Abstract
Pharmacological blockade of cyclic nucleotide phosphodiesterase (PDE) can relax human urinary bladder smooth muscle (UBSM); however, the underlying cellular mechanism is unknown. In this study, we investigated the effects of PDE pharmacological blockade on human UBSM excitability, spontaneous and nerve-evoked contractility, and determined the underlying cellular mechanism mediating these effects. Patch-clamp electrophysiological experiments showed that 3-isobutyl-1-methylxanthine (10 μM), a nonselective PDE inhibitor, caused ∼3.6-fold increase in the transient KCa1.1 channel current frequency and ∼2.5-fold increase in the spontaneous transient hyperpolarization frequency in UBSM-isolated cells. PDE blockade also caused ∼5.6-mV hyperpolarization of the UBSM cell membrane potential. Blocking the KCa1.1 channels with paxilline abolished the spontaneous transient hyperpolarization and the hyperpolarization effect of PDE blockade on the UBSM cell membrane potential. Live cell Ca2+-imaging experiments showed that PDE blockade significantly decreased the global intracellular Ca2+ levels. Attenuation of PDE activity significantly reduced spontaneous phasic contraction amplitude, muscle force integral, duration, frequency, and muscle tone of human UBSM isolated strips. Blockade of PDE also significantly reduced the contraction amplitude, muscle force integral, and duration of the nerve-evoked contractions induced by 20-Hz electrical field stimulation. Pharmacological inhibition of KCa1.1 channels abolished the relaxation effects of PDE blockade on both spontaneous and nerve-evoked contractions in human UBSM-isolated strips. Our data provide strong evidence that in human UBSM PDE is constitutively active, thus maintaining spontaneous UBSM contractility. PDE blockade causes relaxation of human UBSM by increasing transient KCa1.1 channel current activity, hyperpolarizing cell membrane potential, and decreasing the global intracellular Ca2+.
Keywords: overactive bladder, hyperpolarization, patch-clamp, contractility
the large-conductance voltage- and Ca2+-activated K+ channel, KCa1.1, also known as BK channel, is highly expressed in urinary bladder smooth muscle (UBSM) and is a key regulator of human UBSM excitability and contractility (5, 10, 12, 16, 18, 21, 24). Our recent studies demonstrated that the direct pharmacological activation or inhibition of KCa1.1 channels with selective openers or inhibitors, respectively, is highly effective in controlling human UBSM cell membrane potential and thereby UBSM contractility (16, 18). Impaired KCa1.1 channel activity has been shown to be one of the major contributors to detrusor overactivity (DO) in benign prostatic hyperplasia patients, and it also contributes to neurogenic DO (2, 7, 21). These studies indicate clearly that KCa1.1 channels are important regulators of human UBSM function suggesting that KCa1.1 channels can be novel therapeutic targets for the treatment of urinary bladder dysfunction related to DO and potentially urinary retention due to UBSM underactivity (18, 21, 24).
KCa1.1 channels are activated by Ca2+ released from the sarcoplasmic reticulum (SR) through ryanodine receptors (RyRs), known as “Ca2+ sparks” (8, 14, 26). Pharmacological manipulation of intracellular Ca2+ can regulate KCa1.1 channel activity (26). In freshly isolated guinea pig, mouse, and rat UBSM cells, activation of β-adrenergic receptors that activates the cAMP pathway increases Ca2+ sparks which stimulate KCa1.1 channel activity (6, 17, 26). KCa1.1 channel activation results in UBSM cell membrane potential hyperpolarization and UBSM relaxation via cAMP and cAMP-dependent protein kinase A (PKA) (6, 17, 24, 26).
cAMP is hydrolyzed by phosphodiesterase (PDE); thus, PDE pharmacological blockade can readily elevate cellular cAMP levels and activate PKA signaling pathways (3). There are 11 known PDE isoenzymes of which five (PDE1-PDE5) have been reported in the human urinary bladder (4, 20, 30). Previous studies showed that blockade of PDE1, PDE4, and PDE5 can reduce human UBSM contractility (22, 28, 29). However, the underlying cellular mechanism of PDE regulation of human UBSM function is unknown.
Our recent study, using guinea pig as an animal model, demonstrated that nonselective PDE inhibition can reduce UBSM excitability and contractility (32). However, there is no information on PDE regulation of KCa1.1 channels in human UBSM cells, the target species of interest for therapeutic interventions. While animal models are somehow useful for understanding the physiology and pathophysiology of human bladder disorder, such as overactive bladder (OAB), the knowledge gained from animal models cannot be directly applied to humans due to substantial species differences (1, 23). Rodent urination patterns are completely different from humans: male rodents mark their territories of dominance by means of frequent urination (11). Micturition in rodents includes external urethral bursting activity associated with pulsatile urine flow compared with continuous flow in higher mammals, including humans, under normal physiological conditions. Most likely, this distinct rodent behavior is determined by differential regulatory mechanisms, including differences in the regulation of UBSM ion channels. Also, the patterns of action potentials of human and guinea pig UBSM are substantially different (12). Furthermore, studies show that the PDE expression profile in humans is different from guinea pigs (19). PDE1A, PDE1B, PDE3A, PDE3B, PDE4A-C mRNA are expressed in human cardiac myocytes but are not detectable in guinea pig cardiac myocytes (19). The PDE2A, PDE4D, and PDE5A relative mRNA levels in human cardiac myocytes are much lower than in guinea pig myocytes (19). Rolipram, a PDE4-selective inhibitor, inhibits PDE4 in human neutrophil with an IC50 value of 4.52 ± 1.55 μM which is higher than that in guinea pig neutrophil (0.56–1.17 μM) (9). Due to the significant differences between human and guinea pig PDEs, it is important to obtain human data to verify the findings in guinea pig, and to better understand human bladder physiology. The data collected from human UBSM will lay the foundation of further investigation for the therapeutic potential of selective PDE inhibitors in the treatment of bladder dysfunctions.
MATERIALS AND METHODS
Human UBSM tissue collection.
Human UBSM tissue specimens were obtained from routine open bladder surgeries for bladder cancer according to protocol HR 16918, which was reviewed and approved by the Medical University of South Carolina Institutional Review Board. All specimens were obtained from patients without a preoperative history of OAB. We used 13 male and 4 female patients, 12 Caucasian and 5 African American from 46 to 79 years of age (mean 64.9 ± 2.2 yr).
UBSM single cell isolation.
UBSM single cells were freshly isolated as described previously (15, 16, 18). Briefly, 1–2 UBSM strips (5- to 10-mm long, 2- to 4-mm wide) free of mucosa were incubated in 2 ml of dissection solution (DS) at 37°C for 30 min (Solutions and drugs) supplemented with 1 mg/ml bovine serum albumin (BSA), 1 mg/ml papain, and 1 mg/ml dl-dithiothreitol. The UBSM tissues were then transferred to 2 ml of DS supplemented with 1 mg/ml BSA, 0.5 mg/ml collagenase, 0.5 mg/ml trypsin inhibitor, 100 μM CaCl2 and incubated at 37°C for 20–25 min followed by three washouts with DS supplemented with 1 mg/ml BSA. Individual cells were released from the tissue by passing the enzyme-treated strips through a fire-polished Pasteur pipette. The freshly isolated UBSM cells were used within 8 h following isolation.
Electrophysiological recordings.
The amphotericin-B perforated whole cell patch-clamp technique was used for electrophysiological recordings on freshly isolated single human UBSM cells. Only cells that adhered to the glass bottom, which was not coated with poly-l-lysine, of the recording chamber were used in the experiments. Patch-clamp recordings were conducted using a system equipped with Axopatch 200B amplifier controlled with pCLAMP 10.2 software (Molecular Devices, Union City, CA), Digidata 1440A, and an eight-pole Bessel filter 900CT/9L8L (Frequency Devices, Ottawa, IL). The patch-clamp glass pipettes with fire-polished tips were made from borosilicate glass (Sutter Instruments, Novato, CA) using a Narishige PP-830 puller and a Micro Forge MF-830 fire polisher (Narishige Group, Tokyo, Japan). Pipette resistance was 4 to 6 MΩ. The spontaneous transient outward currents (STOCs), which are mediated by the KCa1.1 channels, were recorded at a holding potential of −40 mV and UBSM cell membrane potential was recorded using the current-clamp mode of the patch-clamp technique (16). The average capacitance of 13 cells was 29.4 ± 2.6 pF. All patch-clamp experiments were conducted at room temperature (22–23°C). Voltages were corrected for the junction potential of ∼−10 mV.
Monitoring the change of intracellular Ca2+ levels in freshly isolated human UBSM cells.
The intracellular Ca2+ levels were monitored using a fluorescent probe, fura 2-AM, as previously described (18). Briefly, UBSM cells were placed on a 35-mm glass bottom dish coated with poly-l-lysine and were incubated with 2 μM fura 2-AM in extracellular solution for 30 min in the dark. Fura 2-AM was washed out three times with fresh extracellular solution. Cells were imaged with an Olympus IX81 motorized inverted microscope equipped with a ×40 oil objective and MetaFluor 7.7.2.0 software. The intracellular Ca2+ level was expressed as fura-2 fluorescence emission intensity ratio at 510 nm determined with a 200-ms exposure at 340 and 380 nm (F340/F380) every 3 s. All Ca2+ imaging experiments were carried out at room temperature (∼22–23°C).
Isometric UBSM tension recordings.
Isometric contractions of UBSM-isolated strips were measured as previously described (16, 18). Briefly, UBSM strips were secured to isometric force displacement transducers and were suspended in temperature-controlled (37°C) water-jacketed tissue baths containing 10 ml physiological saline solution (PSS) aerated with 95% O2-5% CO2, pH 7.4. The UBSM strips were subjected to an initial tension of 1 g, and the strips were washed with fresh PSS every 15 min during an equilibration period of 45–60 min. To minimize the potential effects of neurotransmitters released from the UBSM neurons, the experiments on spontaneous UBSM contractions were performed in the presence of 1 μM tetrodotoxin (TTX), a selective blocker of the neuronal Na+ channels.
Nerve-evoked contractions were induced by electrical field stimulation (EFS) using a pair of platinum electrodes mounted in the tissue baths parallel to the UBSM strips. The EFS pulse parameters were as follows: 0.75-ms pulse width, 20-V pulse amplitude, 3-s stimulus duration, and polarity was reversed for alternating pulses. After the equilibration period, the UBSM strips were subjected to continuous repetitive EFS with a frequency of 20 Hz at 1-min intervals. The EFS pulses were generated using a PHM-152I stimulator and the UBSM contractions were recorded using a Myomed myograph system (MED Associates, St. Albans, VT).
Solutions and drugs.
The nominally Ca2+-free DS contained (in mM) 80 monosodium glutamate, 55 NaCl, 6 KCl, 10 glucose, 10 HEPES, and 2 MgCl2, pH 7.3, adjusted with NaOH. The extracellular solution for whole cell patch-clamp experiments contained (in mM) 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH adjusted to 7.4 with NaOH. The pipette solution contained (in mM) 110 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 10 HEPES, and 0.05 EGTA, pH adjusted to 7.2 with NaOH and supplemented with freshly dissolved (every 1–2 h) 200 μg/ml amphotericin-B. The Ca2+-containing PSS was prepared daily and contained (in mM) 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, and 11 glucose. Trypsin inhibitor, BSA, and amphotericin-B were obtained from Thermo Fisher Scientific (Fair Lawn, NJ). Papain was purchased from Worthington Biochemical (Lakewood, NJ). IBMX, paxilline, fura 2-AM, collagenase (type II), and TTX (in citrate buffer) were purchased from Sigma (St. Louis, MO).
Data analysis and statistics.
The membrane potential was analyzed using Clampfit 10.2 (Molecular Devices). The STOCs and spontaneous transient hyperpolarizations (STHs) frequency and amplitude, the UBSM phasic contraction amplitude, muscle force integral, contraction duration, contraction frequency, and muscle tone were analyzed using MiniAnalysis (Synaptosoft, Decatur, GA). Muscle force integral was determined by integrating the area under the phasic contraction force-time baseline curve. The relative change of muscle tone was determined by measuring changes of the phasic contraction baseline curve. The spontaneous and 20-Hz EFS-induced contraction parameters were normalized to the respective control (taken to be 100%). Data were further analyzed with GraphPad Prism 4.03 software (GraphPad Software, La Jolla, CA) and expressed as means ± SE, n = the number of UBSM cells or strips, and N = the number of patients. Statistical significance was tested using paired Student's t-test and P < 0.05 was considered significant.
RESULTS
Blockade of PDE increased the STOCs frequency in human UBSM-isolated cells.
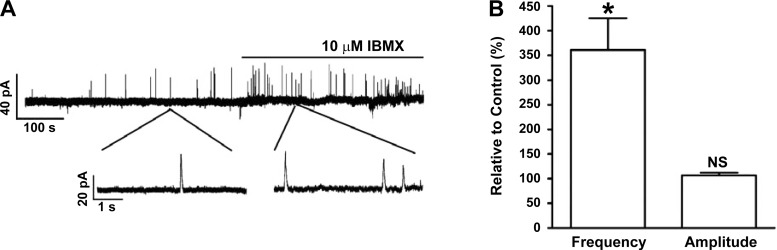
In UBSM cells, localized Ca2+ release from SR RyRs, known as “Ca2+ sparks,” transiently activates the closely located KCa1.1 channels and generates STOCs (16). The STOCs were recorded at a holding potential of −40 mV, which is close to UBSM resting membrane potential (25). In human UBSM-isolated cells, PDE blockade with 3-isobutyl-1-methylxanthine (IBMX; 10 μM) increased the STOCs frequency to 361.1 ± 64.8% of the control activity (n = 4, N = 4; P < 0.05), without a significant effect on the average STOCs amplitude (106.6 ± 5.9%; n = 4, N = 4; P > 0.05; Fig. 1). STOCs cause UBSM cell membrane hyperpolarization. Hence, we next evaluated the effect of PDE blockade on human UBSM cell resting membrane potential.
Fig. 1.
Pharmacological blockade of phosphodiesterase (PDE) with 10 μM 3-isobutyl-1-methylxanthine (IBMX) increased the spontaneous transient outward currents (STOCs) frequency in human urinary bladder smooth muscle (UBSM)-isolated cells. A: original recording illustrating that PDE blockade increased the frequency of STOCs in a single UBSM cell. Portions of the recording are shown on an expanded time scale before and after IBMX application. B: summary data showing that PDE blockade significantly increased the STOCs frequency without a significant effect on the STOCs amplitude [n = 4, N = 4; *P < 0.05; nonsignificant (NS)]. The STOCs frequency and average amplitude under control conditions were taken to be 100%, respectively.
Blockade of PDE increased the STHs frequency and hyperpolarized the membrane potential of isolated human UBSM cells.
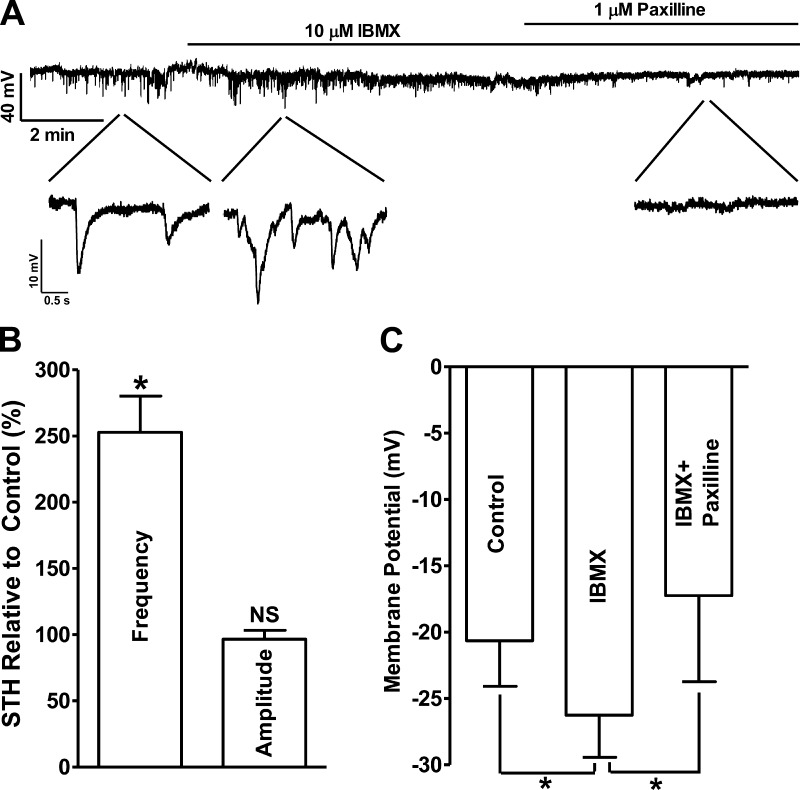
The cell membrane potential was recorded using the perforated patch-clamp technique in the current-clamp mode (Ih = 0). In cells exhibiting STHs, blockade of PDE increased STHs frequency to 252.8 ± 27.3% of the control values (n = 4, N = 4; P < 0.05; Fig. 2, A and B), without any effect on the average STHs amplitude (96.6 ± 6.8% of the control values; n = 4, N = 4; P > 0.05; Fig. 2). Blocking KCa1.1 channels with paxilline (1 μM), following IBMX application, abolished the STHs and depolarized the cell membrane potential. Under control conditions, the UBSM cell membrane potential was −20.6 ± 3.4 mV, and PDE blockade hyperpolarized the membrane potential to −26.3 ± 3.2 mV (n = 5, N = 4; P < 0.05; Fig. 2C). The subsequent addition of 1 μM paxilline reversed the membrane potential hyperpolarization induced by the blockade of PDE and further depolarized the membrane potential to −17.4 ± 6.5 mV (n = 5, N = 4; P < 0.05 vs. IBMX; Fig. 2, A and C).
Fig. 2.
Pharmacological blockade of PDE with 10 μM IBMX increased the spontaneous transient hyperpolarizations (STHs) frequency and hyperpolarized human UBSM cell membrane potential. A: original current-clamp recording illustrating that PDE blockade increased the STHs frequency and hyperpolarized the UBSM cell membrane potential, and paxilline (1 μM) abolished STHs. Portions of the recording are shown on an expanded time scale. B: summary data showing that PDE blockade significantly increased STHs frequency but had no effect on the STHs amplitude (n = 4, N = 4; *P < 0.05; NS). C: summary data showing that PDE blockade significantly hyperpolarized the UBSM cell membrane potential, and paxilline reversed the hyperpolarization effect of IBMX (n = 5, N = 4; *P < 0.05).
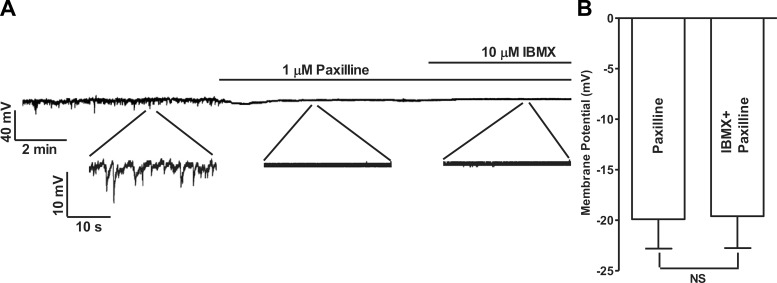
To further confirm that the hyperpolarization effect of PDE blockade was mediated by the KCa1.1 channels, KCa1.1 channels were inhibited with paxilline before the application of IBMX. Paxilline (1 μM) abolished the STHs and eliminated the hyperpolarization effect of PDE blockade in UBSM cells. The membrane potentials were −19.9 ± 2.9 mV in the presence of paxilline and −19.6 ± 3.1 mV in the presence of both paxilline and IBMX [n = 4, N = 4; nonsignificant (NS); Fig. 3]. These results confirmed that the hyperpolarization effect of PDE blockade on UBSM cell membrane potential is mediated by the KCa1.1 channels. Since the human UBSM cell membrane potential directly affects the intracellular Ca2+ levels (18), we next investigated whether the hyperpolarization of the membrane potential induced by PDE blockade influences the intracellular Ca2+ levels.
Fig. 3.
Pharmacological inhibition of KCa1.1 channels with paxilline abolished the hyperpolarizing effect of IBMX in human UBSM-isolated cells. A: original current-clamp recording illustrating that 1 μM paxilline abolished STHs and that the subsequent addition of IBMX (10 μM) did not have any additional effect on the membrane potential. Portions of the recording are shown on an expanded time scale. B: summary data showing that in the presence of 1 μM paxilline, the addition of IBMX did not cause any change in the UBSM cell membrane potential (n = 4, N = 4; NS).
PDE blockade decreased the intracellular Ca2+ levels in human UBSM-isolated cells.
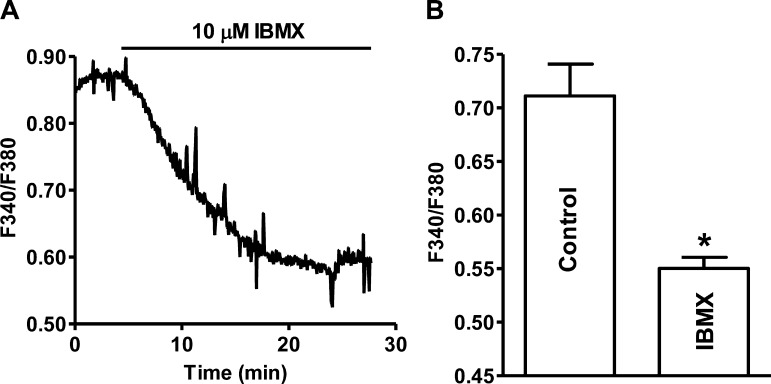
The global Ca2+ levels decreased significantly from 0.71 ± 0.03 under control condition to 0.55 ± 0.01 following the addition of 10 μM IBMX (n = 22, N = 4; P < 0.05; Fig. 4). We next determined how the decrease of Ca2+ levels affects human UBSM spontaneous phasic contractions.
Fig. 4.
Pharmacological PDE blockade with 10 μM IBMX reduced the intracellular Ca2+ levels in freshly isolated human UBSM cells. A: original recording illustrating that PDE blockade decreased the cellular Ca2+ levels in a single UBSM cell shown as a ratio of fura-2 fluorescence emission intensities at 510 nm with excitation at 340 and 380 nm. B: summary data indicating that PDE blockade significantly decreased the intracellular Ca2+ levels (n = 22, N = 4; *P < 0.05).
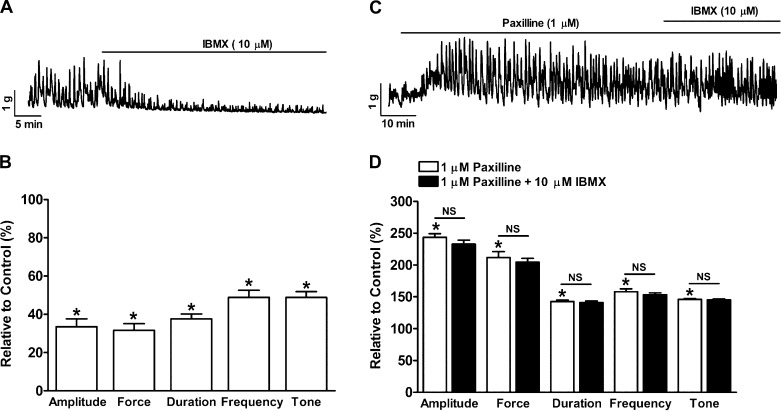
PDE blockade suppressed the spontaneous phasic (myogenic) contractions of human UBSM-isolated strips.
Human UBSM-isolated strips exhibit spontaneous phasic contractions (Fig. 5). IBMX (10 μM) reduced the UBSM phasic contraction amplitude, muscle force integral, contraction duration, contraction frequency, and muscle tone to 33.5 ± 4.1, 31.7 ± 3.4, 37.7 ± 2.5, 48.8 ± 3.7, and 48.8 ± 3.0% of the control activity, respectively (n = 8, N = 4; P < 0.05; Fig. 5, A and B). To determine the role of KCa1.1 channels in PDE-induced inhibition of human UBSM spontaneous contractions, KCa1.1 channels were blocked with paxilline. Paxilline (1 μM) alone increased the UBSM phasic contraction amplitude, muscle force integral, duration, frequency, and tone to 243.6 ± 5.7, 211.7 ± 9.6, 142.6 ± 2.3, 158.1 ± 4.3, and 146.1 ± 0.9% of the control activity, respectively (n = 5, N = 5; P < 0.05; Fig. 5, C and D). In the presence of 1 μM paxilline, the subsequent addition of IBMX had no effect on the phasic contraction amplitude, muscle force integral, duration, frequency, and muscle tone (n = 5, N = 5; P > 0.05 vs. paxilline; Fig. 5, C and D). The fact that pharmacological blockade of the KCa1.1 channels diminished the UBSM relaxation following PDE blockade suggests that KCa1.1 channels are critical to the PDE-mediated suppression of human UBSM spontaneous contractility.
Fig. 5.
PDE blockade with IBMX significantly reduced spontaneous phasic contractions of human UBSM-isolated strips. A: representative recording of a UBSM-isolated strip exhibiting spontaneous phasic contractions and illustrating that IBMX (10 μM) inhibited the contractions. B: summary data showing that PDE blockade reduced the UBSM spontaneous contraction amplitude, muscle force integral, duration, frequency, and tone (n = 8, N = 4; *P < 0.05 vs. control). C: representative recording of a UBSM-isolated strip exhibiting spontaneous phasic contractions showing that the KCa1.1 channel blocker paxilline enhanced the spontaneous phasic contractions and that the subsequent addition of IBMX did not have any further effect on the contractions. D: summary data showing that paxilline increased the UBSM spontaneous contraction amplitude, muscle force integral, duration, frequency, and tone and that the subsequent addition of 10 μM IBMX did not have any further effect on UBSM contraction parameters (n = 5, N = 5; *P < 0.05 vs. control; NS vs. paxilline). All these experiments were performed in the presence of 1 μM tetrodotoxin (TTX).
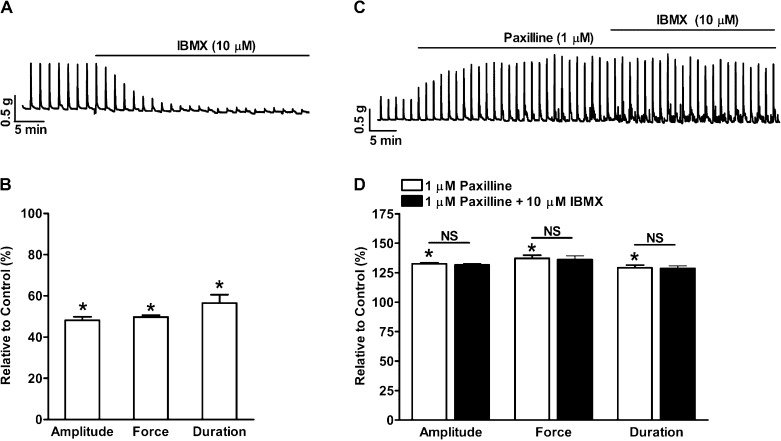
PDE blockade suppressed nerve-evoked contractions of human UBSM-isolated strips.
Nerve-evoked UBSM contractions were induced by 20-Hz EFS. EFS induces acetylcholine and ATP release from bladder nerve terminals and causes an increase in UBSM contractions. PDE blockade significantly reduced the 20-Hz EFS-induced UBSM contraction amplitude, muscle force integral, and duration to 48.2 ± 1.6, 49.7 ± 1.0, and 56.6 ± 4.0% of the control activity, respectively (n = 5, N = 5; P < 0.05; Fig. 6, A and B). Blocking KCa1.1 channels with paxilline (1 μM) increased the 20-Hz EFS-induced contraction amplitude, muscle force integral, and duration to 132.6 ± 0.8, 137.2 ± 2.6, and 129.1 ± 2.3% of the control activity, respectively (n = 4, N = 4; P < 0.05; Fig. 6, C and D). In the presence of 1 μM paxilline, PDE blockade had no effect on the EFS-induced contraction amplitude, muscle force integral, and duration (n = 4, N = 4; P > 0.05 vs. paxilline; Fig. 6, C and D). These data indicate that PDE plays a major role in the suppression of nerve-evoked contractions induced by KCa1.1 channel blockade.
Fig. 6.
PDE blockade with 10 μM IBMX significantly reduced 20-Hz electrical field stimulation (EFS)-induced phasic contractions of human UBSM-isolated strips. A: representative recording illustrating that PDE blockade suppressed the 20-Hz EFS-induced phasic contractions. B: summary data showing that PDE blockade reduced the 20-Hz EFS-induced UBSM contraction amplitude, muscle force integral, and duration (n = 5, N = 5; *P < 0.05 vs. control). C: representative recording showing that paxilline (1 μM) increased the 20-Hz EFS-induced phasic contractions and that the subsequent addition of 10 μM IBMX did not have any further effect on the contractions. D: summary data showing that 1 μM paxilline increased the 20-Hz EFS-induced contraction amplitude, muscle force integral, and duration and that the subsequent addition of 10 μM IBMX did not have any further effect on UBSM contraction parameters (n = 4, N = 4; *P < 0.05 vs. control; NS vs. paxilline).
DISCUSSION
For the first time, we determined the cellular mechanism of PDE regulation of human UBSM function using pharmacological PDE blockade. Our results indicate that in human UBSM, PDE is constitutively active and that pharmacological PDE blockade significantly 1) increases STOCs frequency, 2) enhances STHs frequency and hyperpolarizes the cell membrane potential, 3) decreases the intracellular Ca2+ levels, 4) attenuates human UBSM spontaneous phasic and nerve-evoked contractions, and 5) these effects are mediated by the KCa1.1 channels. The results provide compelling evidence that PDE and KCa1.1 channels are critical for controlling human UBSM excitability and contractility and that PDE pharmacological manipulation can effectively suppress the contractility of human UBSM via KCa1.1 channel activation.
This study, in addition to some earlier studies, demonstrates that blockade of PDEs can induce human UBSM relaxation, and it suggests that PDEs can be potential therapeutic targets for OAB treatment (22, 29, 31). Furthermore, the current study reveals the underlying mechanism of PDE regulation of human UBSM function, which is essential for developing novel strategies to treat OAB by targeting PDE pharmacologically. The data presented here indicate that in human UBSM-isolated cells, PDE blockade increases STOCs and STHs frequency and hyperpolarizes UBSM cell resting membrane potential (Figs. 1 and 2). These results also demonstrate that the hyperpolarization effect of PDE blockade on human UBSM cells is mediated by the KCa1.1 channels. The role of KCa1.1 channels was confirmed by the fact that pharmacological inhibition of KCa1.1 channels with paxilline abolished the hyperpolarization effect of PDE blockade on human UBSM cells (Fig. 3). This study reveals that in human UBSM, PDE is constitutively active and controls the KCa1.1 channels, key mediators of human UBSM function.
KCa1.1 channels have been shown to be fundamental regulators of human UBSM excitability and contractility by controlling the cell membrane potential and repolarization phase of UBSM action potential (13, 16). Selective KCa1.1 channel openers hyperpolarize the cell membrane potential and decrease the global intracellular Ca2+ by limiting the Ca2+ influx through l-type voltage-dependent Ca2+ (CaV) channels in human UBSM cells (16). The indirect activation of KCa1.1 channels upon PDE blockade results in reduction of intracellular Ca2+ (Fig. 4), an effect similar to the one of KCa1.1 channel openers, which leads to the suppression of human UBSM contractility.
The transient elevation of intracellular Ca2+ concentration due to Ca2+ influx through CaV channels during the spontaneous action potentials activates UBSM myogenic phasic contractions (5, 12, 18, 24). The Ca2+ entry was limited by hyperpolarization of membrane potential via KCa1.1 channel activation. For the first time, we demonstrated that PDE blockade significantly reduces the spontaneous phasic contraction amplitude, muscle force integral, duration, frequency, and muscle tone in human UBSM-isolated strips and that KCa1.1 channels mediated these effects (Fig. 5). When KCa1.1 channels were pharmacologically blocked with paxilline, PDE blockade had reduced effect on the human UBSM myogenic contractility, further demonstrating a key role of the interplay between the PDE and KCa1.1 channels in the regulation of human UBSM phasic contractions (Fig. 5).
During bladder voiding, the activation of parasympathetic cholinergic nerves in the UBSM evokes forceful contractions. However, an abnormal neuronal activity could excessively increase UBSM contractility and cause DO (1). KCa1.1 channels are important regulators of the nerve-evoked UBSM contractions (16, 27). Direct pharmacological KCa1.1 channel activation suppresses the nerve-evoked human UBSM contractility (18). The data presented here show that PDE blockade attenuated the nerve-evoked contractions (Fig. 6). Blocking KCa1.1 channels with paxilline increased the nerve-evoked human UBSM contractions and completely abolished the relaxation effect of PDE blockade. This suggests a KCa1.1 channel-dependent mechanism in the suppression of the nerve-evoked human UBSM contractions by PDE blockade (Fig. 6).
This study fills a gap in our understanding of PDE regulation of human UBSM function. Our data suggest that PDEs are constitutively active and contribute to the maintenance of human UBSM spontaneous contractile activity. The results reported here for the first time suggest that human UBSM response to PDE blockade, including UBSM cell hyperpolarization, reduced intracellular Ca2+ levels, and decreased UBSM contractility, results from the activation of the KCa1.1 channels. This study contributes a novel insight to human cell physiology revealing that PDE blockade can effectively suppress human UBSM contractility primarily via KCa1.1 channel activation. Inhibitors for human UBSM-specific PDEs could be an effective approach to reduce abnormal UBSM contractility without the adverse effects of antimuscarinic drugs, the current main pharmacological treatment for OAB.
GRANTS
This study was supported by National Institutes of Health Grant DK084284 to G. V. Petkov.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.X., Q.C., R.P.S., and G.V.P. performed experiments; W.X., Q.C., R.P.S., and G.V.P. analyzed data; W.X., Q.C., R.P.S., E.S.R., and G.V.P. interpreted results of experiments; W.X., Q.C., R.P.S., E.S.R., and G.V.P. prepared figures; W.X., Q.C., R.P.S., E.S.R., and G.V.P. drafted manuscript; W.X., Q.C., R.P.S., E.S.R., and G.V.P. edited and revised manuscript; W.X., Q.C., R.P.S., E.S.R., and G.V.P. approved final version of manuscript; E.S.R. and G.V.P. conception and design of research.
ACKNOWLEDGMENTS
We thank the Medical University of South Carolina (MUSC) Urology staff surgeons: Drs. Thomas Keane, Harry Clarke, Stephen Savage, Ross Rames, Jonathan Picard, and Ahmed M. El-Zawahry, as well as the MUSC Urology Residents: Avi C. Weiss, Gary W. Bong, Kelly Doyle, Matthew McIntyre, Matt Eskridge, Jonathan N. Hamilton, Robin Bhavsar, Timothy R. Yoost, Vinh Q. Trang, Lydia Labocetta, Elizabeth Peacock, Matthew Young, Erin Burns, Vaughan Taylor, and Samuel Walker Nickles for help with human tissue collection; and Drs. John Malysz, Shankar Parajuli, Kiril Hristov, Ms. Amy Smith, and Mr. Serge Afeli for the critical evaluation of the manuscript.
REFERENCES
- 1.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935–986, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Aydin M, Wang HZ, Zhang X, Chua R, Downing K, Melman A, Disanto ME. Large-conductance calcium-activated potassium channel activity, as determined by whole-cell patch clamp recording, is decreased in urinary bladder smooth muscle cells from male rats with partial urethral obstruction. BJU Int doi:10.1111/j.1464–410X.2012.11137.x (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 3.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58: 488–520, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bingham J, Sudarsanam S, Srinivasan S. Profiling human phosphodiesterase genes and splice isoforms. Biochem Biophys Res Commun 350: 25–32, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Brading AF. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol 570: 13–22, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. β-Adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol 295: F1149–F1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang S, Gomes CM, Hypolite JA, Marx J, Alanzi J, Zderic SA, Malkowicz B, Wein AJ, Chacko S. Detrusor overactivity is associated with downregulation of large-conductance calcium- and voltage-activated potassium channel protein. Am J Physiol Renal Physiol 298: F1416–F1423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng H, Lederer WJ. Calcium sparks. Physiol Rev 88: 1491–1545, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Cooper N, Teixeira MM, Warneck J, Miotla JM, Wills RE, Macari DM, Gristwood RW, Hellewell PG. A comparison of the inhibitory activity of PDE4 inhibitors on leukocyte PDE4 activity in vitro and eosinophil trafficking in vivo. Br J Pharmacol 126: 1863–1871, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darblade B, Behr-Roussel D, Oger S, Hieble JP, Lebret T, Gorny D, Benoit G, Alexandre L, Giuliano F. Effects of potassium channel modulators on human detrusor smooth muscle myogenic phasic contractile activity: potential therapeutic targets for overactive bladder. Urology 68: 442–448, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science 182: 939–941, 1973 [DOI] [PubMed] [Google Scholar]
- 12.Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol 140: 146–158, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca2+ sparks to BKCa channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol 280: C481–C490, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Hristov KL, Chen M, Afeli SA, Cheng Q, Rovner ES, Petkov GV. Expression and function of KV2-containing channels in human urinary bladder smooth muscle. Am J Physiol Cell Physiol 302: C1599–C1608, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV. Large-conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol 301: C903–C912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of β3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295: C1344–C1353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hristov KL, Parajuli SP, Soder RP, Cheng Q, Rovner ES, Petkov GV. Suppression of human detrusor smooth muscle excitability and contractility via pharmacological activation of large conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 302: C1632–C1641, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson WB, Katugampola S, Able S, Napier C, Harding SE. Profiling of cAMP and cGMP phosphodiesterases in isolated ventricular cardiomyocytes from human hearts: comparison with rat and guinea pig. Life Sci 90: 328–336, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Lakics V, Karran EH, Boess FG. Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology 59: 367–374, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Oger S, Behr-Roussel D, Gorny D, Bernabe J, Comperat E, Chartier-Kastler E, Denys P, Giuliano F. Effects of potassium channel modulators on myogenic spontaneous phasic contractile activity in human detrusor from neurogenic patients. BJU Int 108: 604–611, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Oger S, Behr-Roussel D, Gorny D, Denys P, Lebret T, Alexandre L, Giuliano F. Relaxation of phasic contractile activity of human detrusor strips by cyclic nucleotide phosphodiesterase type 4 inhibition. Eur Urol 51: 772–780, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Parsons BA, Drake MJ. Animal models in overactive bladder research. Hand Exp Pharmacol 202: 15–43, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9: 30–40, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petkov GV, Heppner TJ, Bonev AD, Herrera GM, Nelson MT. Low levels of KATP channel activation decrease excitability and contractility of urinary bladder. Am J Physiol Regul Integr Comp Physiol 280: R1427–R1433, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by β-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol 288: C1255–C1263, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Soder RP, Petkov GV. Large conductance Ca2+-activated K+ channel activation with NS1619 decreases myogenic and neurogenic contractions of rat detrusor smooth muscle. Eur J Pharmacol 670: 252–259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Truss MC, Stief CG, Uckert S, Becker AJ, Schultheiss D, Machtens S, Jonas U. Initial clinical experience with the selective phosphodiesterase-I isoenzyme inhibitor vinpocetine in the treatment of urge incontinence and low compliance bladder. World J Urol 18: 439–443, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Truss MC, Stief CG, Uckert S, Becker AJ, Wefer J, Schultheiss D, Jonas U. Phosphodiesterase 1 inhibition in the treatment of lower urinary tract dysfunction: from bench to bedside. World J Urol 19: 344–350, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Truss MC, Uckert S, Stief CG, Kuczyk M, Jonas U. Cyclic nucleotide phosphodiesterase (PDE) isoenzymes in the human detrusor smooth muscle. I. Identification and characterization. Urol Res 24: 123–128, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Uckert S, Sigl K, Waldkirch ES, Sandner P, Ulbrich E, Oelke M, Stief CG, Kuczyk MA. Significance of phosphodiesterase isoenzymes in the control of human detrusor smooth muscle function. An immunohistochemical and functional study. Urologe A 48: 764–769, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Xin W, Cheng Q, Soder RP, Petkov GV. Inhibition of phosphodiesterases relaxes detrusor smooth muscle via activation of the large-conductance voltage- and Ca2+-activated K+ channel. Am J Physiol Cell Physiol 302: C1361–C1370, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]