Abstract
The adenylyl cyclase stimulator forskolin (FSK) stimulates UT-A1 phosphorylation, membrane trafficking, and urea transport activity. Here, we found that FSK stimulation induces UT-A1 ubiquitination in UT-A1 Madin-Darby canine kidney (MDCK) cells. This suggests that phosphorylation by FSK also triggers the protein degradation machinery for UT-A1. UT-A1-MDCK cells were treated with 100 μg/ml cycloheximide to inhibit protein synthesis, with or without 10 μM FSK. Total UT-A1 protein abundance was significantly reduced after FSK treatment, concomitantly ubiquitinated UT-A1 was increased. We then specifically investigated the effect of FSK on UT-A1 expressed on the cell plasma membrane. FSK treatment accelerated UT-A1 removal from the cell plasma membrane by increasing UT-A1 endocytosis as judged by biotinylation/MesNa treatment and confocal microscopy. We further found that inhibition of the clathrin-mediated endocytic pathway, but not the caveolin-mediated endocytic pathway, significantly blocks FSK-stimulated UT-A1 endocytosis. The PKA inhibitor H89 and the proteasome inhibitors MG132 and lactacystin reduced FSK-induced membrane UT-A1 reduction. Our study shows that FSK activates the UT-A1 urea transporter and the activation/phosphorylation subsequently triggers the downregulation of UT-A1, which represents an important mechanism for the cell to return to the basal conditions after vasopressin stimulation.
Keywords: urine-concentrating mechanism, protein kinase A, phosphorylation, clathrin, vasopressin
urea accumulation in the kidney inner medulla plays a critical role in the urinary concentration mechanism and therefore in the regulation of water homeostasis. Urea reabsorption is mainly mediated by the urea transporter UT-A1 in the inner medullary collecting duct (IMCD) epithelial cells (7, 20, 21). An UT-A1/A3 knockout mouse has impaired urea clearance and urinary concentrating ability (8).
Vasopressin [AVP; also known as antidiuretic hormone (ADH)], synthesized in the hypothalamus and stored in vesicles at the posterior pituitary, is the major hormone in vivo that regulates both water (aquaporin-2) and urea (UT-A1) permeability of the IMCDs. In response to a reduction in plasma volume and an increase in the plasma osmolality, vasopressin is released from the posterior pituitary and causes an increased urea concentration in the papillary tip, which contributes to the efficiency of the urinary concentrating mechanism (2, 21). Vasopressin rapidly increases urea permeability in isolated rat terminal IMCDs within 5–10 min (26).
Vasopressin binds to the IMCD cell V2 receptor, activates the heterotrimeric G protein Gαs, and stimulates adenylyl cyclases III and VI, which convert ATP into cAMP (1, 21). Increased cAMP levels activate cAMP-dependent protein kinase A (PKA) and lead to UT-A1 phosphorylation (21, 29). The study by Zhang et al. (29) revealed that vasopressin increases the phosphorylation of UT-A1 protein at 2 min, which peaks at 5–10 min and remains elevated for up to 30 min (29). The time course of UT-A1 phosphorylation is similar to vasopressin-induced stimulation of urea permeability observed by Wall et al. (26) in 1992 in perfused rat terminal IMCDs. Vasopressin-stimulated UT-A1 phosphorylation is blocked by H89 or a specific peptide inhibitor of PKA (29). Further studies showed that vasopressin, or directly activating adenylyl cyclase with forskolin, also increases UT-A1 accumulation in the apical plasma membrane, both in freshly isolated suspensions of rat IMCDs (16) and stably transfected UT-A1-Madin-Darby canine kidney (MDCK) cells (3). Increasing UT-A1 in the apical plasma membrane (3, 16) and UT-A1 phosphorylation (1, 29) are the two major mechanisms for vasopressin stimulation of kidney urea transport activity.
The long-term effect of vasopressin on UT-A1 is controversial. Vasopressin (dDAVP) infusion for 3–7 days is shown to decrease UT-A1 mRNA and protein abundance in the renal medulla from Brattleboro rats but not from normal rats (22). However, a study from Brooks' group (2) reported that UT-A1 mRNA and protein abundance is increased in the medullas of wild-type and AQP1 knockout mice following a 7-day dDAVP infusion.
Previous studies show that forskolin, which directly activates the catalytic subunit of adenylyl cyclase, increases urea flux in UT-A1-MDCK cells through increasing UT-A1 phosphorylation and membrane trafficking (3, 9, 16). In the current study, we made the new finding that forskolin also initiates the UT-A1 downregulation machinery by promoting UT-A1 ubiquitination, endocytosis, and protein degradation. The negative feedback of UT-A1 activation may have important physiological roles to limit the reaction by bringing down UT-A1 membrane expression and increasing protein degradation and to allow the cell to come back to the basal condition in response to vasopressin stimulation.
MATERIALS AND METHODS
Construct.
The FLAG-Tac fragment was PCR amplified from pcDNA3 FLAG-Tac-DAT (kindly provided by Dr. Ulrik Gether, University of Copenhagen) (6) and subcloned into pcDNA3-UT-A1, denoted as pcDNA3 FLAG-Tac-UT-A1.
Cell culture, transfection, and treatment.
UT-A1 MDCK cells (9) were grown in DMEM supplemented with 10% FCS, 25 mM HEPES and antibiotics in a 5% CO2 incubator at 37°C. After reaching confluency, the cells were treated with the different compounds alone or in combination as described in detail in the relevant experiments. Cells were then processed for Western blot, immunoprecipation, cell surface biotinylation, or internalization assay. Forskolin, PKA inhibitor H89, filipin, nystatin, and chlorpromazine were purchased from Sigma. Cycloheximide (CHX), lactacystin, and MG132 were purchased from Calbiochem.
For potassium depletion, the cells were washed three times with potassium-free buffer containing 140 mM NaCl, 20 mM HEPES pH 7.4, 1 mM CaCl2, 1 mM MgCl2, and 1 mg/ml d-glucose, followed by 2-min incubation with hypotonic buffer (potassium-free buffer diluted with water 1:1) for 1 h. After being washed with PBS, the cells were ready for further (forskolin) treatment.
The transient transfection of pcDNA3 FLAG-Tac-UT-A1 into MDCK cells was carried out using GenJet In Vitro DNA Transfection Reagent (SignaGen Laboratories). Eight microliters of GenJet and 2 μl of DNA (1 μg/μl) were mixed and incubated at room temperature for 15 min. MDCK cells from one well of a six-well plate were trypsinized. After spin, the cell pellets were resuspended immediatedly with the transfection mixture and incubated at 37°C for 20 min. The prewarmed 10% FCS DMEM was added and the cells were then seeded on a four-chamber slide (BD Biosciences). After 48-h incubation, transfected cells were processed for immunohistochemical staining.
Cell surface biotinylation.
Cell surface biotinylation assays were performed as described before (3). Briefly, after treatment, the cells were incubated twice with a freshly prepared solution of 1.0 mg/ml EZ-Link sulfo-N-hydroxysuccinimide disulfide-biotin (Pierce) in borate buffer for 30 min at 4°C. The biotin reaction was quenched for 5 min with 0.1 mM lysine (Sigma). After being washed with PBS, the cells were lysed in radioimmunoprecipitation assay (RIPA) buffer. Equal amounts of cleared lysate protein (0.5–1 mg) were incubated with 25 μl of immobilized streptavidin-agarose beads (Pierce) overnight at 4°C with gentle shaking. The beads were washed four times with RIPA buffer. Biotin-labeled proteins were eluted in 35 μl of Laemmli sample buffer and analyzed for UT-A1 expression by Western blotting.
MesNa treatment and internalization assay.
To measure UT-A1 endocytosis, cells were labeled with biotin as described above. After being washed with PBS, cells were added to prewarmed culture medium (with or without treatments) and incubated at 37°C to allow protein endocytosis for the indicated period. The control cells without endocytosis were kept on ice. The noninternalized biotin was cleaved three times with the small cell-impermeant reducing agent sodium 2-mercaptoethane sulfonate (MesNa) solution (50 mM MesNa, 1 mM EDTA, 0.2% BSA in 50 mM Tris, pH 8.6) for 20 min at 4°C on a rocking platform. The biotin bound to the endocytosed proteins is protected from MesNa cleavage. After being quenched with iodoacetamide and PBS washing, cells were solubilized in RIPA buffer. The biotinylated proteins were retrieved by streptavidin-agarose affinity precipitation and detected by Western blotting with UT-A1 antibody.
Confocal microscopy.
MDCK cells transfected with pcDNA3 FLAG-Tac-UT-A1 were washed with ice-cold PBS and then incubated with FLAG monoclonal antibody (1:200 from Sigma) and Rab5 polyclonal antibody (1:200 from Santa Cruz Biotechnology) in DMEM without serum for 60 min at 4°C. Unbound antibody was removed with three washes of ice-cold PBS. Prewarmed complete culture medium with or without forskolin was added. The cells were then switched to 37°C incubation for 60 min to allow internalization. The control cells without endocytosis were kept on ice. The internalization was stopped by washing with ice-cold PBS followed by fixation with 3% paraformaldehyde. The cells were permeabilized with 0.2% Tween for 5 min and incubated with Texas Red-conjugated anti-mouse antibody and FITC-conjugated anti-rabbit antibody (1:200 from InVitrogen) for 60 min. Nuclei were counterstained with TOPRO-3 (Molecular Probes). Cells were mounted with Vectashield (Vector Laboratories) and examined under a microscope. The images were captured by confocal microscopy at identical microscopic settings.
Cell lysate preparation, immunoprecipitation, and Western blot.
After treatment, MDCK cells were lysed in a modified RIPA buffer (150 mM NaCl, 10 mM Tris·HCl, pH 7.5, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, and protease inhibitors). The protein concentration of the cleared lysate was determined using the BCA Protein Assay (Pierce). For the immunoprecipitation, equal amounts (0.5–1 mg) of total proteins were incubated with 6 μl of UT-A1 antibody at 4°C overnight with gentle mixing, followed by the addition of 10 μl of protein A beads (Pierce) and continued incubation for another 2 h. The beads were pelleted by centrifugation at 3,000 rpm for 1 min and washed three times with RIPA buffer. The precipitated proteins were eluted in 40 μl of Laemmli sample buffer. For Western blot analysis, the proteins were separated by 4–15% SDS-PAGE and electrotransferred to polyvinylidene difluoride membranes (Bio-Rad). The membranes were routinely processed by blocking with 5% milk/PBS, incubation overnight with primary antibody, and incubation for 1 h with horseradish peroxidase-conjugated secondary antibody. Immunoreacting proteins were detected using an enhanced chemiluminescence (ECL) Kit (Amersham). The following antibodies were used in this study: UT-A1 antibody (1), Ub (P4D1) antibody (Santa Cruz Biotechnology), FLAG antibody and actin antibody (Sigma), secondary horseradish peroxidase-conjugated goat anti-rabbit IgG (Amersham), secondary horseradish peroxidase-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch). Western blot band densities were quantitated with the ImageJ program (U.S. National Institutes of Health, Bethesda, MD).
Oocyte experiments and urea flux measurement.
Capped cRNAs of UT-A1 and FLAG-Tac-UT-A1 were transcribed with T7 polymerase using the mMESSAGE mMACHINE T7 Ultra Kit (Ambion). UT-A1 urea transport activity was measured in Xenopus laevis oocytes by measuring urea flux as described before (13).
Statistical analysis.
Densitometry quantification and urea flux data were expressed as means ± SD. Statistical analysis of the data was performed by ANOVA followed by Tukey's HSD tests. Differences were considered as significant at *P < 0.05 or **P < 0.01; NS, not significant.
RESULTS
Forskolin stimulation induces UT-A1 ubiquitination.
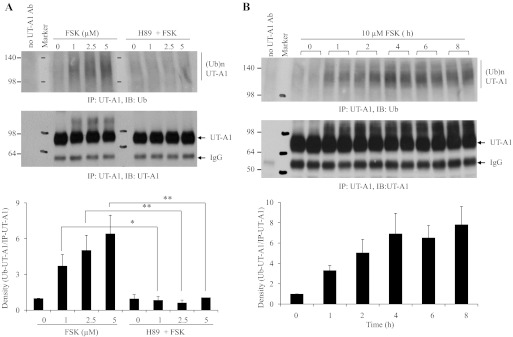
The initial aim of our study was to investigate UT-A1 degradation by ubiquitination. Unexpectedly, we observed that addition of forskolin to UT-A1 MDCK cells significantly induces UT-A1 ubiquitination as seen in cells treated with proteosome inhibitor MG132 (data not shown). To further confirm the result, we did dose- and time-dependent experiments. UT-A1-MDCK cells were treated with different doses of forskolin for 1 h. Figure 1A shows that forskolin dose dependently induces UT-A1 ubiquitination and this effect was significantly prevented by pretreatment with the PKA inhibitor H89. We also treated UT-A1-MDCK cells with 10 μM forskolin for different lengths of time and probed the UT-A1 immunoprecipitates with anti-ubiquitin (Fig. 1B). Forskolin treatment time dependently increases UT-A1 ubiquitination.
Fig. 1.
Forskolin (FSK) dose and time dependently induces UT-A1 ubiquitination. UT-A1 Madin-Darby canine kidney (MDCK) cells were treated with different doses of FSK for 1 h (A) or with 10 μM FSK for different time periods (B). Some cells as indicated in A were pretreated with 20 μM H89 for 1 h. After treatment, cells were lysed with radioimmunoprecipitation assay (RIPA) buffer. Equal amounts of lysates were immunoprecipitated with UT-A1 antibody and Western blotted with anti-ubiquitin (Ub) P4D1. Immunoprecipitation without primary (UT-A1) Ab was set as a negative control. The bands were quantified with NIH ImageJ from 3 different experiments. The ubiquitinated UT-A1 was normalized to the immunoprecipitated UT-A1. The relative density of control (or time point 0) was set as 1. *P < 0.05. **P < 0.01.
Forskolin stimulation promotes UT-A1 degradation.
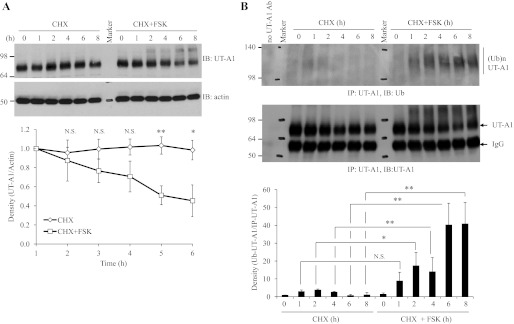
The finding that forskolin stimulation induces UT-A1 ubiquitination prompted us to investigate whether increased UT-A1 ubiqiutination is linked to an increase in UT-A1 protein turnover. UT-A1-MDCK cells were treated with CHX to block new protein synthesis. Protein degradation was observed for 8 h. As we can see in Fig. 2A, the total amount of UT-A1 was significantly reduced after forskolin treatment. This is accompanied by the increased smear of higher molecular weight protein representing ubiquitinated UT-A1. This was further confirmed in Fig. 2B by probing UT-A1 immunoprecipitates with ubiquitin antibody. Our result demonstrated a link between forskolin-induced ubiquitination and protein downregulation.
Fig. 2.
UT-A1 degradation upon FSK stimulation. UT-A1 MDCK cells were treated with 100 μg/ml cycloheximide (CHX) and with or without 10 μM FSK for the indicated time. The cells were lysed with RIPA buffer. A: total UT-A1 protein abundance was analyzed by Western blot with UT-A1 antibody. The same blot was stripped and reprobed with actin antibody. B: ubiquitinated UT-A1 was examined by UT-A1 immunoprecipitation followed by immunoblotting with ubiquitin antibody. The bands were quantified (n = 3). The total UT-A1 from cell lysates was normalized to actin and ubiquitinated UT-A1 was normalized to the immunoprecipitated UT-A1. The relative density of the control (time point 0) was set as 1. NS, not significant. *P < 0.05. **P < 0.01.
Forskolin stimulation increases UT-A1 endocytosis.
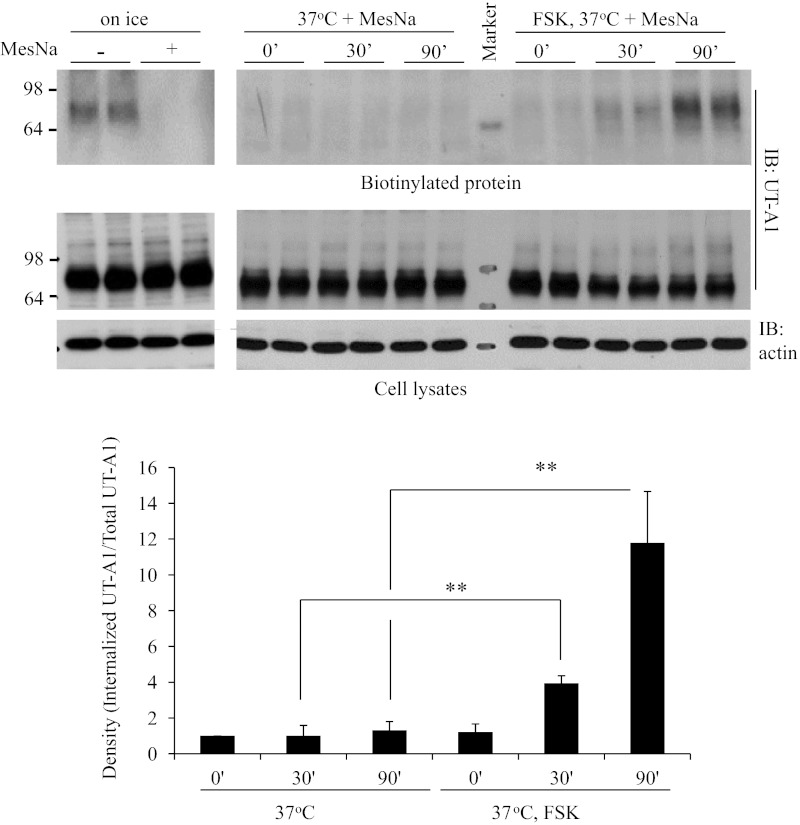
We then specifically investigated the effect of forskolin on UT-A1 expressed on the cell plasma membrane. To examine whether forskolin treatment accelerates UT-A1 removal from the cell plasma membrane, we performed the UT-A1 internalization assay. UT-A1 MDCK cells were first biotinylated and then rewarmed at 37°C in the presence of forskolin for different times. The noninternalized biotin on the cell surface was stripped by MesNa treatment. The internalized UT-A1 bands were quantified and normalized to the total UT-A1. Actin was used to evaluate the same amounts of total proteins applied for the experiments. As seen in Fig. 3, forskolin stimulation significantly promotes UT-A1 endocytosis.
Fig. 3.
UT-A1 internalization assay. UT-A1 MDCK cells were first biotinylated and then rewarmed at 37°C in the presence or absence of FSK for the different times. The noninternalized biotin on the cell surface was stripped by sodium 2-mercaptoethane sulfonate (MesNa) treatment. The cells were lysed in RIPA buffer and the total lysates were used for Western blot with UT-A1 and actin antibodies. Internalized proteins were recovered by streptavidin beads and processed for Western blotting with UT-A1 antibody. The bands were quantified (n = 3). The internalized UT-A1 was normalized to the UT-A1 from total lysates. The relative density of the control (time point 0) was set as 1. **P < 0.01.
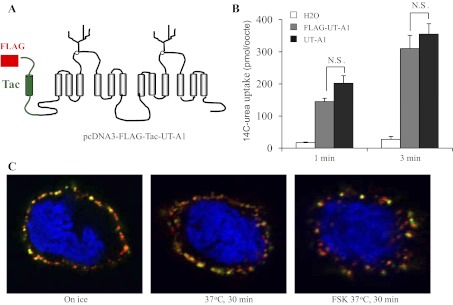
To directly visualize UT-A1 endocytosis, we generated FLAG-Tac-fused UT-A1. FLAG-Tac gene was obtained from Dr. Ulrik Gether and fused to UT-A1's NH2 terminus. The recombinant FLAG NH2-terminal end is present in the extracellular space (Fig. 4A). To assess whether the FLAG-tagged UT-A1 behaves as normal UT-A1, Flag-UT-A1 and nontagged UT-A1 cRNAs were prepared and injected into oocytes. The activity was measured by 14C-urea flux. The Flag-UT-A1 demonstrated urea transport activity (Fig. 4B). Then, this external FLAG-tagged UT-A1 was used to investigate UT-A1 endocytosis. The FLAG-Tac-UT-A1 was transfected into MDCK cells and the cell surface UT-A1 was labeled by FLAG antibody in living cells at 4°C for 1 h. After the unbound FLAG antibody was washed, the cells were switched to 37°C for 30 min. Rab5 localized at the plasma membrane and early endosomes (29) were used as a subcellular marker. Figure 4C demonstrated that UT-A1 staining that was on the cell surface mostly overlapped with Rab5 when there is no endocytosis (on ice). After 30-min incubation at 37°C, internalized UT-A1 was observed in the subplasma membrane area. Forskolin treatment increased UT-A1 endocytosis and the endocytic UT-A1 was located deeper within the cells.
Fig. 4.
Analysis of FLAG-tagged UT-A1 endocytosis by confocal microscopy. A: illustration of external Flag-tagged UT-A1. B: urea uptake experiment. cRNAs of UT-A1 and FLAG-UT-A1 were prepared and microinjected into oocytes. After 3 days, urea transport activity was measured by 14C-urea uptake for 1 and 3 min (n = 6 oocytes/timepoint). Data were expressed as means ± SD. Statistical analysis of the data was performed by ANOVA. C: MDCK cells were transiently transfected with pcDNA3 FLAG-Tac-UT-A1. After 48 h, cell surface UT-A1 was labeled with anti-FLAG and Rab5 antibodies at 4°C for 1 h. Cells were then switched to 37°C for 30 min to allow endocytosis in the presence or absence of 10 μM FSK. The internalized UT-A1 was detected by the fluorescent secondary antibodies and examined under confocal microscope as described in materials and methods.
Forskolin-stimulated UT-A1 endocytosis is mainly through the clathrin-mediated endocytic pathway.
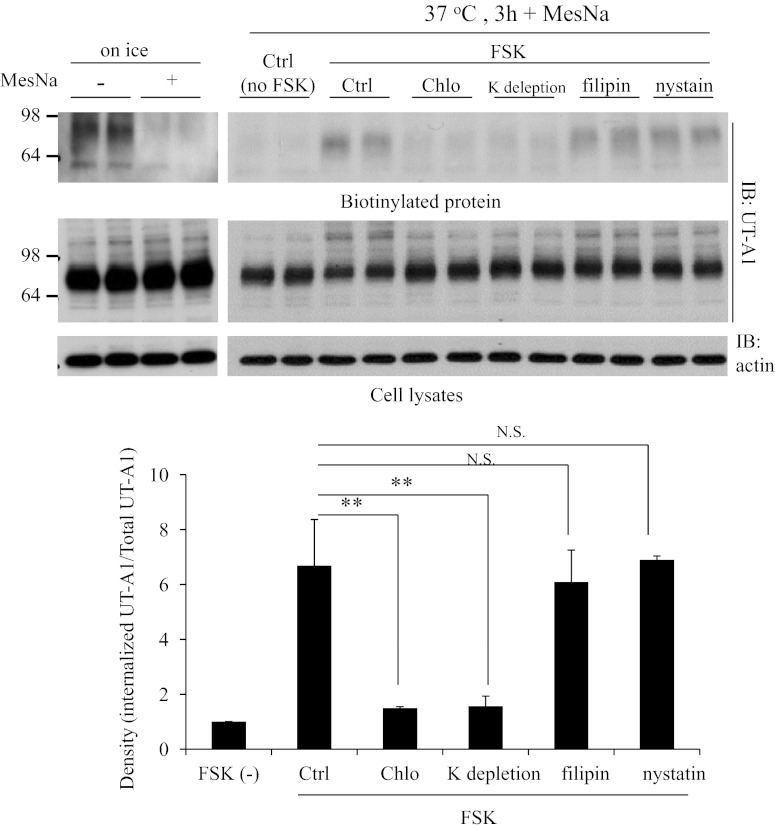
The caveolin-mediated pathway and clathrin-mediated pathway are the two major endocytic pathways. We previously reported that UT-A1 endocytosis is through both pathways (13). To investigate the UT-A1 endocytic route under forskolin stimulation, we used chlorpromazine and K depletion to block the clathrin endocytic pathway, and filipin and nystatin to inhibit the caveolin endocytic pathway. UT-A1 internalization was then evaluated as above. Figure 5 shows that inhibition of the clathrin pathway significantly inhibits forskolin-induced internalization of UT-A1. By contrast, UT-A1 internalization was not affected by the caveolin pathway blocker filipin and nystatin. These findings indicate that forskolin-induced UT-A1 internalization is predominantly mediated by the clathrin pathway.
Fig. 5.
Inhibition of clathrin and caveolin endocytic pathways on UT-A1 endocytosis. UT-A1-MDCK cells were pretreated with 10 μg/ml chlorpromazine (Chlo), potassium depletion, 1 μM filipin, or 30 μM nystatin for 1 h. UT-A1 internalization was examined by biotinylation and MesNa treatment was followed by Western blotting with UT-A1 antibody. The bands were quantified from 3 independent experiments. The internalized UT-A1 was normalized to the UT-A1 from total lysates. The relative density of the control was set as 1. **P < 0.01.
Proteasome inhibitor reduces forskolin-induced UT-A1 endocytosis.
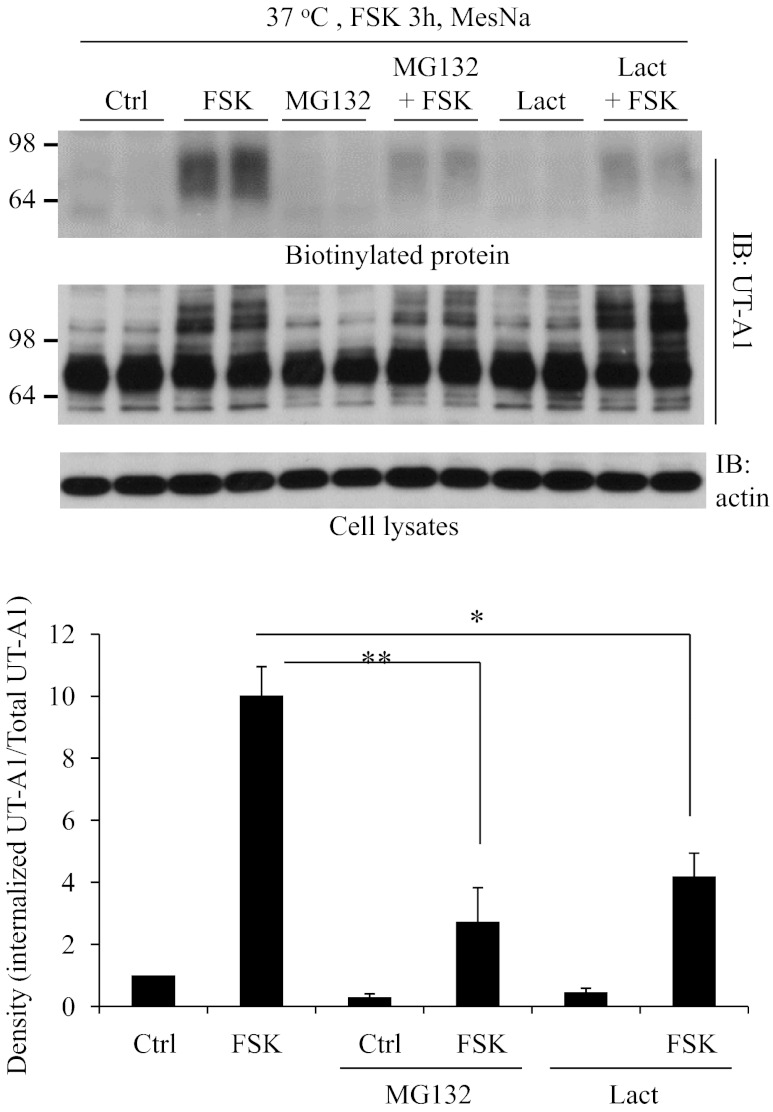
Forskolin stimulation induces UT-A1 ubiquitination (Fig. 1) and promotes UT-A1 endocytosis (Figs. 3 and 4). To examine whether the forskolin-stimulated internalization of UT-A1 depends on its ubiquitination, UT-A1-MDCK cells were pretreated with the proteasome inhibitor MG132 or lactacystin and internalization assays were performed (Fig. 6). As seen in Fig. 7, MG132 and lactacystin significantly inhibit forskolin-induced membrane endocytosis of UT-A1.
Fig. 6.
Effect of proteasome inhibitor on UT-A1 endocytosis. UT-A1-MDCK cells were pretreated with 10 μM MG132 or 10 μM lactacystin (Lact) for 1 h and then treated with or without 10 μM FSK for 3 h. UT-A1 internalization was examined by biotinylation and MesNa treatment was as above. The bands were quantified (n = 3). The internalized UT-A1 was normalized to the UT-A1 from total lysates. The relative density of the control (without any treatment) was set as 1. *P < 0.05. **P < 0.01.
Fig. 7.
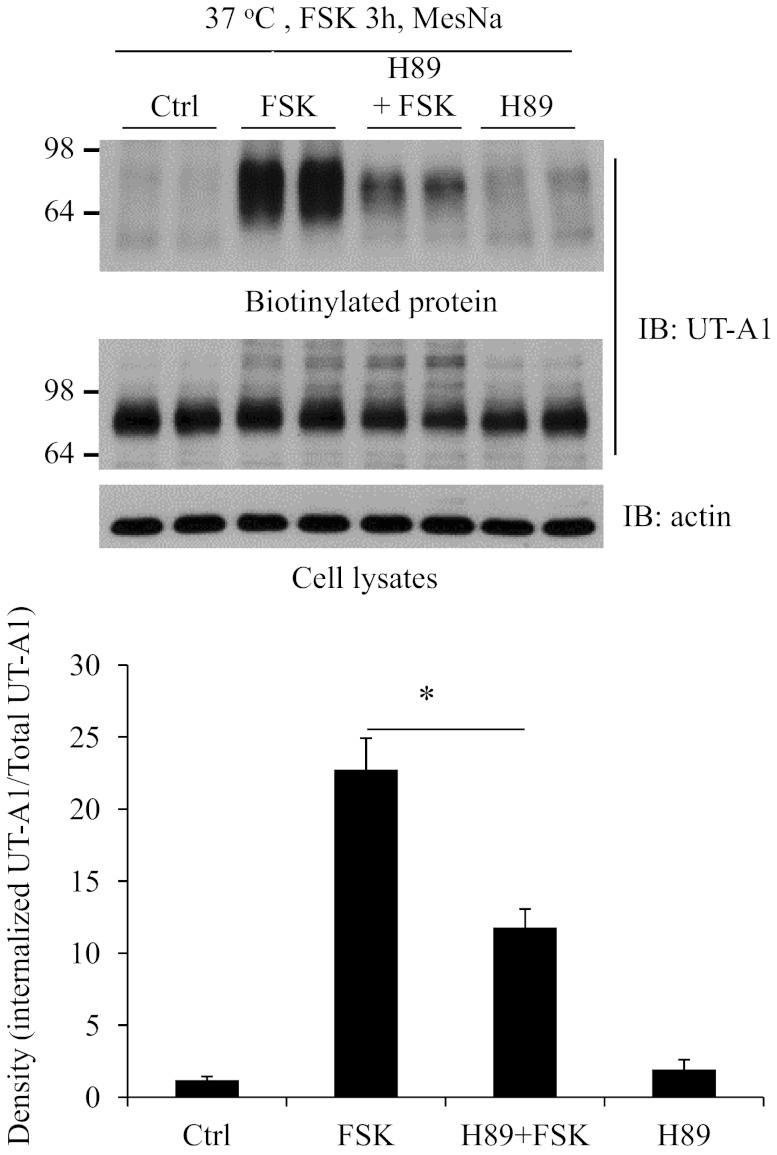
Effect of protein kinase A (PKA) inhibitor H89 on UT-A1 endocytosis. UT-A1-MDCK cells were pretreated with 20 μM H89 for 1 h. FSK-stimulated UT-A1 internalization was examined by biotinylation and MesNa treatment. The signals were quantified (n = 3). The internalized UT-A1 was normalized to the UT-A1 from total lysates. The relative density of the control (without any treatment) was set as 1. *P < 0.05.
PKA inhibitor H89 attenuates forskolin-stimulated UT-A1 endocytosis.
Forskolin directly stimulates adenylyl cyclases and increases cAMP levels. The increased cAMP levels can activate both PKA and non-PKA signaling pathways (27). To examine whether forskolin-stimulated UT-A1 endocytosis is mainly through the PKA pathway, UT-A1-MDCK cells were pretreated with the PKA inhibitor H89. As seen in Fig. 8, H89 pretreatment significantly blocks forskolin-stimulated UT-A1 endocytosis. It suggests that PKA-mediated UT-A1 phosphorylation is required for efficient UT-A1 internalization and downregulation.
Fig. 8.
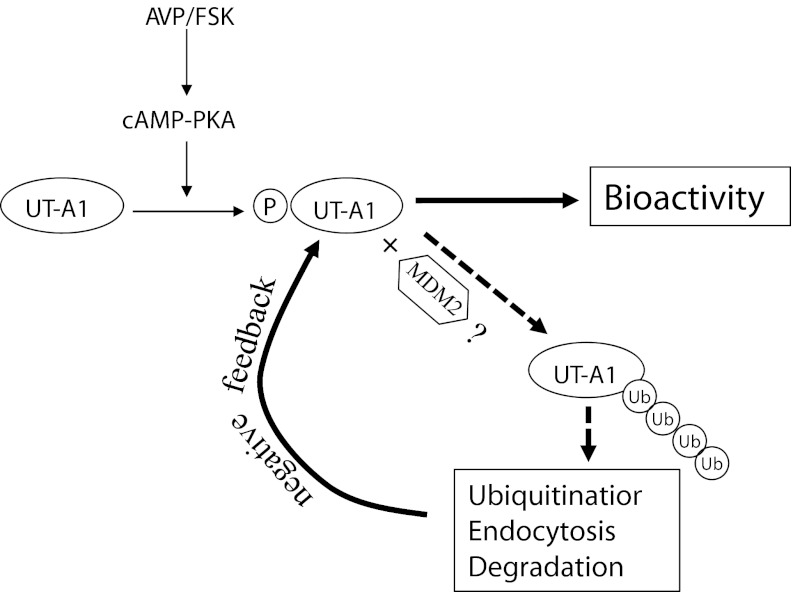
Illustration of UT-A1 activation and degradation after vasopressin treatment. Vasopressin in vivo (FSK in vitro) increases cAMP levels and activates PKA-mediated phosphorylation. UT-A1 becomes an active form with high-urea transport activity. Meanwhile, UT-A1 phosphorylation recruits the ubiquitination enzyme (MDM2) resulting in UT-A1 ubiquitination, endocytosis, and degradation.
DISCUSSION
Ubiquitination is a posttranslational modification carried out by a set of three enzymes, E1, E2, and E3. After being activated by ubiquitin-activating enzyme E1 and passed by ubiquitin-conjugating enzyme E2, ubiquitin is attached to the specific substrate mediated by a specific ubiquitin protein ligase E3 (11). Mature membrane proteins on the cell surface can be modified by the addition of either monoubiquitin or polyubiquitin chains (11). Different ubiquitin linkages are associated with distinct functions. Monoubiquitination is implicated in membrane protein internalization and endocytosis. Polyubiquitination can have distinct functions depending on the lysine linkage (11).
We (4) and others (23) previously reported that the urea transporter UT-A1 undergoes ubiquination and proteasome-dependent degradation. Ubiquitination regulates UT-A1 cell surface accumulation, protein stability, and urea permeability. In the current study, we found that forskolin, the adenylyl cyclase stimulator that is often used for in vitro experiments to stimulate UT-A1 urea transport activity, can induce UT-A1 urea transporter ubiquitination. This conveys important information that activation of cAMP/PKA pathway also triggers the UT-A1 ubiquitination and degradation systems.
Ubiquitination is a highly regulated posttranslational modification process. Our finding suggests that phosphorylation by forskolin stimulation may play an important role in subsequent UT-A1 ubiquitination. This could be the general mechanism for many membrane protein ubiquitination processes, particularly in the ligand-induced tyrosine kinase receptor ubiquitination (10, 14, 19, 24). Upon epidermal growth factor (EGF) stimulation, EGF receptor (EGFR) undergoes rapid dimerization, activation of its intrinsic tyrosine kinase activity, and autophosphorylation at multiple tyrosine sites within its cytoplasmic tail. Tyrosine phosphorylation then recruits ubiquitin ligase C-Cbl to EGFR and results in ubiquitination of EGFR. The ubiquitinated EGFR is then rapidly internalized and degraded (14, 24). Similar to EGFR, insulin-like growth factor I receptor (IGF-IR) phosphorylation is reported to be crucial for IGF-I-mediated receptor ubiquitination (19). In our study, pretreatment with the PKA inhibitor H89 significantly inhibits forskolin-induced UT-A1 ubiquitination (Fig. 1A), indicating phosphorylation is required for efficient UT-A1 ubiquitination.
The importance of ubiquitination in the endocytosis of various membrane proteins has been appreciated in recent years (5, 17, 18, 25). Both monoubiquitination and polyubiquitination have been shown to serve as efficient internalization signals (15, 17, 18, 25). Ubiquitination causes EGFR to be rapidly internalized and transfered to early endosomes. There, the EGFR can be further sorted to the lysosomes for degradation. Data from our study showed that UT-A1 endocytosis is increased after activation by forskolin stimulation. Furthermore, pretreatment with MG132, lactacystin, or H89 significantly reduces forskolin-induced UT-A1 endocytosis suggesting that, similar to EGFR, phosphorylation and ubiquitination are required for efficient UT-A1 internalization and downregulation in MDCK cells.
Endocytosis is a complex cell process with multiple routes. Clathrin-mediated endocytosis and caveolin-mediated endocytosis are the two major endocytic pathways. The same cargo protein can be internalized via one or multiple endocytic pathways depending on the different cellular situations (5). Internalization of UT-A1 is dynamin dependent and mediated by both clathrin- and caveolin-mediated pathways (13). However, the mechanisms by which UT-A1 enters these two different pathways and what is the fate of UT-A1 in these two different pathways are still unclear. In this study, we employed pharmacological inhibition of either clathrin or caveolin pathways and found that chlorpromazine, as well as K depletion, but not filipin and nystatin, inhibits forskolin-induced UT-A1 endocytosis. This result shows that forskolin-stimulated UT-A1 internalization is mainly mediated by a clathrin-mediated mechanism. Based on this finding, we can speculate on the possible roles of these two pathways in UT-A1 endocytosis: the clathrin pathway may mediate vasopressin-regulated UT-A1 internalization; while the caveolin pathway is responsible for constitutive UT-A1 internalization. Further study is required to prove it. In addition, it will also be very interesting to know, in the future, whether UT-A1 (phosphorylated and ubiquitinated) entering the clathrin pathway is destined for degradation, whereas UT-A1 internalized through the caveolin pathway is destined for recycling back to the plasma membrane.
In summary, over the past decade, studies have shown that vasopressin rapidly increases PKA-mediated phosphorylation of the UT-A1 urea transporter and increases urea permeability in rat IMCDs (1, 16, 26, 29). We here provide new evidence showing the coin's other side of vasopressin's effects on UT-A1. Activation/phosphorylation of the UT-A1 urea transporter by the adenylyl cyclase stimulator forskolin also initiates the urea transporter downregulation mechanism. Figure 8 illustrates the scenarios of UT-A1 regulation by vasopressin. Vasopressin in vivo (forskolin in vitro) stimulates adenylyl cyclase, increases cAMP levels, and activates PKA-mediated phosphorylation. UT-A1 is therefore activated to mediate high-urea transport activity. However, once UT-A1 is phosphorylated, ubiquitination enzymes (particularly the ubiquitin E3 ligase MDM2) are recruited and activated in the same way as EGFR phosphorylation recruits the ubiquitin ligase C-Cbl. Subsequently, a series of UT-A1 downregulation steps is initiated including UT-A1 ubiquitination, clathrin-mediated endocytosis, and protein degradation. Thus, the vasopressin effect on UT-A1 is eventually terminated. It is common to many proteins that, when they are phosphorylated, they are degraded rapidly (14, 19, 24). Obviously, this process is important for the cells to limit the reaction in response to stimulation and enables the cells to return to basal conditions. In fact, in 1986 Harris et al. (12) observed that addition of vasopressin to toad urinary bladder caused a prompt increase in osmotic water permeability and this effect diminished within minutes. This could be by the same mechanism as we observed here for UT-A1, i.e., that vasopressin activated the water channel and also activated its downregulation to bring the cells quickly back to normal conditions.
GRANTS
This work was supported by National Institutes of Health Grants R01-DK087838 (to G. Chen) and R01-DK041707 (to J. M. Sands).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.S. and G.C. conception and design of research; H.S., C.B.C., and O.L. performed experiments; H.S., J.M.S., and G.C. analyzed data; H.S. and G.C. prepared figures; J.M.S. and G.C. interpreted results of experiments; J.M.S. and G.C. edited and revised manuscript; G.C. drafted manuscript; G.C. approved final version of manuscript.
REFERENCES
- 1. Blount MA, Mistry AC, Fröhlich O, Price SR, Chen G, Sands JM, Klein JD. Phosphorylation of UT-A1 urea transporter at serines 486 and 499 is important for vasopressin-regulated activity and membrane accumulation. Am J Physiol Renal Physiol 295: F295– F299, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cai Q, Nelson SK, McReynolds MR, Diamond-Stanic MK, Elliott D, Brooks HL. Vasopressin increases expression of UT-A1, UT-A3, and ER chaperone GRP78 in the renal medulla of mice with a urinary concentrating defect. Am J Physiol Renal Physiol 299: F712– F719, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen G, Fröhlich O, Yang Y, Klein JD, Sands JM. Loss of N-linked glycosylation reduces urea transporter UT-A1 response to vasopressin. J Biol Chem 281: 27436– 27442, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Chen G, Huang H, Fröhlich O, Yang Y, Klein JD, Price SR, Sands JM. MDM2 E3 ubiquitin ligase mediates UT-A1 urea transporter ubiquitination and degradation. Am J Physiol Renal Physiol 295: F1528– F1534, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Juan-Sanz J, Zafra F, López-Corcuera B, Aragón C. Endocytosis of the neuronal glycine transporter GLYT2: role of membrane rafts and protein kinase C-dependent ubiquitination. Traffic 12: 1850– 1867, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Eriksen J, Bjørn-Yoshimoto WE, Jørgensen TN, Newman AH, Gether U. Postendocytic sorting of constitutively internalized dopamine transporter in cell lines and dopaminergic neurons. J Biol Chem 285: 27289– 27301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fenton RA. Essential role of vasopressin-regulated urea transport processes in the mammalian kidney. Pflügers Arch 458: 169– 177, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Fenton RA, Chou CL, Stewart GS, Smith CP, Knepper MA. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci USA 101: 7469– 7474, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fröhlich O, Klein JD, Smith PM, Sands JM, Gunn RB. Urea transport in MDCK cells that are stably transfected with UT-A1. Am J Physiol Cell Physiol 286: C1264– C1270, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Guo HB, Johnson H, Randolph M, Lee I, Pierce M. Knockdown of GnT-Va expression inhibits ligand-induced downregulation of the epidermal growth factor receptor and intracellular signaling by inhibiting receptor endocytosis. Glycobiology 19: 547– 559, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haglund K, Stenmark H. Working out coupled monoubiquitination. Nat Cell Biol 8: 1218– 1219, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Harris HW, Jr, Wade JB, Handler JS. Transepithelial water flow regulates apical membrane retrieval in antidiuretic hormone-stimulated toad urinary bladder. J Clin Invest 78: 703– 712, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang H, Feng X, Zhuang J, Fröhlich O, Klein JD, Cai H, Sands JM, Chen G. Internalization of UT-A1 urea transporter is dynamin dependent and mediated by both caveolae and clathrin-coated pit pathways. Am J Physiol Renal Physiol 299: F1389– F1395, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol Cell 21: 737– 748, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Kamsteeg EJ, Hendriks G, Boone M, Konings IB, Oorschot V, van der Sluijs P, Klumperman J, Deen PM. Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc Natl Acad Sci USA 103: 18344– 18349, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klein JD, Frohlich O, Blount MA, Martin CF, Smith TD, Sands JM. Vasopressin increases plasma membrane accumulation of urea transporter UT-A1 in rat inner medullary collecting ducts. J Am Soc Nephrol 17: 2680– 2686, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Ko B, Kamsteeg EJ, Cooke LL, Moddes LN, Deen PM, Hoover RS. RasGRP1 stimulation enhances ubiquitination and endocytosis of the sodium-chloride cotransporter. Am J Physiol Renal Physiol 299: F300– F309, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin DH, Yue P, Pan CY, Sun P, Zhang X, Han Z, Roos M, Caplan M, Giebisch G, Wang WH. POSH stimulates the ubiquitination and the clathrin-independent endocytosis of ROMK1 channels. J Biol Chem 284: 29614– 29624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mao Y, Shang Y, Pham VC, Ernst JA, Lill JR, Scales SJ, Zha J. Polyubiquitination of insulin-like growth factor I receptor (IGF-IR) activation loop promotes antibody-induced receptor internalization and downregulation. J Biol Chem 286: 41852– 41861, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nielsen S, Terris J, Smith CP, Hediger MA, Ecelbarger CA, Knepper MA. Cellular, and subcellular localization of the vasopressin-regulated urea transporter in rat kidney. Proc Natl Acad Sci USA 93: 5495– 5500, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sands JM. Critical role of urea in the urine-concentrating mechanism. J Am Soc Nephrol 18: 670– 671, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Shayakul C, Smith CP, Mackenzie HS, Lee WS, Brown D, Hediger MA. Long-term regulation of urea transporter expression by vasopressin in Brattleboro rats. Am J Physiol Renal Physiol 278: F620– F627, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Stewart GS, O'Brien JH, Smith CP. Ubiquitination regulates the plasma membrane expression of renal UT-A urea transporters. Am J Physiol Cell Physiol 295: C121– C129, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Umebayashi K, Stenmark H, Yoshimori T. Ubc4/5 and c-Cbl continue to ubiquitinate EGF receptor after internalization to facilitate polyubiquitination and degradation. Mol Biol Cell 19: 3454– 3462, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vina-Vilaseca A, Bender-Sigel J, Sorkina T, Closs EI, Sorkin A. Protein kinase C-dependent ubiquitination and clathrin-mediated endocytosis of the cationic amino acid transporter CAT-1. J Biol Chem 286: 8697– 8706, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wall SM, Han JS, Chou CL, Knepper MA. Kinetics of urea and water permeability activation by vasopressin in rat terminal IMCD. Am J Physiol Renal Fluid Electrolyte Physiol 262: F989– F998, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, Klein JD, Liedtke CM, Sands JM. Protein kinase C regulates urea permeability in the rat inner medullary collecting duct. Am J Physiol Renal Physiol 299: F1401– F1406, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107– 117, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Zhang C, Sands JM, Klein JD. Vasopressin rapidly increases phosphorylation of UT-A1 urea transporter in rat IMCDs through PKA. Am J Physiol Renal Physiol 282: F85– F90, 2002. [DOI] [PubMed] [Google Scholar]