Abstract
Changes in apoplastic carbohydrate concentrations and activities of carbohydrate-degrading enzymes were determined in crown tissues of oat (Avena sativa L., cv Wintok) during cold hardening. During second-phase hardening (−3°C for 3 d) levels of fructan, sucrose, glucose, and fructose in the apoplast increased significantly above that in nonhardened and first-phase-hardened plants. The extent of the increase in apoplastic fructan during second-phase hardening varied with the degree of fructan polymerization (DP) (e.g. DP3 and DP4 increased to a greater extent than DP7 and DP > 7). Activities of invertase and fructan exohydrolase in the crown apoplast increased approximately 4-fold over nonhardened and first-phase-hardened plants. Apoplastic fluid extracted from nonhardened, first-phase-hardened, and second-phase-hardened crown tissues had low levels, of symplastic contamination, as determined by malate dehydrogenase activity. The significance of these results in relation to increases in freezing tolerance from second-phase hardening is discussed.
Cold-hardening winter cereals such as rye (Secale cereale), wheat (Triticum aestivum), barley (Hordeum vulgare), and oat (Avena sativa) is generally accomplished by exposure to temperatures just above freezing. As early as 1935, Tumenov (cited by Trunova, 1965) reported that an additional phase of hardening by exposure of cold-hardened plants to nonlethal, below-freezing temperatures resulted in significant increases in cold hardiness. Tumenov called this 2PH. With a 2PH treatment, Trunova (1965) and Siminovich (cited by Steponkus, 1978) induced an increase in freezing tolerance of wheat significantly beyond that achieved from cold hardening above freezing (1PH). Olien (1984) reported similar results in rye and barley, and Livingston (1996) reported a decrease in the LT50 of oats from −13°C with 1PH only to −18°C after a 3-d 2PH period at −3°C.
After 2PH Trunova (1965) found decreased levels of fructan and increased levels of Glc, Fru, and Suc in whole crown tissue of wheat. He suggested that the sugar increase during 2PH was providing osmotic protection to cells in the over-wintering organ (crown) of the plant. Olien (1984), using a perfusion technique, reported an increase in apoplastic sugars during 2PH and suggested that these sugars helped prevent adhesion of ice to critical cellular tissue during freezing.
In the present study we wanted to determine the percent distribution of fructan exohydrolase and invertase in the apoplast and symplast of oat crowns, and what the effect of 1PH and 2PH would be on their activities in the respective locations. Additionally, we wanted to determine if fructan was present in the apoplast and the effect of cold hardening on its presence. Finally, we wanted to re-examine the effect of cold hardening on simple sugar levels in the apoplast using a different technique than that used by Olien (1984).
MATERIALS AND METHODS
Plant Culture
Seeds of the oat (Avena sativa L.) cv Wintok were planted in Scotts Metromix 2201 (Scotts-Sierra Horticultural Products Co., Marysville, OH) in plastic tubes (2.5 cm in diameter × 16 cm in height) with holes in the bottom to allow drainage. The tubes were suspended in a grid that held 100 tubes. Plants were watered three times weekly with a complete nutrient solution (Livingston, 1991) and flushed three times weekly with tap water. Plants were grown for 5 weeks at a day/night temperature regime of 13/10°C with a 12-h photoperiod in a growth chamber with 240 μmol m−2 s−1 PAR (80% cool fluorescent and 20% incandescent). These were the NH plants. Five weeks after planting, plants were transferred to a chamber at 2°C with an 12-h photoperiod at 310 μmol m−2 s−1 for 3 weeks. This 3-week period constituted 1PH.
Second-Phase Hardening
After 1PH plants were at the three-leaf stage with two to three tillers each. They were removed from tubes and washed free of planting medium in ice water. Roots were trimmed to 2 cm and the plants were placed in a plastic bag, inoculated with ice, and sealed to help prevent desiccation. To induce 2PH, plastic bags containing plants were placed in a freezer at −3°C in the dark for 3 d. Thermocouples placed near plants were used to monitor temperatures.
Carbohydrate Extraction, Whole Tissue
Six crowns (the tissue remaining after removing leaves and stems to within 2 cm of the stem base) were bulked and ground for approximately 30 s using a stainless-steel grinder (Livingston, 1990). Measurements were conducted in triplicate. Ten milliliters of HPLC-grade water at 1°C was used to rinse plant tissue into a beaker. A 2-mL aliquot was filtered through a 0.45-μm filter and placed on dry ice. These aliquots were used for determining osmolyte concentration and the activity of invertase, MDH, and FEH. The remaining extract was heated to 95°C for 10 min. The lapsed time from plant harvest to either freezing on dry ice or heating to 95°C was approximately 3 min. Heat-treated solution was filtered through a 0.45-μm filter in preparation for HPLC analysis. No changes in carbohydrate composition were observed for 36 h at 2°C in filtered, heat-treated samples.
Apoplast Extraction
Intact crowns from 15 plants were placed in the bottom 3 cm of a 50-mL syringe barrel. A 4-mL HPLC insert vial was placed on the syringe tip to collect apoplastic solution. The syringe barrel containing crown tissue with the 4-mL vial attached was placed in a 50-mL centrifuge tube and centrifuged at 500g for 10 min at 3°C.
An aliquot of the apoplastic fluid was used for determining osmolyte concentration and the activities of MDH, FEH, and invertase. The remaining solution was heated for 10 min at 95°C and analyzed by HPLC.
The volume of liquid centrifuged averaged around 5 to 7% of the total liquid in the whole crown tissue (data not shown). By infiltrating the apoplast with an indicator dye, Tetlow and Farrar (1993) found apoplast volumes to be about 8% of the total volume in healthy barley (Hordeum vulgare) leaves.
The viability of crowns after their apoplastic fluid was removed was examined by observing the regrowth of roots and shoots after a 3-week growth period under the initial growing conditions described above. One-hundred percent survival occurred for all three treatments.
Guttation
Guttation was induced in oat plants by placing racks containing 1PH plants in a covered container in the dark with a 4-cm layer of water in the bottom. After 10 h about 10 droplets of liquid found on leaf tips of separate plants were pooled for HPLC analysis of carbohydrates.
Carbohydrate Separation and Quantification
Fructans were separated according to DP using a modified silver-based analytical HPLC column (7.8 × 300 mm, Aminex HPX-42A, Bio-Rad). This column was found to hydrolyze fructans and Suc and was therefore permanently modified by passing 0.5 m NaNO3, at a rate of 2 mL min−1, through the column for approximately 18 h. This treatment eliminated the hydrolysis of smaller fructans and Suc and improved the resolution of smaller sugars. Resolution of larger (DP5 and DP6) fructans, however, was slightly reduced in the modified column. Because samples were not desalted prior to injection, a cation and anion-exchange guard column immediately preceding the analytical column was used to prevent co-elution of small ionic compounds with carbohydrates. The mobile phase was HPLC-grade water at a flow rate of 0.4 mL min−1.
Separated carbohydrates were detected by a 410 refractive index detector (Waters). Unknown peaks were identified by co-chromatography with external standards. Oligomers were quantified by comparison of unknown peak areas to peak area-response curves derived from standard solutions of varying concentrations (Livingston et al., 1993). Peak area was measured by a Millennium 2000 chromatography workstation (Waters) with a microcomputer.
Fructan Collection and Hydrolysis
Each size class of fructan is composed of more than one isomer. In 1PH oats DP3 is composed of 1-kestose and neokestose, DP4 is composed of four isomers, and DP5 is composed of seven isomers (Livingston et al., 1993). The DP6 and DP7 isomers in oats have not yet been identified but each size class is composed of numerous isomers.
To confirm that quantified peaks from the apoplastic fluid were Fru polymers, individual peaks were collected from the 42A column and hydrolyzed for 1.5 h at 95°C in 0.16 n HCl. These conditions were found to be ideal for measuring the DP of fructans (D. P. Livingston, unpublished data). Suc was hydrolyzed with the samples to confirm that conditions were not too stringent. Hydrolyzed samples were then separated by HPLC using an Aminex 87H column (Bio-Rad). Glc and Fru residues were quantified as above. All peaks from DP3 to DP7 produced Glc and Fru in ratios consistent with their suspected DP. The DP > 7 peak is composed of a mixture of sizes, therefore, its DP could not be determined. No evidence was found from the hydrolysis data that any of the peaks from whole-tissue extracts or apoplastic fluid were glucans.
Osmolality, MDH, FEH, and Invertase
Osmolyte concentrations were measured with a vapor pressure osmometer (model 5100C, Wescor, Logan, UT) calibrated with 100 and 290 mmol/kg NaCl standards. Measurements were conducted in triplicate and data are expressed as millimoles/gram fresh weight.
NAD-linked MDH (EC 1.1.1. 37) activities were assayed by measuring the oxaloacetate-dependent oxidation of NADH on a recording spectrophotometer (model 2101-PC, Shimadzu, Tokyo, Japan) at 30°C. Assays were conducted as described by Duke et al. (1975).
FEH activity was assayed using neokestin as the substrate, with detection of released Fru as described by Henson and Livingston (1996). Neokestin is a mixture of neokestose-based fructan isomers of DP7 to 14 that is aqueously extracted from oat leaves and precipitated by ethanol (Livingston, 1989). Invertase activity was measured as described by Henson (1989). Enzyme activities are expressed as nanomoles or micromoles of product generated per minute per gram fresh weight of crown tissue.
RESULTS AND DISCUSSION
Carbohydrates in Whole Tissue
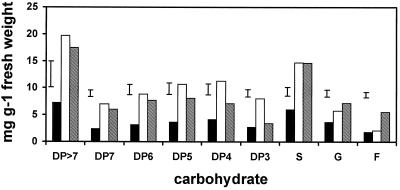
The concentrations of Suc and all fructans increased nearly 3-fold in whole crown tissue during 1PH (Fig. 1), which is consistent with results reported earlier in oats and other winter cereals (Livingston, 1991; Pollock and Cairns, 1991).
Figure 1.
Carbohydrate concentrations in whole-crown tissue from the winter oat cv Wintok. Plants were grown under NH (5 weeks at 13°C, black bars), 1PH (3 weeks at 2°C, open bars), or 2PH (1PH plants were placed in a −3°C freezer for 3 d in the dark, shaded bars) conditions. The bar above each group of three bars is the lsd. P = 0.05; n = 3. S, Suc; G, Glc; F, Fru.
During 2PH the concentration of all size classes of fructan decreased in whole crown tissue, whereas the concentration of Glc and Fru increased. The largest decrease was in DP3 and DP4 fructan, and the largest increase was in Fru (Fig. 1). This decrease in fructan and increase in sugars (Suc excepted, Fig. 1) during 2PH agrees with earlier reports in oats, barley, wheat (Triticum aestivum), and rye (Secale cereale) (Trunova, 1965; Olien, 1984; Livingston, 1996). Although the increased sugars in whole crown tissue would lower the freezing point of the cytoplasm by only a fraction of a degree, if sugars were concentrated in specific regions of the crown, the freezing point of those regions could be lowered enough to increase the freezing survival of the whole plant.
Indicators of Cellular Leakage
MDH activity has frequently been used as a specific marker for cellular integrity, since buffer-soluble MDH activity is inside the cell when the plasmalemma is intact. MDH activity is readily detectable in extracellular fluids when the plasmalemma is damaged. The usefulness of MDH activity as a specific marker of cellular integrity has been demonstrated in studies of imbibition-induced damage of seeds with genetically altered testa (Duke and Kakefuda, 1981; Duke et al., 1986) and in studies of apoplastic fluid composition. In apoplastic preparations from pea, Brassica napus, and barley (Beers and Duke, 1988; Tetlow and Farrar, 1993; Husted and Schjoerring, 1995), levels of MDH contamination of less than or equal to 1% were reported. In our study the percentages of total MDH activity recovered in apoplastic preparations from NH, 1PH, and 2PH tissues ranged from 0.7 to 2.8% (Table I), indicating little cellular rupture. MDH leakage from cells of alfalfa crowns and roots was 4-fold higher when plants were frozen at −8°C than when they were frozen at −4°C (Sulc et al., 1991), suggesting an increase in cellular leakage as the temperature was lowered. The small increase in intracellular leakage from our 2PH treatment could have occurred prior to or during apoplast extraction, possibly from cellular membranes that were more susceptible to leakage from centrifugation.
Table I.
MDH activity and osmolyte concentration of apoplast and whole crown tissue from plants that were subjected to three hardening treatments
| Tissue | NH | 1PH | 2PH | lsd (P = 0.05) |
|---|---|---|---|---|
| Osmolytes | ||||
| (mmoles/g fresh wt) | ||||
| Crown | 0.624 | 0.7278 | 0.706 | nsa |
| Apoplast | 0.006 (1.0%) | 0.009 (1.2%) | 0.056 (7.9%) | 0.025 |
| MDH | ||||
| (μmoles malate produced/g fresh wt) | ||||
| Crown | 47.2 | 55.2 | 51.8 | ns |
| Apoplast | 0.328 (0.7%) | 0.639 (1.1%) | 1.43 (2.8%) | 0.25 |
Shown in parentheses is the amount of marker in the apoplast as a percentage of the total marker in the crown.
Treatments in this row are not significantly different from each other at P = 0.05.
The second method we used to assess cellular integrity was to compare the concentration of osmolytes in the apoplast with that of the whole tissue. Changes in osmolyte concentrations are analogous to changes in electrical conductivity, which is commonly used as a measure of cell rupture because the conductivity of extracellular fluids under nonstressed conditions is low and increases significantly upon loss of cellular integrity (Dexter et al., 1932; Sulc et al., 1991). Percentages of total osmolytes present in apoplastic fluid extracted from NH and 1PH tissues were not significantly different from each other (Table I). Even though we found no significant difference in osmolytes in the apoplasts of NH and 1PH oat crowns, others (Marentes et al., 1993; Antikainen et al., 1996) have shown that quantitative changes in proteins do occur in the apoplast of rye leaves during 1PH.
We conclude that 2.8% of the cells experienced damage severe enough to result in the release of macromolecules of at least the size of MDH because 2.8% of the total MDH was present in the apoplast of 2PH tissues. This amount of damage was not lethal to the plants, as evidenced by complete re-growth of roots and stems after apoplast extraction (data not shown). This rupture of 2.8% of the cells undoubtedly contributed to the osmolytes present in the apoplast of 2PH tissues. However, other factors probably contributed to the observed increase in osmolytes during 2PH. For example, nonlethal perturbations in the plasmalemma that allow molecules smaller than MDH, such as carbohydrates (Fig. 2 ), to pass into the apoplast could have occurred during 2PH. Such leakage would contribute to the observed increase in osmolytes during 2PH and may have been the result of specific adaptive responses to the hardening treatment. Some or all of these adaptive responses may account for the clear increase in freezing tolerance of oats (Livingston, 1996), as well as other winter cereals (Trunova, 1965; Olien, 1984).
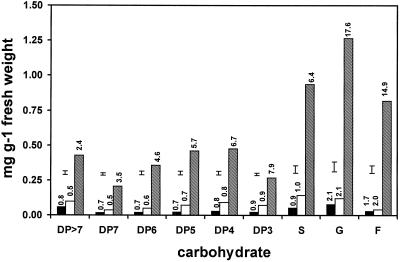
Figure 2.
Carbohydrate concentrations in apoplastic fluid from crown tissue of the winter oat cv Wintok. Plants were grown under NH (5 weeks at 13°C, black bars), 1PH (3 weeks at 2°C, open bars), or 2PH (1PH plants were placed in a −3°C freezer for 3 d in the dark, shaded bars) conditions. The error bar above each group of three bars is the lsd. P = 0.05; n = 3. Numbers above the bars indicate the percentage of the total carbohydrate (taken from Fig. 1) that was found in the apoplast. S, Suc; G, Glc; F, Fru.
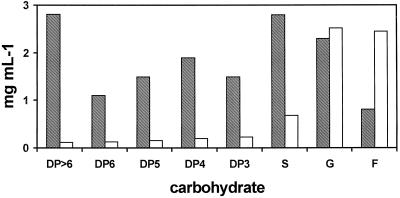
Since hydathodes are apparently in direct contact with the apoplast (Dieffenbach et al., 1980), sampling guttated liquid could be viewed as an apoplast-sampling method with the least possible perturbation of the plant. The concentration of Suc in guttated liquid was about 25% of that in the apoplast. The concentration of Glc in the guttate was slightly higher than it was in the apoplast, and the concentration of Fru in guttated liquid was three times that found in the apoplast. Small quantities of all fructan size classes found in the apoplast were also found in the solution collected from leaf tips (Fig. 3). Fructan has been found in the guttated liquid from cold-hardened barley plants, although individual DPs were not quantified (C.R. Olien, unpublished data). The presence of fructan in the guttate (Fig. 3) suggests that at least some level of fructan in the apoplast is not an artifact of extraction.
Figure 3.
Carbohydrate concentrations in apoplastic fluid (shaded bars) in crown tissue in comparison to guttated liquid (open bars) from the 1PH winter oat cv Wintok. S, Suc; G, Glc; and F, Fru.
The differences in the composition of fructan, Suc, and Fru in guttated liquid from that in the apoplast (Fig. 3) could be explained by the presence of active invertase and FEH in the guttated liquid in contrast to the apoplastic fluid, which had been heat inactivated. Although we did not assay for FEH or invertase in guttated liquid, since guttated liquid passes through hydathodes that are reportedly in contact with the apoplast (Dieffenbach et al., 1980), it would not be surprising to find both FEH and invertase in guttated liquid. In addition, the apoplastic fluid was sampled at the crown and the relative concentrations of sugars would likely change as they approach photosynthesizing cells.
Carbohydrates in Apoplast
The amount of Suc, Glc, and Fru in the apoplast of NH and IPH plants as a percentage of the total in whole crown tissue is similar to the distribution of osmolytes and MDH. This suggests that their presence could be attributed to cellular leakage or rupture. A lack of change during 1PH in the percentage of apoplastic carbohydrates (Fig. 2) suggests that this is not a factor in the universally recognized increase in freezing tolerance of winter cereals during 1PH; other mechanisms must be responsible for this adaptation.
In contrast to 1PH, during 2PH the percentage of total sugars in the apoplast increased to 17.6% for Glc (a 10-fold increase), 14.9% for Fru (a 20-fold increase), and 6.4% for Suc (a 6-fold increase) (calculated from Figs. 1 and 2). The increases in these sugars beyond levels attributable to cellular rupture may be part of a specific response to the 2PH treatment.
Carbohydrate increases during 2PH do not increase the molarity of the apoplastic solution enough to lower the freezing point of the apoplast by more than a fraction of a degree. However, Canny (1995) showed that the solute concentration in the apoplast is not uniform, but that solutes tend to accumulate in discrete regions termed sumps. In addition to sugars being distributed unevenly in the apoplast, the layer of liquid water into which sugars have apparently been released would be very small due to the presence of apoplastic ice at −3°C (Gusta and Fowler, 1977; Single and Marcellos, 1981; Pearce and Ashworth, 1992). This could lead to regions of very high sugar concentrations in the apoplast of crown tissue, which could prevent ice adhesions and possibly provide other forms of protection to cell walls and membranes.
In both NH and 1PH plants the percentage of fructan in the apoplast was below that of MDH and osmolytes, suggesting that its presence in the apoplast could also be attributed to cellular leakage or rupture.
During 2PH all fructans increased 5-fold or more (Fig. 2), but in a manner that suggested some form of differential leakage into the apoplast. For example, in NH and 1PH apoplastic fluid the percentage of fructan did not vary according to size and was always below 1% (Fig. 2). However, after 2PH the percentage of fructan in the apoplast was inversely related to DP (Fig. 2). In addition to differential leakage, it is possible that changes in fructan concentrations were influenced by changes in FEH activity in the apoplast.
Fructan is considered primarily to be a storage carbohydrate, and its function as a cryoprotectant has always been controversial. Since freezing in plants begins in the apoplast, it is somewhat easier to envision a role for fructan in freezing protection, at least where 2PH is concerned, with the discovery of its presence in the apoplast.
FEH and Invertase in Apoplast and Whole Tissue
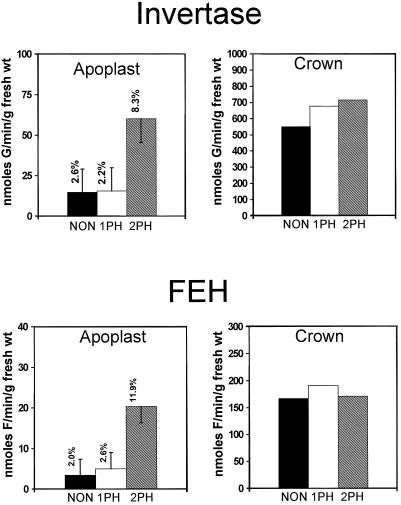
Apoplast fluid extracted from crowns at all three stages of hardening contained readily detectable FEH activity. Although the location of FEH is generally accepted to be vacuolar (Pollock and Chatterton, 1988), we considered that it may be present in the apoplast for several reasons: (a) we found extracellular fructans present in oat crowns of all three hardening treatments, (b) numerous carbohydrate- hydrolyzing enzymes (acid invertase, α-amylase, β-amylase, α-glucosidase, and β-glucosidases) are known to be present in the apoplast of many plant species (Hatch et al., 1963; Hawker and Hatch, 1965; Beers and Duke, 1988; Li and McClure, 1989; Beers et al., 1990), and (c) previous localizations of FEH activity relied upon isolation of vacuoles from protoplasts using a protocol that did not account for extracellular enzyme activities (Wagner and Wiemken, 1986). The levels of FEH activities that we found in apoplastic fluid from crowns of NH and 1PH oat were not significantly different from each other and were about 2% of the total activity in the tissue. This amount of enzyme activity in the apoplast was probably not significantly different from that resulting from cell leakage or rupture (Table I). However, FEH in the apoplast of 2PH plants increased to approximately 11% of the total, which is greater than that predicted to result simply from cellular rupture. The portion not attributable to rupture may result from activation of the enzyme already present in the apoplast, or it may be due to increased secretion of FEH into the apoplast or a combination of both mechanisms.
FEH activity in whole crown tissue did not significantly (P = 0.05) change as a function of 2PH (Fig. 4), even though all fructan size classes were lower in whole crown tissue from 2PH plants (Fig. 1). FEH activities reported here cannot be directly correlated with the appearance or disappearance of specific fructans either in whole tissue or in apoplast fluid for several reasons. First, neokestin, the substrate used in the in vitro assays, is a mixture of fructan polymers; we measured only the release of free Fru and did not identify the sizes of individual fructans resulting from the hydrolysis. Second, the plant extracts assayed were crude and contained more than one fructan-hydrolyzing enzyme (C. A. Henson, unpublished data). Third, the in vitro assays were conducted over several hours, in contrast to the several weeks of hardening treatments that resulted in the fructan distribution found in whole crown tissue.
Figure 4.
Activity of FEH and invertase in apoplast fluid and whole-crown tissue from the winter oat cv Wintok. Plants were grown under NH (5 weeks at 13°C, NON), 1PH (3 weeks at 2°C), or 2PH (1PH plants were placed in a −3°C freezer for 3 d in the dark) conditions. The bars represent the lsd at P = 0.05 (n = 3). The percentages above the bars in the apoplast frames indicate the percentage of total activity measured in the apoplast. G, Glc; fresh wt, Fresh weight; F, Fru. No significant differences were found in the three treatments of whole crown tissue.
Apoplastic fluid extracted from crowns at all three stages of hardening contained invertase activity. Invertase activities in apoplastic fluid from crowns of NH and 1PH plants were not significantly different from each other and were about 2 to 2.5% of the total activity in the tissue. Invertase activity in the apoplast from crowns of 2PH plants increased to approximately 8% of the total activity in the tissue. This increase in invertase activity partly explains the increase in Glc and Fru in the apoplast of 2PH plants (Fig. 2). Invertase activities in whole crown tissue increased slightly, although not significantly, as a function of hardening (Fig. 4).
The leakage or secretion of FEH and invertase into the apoplast or the alteration of their activities during 2PH may be a specific adaptive response that helps the plant survive freezing temperatures. The presence of fructan and Suc in the apoplast during 2PH certainly provides substrates for both enzymes, the products of which are known cryoprotectants. However, the changes we measured in this study may also be coincidental to the increase in freezing tolerance during 2PH. There are undoubtedly other alterations we did not measure that will help provide answers to the exact nature of the increased freezing survival during 2PH.
Abbreviations:
- DP
degree of polymerization
- FEH
fructan exohydrolase
- LT50
temperature at which 50% of a population survives
- MDH
malate dehydrogenase
- NH
nonhardened
- 1PH
first phase of cold hardening
- 2PH
second phase of cold hardening
Footnotes
Mention of a proprietary product does not constitute an endorsement or recommendation for its use by the U.S. Department of Agriculture.
LITERATURE CITED
- Antikainen M, Griffith M, Zhang J, Hon W-C, Yang DSC, Pihakaski-Maunsbach K. Immunolocalization of antifreeze proteins in winter rye leaves, crowns, and roots by tissue printing. Plant Physiol. 1996;110:845–857. doi: 10.1104/pp.110.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers EP, Duke SH. Localization of α-amylase in the apoplast of pea (Pisum sativum L.) stems. Plant Physiol. 1988;87:799–802. doi: 10.1104/pp.87.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers EP, Duke SH, Henson CA. Partial characterization and subcellular localization of three α-glucosidase isoforms in pea (Pisum sativum L.) seedlings. Plant Physiol. 1990;94:738–744. doi: 10.1104/pp.94.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canny MJ. Apoplastic water and solute movement: new rules for an old space. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:215–236. [Google Scholar]
- Dexter ST, Tottingham WE, Graber LF. Investigations of the hardiness of plants by measurement of electrical conductivity. Plant Physiol. 1932;7:63–78. doi: 10.1104/pp.7.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieffenbach H, Kramer D, Luttge U. Release of guttation fluid from passive hydathodes of intact barley plants. I. Structure and cytological aspects. Ann Bot. 1980;45:397–401. [Google Scholar]
- Duke SH, Kakefuda G. Role of the testa in preventing cellular rupture during imbibition of legume seeds. Plant Physiol. 1981;67:449–456. doi: 10.1104/pp.67.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke SH, Kakefuda G, Henson CA, Loeffler NL, Van Hulle NM. Role of the testa epidermis in the leakage of intracellular substances from imbibing soybean seeds and its implications for seedling survival. Physiol Plant. 1986;68:625–631. [Google Scholar]
- Duke SH, Koukkari WL, Soulen TK. Glutamate dehydrogenase activity in roots: distribution in a seedling and storage root, and the effects of red and far-red illuminations. Physiol Plant. 1975;34:8–13. [Google Scholar]
- Gusta LV, Fowler DB. Factors affecting the cold survival of winter cereals. Can J Plant Sci. 1977;57:213–219. [Google Scholar]
- Hatch MD, Sacher JA, Glasziou KY. Sugar accumulation cycle in sugarcane. I. Studies on enzymes of the cycle. Plant Physiol. 1963;38:344–348. doi: 10.1104/pp.38.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker JS, Hatch MD. Mechanism of sugar storage by mature stem tissue of sugarcane. Physiol Plant. 1965;18:444–453. [Google Scholar]
- Henson CA. Purification and properties of barley stem fructan exohydrolase. J Plant Physiol. 1989;134:186–191. [Google Scholar]
- Henson CA, Livingston DP., III Purification and characterization of an oat fructan exohydrolase that preferentially hydrolyzes β-2,6-fructans. Plant Physiol. 1996;110:639–644. doi: 10.1104/pp.110.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husted S, Schjoerring JK. Apoplastic pH and ammonium concentration in leaves of Brassica napus L. Plant Physiol. 1995;109:1453–1460. doi: 10.1104/pp.109.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZC, McClure JW. Contamination of oat mesophyll protoplasts by apoplastic polyamine oxidase. Physiol Plant. 1989;77:347–351. [Google Scholar]
- Livingston DP., III Fructan precipitation from a water/ethanol extract of oats and barley. Plant Physiol. 1989;92:767–769. doi: 10.1104/pp.92.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston DP., III A device for grinding cereal crowns. Crop Sci. 1990;30:737–739. [Google Scholar]
- Livingston DP., III Non-structural carbohydrate accumulation in winter oat crowns before and during cold hardening. Crop Sci. 1991;31:751–755. [Google Scholar]
- Livingston DP., III The second phase of cold hardening: freezing tolerance and fructan isomer changes in winter cereal crowns. Crop Sci. 1996;36:1568–1573. [Google Scholar]
- Livingston DP, III, Chatterton NJ, Harrison PA. Structure and quantity of fructan oligomers in oat (Avena spp) New Phytol. 1993;123:725–734. [Google Scholar]
- Marentes E, Griffith M, Mlynarz A, Brush RA. Proteins accumulate in the apoplast of winter rye leaves during cold acclimation. Physiol Plant. 1993;87:499–507. [Google Scholar]
- Olien CR. An adaptive response of rye to freezing. Crop Sci. 1984;24:51–54. [Google Scholar]
- Pearce RS, Ashworth EN. Cell shape and localization of ice in leaves of overwintering wheat during frost stress in the field. Planta. 1992;188:324–331. doi: 10.1007/BF00192798. [DOI] [PubMed] [Google Scholar]
- Pollock CJ, Cairns AJ. Fructan metabolism in grasses and cereals. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:77–101. [Google Scholar]
- Pollock CJ, Chatterton NJ. Fructans. In: Preiss J, editor. The Biochemistry of Plants, Vol 14. San Diego, CA: Academic Press; 1988. pp. 109–140. [Google Scholar]
- Single WV, Marcellos H (1981) Ice formation and freezing injury in actively growing cereals, In CR Olien, MN Smith, eds, Analysis and Improvement of Plant Cold Hardiness. CRC Press, Boca Raton, FL, pp 35–59
- Steponkus PL. Cold hardiness and freezing injury of agronomic crops. Adv Agron. 1978;30:51–98. [Google Scholar]
- Sulc RM, Albrecht KA, Palta JP, Duke SH. Leakage of intracellular substances from alfalfa roots at various subfreezing temperatures. Crop Sci. 1991;33:1575–1578. [Google Scholar]
- Tetlow IJ, Farrar JF. Apoplastic sugar concentration and pH in barley leaves infected with brown rust. J Exp Bot. 1993;44:929–936. [Google Scholar]
- Trunova TL. Light and temperature systems in the hardening of winter wheat and the significance of oligosaccharides for frost resistance. Fiziol Rast. 1965;12:70–77. [Google Scholar]
- Wagner W, Wiemken A. Properties and subcellular localization of fructan hydrolase in the leaves of barley (Hordeum vulgare L. cv. Gerbel) J Plant Physiol. 1986;123:429–439. [Google Scholar]