-porin P1.2 (1:1,000 dilution) protected against 8047 and 8047 lpt3 (Fig. 5e). No protection was observed with LPT3-1 against 2996 or 2996 lpt3 (data not shown).
Hence, the results of the functional assays show that L2-16, despite binding to whole cells and having opsonic activity, is not bactericidal and not able to protect in vivo using an infant rat model of infection. In comparison, LPT3-1, which preferentially binds to truncated galE mutants rather than wild type, is bactericidal and may partially protect in vivo against MC58 lpt3 galE.
DISCUSSION
This work describes different inner core LPS structures of NmB reflecting variations in substitution of HepII (Table 3). During the course of this study, six MAbs specific to the inner core LPS of N. meningitidis were obtained which exhibit specific recognition patterns corresponding to these distinct inner core structures. Of these MAbs, two (L2-16, LPT3-1) were studied in greater detail and extend our previous studies with L3B5 (32-35).
All N. meningitidis strains tested from a genetically diverse collection of invasive isolates representing all major serogroups (global strain collection) were recognized using one or more of a panel of three inner LPS MAbs (L3B5, L2-16, and LPT3-1). There are a few exceptions, including strain NM93 from a set of 26 United Kingdom invasive isolates and two strains from a collection of Czech carriage isolates (425/93 and 30/93). Nonetheless, this set of inner core LPS MAbs provides a robust tool for immunotyping the inner core LPS of N. meningitidis strains and can be used to identify candidate epitopes of relevance to the development of a vaccine against hypervirulent N. meningitidis strains. In assessing the binding of these MAbs to N. meningitidis strains, we have established a correlation between WCE, IBs, and surface accessibility using IF microscopy. We conclude that WCE and/or IBs using fixed N. meningitidis strains (whole cells or whole-cell lysates) correlate strongly with surface accessibility of inner core LPS epitopes of fully encapsulated live N. meningitidis bacteria.
Using MAbs L2-16 and LPT3-1, SB, OPAs, and an infant rat model of passive protection have demonstrated that the ability to bind to the surface of whole cells did not necessarily correlate with functional in vitro activity or in vivo. L2-16 (IgG2b) was not bactericidal and not able to protect in vivo despite binding to and having opsonic activity against L2 (35E) galE, L2 (35E) wild-type, and L4 (89I) galE N. meningitidis strains. In addition, L2-16 failed to have any bactericidal activity against a number of L2 immunotype strains, representing both serogroups B and C that bound L2-16 strongly (unpublished data). In comparison, LPT3-1 (IgG2a) was opsonic and bactericidal against the double mutant MC58 lpt3 galE but not the wild-type MC58 lpt3 strain. In an infant rat model of infection, LPT3-1 reduced bacteremia against a challenge infection with the 8047 lpt3 mutant compared to untreated controls (Fig. 5e).
Previous studies with L3B5 (IgG3) have demonstrated bactericidal activity and passive protection in vivo against N. meningitidis strains with mainly truncated glycoforms and opsonic activity against N. meningitidis expressing wild-type and truncated glycoforms (35).
The reasons for the differences in functional activity in vitro and in vivo between these MAbs may be due to the membrane attack complex stability, inability to bind due to low-affinity antibodies, or accessibility problems due to steric hindrance in extended LPS structure. Studies are currently under way to determine the binding of complement components of the classical pathway (C4, C5b-9) to determine at what stage the pathway may be deficient in mediating bactericidal activity. Opsonic activity may be present without SB activity in vitro, and it is not known whether opsonic activity may correlate with protection in the infant rat model (present work). There are examples historically where MAbs raised to Neisseria gonorrhoeae were nonbactericidal against some strains (serum resistant) and bactericidal against others (serum sensitive). The differences in bactericidal activity were shown to be due to the molecular configuration of C5b-9 bound to distinctive outer membrane proteins on the surface of the bacteria (17, 18).
Using a Metropolis Monte Carlo approach, three-dimensional space-filling molecular models of galE LPS structures were constructed to compare the seven common inner core LPS immunotypes in an attempt to understand the interactions of inner core epitopes for MAbs L3B5, L2-16, and LPT3-1 (Fig. 1). The models show the relative accessibility of PEtn and N-acetylglucosamine (GlcNAc) with or without glucose in the different glycoforms. In L3 galE, the PEtn is situated at position 3 of HepII, and this group together with the Glc and GlcNAc form the key groups that are necessary for optimal epitope required for L3B5 binding (33) (Fig. 1c). By comparison the PEtn is situated at position 6 in L2 galE (Fig. 1b), where it appears less predominant than the GlcNAc and Glc. In L4 galE (Fig. 1d), where there is no Glc present at position 3, the PEtn at position 6 seems more exposed (than L2 galE), which is consistent with the absolute requirement for this group in L2-16 binding. In L5 galE, the Glc and GlcNAc substituent at HepII are the most prominent features when PEtn is absent from the inner core (Fig. 1e). In the galE mutant of MC58 lpt3, where PEtn and Glc are absent from HepII, the GlcNAc moiety and the Glc at HepI are the most prominent structural features, with the heptose backbone being relatively exposed (Fig. 1f). In the glycoform with two PEtn, e.g., 2220Y galE, (Fig. 1h), both PEtn groups are prominent and would cooperatively mask epitopes recognized by MAbs L3B5, L2-16, and LPT3-1. In the novel glycoforms with an additional Glc at HepI (e.g., in 1000 galE), GlcNAc is the most predominant group and the Hep backbone is less exposed due to the two Glc at HepI (Fig. 1g).
In summary, this work describes and characterizes a panel of inner core LPS MAbs for immunotyping the inner core LPS of N. meningitidis strains. These MAbs have been used to identify novel LPS structures and offer a robust tool for further work on the development of an LPS-based vaccine against NmB strains.
Acknowledgments
This work was supported by Chiron Vaccines S.p.a., Medical Research Council, and National Meningitis Trust, Stroud, Gloucestershire, United Kingdom.
We thank Adele Martin for isolation and purification of LPS.
Editor: J. N. Weiser
REFERENCES
- 1.Abdillahi, H., and J. T. Poolman. 1988. Typing of group-B Neisseria meningitidis with monoclonal antibodies in the whole-cell ELISA. J. Med. Microbiol. 26:177-180. [PubMed] [Google Scholar]
- 2.Banerjee, A., R. Wang, S. N. Uljon, P. A. Rice, E. C. Gotschlich, and D. C. Stein. 1998. Identification of the gene (lgtG) encoding the lipooligosaccharide beta chain synthesizing glucosyl transferase from Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. USA 95:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox, A. D., J. C. Wright, M. A. J. Gidney, S. Lacelle, J. S. Plested, A. Martin, E. R. Moxon, and J. C. Richards. 2003. Identification of a novel inner core oligosaccharide structure in Neisseria meningitidis lipopolysaccharide. Eur. J. Biochem. 270:1759-1766. [DOI] [PubMed] [Google Scholar]
- 4.Cox, A. D., J. Li, J. R. Brisson, E. R. Moxon, and J. C. Richards. 2002. Structural analysis of the lipopolysaccharide from Neisseria meningitidis strain BZ157 galE: localisation of two phosphoethanolamine residues in the inner core oligosaccharide. Carbohydr. Res. 337:1435-1444. [DOI] [PubMed] [Google Scholar]
- 5.Di Fabio, J. L., F. Michon, J. R. Brisson, and H. J. Jennings. 1990. Structure of L1 and L6 core oligosaccharide epitopes of Neisseria meningitidis. Can. J. Chem. 68:1029-1034. [Google Scholar]
- 6.Finne, J., M. Leinonen, and P. H. Makela. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development. Lancet ii:355-357. [DOI] [PubMed] [Google Scholar]
- 7.Frasch, C. E., L. van Alphen, J. Holst, J. T. Poolman, and E. Rosenqvist. 2001. Outer membrane protein vesicle vaccines for meningococcal disease, p. 81-107. In A. J. Pollard and M. C. J. Maiden (ed.), Meningococcal vaccines. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 8.Gamian, A., M. Beurret, F. Michon, J. R. Brisson, and H. J. Jennings. 1992. Structure of L2 lipopolysaccharide core oligosaccharides of Neisseria meningitidis. J. Biol. Chem. 267:922-925. [PubMed] [Google Scholar]
- 9.Gotschlich, E. C. 1994. Genetic locus for the biosynthesis of the variable portion of Neisseria gonorrhoeae lipooligosaccharide. J. Exp. Med. 180:2181-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison, O. B., B. D. Robertson, S. N. Faust, M. A. Jepson, R. D. Goldin, M. Levin, and R. S. Heyderman. 2002. Analysis of pathogen-host cell interactions in purpura fulminans: expression of capsule, type IV pili, and PorA by Neisseria meningitidis in vivo. Infect. Immun. 70:5193-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holten, E. 1979. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J. Clin. Microbiol. 9:186-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jennings, H. J., K. G. Johnson, and L. Kenne. 1983. The structure of an R-type oligosaccharide core obtained from some lipopolysaccharides of Neisseria meningitidis. Carbohydr. Res. 121:233-241. [DOI] [PubMed] [Google Scholar]
- 13.Jennings, M. P., D. W. Hood, I. R. A. Peak, M. Virji, and E. R. Moxon. 1995. Molecular analysis of a locus for the biosynthesis and phase-variable expression of the lacto-N-neotetraose terminal lipopolysaccharide structure in Neisseria meningitidis. Mol. Microbiol. 18:729-740. [DOI] [PubMed] [Google Scholar]
- 14.Jennings, M. P., M. Bisercic, K. L. Dunn, M. Virji, A. Martin, K. E. Wilks, J. C. Richards, and E. R. Moxon. 1995. Cloning and molecular analysis of the lsi1 (rfaF) gene of Neisseria meningitidis which encodes a heptosyl-2-transferase involved in LPS biosynthesis: evaluation of surface exposed carbohydrates in LPS mediated toxicity for human endothelial cells. Microb. Pathog. 19:391-407. [DOI] [PubMed] [Google Scholar]
- 15.Jennings, M. P., P. van der Ley, K. E. Wilks, D. J. Maskell, J. T. Poolman, and E. R Moxon. 1993. Cloning and molecular analysis of the galE gene of Neisseria meningitidis and its role in lipopolysaccharide biosynthesis. Mol. Microbiol. 10:361-369. [PubMed] [Google Scholar]
- 16.Jennings, M. P., Y. N. Srikhanta, E. R. Moxon, M. Kramer, J. T. Poolman, B. Kuipers, and P. van der Ley. 1999. The genetic basis of the phase variation repertoire of lipopolysaccharide immunotypes in Neisseria meningitidis. Microbiology 145:3013-3021. [DOI] [PubMed] [Google Scholar]
- 17.Joiner, K. A., K. A. Warren, C. Hammer, and M. M. Frank. 1985. Bactericidal but not non-bactericidal C5b-9 is associated with distinctive outer membrane proteins in Neisseria gonorrhoeae. J. Immunol. 134:1920-1925. [PubMed] [Google Scholar]
- 18.Joiner, K. A., K. A. Warren, E. J. Brown, J. Swanson, and M. M. Frank. 1983. Studies on the mechanism of bactericidal resistance to complement-mediated killing. IV. C5b-9 forms high molecular weight complexes with bacterial outer membrane constituents on serum-resistant but not on serum-sensitive Neisseria gonorrhoeae. J. Immunol. 131:1443-1451. [PubMed] [Google Scholar]
- 19.Jolley, K. A., J. Kalmosova, E. J. Feil, S. Gupta, M. Musilek, P. Kriz, and M. C. Maiden. 2000. Carried meningococci in the Czech Republic: a diverse recombining population. J. Clin. Microbiol. 38:4492-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahler, C. M., and D. S. Stephens. 1998. Genetic basis for biosynthesis, structure and function of meningococcal lipooligosaccharide (endotoxin). Crit. Rev. Microbiol. 24:281-334. [DOI] [PubMed] [Google Scholar]
- 21.Kahler, C. M., R. W. Carlson, M. M. Rahman, L. E. Martin, and D. S. Stephens. 1996. Inner core biosynthesis of lipooligosaccharide (LOS) in Neisseria meningitidis serogroup B: identification and role in LOS assembly of the α1,2 N-acetylglucosamine transferase (RfaK). J. Bacteriol. 178:1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahler, C. M., R. W. Carlson, M. M. Rahman, L. E. Martin, and D. S. Stephens. 1996. Two glycosyltransferase genes, lgtF and rfaK, constitute the lipooligosaccharide ice (inner core extension) biosynthesis operon of Neisseria meningitidis. J. Bacteriol. 178:6677-6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kogan, G., D. Uhrin, J. R. Brisson, and H. J. Jennings. 1997. Structural basis of the Neisseria meningitidis immunotypes including L4 and L7 immunotypes. Carbohydr. Res. 298:191-199. [DOI] [PubMed] [Google Scholar]
- 24.Mackinnon, F. G., A. D. Cox, J. S. Plested, C. M. Tang, K. Makepeace, P. A. Coull, J. C. Wright, R. Chalmers, D. W. Hood, J. C. Richards, and E. R. Moxon. 2002. Identification of a gene (lpt-3) required for the addition of phosphoethanolamine to the lipopolysaccharide inner core of Neisseria meningitidis and its role in mediating susceptibility to bactericidal killing and opsonophagocytosis. Mol. Microbiol. 43:931-943. [DOI] [PubMed] [Google Scholar]
- 25.Maiden, M. C. J., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. S. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masignani, V., M. Comanducci, M. M. Giulani, S. Bambini, J. Adu-Bobie, B. Arico, B. Brunelli, A. Pieri, L. Santini, S. Savino, D. Serruto, D. Litt, S. Kroll, J. A. Welsch, D. M. Granoff, R. Rappuoli, and M. Pizza. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197:789-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michon, F., M. Beurret, A. Gamian, J. R. Brisson, and H. J. Jennings. 1990. Structure of the L5 lipopolysaccharide core oligosaccharides of Neisseria meningitidis. J. Biol. Chem. 265:7243-7247. [PubMed] [Google Scholar]
- 28.Moe, F. R., P. Zuno-Mitchell, S. N. Hammond, and D. M. Granoff. 2002. Sequential immunization with vesicles prepared from heterologous Neisseria meningitidis strains elicits broadly protective serum antibodies. Infect. Immun. 70:6021-6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliver, K. J., K. M. Reddin, P. Bracegirdle, M. J. Hudson, R. Borrow, I. M. Feavers, A. Robinson, K. Cartwright, and A. R. Gorringe. 2002. Neisseria lactamica protects against experimental meningococcal infection. Infect. Immun. 70:3621-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavliak, V., J. R. Brisson, F. Michon, D. Uhrin, and H. J. Jennings. 1993. Structure of the sialylated L3 lipopolysaccharide of Neisseria meningitidis. J. Biol. Chem. 268:14146-14152. [PubMed] [Google Scholar]
- 31.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Arico, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816-1820. [DOI] [PubMed] [Google Scholar]
- 32.Plested, J. S., B. L. Ferry, P. A. Coull, K. Makepeace, A. K. Lehmann, F. G. MacKinnon, H. G. Griffiths, M. A. Herbert, J. C. Richards, and E. R. Moxon. 2001. Functional opsonic activity of human serum antibodies to inner core lipopolysaccharide (galE) of serogroup B meningococci measured by flow cytometry. Infect. Immun. 69:3203-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plested, J. S., K. Makepeace, M. P. Jennings, M. A. J. Gidney, S. Lacelle, J. R. Brisson, A. D. Cox, A. Martin, A. G. Bird, C. M. Tang, F. M. Mackinnon, J. C. Richards, and E. R. Moxon. 1999. Conservation and accessibility of an inner core lipopolysaccharide epitope of Neisseria meningitidis. Infect. Immun. 67:5417-5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plested, J. S., M. A. J. Gidney, P. A. Coull, M. A. Herbert, A. G. Bird, J. C. Richards, and E. R. Moxon. 2000. Enzyme linked immunosorbent assay (ELISA) for the detection of serum antibodies to the inner core lipopolysaccharide of Neisseria meningitidis group B. J. Immunol. Methods 237:73-84. [DOI] [PubMed] [Google Scholar]
- 35.Plested, J. S., S. L. Harris, J. C. Wright, P. A. Coull, K. Makepeace, M. A. J. Gidney, J. R. Brisson, J. C. Richards, D. M. Granoff, and E. R. Moxon. 2003. Highly conserved Neisseria meningitidis inner-core lipopolysaccharide epitope confers protection against experimental meningococcal bacteremia. J. Infect. Dis. 187:1223-1234. [DOI] [PubMed] [Google Scholar]
- 36.Pollard, A. P., and E. R. Moxon. 2002. The meningococcus tamed? Arch. Dis. Child. 87:13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scholten, R. J., B. Kuipers, H. A. Valkenburg, J. Dankert, W. D. Zollinger, and J. T. Poolman. 1994. Lipo-oligosaccharide immunotyping of Neisseria meningitidis by whole-cell ELISA with monoclonal antibodies. J. Med. Microbiol. 41:236-243. [DOI] [PubMed] [Google Scholar]
- 38.Seiler, A., R. Reinhardt, J. Sakart, D. A. Caugant, and M. Achtman. 1996. Allelic polymorphisms and site-specific recombination in the opc locus of Neisseria meningitidis. Mol. Microbiol. 19:841-856. [DOI] [PubMed] [Google Scholar]
- 39.Stojiljkovic, I., V. Hwa, J. Larson, L. Lin, M. So, and X. Nassif. 1997. Cloning and characterisation of the Neisseria meningitidis rfaC gene encoding alpha-1,5 heptosyltransferase I. FEMS Microbiol. Lett. 151:41-49. [DOI] [PubMed] [Google Scholar]
- 40.Sun, Y. H., S. Bakshi, R. Chalmers, and C. M. Tang. 2000. Functional genomics of Neisseria meningitidis pathogenesis. Nat. Med. 6:1269-1273. [DOI] [PubMed] [Google Scholar]
- 41.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 42.Virji, M., H. Kayhty, D. J. P. Ferguson, J. E. Heckels, and E. R. Moxon. 1991. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol. Microbiol. 5:1831-1841. [DOI] [PubMed] [Google Scholar]
- 43.Wakarchuk, W. W., M. Gilbert, A. Martin, Y. Wu, J. R. Brisson, P. Thibault, and J. C. Richards. 1998. Structure of an alpha-2,6-sialylated lipooligosaccharide from Neisseria meningitidis immunotype L1. Eur. J. Biochem. 254:626-633. [DOI] [PubMed] [Google Scholar]