Abstract
Renal ischemia-reperfusion injury is a major cause of acute kidney injury that carries a high mortality rate and increases the risk of later development of hypertension and chronic kidney disease. Although mouse models have contributed much to our understanding of the mechanisms involved, studying aspects of the injury process in vivo remains technically challenging. This study validates the use of noninvasive ultrasound imaging to assess both renal perfusion and vascular adhesion molecule expression following 1-h unilateral renal ischemia in male and female mice. Pulsed-wave Doppler measurements of renal arterial blood velocity revealed renal perfusion recoveries of 56 ± 9% in male and 69 ± 10% in female mice 1 h after the commencing of reperfusion, which is similar to what we have previously published using conventional invasive methodology. At 24 h postischemia, renal perfusion was 40 ± 8% in male and 46 ± 7% in female mice, representing a further significant reduction of perfusion (PTime < 0.001). Using ultrasound imaging of a P-selectin-targeted contrast agent, a significant increase in vascular P-selectin protein expression was observed after 1-h reperfusion in the cortex of the postischemic compared with contralateral kidney in both male and female mice (18 ± 5 vs. 3 ± 3 intensity units in male and 30 ± 6 vs. 0 ± 4 in female mice, PIschemia < 0.01). An approximately sixfold increase in P-selectin mRNA was observed ex vivo in the renal vasculature of male and female mice at this time point (P < 0.01). In conclusion, ultrasound represents an effective and noninvasive method for the measurement of both renal perfusion and vascular adhesion molecule expression in mice.
Keywords: kidney, blood flow
ischemia and reperfusion of the kidney is a major cause of acute kidney injury and thought to contribute to impaired function of transplanted kidneys (24, 31, 42). Ischemic insults to the kidney promote a host of pathological changes to both the vasculature and tubular cells, resulting in reduced glomerular filtration rate, impaired urinary concentrating ability, and structural damage, including reduced microvascular density, tubular cell death, formation of tubular casts, and fibrosis (4, 35). Acute kidney injury or acute renal failure has a mortality rate of ∼50% (27), and both clinical and animal studies suggest that survivors recover renal function to varying degrees but are at greater risk of subsequently developing hypertension and chronic kidney disease (3, 5). No specific treatments for acute kidney injury exist, and the mechanisms of both acute kidney injury and its long-term consequences on the kidney are incompletely understood. Accordingly, noninvasive techniques that can be used repeatedly within the same subject to study the renal injury and repair processes are highly attractive, especially if they are amenable to translation to clinical use.
Mouse models of renal ischemia-reperfusion injury have contributed much to our understanding of the mechanisms involved; however, studying certain aspects of the injury and recovery process in vivo presents technical challenges. For example, the postischemic kidney displays hemodynamic abnormalities, including increased vasoconstriction, which is thought to exacerbate tissue hypoxia and further contribute to injury (35). The ability to assess the effects of potential treatments on renal hemodynamics over days to weeks would clearly be advantageous, but instrumenting and maintaining mice with perivascular or laser-Doppler flow probes for measurements over extended periods is no small feat. For this reason, the use of contrast-enhanced ultrasound imaging is an attractive alternative for extended longitudinal studies, allowing for noninvasive data collection at multiple time points within the same animal.
Renal ischemia-reperfusion injury induces an inflammatory response both within the kidney and systemically. Neutrophil and monocyte/macrophage infiltration occurs following ischemia, and depletion of these cells attenuates renal ischemia-reperfusion injury (20, 43). T cell infiltration also occurs, and different T cell subsets play divergent roles in injury and repair (10, 16). An early event in the inflammatory response is the expression of cellular adhesion molecules on the surface of the activated endothelium. These adhesion molecules, including P-selectin, play a critical role in the adhesion of circulating inflammatory cells to the endothelium and ultimately extravasation into underlying tissues. Highlighting the importance of P-selectin in renal ischemia-reperfusion injury, total body knockout of P-selectin or treatment with P-selectin neutralizing antibodies have been shown to reduce renal injury following ischemia (14, 36). The ability to assess expression of adhesion molecules such as P-selectin in vivo would clearly be useful in determining the impact of potential treatments on the inflammatory response following ischemia and reperfusion.
Previously, Andonian and colleagues (1) and Chen and colleagues (11) provided promising but limited data regarding the use of high-resolution ultrasound in conjunction with a P-selectin-targeted echogenic gas-filled microbubble contrast agent in mouse models of renal ischemia-reperfusion injury. A notable limitation of the study by Andonian and colleagues (1) was that the kidney was surgically exposed during ultrasound imaging, negating a major advantage of this ostensibly noninvasive technique. Moreover, apparent changes in P-selectin expression observed in vivo appeared similar to data obtained with the contrast agent conjugated to isotype control antibody and were not corroborated ex vivo. An additional limitation was that the studies utilized only female mice, and sex differences in renal ischemia-reperfusion injury and acute kidney injury have been reported in both humans (18) and experimental models (30, 41). Accordingly, the goals of the present study were to determine if high-resolution ultrasound could be used noninvasively to assess restoration of perfusion and induction of vascular P-selectin expression in vivo following renal ischemia and reperfusion and whether these responses differed in male and female mice.
METHODS
Experimental design.
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved and monitored by the Georgia Health Sciences University Institutional Animal Care and Use Committee. Studies were performed on male and female C57Bl/6 mice (10–12 wk; Jackson Laboratories, Bar Harbor, ME). For determination of recovery of renal perfusion over time, mice underwent pulsed-wave Doppler ultrasound imaging before and at 1 and 24 h following unilateral renal ischemia-reperfusion injury (see below). To assess renal vascular P-selectin protein expression in vivo, separate groups of mice underwent contrast-enhanced ultrasound imaging at 1 h after ischemia-reperfusion injury as described below. To provide additional confirmation that vascular P-selectin expression is increased in the postischemic kidney at 1 h following injury, additional groups of mice underwent ischemia-reperfusion injury without imaging. After 1 h reperfusion, renal vascular tissue was isolated by gently sieving the kidney through 100 μm mesh and collecting the vessels retained for ex vivo analysis of renal vascular P-selectin expression. RNA was extracted from this renal vascular tissue using an RNeasy Mini kit (Qiagen, Valencia, CA) and reverse transcribed to cDNA using a QuantiTect Reverse Transcription kit. Expression of P-selectin mRNA relative to GAPDH was assessed using QuantiTect Primer Assays (Qiagen), and relative fold expression was calculated as 2−(ΔΔCT) as described previously (8).
Renal ischemia-reperfusion injury.
Each mouse was anesthetized with isoflurane (∼2% by inhalation; Baxter, Deerfield, IL) and placed on a servo-controlled heating table in a prone position. The right kidney was approached via a retroperitoneal incision, and a small microvascular clamp was applied to the renal artery, stopping blood flow. After a period of 1 h, the clamp was removed, allowing reperfusion; 0.4 ml warmed sterile saline was instilled intraperitoneally, and the retroperitoneal incision was closed using sterile sutures and surgical staples. Anesthesia was withdrawn, and the mouse was allowed to recover.
Ultrasound imaging.
As described previously (37), mice were anesthetized with isoflurane (∼1.5% by inhalation) and placed in a supine position on the THM100 MousePad imaging platform (Indus Instruments, Houston, TX). Appendages were secured to electrocardiogram pads to allow constant monitoring of heart rate and body temperature, which was maintained at 37.5°C. Depilatory cream (Nair; Carter-Horner, Mississauga, ON, Canada) was used to remove fur from the abdominal skin, and medical ultrasound acoustic gel (Other-Sonic, Pharmaceutical Innovations, Newark, NJ) was used as a coupling fluid between the real-time microvisualization (RMV) scanhead and the skin. Ultrasound imaging was performed using the Vevo 770 system (VisualSonics, Toronto, Canada) with an RMV-706 scanhead (40 MHz; 6 mm focal length; lateral and axial resolutions of 68.2 and 38.5 μm, respectively) that was positioned and held immobile using the VisualSonics Vevo Integrated Rail System II.
Pulsed-wave Doppler measurement of renal blood velocity.
Peak systolic renal artery blood velocity was measured using pulsed-wave Doppler (PW mode). Following a brief stabilization period, pulsed-wave Doppler blood velocity was obtained in the renal artery before it enters the kidney to provide a measure of total renal blood flow. Values from 5 to 10 cardiac cycles were averaged in each kidney.
Molecular-targeted contrast imaging of the kidney.
The VisualSonics Vevo MicroMarker Target-Ready Contrast Agent Kit (Toronto, Canada) was used for the in vivo detection of vascular endothelial P-selectin. The target-ready contrast agent was reconstituted in 0.5 ml of sterile saline according to the manufacturer's directions. The contrast agent is a blood-pool marker that consists of a gas-filled (nitrogen and perflurobutane) microbubble (2.3–2.9 μm diameter) that has strepavidin incorporated into the lipid shell of the contrast agent that can be conjugated to a biotinylated ligand. Twenty micrograms of P-selectin antibody (Sc25771; Santa Cruz Biotechnology, Santa Cruz, CA) were diluted in 0.4 ml sterile saline and then added to the reconstituted contrast agent, gently mixed, and allowed to rest at room temperature for 15 min. After 1 h reperfusion, the contrast agent was injected via an acutely placed jugular vein catheter as a 70-μl bolus at a rate of 400 μl/min for 15 s using a syringe pump (Harvard Apparatus, Holliston, MA). The contrast agent circulated for 4 min without imaging to allow time for dispersal through the circulation and P-selectin antibody binding to the vascular endothelium. Using contrast-mode imaging, the RMV-706 scanhead was then used to view the mouse kidney, and an 800-frame cine loop (14 frames/s) was collected, with a high-power, low-frequency destruction sequence applied at frame 240 to disrupt all microbubbles within the imaging plane. Intensity before the destruction sequence reflects the sum of circulating and endothelial-bound contrast agent, and the intensity after destruction reflects circulating contrast agent only. To reduce variability, imaging parameters were held constant throughout each experiment with focus and depth optimized at the beginning of the imaging session for each animal. Images in different kidneys and mice were obtained using approximately the same scan plane as determined by anatomical markers. Additional control experiments were performed in an identical manner using either an isotype control antibody-conjugated contrast agent (Sc2027; Santa Cruz Biotechnology) or Vevo MicroMarker Non-Targeted Contrast Agent.
Image analysis.
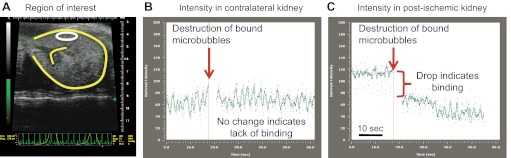
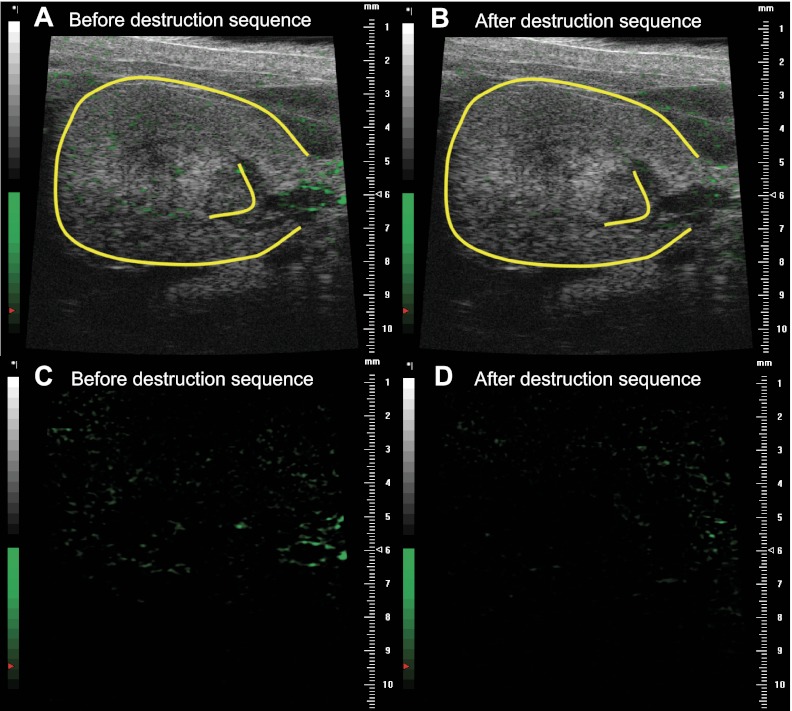
Reference-subtracted intensity data were analyzed offline on a personal computer. As shown in Fig. 1, the 800-frame cine loop was used to generate contrast agent time-intensity curves in user-defined regions of interest (ROI) in the renal cortex (∼0.6 mm2) and medulla (∼1.4 mm2) using Vevo 770 software allowing for a qualitative assessment of P-selectin expression. Contrast agent intensity in each ROI was averaged over the 10 s immediately preceding and immediately following the destruction sequence, with the difference nominally representing endothelial-bound contrast agent. A similar-sized ROI was selected for each mouse. The representative image shown in Fig. 1A depicts the ROI in the renal cortex of a postischemic kidney before the destruction sequence to burst adherent contrast agent. To quantify P-selectin expression, a reference frame is generated from the final 100 frames of the cine loop following the destruction sequence, and signal intensity within this reference frame represents circulating contrast agent only. This reference frame is then subtracted from the entire cine loop, thereby allowing for the quantification of the intensity of bound contrast agent only. Representative time-intensity curves for the ROI in Fig. 1A are depicted in Fig. 1, B and C. Figure 2 depicts a representative contrast-mode image of a postischemic kidney before (Fig. 2, A and C) and after the destruction sequence to burst all contrast agent (Fig. 2, B and D). In all images, the circulating contrast agent has been subtracted, showing adherent contrast agent only; images are shown both in gray scale (Fig. 2, A and B) and green scale (Fig. 2, C and D). Therefore, the green in the images represents bound contrast agent.
Fig. 1.
A: representative ultrasound image of the kidney (outlined in yellow), with an example of a user-defined region of interest shown in white. B: contrast agent time-intensity curve in the contralateral kidney, with data shown before and after contrast agent destruction. C: contrast agent time-intensity curve in the postischemic kidney, with data shown before and after contrast agent destruction.
Fig. 2.
Representative contrast-mode ultrasound image of a postischemic kidney (outlined in yellow) before (A and C) and after (B and D) application of the destruction sequence. In all images, the circulating contrast agent has been subtracted, thus showing adherent contrast agent only. Images are shown both in gray scale (A and B) and green scale, depicting adherent contrast agent only (C and D).
Statistical analysis.
Data were compared by two-factor ANOVA (F statistics with between- and within-groups degrees of freedom are given in the text) or paired Student's t-test using Prism 5 for Mac OSX, version 5.0c (GraphPad Software, San Diego, CA), and are expressed as means ± SE. P values <0.05 were considered statistically significant.
RESULTS
Recovery of renal perfusion is incomplete following unilateral renal ischemia.
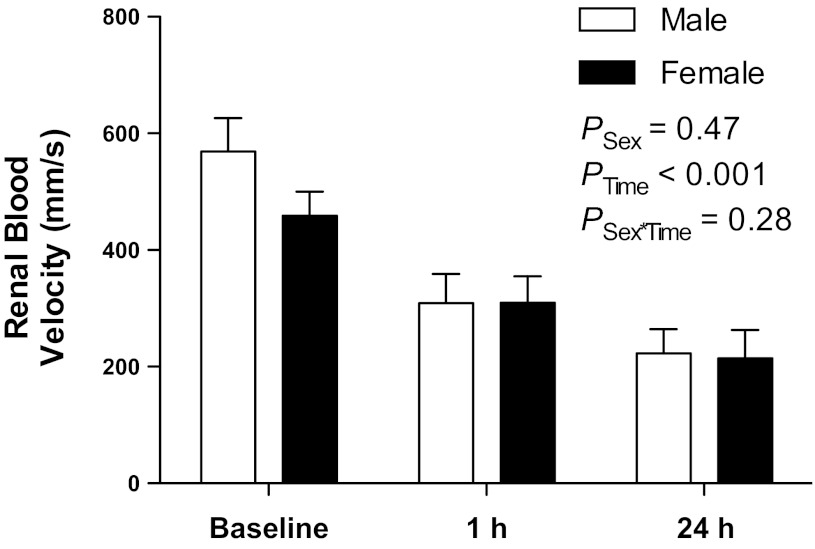
As shown in Fig. 3, peak systolic renal blood velocities measured noninvasively by pulse-wave Doppler ultrasound before ischemia-reperfusion surgery (baseline) were not significantly different between male (569 ± 57 mm/s) and female (459 ± 41 mm/s) mice. Following 1 h unilateral renal ischemia, postischemic kidneys of both male and female mice displayed significantly impaired recovery of renal perfusion [F(2,22) = 32.6; PTime < 0.001, Fig. 3], with perfusion returning to 56 ± 9 and 69 ± 10% of baseline values at 1 h, and to 40 ± 8 and 46 ± 7% at 24 h in male and female mice, respectively. There was no significant main effect of sex on these responses [F(1,22) = 0.55; PSex = 0.5, Fig. 3], nor did the effect of ischemia differ significantly between males and females [F(2,22) = 1.35; PSex×Ischemia = 0.28]. In a subset of animals, renal blood velocity was measured in the contralateral kidney at these time points and showed no significant change from baseline values (data not shown).
Fig. 3.
Peak systolic renal blood velocities measured by pulsed-wave Doppler ultrasound in the right kidney of male (n = 7) and female (n = 6) mice before 1 h ischemia (baseline) and following 1 and 24 h reperfusion. Data are presented as means ± SE and were analyzed by two-factor ANOVA, testing for main effects of sex (PSex), time (PTime), and the interaction between the two (PSex×Time).
P-selectin is upregulated following renal ischemia and reperfusion: Ex vivo and in vivo demonstration.
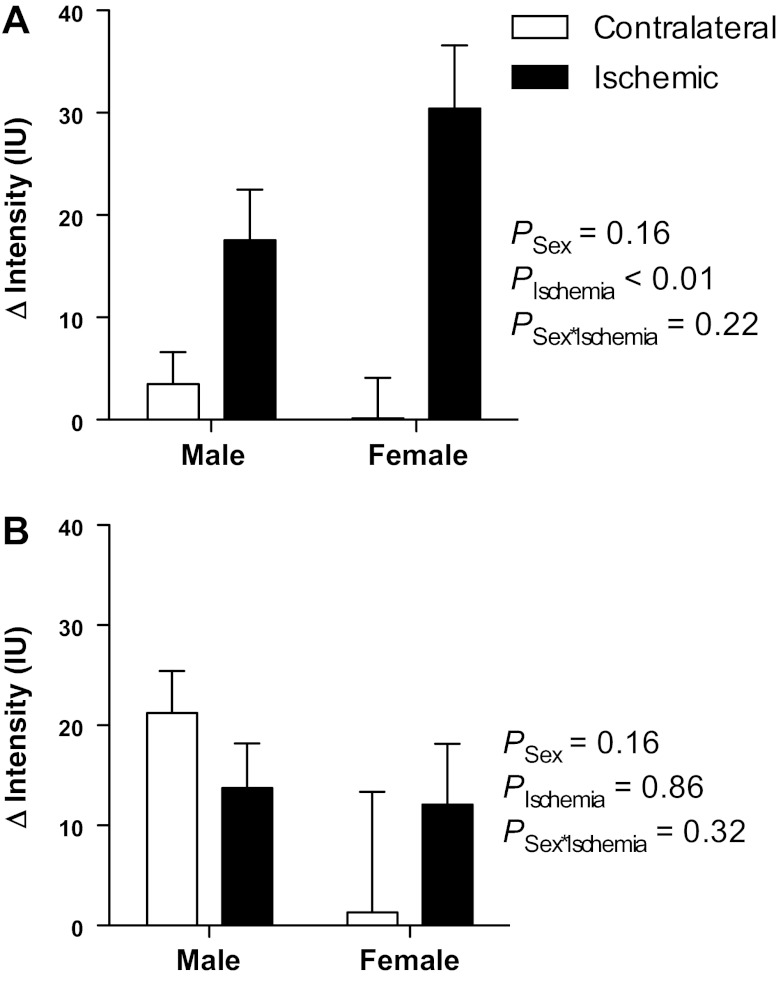
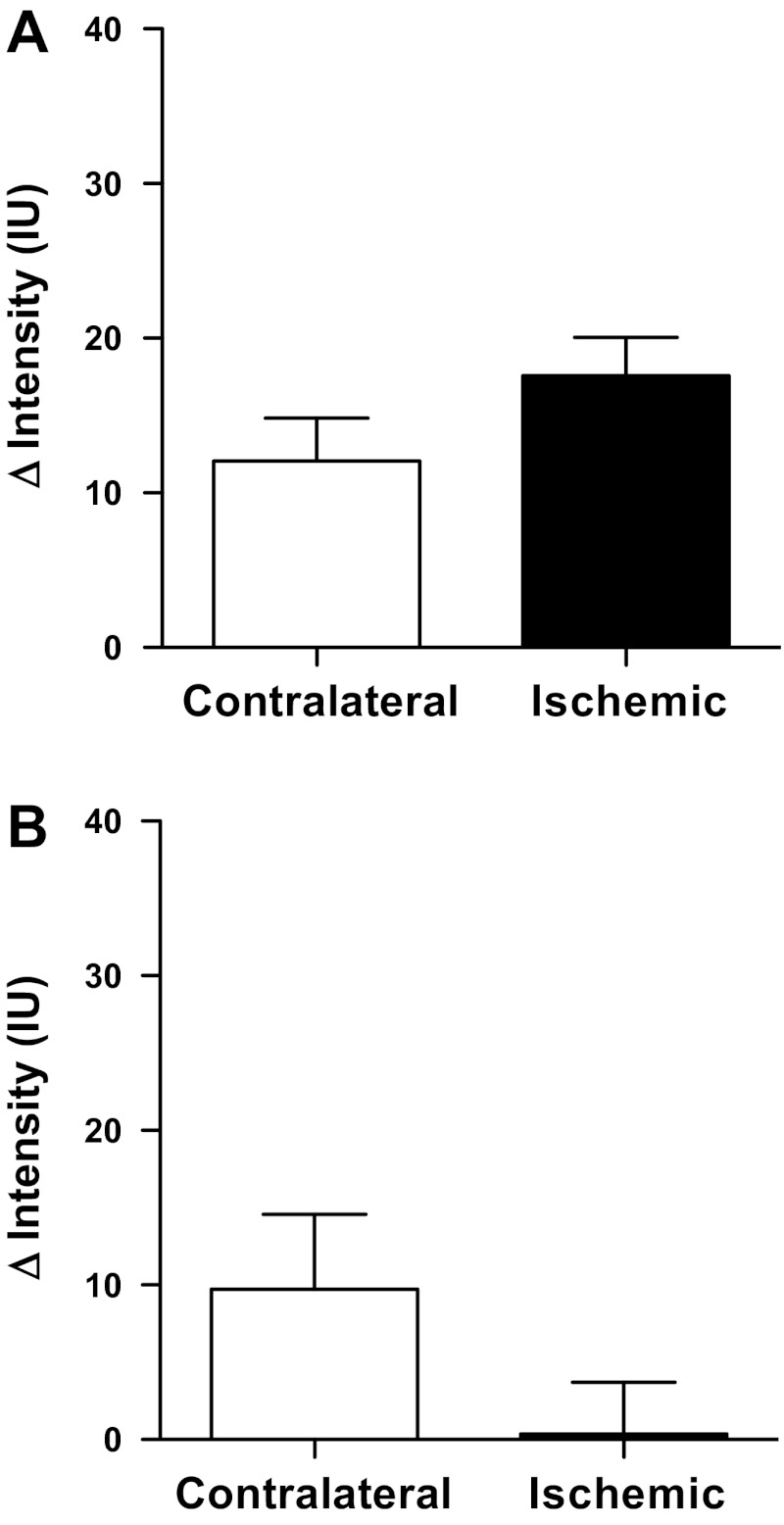
Previous studies have shown that P-selectin is rapidly upregulated following renal ischemia and reperfusion, peaking at 1–4 h postischemia in a bilateral model (14). Accordingly, renal vascular expression of P-selectin was assessed noninvasively in vivo using molecular-targeted contrast imaging after 1 h of reperfusion. The difference in contrast agent intensity before and after application of a destruction sequence was calculated for separate user-defined ROIs in the cortex and medulla of both the postischemic and contralateral kidneys. This difference in intensities reflects the amount of adherent contrast agent. Both male and female mice displayed significantly greater changes in renal cortical contrast intensity following contrast agent destruction in the postischemic kidney than in the contralateral kidney [F(1,9) = 13.17; PIschemia = 0.0055; Fig. 4A], indicating that ischemia and reperfusion induced a significant upregulation of renal cortical vascular P-selectin protein expression. There was no statistically significant sex difference in P-selectin expression overall [F(1,9) = 2.37; PSex = 0.2] or in the upregulation of P-selectin in response to ischemia [F(1,9) = 1.77; PSex×Ischemia = 0.22]. Changes in contrast agent intensity were observed in the medulla following the destruction sequence in both male and female mice (Fig. 4B); however, this effect was not significantly different in the ischemic compared with contralateral kidney [F(1,9) = 0.03; PIschemia = 0.9]. These data suggest that, in contrast to the renal cortex, the outer medulla of both kidneys appears to show P-selectin protein expression following unilateral renal ischemia and reperfusion. There was no significant main effect of sex on medullary P-selectin expression [F(1,9) = 2.33; PSex = 0.16] nor of sex on the effect of ischemia [F(1,9) = 1.09; PSex×Ischemia = 0.32], with the change in intensity closely similar in the ischemic kidney in both male and female mice (14 ± 4 and 12 ± 6 arbitrary units). The medullary values in the contralateral kidney appeared less similar between sexes, but this was likely because of a high degree of variability in values obtained from the female mice (range of −48 to +39 for the contralateral kidney and −5 to +31 for the ischemic kidney; Fig. 4B).
Fig. 4.
Renal P-selectin expression assessed in vivo by contrast-enhanced ultrasound imaging. Data are presented as the absolute change in contrast intensity (ΔIntensity; IU, intensity units) following application of a high-power, low-frequency destruction sequence. Intensity data were obtained from user-defined regions of interest in the renal cortex (A) and medulla (B) of male (n = 5) and female (n = 6) mice following 1 h unilateral ischemia and 1 h of reperfusion. Data are presented as means ± SE and were analyzed by two-factor ANOVA, testing for main effects of sex (PSex), time (PTime), and the interaction between the two (PSex×Time).
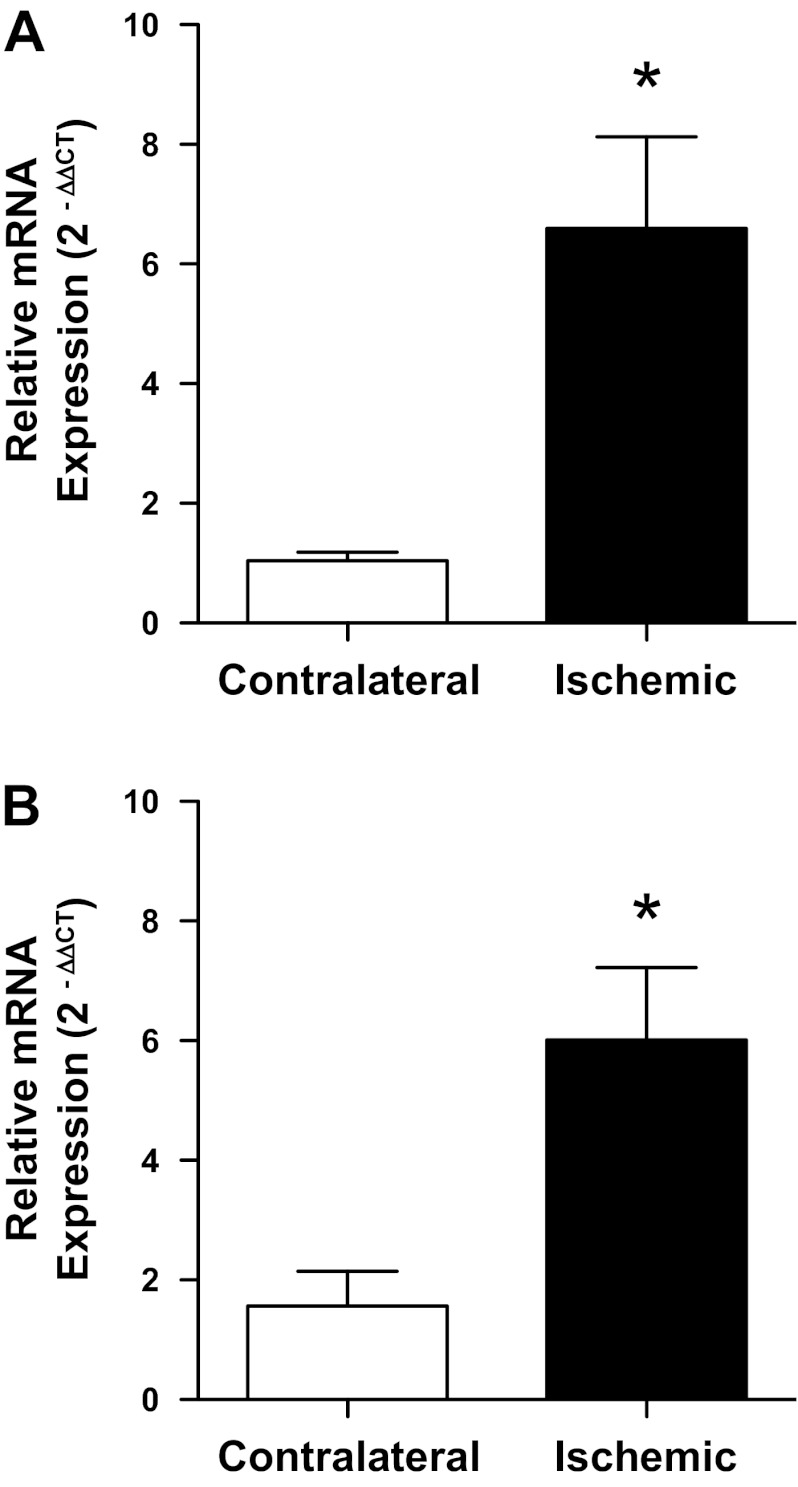
To provide additional confirmation that P-selectin is upregulated after 1 h ischemia and 1 h reperfusion in our model, we used quantitative real-time RT-PCR to measure P-selectin mRNA levels ex vivo in renal vascular preparations from contralateral and postischemic kidneys of male and female mice after 1 h reperfusion (Fig. 5). In both sexes, P-selectin mRNA was approximately sixfold higher in the ischemic kidney compared with the contralateral kidney of the same sex (P = 0.01 in male and P = 0.006 in female mice).
Fig. 5.
Relative expression of P-selectin mRNA in renal vessels isolated from ischemic and contralateral kidneys of male (A) and female (B) mice following 1 h unilateral renal ischemia and 1 h reperfusion. Values are means ± SE for n = 6 in each group. *P < 0.05 vs. contralateral kidney by paired Student's t-test.
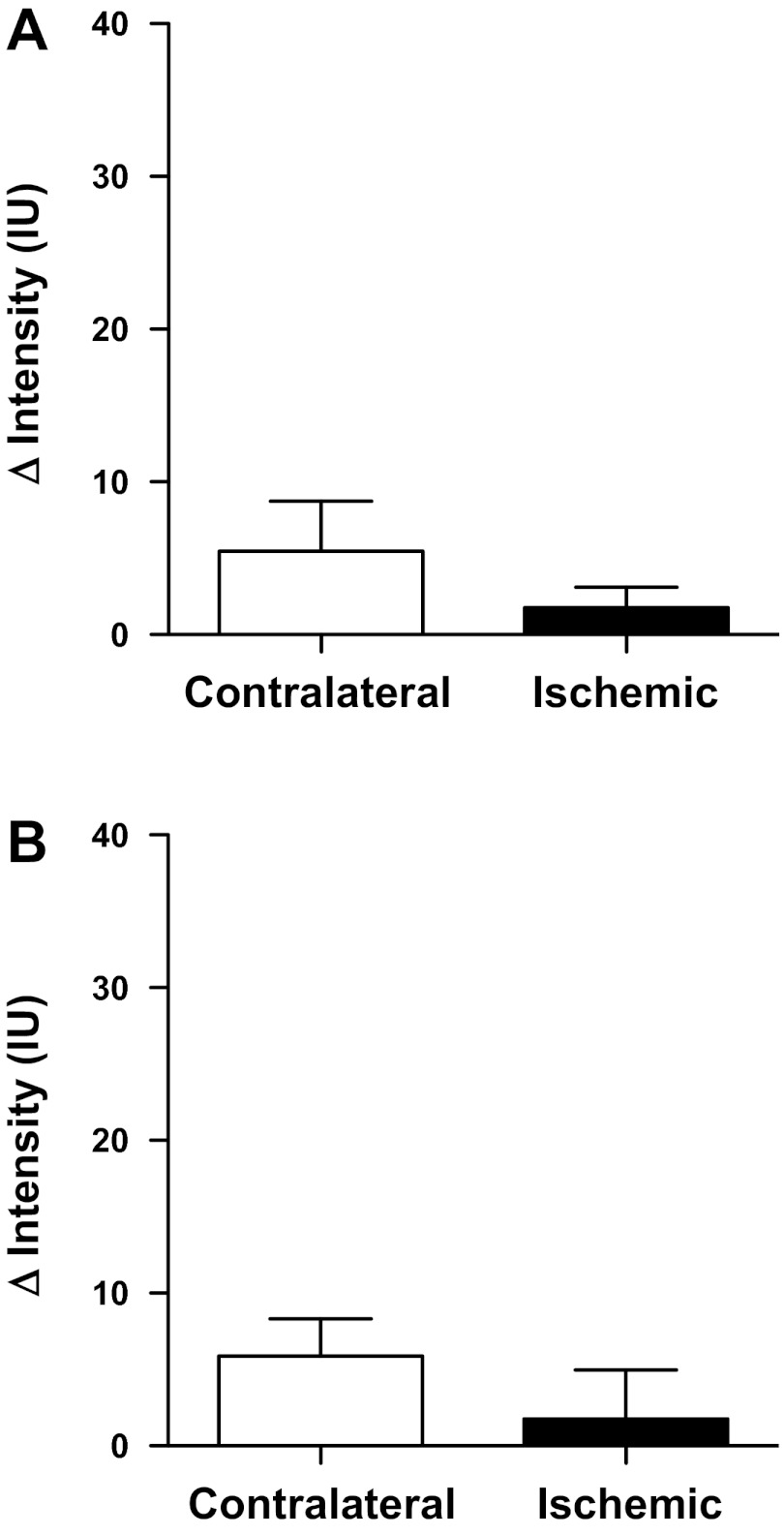
In separate groups of male mice, the kidney was imaged after 1 h unilateral ischemia and 1 h recovery, with an isotype control antibody-conjugated contrast agent used to assess nonspecific binding of molecular-targeted contrast agent. There was no significant difference between ischemic and contralateral kidneys in terms of contrast agent binding in the renal cortex (P = 0.15; Fig. 6A) or medulla (P = 0.12; Fig. 6B). To further verify that any changes in signal intensity observed in the postischemic kidney are not the result of trapping of contrast agent due to vascular congestion following ischemia and reperfusion, additional male mice underwent 1 h unilateral ischemia and 1 h recovery, followed by imaging using nontargeted contrast agent. The changes in signal intensity following the destruction sequence in the postischemic kidney were minimal, averaging 2 ± 1 in the renal cortex and 2 ± 3 intensity units in the renal medulla, which were not significantly different from the contralateral kidney in either case (P = 0.20 and P = 0.13 respectively; Fig. 7).
Fig. 6.
Binding of isotype control antibody-targeted contrast agent following unilateral renal ischemia and reperfusion. Data are presented as the absolute change in contrast intensity (ΔIntensity) following application of a high-power, low-frequency destruction sequence. Intensity data were obtained from user-defined regions of interest in the renal cortex (A) and medulla (B) of male mice (n = 4) following 1 h unilateral ischemia and 1 h of reperfusion and are presented as means ± SE.
Fig. 7.
Binding of nontargeted contrast agent following unilateral renal ischemia-reperfusion. Data are presented as the absolute change in contrast intensity (ΔIntensity) following application of a high-power, low-frequency destruction sequence. Intensity data were obtained from user-defined regions of interest in the renal cortex (A) and medulla (B) of male mice (n = 3) following 1 h unilateral ischemia and 1 h of reperfusion and are presented as means ± SE.
DISCUSSION
The wide availability of whole animal, conditional, and inducible gene knockout technology in mice has allowed investigators to ask highly specific questions regarding the involvement of specific genes in disease processes. To fully capitalize on these advances in gene-targeting technology, there is a need for methodologies to be developed to better study disease progression in vivo. Herein, we report the use of noninvasive ultrasound imaging to assess both renal perfusion and vascular adhesion molecule expression following 1 h unilateral renal ischemia in male and female mice. Our overall finding was that use of this technology is not only feasible, providing the potential for time-course studies to be conducted within the same animal, but gives comparable results to existing invasive methodologies.
In our previous study, restoration of renal perfusion 1 h following 1 h ischemia was found to be impaired in male C57Bl/6 mice (34). In that study, renal blood flow, as measured invasively with a perivascular transit-time ultrasonic flow probe placed acutely around the renal artery, was found to return to only 70 ± 4% of baseline after 1 h reperfusion (34). In the current study, we report that renal arterial blood velocity, as measured by pulsed-wave Doppler ultrasound, shows an almost identical percentage reduction at 1 h, with a further reduction in perfusion seen at 24 h following ischemia and reperfusion. Bonnin and colleagues (9) have also used noninvasive high-frequency ultrasound to detect differences in renal artery blood velocity following an episode of systemic hypoxia in a transgenic mouse model of sickle cell disease and their wild-type counterparts. Together, these studies indicate that noninvasive ultrasound imaging offers a valid means to assess changes in renal perfusion over extended periods of time in mice, allowing for longitudinal studies of disease progression or recovery without the requirement of chronic instrumentation. These measurements of perfusion would provide functional data to complement morphological measurements of the kidney made via ultrasound, such as has been demonstrated previously with polycystic kidney disease (32) and tumor progression (19).
In addition to being a noninvasive technique, thus permitting repeated measurements to be performed in the same subject in vivo, ultrasound imaging provides information in real time, without the need for extensive biological sample processing and analysis. The real-time nature of ultrasound imaging could have an advantage over existing methodologies for detection of acute kidney injury, such as measurement of serum creatinine, which shows a delayed rise and peaks at 24–48 h postinjury. An investigation of the prognostic ability of ultrasound measurements of renal perfusion postischemia to predict subsequent development of renal failure was not the focus of this study but would be an interesting avenue to explore in future studies.
Clinically, there is great interest in discovering novel urinary biomarkers of renal injury as a noninvasive means to diagnose and to monitor both acute kidney injury and chronic kidney disease progression. In the acute kidney injury field, many potential biomarkers that are being actively investigated, such as neutrophil gelatinase-associated lipocalin (28) and netrin (40), are produced by tubular epithelium following injury and, accordingly, may not necessarily be indicative of vascular damage. Urinary albumin excretion has been a mainstay for detecting glomerular injury, particularly in models of chronic renal injury such as diabetes and hypertension; however, other indexes of renal vascular injury have been lacking. Microvascular congestion, with leukocyte adhesion and red cell stacking in the peritubular capillaries, is thought to play a key role in injury to the corticomedullary region in acute kidney injury (38). Accordingly, a noninvasive means to assess endothelial cell activation and adhesion molecule expression in the kidney would provide a tool to monitor this process and gauge the impact of therapeutic interventions.
In the current study, we have demonstrated that a molecular-targeted contrast agent can be used to detect upregulation of P-selectin protein expression noninvasively in the kidney in vivo following renal ischemia-reperfusion injury. Our study supports and extends the findings of previous studies utilizing P-selectin-targeted contrast agent in the kidney to assess expression following ischemia and reperfusion (1, 11, 25), by showing that this technique can be used noninvasively and can be used to analyze effects on the renal cortex and medulla. Immunohistological approaches could also be used to assess P-selectin upregulation in renal tissue, although this requires biopsy, nephrectomy, or death to allow tissue collection. Accordingly, contrast-enhanced ultrasound measurement of P-selectin expression carries the advantages of being noninvasive and much more amenable to repeated use within the same subject to monitor disease progression or the impact of therapeutic interventions on recovery.
Using the more conventional methodology of quantitative real-time RT-PCR, we demonstrated an approximately sixfold increase in P-selectin mRNA expression in renal vascular tissue 1 h postischemia, providing qualitative corroboration of our findings using ultrasound imaging. Although mRNA levels and levels of antibody binding to protein are not directly comparable, these data nonetheless support the validity of molecular-targeted ultrasound as a noninvasive approach to measure adhesion molecule expression in vivo. Chen and colleagues (11) also provided confirmation of P-selectin upregulation ex vivo in their study via immunohistochemical methods. Together, these studies indicate that this methodology could also prove useful in assessing progression or treatment of renal injury due to other causes, since renal inflammation occurs in a variety of disease states, including chronic renal failure (33), hypertension (7, 13), diabetes (15), glomerulonephritis (39), and autoimmune diseases such as systemic lupus erythematosus (22). In addition, ultrasound in conjunction with specialized contrast agent destruction has been used as an efficient means of gene transfer in the kidney (21, 23). If combined with the use of antibodies directed against activated endothelium, this technology could provide a means to target therapeutic compounds to sites of injury. Indeed, emerging technologies such as E-selectin-targeted liposomes, which specifically target activated endothelial cells (2), and molecular-targeted microbubbles (12) have the potential to exploit cell type-specific mechanisms for therapeutic gain.
Differential binding of the contrast agent to the ischemic vs. contralateral kidney appeared more robust in the renal cortex than in the renal medulla. In our previous study using contrast-enhanced ultrasound to measure regional perfusion of the kidney, we noted a relatively low signal and high degree of variability of measurements for inner medullary perfusion (37). This is perhaps not surprising, given that the inner medulla receives a very small percentage of total renal blood flow, whereas the cortex receives ∼90% (29). In the current study, we also observed less consistent image quality and a high degree of variability of intensity data in the inner medulla and so have not included analysis of this region. Accordingly, we would urge caution in interpreting contrast-enhanced ultrasound data from the inner medulla in future studies. A potential caveat to the use of molecular-targeted ultrasound is that slower blood flow could perhaps facilitate antibody binding. On the other hand, lower blood flow may also reduce the delivery and thus availability of antibody for binding. Our mRNA data from the current study and the work of Chen et al. (11) using immunohistochemistry to detect enhanced P-selectin protein expression in the reperfused kidney both support upregulation of P-selectin in the postischemic kidney compared with contralateral kidney. Together, these studies indicate that the enhanced signal in the postischemic cortex is at least in part the result of increased P-selectin expression rather than generated by a change in hemodynamics that facilitates antibody binding in the absence of an actual change in P-selectin expression.
A number of studies have shown evidence of a sexual dimorphism in the symptoms, incidence, and severity of ischemia-reperfusion injury, including to the heart (44) and brain (26). As reviewed by Hutchens and colleagues (18), women are generally at lower risk of perioperative acute kidney injury than men, except following cardiac surgery. In experimental models of renal ischemia-reperfusion injury, several studies have shown that females are protected relative to males with regard to factors such as histological injury (30), functional impairment, and mortality, although females may be more sensitive to nephrotoxic models of acute injury (41). In the current study, there was no statistically significant evidence of a sexual dimorphism in either the impairment of reperfusion or upregulation of P-selectin protein expression, at least in the renal cortex. The use of a relatively long ischemic period (1 h), subsequently inducing severe renal injury, may have reduced the sensitivity with which to detect any protective effect of female sex on renal injury in our model.
A limitation of the current study is that P-selectin is also produced by platelets. Accordingly, we cannot rule out the possibility that some of the P-selectin we measured by either technique was contributed by platelets rather than purely representing activated endothelium. Future studies should consider the use of alternative, more vascular-specific adhesion molecules, such as E-selectin. Another observation in the current study was that there appeared to be significant P-selectin-targeted contrast agent binding in the contralateral renal medulla (Fig. 4B). This could be due to some degree of inflammation occurring in the contralateral kidney as a result of circulating factors released by the injured kidney, since renal ischemia and reperfusion can produce inflammation and injury in remote organs, including the contralateral kidney (6, 17). There was also some binding of isotype control antibody-targeted contrast observed (Fig. 6). This may be due to immunoglobulin binding by leukocytes, as suggested previously by Lindner and colleagues (25). Notably, very little apparent binding of nontargeted contrast agent occurred (Fig. 7), indicating that the contrast agent itself does not show significant nonspecific binding.
In conclusion, these studies highlight the utility of noninvasive high-resolution ultrasound for assessing renal perfusion noninvasively in mice at multiple time points in vivo. Furthermore, these studies support the use of a molecular-targeted contrast agent to noninvasively assess renal cortical expression of adhesion molecules in vivo. Both approaches are highly amenable to studying aspects of renal disease progression or treatment over time in mice and should be considered in future studies.
GRANTS
This work was supported by a Medical College of Georgia Cardiovascular Discovery Institute Seed Award (E. I. Boesen and J. C. Sullivan), an American Heart Association Scientist Development Grant (E. I. Boesen), and National Heart, Lung, and Blood Institute Grant HL-093271.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.I.B. and J.C.S. conception and design of research; E.I.B., G.R.C., and J.C.S. performed experiments; E.I.B. and G.R.C. analyzed data; E.I.B. and J.C.S. interpreted results of experiments; E.I.B. and J.C.S. prepared figures; E.I.B. and J.C.S. drafted manuscript; E.I.B. and J.C.S. edited and revised manuscript; E.I.B., G.R.C., and J.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Ryan A. Harris for helpful discussions regarding statistics.
REFERENCES
- 1. Andonian S, Coulthard T, Smith AD, Singhal PS, Lee BR. Real-time quantitation of renal ischemia using targeted microbubbles: in-vivo measurement of P-selectin expression. J Endourol 23: 373– 378, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Asgeirsdottir SA, Zwiers PJ, Morselt HW, Moorlag HE, Bakker HI, Heeringa P, Kok JW, Kallenberg CG, Molema G, Kamps JA. Inhibition of proinflammatory genes in anti-GBM glomerulonephritis by targeted dexamethasone-loaded AbEsel liposomes. Am J Physiol Renal Physiol 294: F554– F561, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 69: 184– 189, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int 72: 151– 156, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887– F899, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Basile DP, Leonard EC, Tonade D, Friedrich JL, Goenka S. Distinct effects on long-term function of injured and contralateral kidneys following unilateral renal ischemia-reperfusion. Am J Physiol Renal Physiol 302: F625– F635, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boesen EI, Krishnan KR, Pollock JS, Pollock DM. ETA activation mediates angiotensin II-induced infiltration of renal cortical T cells. J Am Soc Nephrol 22: 2187– 2192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boesen EI, Sasser JM, Saleh MA, Potter WA, Woods M, Warner TD, Pollock JS, Pollock DM. Interleukin-1β but not interleukin-6 enhances renal and systemic endothelin production in vivo. Am J Physiol Renal Physiol 295: F446– F453, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonnin P, Sabaa N, Flamant M, Debbabi H, Tharaux PL. Ultrasound imaging of renal vaso-occlusive events in transgenic sickle mice exposed to hypoxic stress. Ultrasound Med Biol 34: 1076– 1084, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O'Donnell MP, Rabb H. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest 108: 1283– 1290, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen SM, Yang L, Bin JP, Wu JF, Zheng DW, Liu YL. Evaluation of renal tissue ischemia-reperfusion injury with microbubbles targeted to P-selectin and contrast ultrasound. Chinese J Med Imaging Technol 7: 985– 988, 2008 [Google Scholar]
- 12. Deelman LE, Decleves AE, Rychak JJ, Sharma K. Targeted renal therapies through microbubbles and ultrasound. Adv Drug Deliv Rev 62: 1369– 1377, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Miguel C, Das S, Lund H, Mattson DL. T-lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136– R1142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farrar CA, Wang Y, Sacks SH, Zhou W. Independent pathways of P-selectin and complement-mediated renal ischemia/reperfusion injury. Am J Pathol 164: 133– 141, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fornoni A, Ijaz A, Tejada T, Lenz O. Role of inflammation in diabetic nephropathy. Curr Diabetes Rev 4: 10– 17, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Soloski MJ, Crow MT, Rabb H. Mycophenolate mofetil modifies kidney tubular injury and Foxp3+ regulatory T cell trafficking during recovery from experimental ischemia-reperfusion. Transpl Immunol 23: 45– 52, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grams ME, Rabb H. The distant organ effects of acute kidney injury. Kidney Int 81: 942– 948, 2012 [DOI] [PubMed] [Google Scholar]
- 18. Hutchens MP, Dunlap J, Hurn PD, Jarnberg PO. Renal ischemia: does sex matter? Anesth Analg 107: 239– 249, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Jouannot E, Duong-Van-Huyen JP, Bourahla K, Laugier P, Lelievre-Pegorier M, Bridal L. High-frequency ultrasound detection and follow-up of Wilms' tumor in the mouse. Ultrasound Med Biol 32: 183– 190, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest 97: 1056– 1063, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koike H, Tomita N, Azuma H, Taniyama Y, Yamasaki K, Kunugiza Y, Tachibana K, Ogihara T, Morishita R. An efficient gene transfer method mediated by ultrasound and microbubbles into the kidney. J Gene Med 7: 108– 116, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Kuroiwa T, Lee EG. Cellular interactions in the pathogenesis of lupus nephritis: the role of T cells and macrophages in the amplification of the inflammatory process in the kidney. Lupus 7: 597– 603, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Lan HY, Mu W, Tomita N, Huang XR, Li JH, Zhu HJ, Morishita R, Johnson RJ. Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. J Am Soc Nephrol 14: 1535– 1548, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Liano F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 50: 811– 818, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Lindner JR, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation 104: 2107– 2112, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Martinez-Sanchez P, Fuentes B, Fernandez-Dominguez J, Ortega-Casarrubios Mde L, Aguilar-Amar MJ, Abenza-Abildua MJ, Idrovo-Freire L, Diez-Tejedor E. Young women have poorer outcomes than men after stroke. Cerebrovasc Dis 31: 455– 463, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Mehta RL. Outcomes research in acute renal failure. Semin Nephrol 23: 283– 294, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534– 2543, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Pallone TL, Robertson CR, Jamison RL. Renal medullary microcirculation. Physiol Rev 70: 885– 920, 1990 [DOI] [PubMed] [Google Scholar]
- 30. Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem 279: 52282– 52292, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet 364: 1814– 1827, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Pollard R, Yunis R, Kultz D, Martin P, Griffey S, Ferrara K. Ultrasound detection and characterization of polycystic kidney disease in a mouse model. Comp Med 56: 215– 221, 2006 [PubMed] [Google Scholar]
- 33. Rodriguez-Iturbe B, Garcia Garcia G. The role of tubulointerstitial inflammation in the progression of chronic renal failure. Nephron Clin Pract 116: c81– c88, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Schneider MP, Sullivan JC, Wach PF, Boesen EI, Yamamoto T, Fukai T, Harrison DG, Pollock DM, Pollock JS. Protective role of extracellular superoxide dismutase in renal ischemia/reperfusion injury. Kidney Int 78: 374– 381, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 114: 5– 14, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singbartl K, Green SA, Ley K. Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure. FASEB J 14: 48– 54, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Sullivan JC, Wang B, Boesen EI, D'Angelo G, Pollock JS, Pollock DM. Novel use of ultrasound to examine regional blood flow in the mouse kidney. Am J Physiol Renal Physiol 297: F228– F235, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 62: 1539– 1549, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Wada T, Matsushima K, Kaneko S. The role of chemokines in glomerulonephritis. Front Biosci 13: 3966– 3974, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Wang W, Reeves WB, Ramesh G. Netrin-1 and kidney injury. I. Netrin-1 protects against ischemia-reperfusion injury of the kidney. Am J Physiol Renal Physiol 294: F739– F747, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wei Q, Wang MH, Dong Z. Differential gender differences in ischemic and nephrotoxic acute renal failure. Am J Nephrol 25: 491– 499, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17: 1135– 1142, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Yu SJ, Oh DJ, Yu SH. The investigation of macrophage infiltration in the early phase of ischemic acute renal failure in mice. Korean J Intern Med 23: 64– 71, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zbierajewski-Eischeid SJ, Loeb SJ. Myocardial infarction in women: promoting symptom recognition, early diagnosis, and risk assessment. Dimens Crit Care Nurs 28: 1– 6, 2009 [DOI] [PubMed] [Google Scholar]