Abstract
Large conspicuous eyespots have evolved in multiple taxa and presumably function to thwart predator attacks. Traditionally, large eyespots were thought to discourage predator attacks because they mimicked eyes of the predators’ own predators. However, this idea is controversial and the intimidating properties of eyespots have recently been suggested to simply be a consequence of their conspicuousness. Some lepidopteran species include large eyespots in their antipredation repertoire. In the peacock butterfly, Inachis io, eyespots are typically hidden during rest and suddenly exposed by the butterfly when disturbed. Previous experiments have shown that small wild passerines are intimidated by this display. Here, we test whether eyespots also intimidate a considerably larger bird, domestic fowl, Gallus gallus domesticus, by staging interactions between birds and peacock butterflies that were sham-painted or had their eyespots painted over. Our results show that birds typically fled when peacock butterflies performed their display regardless of whether eyespots were visible or painted over. However, birds confronting butterflies with visible eyespots delayed their return to the butterfly, were more vigilant, and more likely to utter alarm calls associated with detection of ground-based predators, compared with birds confronting butterflies with eyespots painted over. Because production of alarm calls and increased vigilance are antipredation behaviors in the fowl, their reaction suggests that eyespots may elicit fear rather than just an aversion to conspicuous patterns. Our results, therefore, suggest that predators perceive large lepidopteran eyespots as belonging to the eyes of a potential predator.
Key words: chicken, predator–prey interactions, startle display
Introduction
The strong selection pressure predation imposes on prey animals have resulted in the evolution of a wide range of antipredator devices encompassing a range of behavioral and morphological traits (Cott 1940; Edmunds 1974; Ruxton et al. 2004). Some traits reduce the risk of predator attacks, such as cryptic coloration, Batesian, or Müllerian mimicry, whereas other traits, for example startle displays, improve prey survival during an attack (Edmunds 1974). Startle displays may occur in both cryptic and aposematic species, and can range from auditory alarm signals to visual threat displays (Edmunds 1974; Ruxton et al. 2004).
The essence of a threat display is to intimidate a predator so that it aborts or delays the attack, thus enabling the displaying prey to escape. A threat display is functionally intuitive when performed by toxic or otherwise unprofitable prey, but why predators should refrain from attacking palatable and harmless prey when performing threat displays is still debated (Ruxton et al. 2004; Olofsson et al. 2011). One hypothesis posits that these kinds of “bluff” displays are targeted toward predators that are subject to attacks from predators themselves (i.e., mesopredators), such as small passerines and rodents (cf. Edmunds 1974; Olofsson et al. 2011) because risking its own life by calling a possible bluff may not be worth the cost to a mesopredator (Ruxton 2005).
One kind of threat display that has aroused considerable research interest recently is the display provided by palatable harmless lepidopterans that have large conspicuous eyespots on their wings (Stevens 2005). Some lepidopterans, for example the emperor moth (Saturnia pavonia), display their eyespots constantly, whereas others, such as the eyed hawkmoth (Smerinthus ocellata), keep their eyespots hidden at rest and display their eyespots only when attacked by a predator (Vallin et al. 2010). Experiments have shown that both the constant display and the startle display can discourage attacks from passerine birds (Vallin et al. 2005 , 2006; Kodandaramaiah et al. 2009; Vallin et al. 2010; Merilaita et al. 2011). One species, the peacock butterfly (Inachis io), seems very effective in thwarting predators and exhibits a multimodal defense by incorporating both auditory and visual defenses (Blest 1957; Mohl and Miller 1976; Olofsson et al. 2012). Although the peacock may expose its dorsal wing surface constantly during sun basking and foraging, it keeps its wings closed during rest. When disturbed during rest, the butterfly flicks its wings open thereby displaying two pairs of large eyespots on the dorsal wing surface and simultaneously producing ultrasonic clicks and a stridulatory hissing sound (Blest 1957; Mohl and Miller 1976; Olofsson et al. 2011). The signaling in the peacock butterfly appears not to be aposematic as they seem palatable to bats, rodents, and birds (Mohl and Miller 1976; Vallin et al. 2005; Olofsson et al. 2012). Recent experiments show that the visual defense is an efficient defense against small passerines (Vallin et al. 2005 , 2006) and that the auditory defense discourages rodents (Olofsson et al. 2011 , 2012).
Traditionally, the rationale why passerines are intimidated by the eyespots of the peacock butterfly posits that a pair of large eyespots mimics the eyes of the birds’ own predators, such as those of an owl (“Eye-mimicry hypothesis”; Blest 1957; Stevens 2005). However, this interpretation has been challenged by Stevens (2005), who has advanced the hypothesis that the conspicuousness of the eyespots per se can fully explain why predator attacks are thwarted (“Conspicuous signal hypothesis”; Stevens et al. 2008a). The “Conspicuous signal hypothesis” posits that lepidopteran eyespots comprise novel elements, developed to produce maximally salient visual stimuli, and so provoking neophobia and/or dietary conservatism in birds (Stevens et al. 2008a).
Recent experiments have shown that eyespots on the wings of butterflies may discourage attacks from small—experienced as well as naïve—passerine birds (Vallin et al. 2005; Kodandaramaiah et al. 2009; Merilaita et al. 2011). In this paper, our objective was to test whether the threat display of the peacock butterfly is effective also against a larger, predatory bird, by staging interactions between peacock butterflies with their eyespots visible or painted over and naïve adult domestic fowl of an old Swedish breed of Gallus gallus domesticus. Domestic fowl are interesting predators from the perspective that they are social and have a variety of antipredator behaviors. These include alarm calls that are used to warn members of the flock when a predator is approaching; moreover, different alarm calls are used to warn for aerial and terrestrial predators, respectively (Collias and Joos 1953; Collias 1987; Gyger et al. 1987; Evans et al. 1993). Domestic fowl produce abrupt “cut-cut-cut … cut KAAAH!” (sensu Collias and Joos 1953) when facing terrestrial threats, whereas airborne threats result in longer, continuous screams (Collias and Joos 1953). Hence, another objective was to investigate whether the butterfly threat display would elicit the former alarm call in the domestic fowl, which would provide a deeper insight of the cognitive aspects of the predators’ response toward large eyespots and so indicate whether they conceived of a displaying butterfly as a predatory threat or not.
METHODS
Study species
The peacock butterfly is a long-lived univoltine species; in central Sweden, the adult butterflies typically eclose from the pupa in July and enter hibernation in August/September and thereafter emerge in April/May to reproduce (Wiklund et al. 2008). The butterflies used in this study descended from four butterflies wild-caught in the vicinity of Stockholm, two males and two females, whose offspring were reared on the natural host plant stinging nettle (Urtica dioica). After eclosion, the butterflies were housed in 0.5 × 0.5 × 0.5-m cages that were lit by 100-W incandescent lamps on a 8:16-h light:dark photoperiod and allowed to feed during 2 weeks on a 20% sucrose solution provided from a sponge fitted into a 150-ml plastic cup. After 2 weeks, the butterflies were transferred to a 50-ml plastic cup and thereafter housed in a cold room maintained at 10 °C until the onset of the experiment. To test the intimidating effect of the eyespots, about half of the butterflies (N = 19) had their four eyespots painted over with a black permanent marker (Pilot Marker Noxylene black), whereas the rest (N = 23) were painted with black marker on an approximately equal (noncircular) dorsal wing surface area on all four wings, closer to the butterfly’s body, without obscuring the eyespots (see Vallin et al. 2005 for further details). It should be mentioned that when performing experiments with real butterflies, or wings of butterflies (e.g., Kodandaramaiah et al. 2009; Merilaita et al. 2011; this study), painting the wings is necessary both to obscure the eyespots as well as to create controls. Vallin and colleagues (2005) included, in addition to sham-painted, unpainted peacock butterflies also as controls in their experiment, and these were not less effective than sham-painted controls in intimidating blue tits, which suggests that sham-painting is an adequate method to create control butterflies in this type of experiments.
The predators used in the study were 22 males and 22 females from a population of domestic fowl of an old Swedish game breed of chicken, “Gammalsvensk dvärghöna,” kept at Tovetorp Zoological Research Station at Stockholm University where the experiments were conducted between 18 February and 26 March 2009. Individuals from this population are similar to their wild ancestor the red junglefowl, Gallus gallus ssp., in both morphology and behavior (Collias et al. 1966; Collias and Collias 1996; see Pizzari and Birkhead 2001 for further discussion; Schütz and Jensen 2001). The birds were 8–9 months old and hence sexually mature when used in the experiment (Johnsgard 1999); moreover, they had been housed indoors their whole lives and were, therefore, naïve with respect to both butterflies and predators. Birds were housed indoors in rooms measuring 3 × 3 × 2.5 m and containing wooden chips, sand, and perches and were provided commercial poultry feed and water supplied ad libitum. The lighting regime in the rooms was maintained on a 9:15-h light:dark regime; lights were lit between 8 AM and 5 PM, to correspond to prevailing light conditions outdoors during the experimental period. Experiments were conducted during the hours of the day when the birds were active (Løvlie and Pizzari 2007). When the birds were caught prior to training and experiment, lights were turned off to minimize stress and to reduce potential differences in the handling of the birds. Housing and handling of birds, together with experiments were conducted in accordance with ethical requirements in Sweden (ethical permission number 60-10, Linköping Ethical Committee).
Experimental room
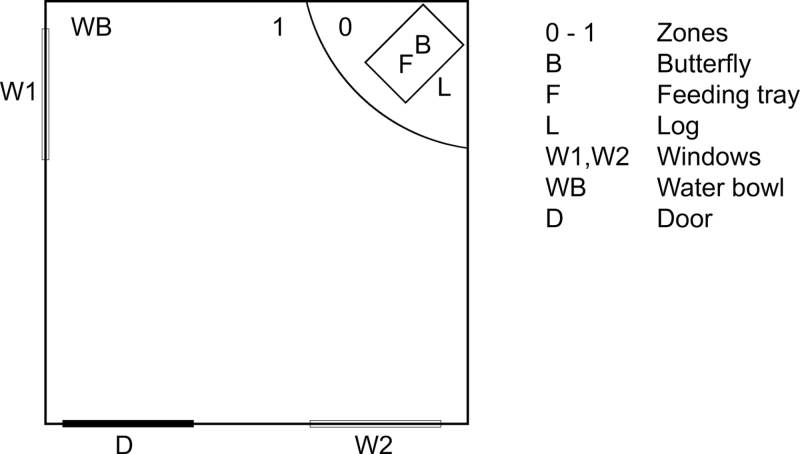
Training and experiments were conducted in a room measuring 2.4 × 2.3 × 1.9 m. The room was lit by eight fluorescent tubes (Philips TL-D 90 Graphica Pro 36W/950) and was provided with two one-way mirrors on two of the walls through which observations could be made without disturbing the birds. The temperature was kept low, between 8 and 10 °C, to prevent the butterflies from taking flight and to encourage them to maintain resting. The room had wooden chips on the floor, similar to the room where the birds were housed, to reduce the novelty of the room to the birds. One corner was defined as zone 0 and shaped as a quadrant with a radius of 0.6 m that was marked with duct tape. The remaining part of the room, outside zone 0, was defined as zone 1. In the corner was a piece of a 0.45-m long willow log (Salix caprea), on which we had nailed a transparent plastic box (from here on referred to as “feeding tray”) measuring 8.5 × 3cm containing live mealworms (Figure 1). The butterfly was placed just above the feeding tray immediately prior to an experimental trial. Events in the room were monitored with two cameras (Grandtech Grand Wi-Fi Camera Pro and Sony DCR-VX100E). The Sony camera was attached to the ceiling above the log and zoomed in to catch the movements of the butterfly and the bird’s initial reaction to the display of the butterfly. The Grandtech camera was placed on the opposing wall where it could record the movements of the birds in the experimental room. An external microphone was attached to the ceiling above the butterfly to record vocalizations from the birds. Birds had access to water ad libitum during training and experimental trials.
Figure 1 .
Outline of the experimental room. The floor was divided into two zones with the log and the butterfly placed in zone 0; zone 1 consisted of the remaining part of the room.
Training and experiment
All birds were individually taught that the feeding tray on the log contained mealworms with the aim of attracting them to this item during the experiment. Individual birds were randomly assigned to being given butterflies, which had their eyespots visible, or butterflies which had their eyespots painted over. Prior to the training session, individual birds were caught and transferred from their home pen and released in the experimental room, which was at that time completely dark. A training session started when the lights were turned on. If the bird did not approach the log and start feeding from the feeding tray within 45min, it was trained again the following day until the task of eating from the feeding tray was completed. The training was regarded as completed when a bird had eaten mealworms from the tray on the log, and a minimum of 20min of training time had elapsed to familiarize the bird to the experimental room. Two males that had not completed their task after five training sessions were excluded, leaving 42 birds in the experiment.
The experiment started immediately after a bird had completed a successful training session. The lights were turned off and the bird was placed outside the experimental room during the time it took to place a butterfly on the log and new mealworms in the feeding tray (2–5min). A transparent plastic lid was placed in the feeding tray with half of the mealworms underneath. This was done to encourage the birds to stay close to the feeding tray and not leave the vicinity of the tray before the butterfly had been disturbed and started performing its warning display. The butterfly was placed a few centimeters above the feeding tray giving it enough space to perform its wing-flicking behavior without touching the tray.
When the butterfly and the mealworms were in place, the bird was placed in the room with the lights turned off. An experimental trial started when the lights were switched on. After a short period of standing still and being vigilant, the bird typically walked directly toward the log and pecked at the mealworms. The pecking almost invariably disturbed the butterfly so that it started performing its warning display. The video recordings were later analyzed, and after the first display of the butterfly, we recorded the reaction of the bird noting 1) whether the bird left zone 0 (i.e., the zone that was closest to the displaying butterfly) within 10 s, 2) the latency until the bird returned to zone 0 (measured in seconds), 3) latency until the bird started feeding again (performing at least one peck on the floor or elsewhere, measured in seconds), and 4) whether the bird uttered alarm calls. We also noted which type of alarm call was uttered by the birds (aerial or terrestrial). Our criterion for an alarm call was that the domestic fowl uttered at least one monosyllabic call (“cut”) within 30 s from the butterfly’s display. We also used a more conservative criterion of what should be considered as an alarm call, defined as at least a trisyllabic call (“cut-cut-cut”) within 10 s from the butterfly’s display. This type of call has been acknowledged as a “ground predator warning” and is easy to distinguish from the sustained alarm screams elicited when domestic fowl perceive airborne threats (Collias and Joos 1953).
Further, to elucidate whether our treatment of the butterflies had affected their motivation or ability to perform their display, we examined the behavior of the butterflies when their display was elicited the first time and we extracted the following data from the video recordings: 1) the time the fowl had to spend in zone 0 (measured in seconds) until the butterfly was disturbed and initiated its display, 2) the number of wing-flicks within 30 s from the onset of the display, and 3) the time (measured in seconds) it took a butterfly to expose the dorsal side of its wings; this was done by studying the video sequences, frame-by-frame, from the first movement of the butterfly until the dorsal wing surface was exposed. It was apparent that two of the butterflies, which had their eyespots painted over, differed markedly in their willingness to display, compared with the other butterflies. These two butterflies only partly and slowly opened their wings when they were disturbed by the fowl, hence exposing the fowl only to modest startling and visual stimuli. As expected, the birds showed very little reaction to these two butterflies. Therefore, these two trials were excluded, leaving 40 trials (17 butterflies with eyespots painted over and 23 butterflies with visible eyespots) for further analysis.
An experimental trial was terminated 30min after the first display of a butterfly, after which the bird was caught and released to its home pen. Each bird and butterfly was only used in one experimental trial. On five occasions, the butterfly started walking before the bird approached the log. When this happened, the lights were turned off and the butterfly was replaced in its original position before the trial was restarted. The butterflies that were replaced at the original position remained still when the trials were restarted, and the remaining 37 butterflies did not move until the bird disturbed them.
Statistical analyses
Data on latency until the birds returned to zone 0 after being subjected to the butterfly’s display were skewed and inflated by zeros, and did not meet the assumptions for parametric testing and were, therefore, analyzed using Wilcoxon rank-sum test. Data on latency until the birds resumed foraging after the butterfly’s first display were log-transformed to meet the assumptions for parametric testing and were analyzed using t-test. Whether the birds uttered alarm calls or not was analyzed using Fisher’s Exact test.
The behavioral parameters of the butterflies (see Training and experiment) were all analyzed using Wilcoxon rank-sum tests. All analyses were performed in R, version 2.10.1 (R Development Core Team 2009).
RESULTS
All of the 40 domestic fowl reacted the first time the butterfly performed its display. The behavioral responses included flinching, ceasing foraging, alarm calling, and varying speeds of withdrawal from the butterfly. Overall, a majority of the birds fled from zone 0 (30 of 40 individuals; binomial test: P = 0.0022) within 10 s from the onset of the butterfly’s display, but there was no difference between birds that had been confronted with butterflies with their eyespots visible (19 of 23) compared with birds that had been confronted with butterflies with their eyespots painted over (11 of 17; N = 40; Fisher’s exact: P = 0.27). However, birds that had been confronted with butterflies with their eyespots visible took longer until they returned to zone 0 (N = 23, median = 217 s, 1st Q = 6.5 s, 3rd Q = 318.5 s) compared with those birds that had been confronted with butterflies with their eyespots painted over (N = 17, median = 7 s, 1st Q = 0 s, 3rd Q = 93 s) (Wilcoxon rank-sum test: N = 40, W = 106, P = 0.014). Furthermore, the fowl that had been confronted with butterflies with their eyespots visible took longer until they resumed foraging (N = 23, median = 137 s, 1st Q = 77.5 s, 3rd Q = 246 s) compared with birds that had been confronted with butterflies with their eyespots painted over (N = 17, median = 58 s, 1st Q = 29 s, 3rd Q = 96 s) (t-test: N = 40, t = −3.42, degrees of freedom = 27.8, P = 0.0019).
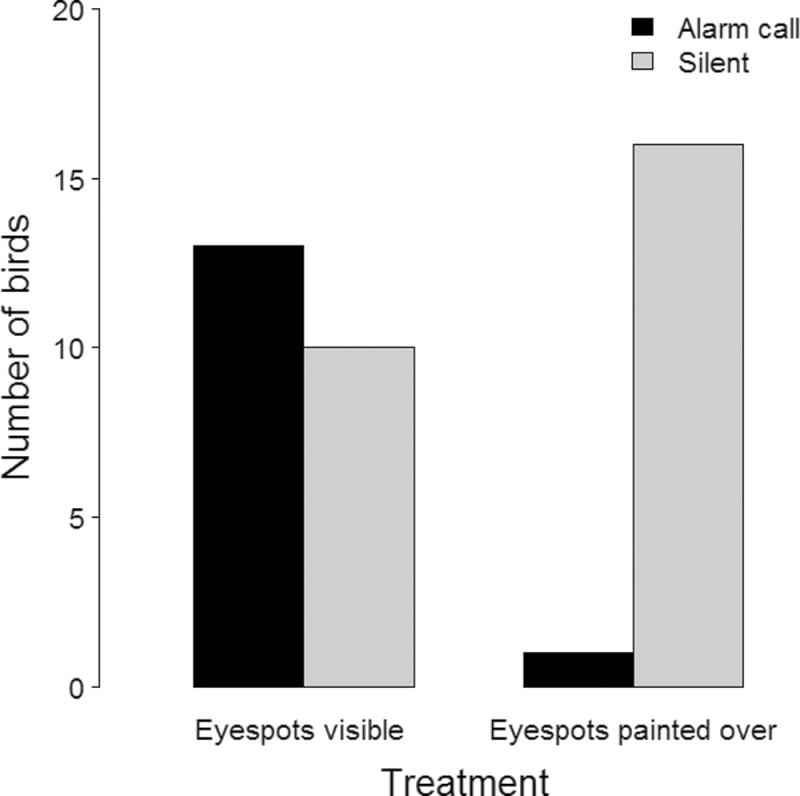
Domestic fowl were more likely to utter at least one monosyllabic alarm call (“cut”) within 30 s from the onset of the butterfly’s display when confronted with butterflies with their eyespots visible (13 of 23), compared with those birds that had been confronted with butterflies with eyespots painted over (1 of 17) (Fisher’s Exact test: N = 40; P = 0.00094) (Figure 2). An analysis of the stricter definition of alarm calls (i.e., at least a trisyllabic alarm call [“cut-cut-cut”] within 10 s from the onset of the butterfly’s display) hinted in the same direction with 8 of 23 fowl uttering alarm calls when confronted with butterflies with visible eyespots and only 1 of 17 uttering alarm calls when confronted with butterflies with eyespots painted over (N = 40; Fisher’s Exact test: P = 0.054) (see Supplementary Material, Video). It is noteworthy that all alarm calls uttered during the experiments exclusively consisted of “ground predator warning” calls (sensu Collias and Joos 1953). Only a few of the butterflies were actually seized by the birds and were always dropped within a second or two, and all butterflies remained alive at the termination of the experiment. The reason why so few of the butterflies were seized and none were consumed by the domestic fowl was probably due to the fact that these birds were naïve with respect to butterflies and, therefore, reluctant to include novel food in their diet (cf. Mappes et al. 2005).
Figure 2 .
The number of birds that produced at least one monosyllabic alarm call (“cut”) within 30 s from the peacock’s first display, when confronting a butterfly with visible eyespots or a butterfly with its eyespots painted over.
There were no differences in any behaviors of the butterflies in the two butterfly groups (eyespots visible/eyespots painted over). There was no difference in the amount of time the fowl had to spend close to the butterfly (i.e., in zone 0) until the butterfly’s display was elicited the first time (butterflies with visible eyespots: N = 23, median = 16 s, 1st Q = 8 s, 3rd Q = 29.5 s; butterflies with eyespots painted over: N = 17, median = 8 s, 1st Q = 6 s, 3rd Q = 38 s [Wilcoxon rank-sum test: N = 40, W = 174.5, P = 0.57]). There was no difference in wing-flicking rate (butterflies with visible eyespots: N = 23, median = 11 flicks per 30 s, 1st Q = 8.5, 3rd Q = 12; butterflies with eyespots painted over: N = 17, median = 11 flicks per 30 s, 1st Q = 7, 3rd Q = 13 [Wilcoxon rank-sum test: N = 40, W = 203.5, P = 0.84]). There was no difference in the time it took for the butterflies to reveal their dorsal wing surface in the initial phase of the display (butterflies with visible eyespots: N = 23, median = 0.2 s, 1st Q = 0.15, 3rd Q = 0.23; butterflies with eyespots painted over: N = 17, median = 0.17 s, 1st Q = 0.13, 3rd Q = 0.23 s [Wilcoxon rank-sum test: N = 40, W = 188, P = 0.85]).
DISCUSSION
In this study, we have shown that domestic fowl typically fled when peacock butterflies performed a display, regardless of whether the eyespots of the butterflies were visible or painted over. Hence, the startle display per se had a definite intimidating effect on the birds. However, the domestic fowl were even more intimidated by peacock butterflies that had their eyespots visible; birds took longer to return to the vicinity of the butterfly/food, were more likely to utter alarm calls and took longer to resume foraging after confronting a peacock butterfly with its eyespots visible compared with when confronting a peacock with its eyespots painted over. Therefore, the eyespots per se definitely enhanced the intimidating effect of the butterflies’ startle display. Our manipulation of the butterflies did not seem to interfere with either their propensity or ability to perform their display, which is crucial for two reasons: first, eyespots are only shown to the predator when the butterfly flicks its wings open, and second, the intimidation of predators is likely a combined effect of the act of wing-flicking and the visual appearance of eyespots (cf. Vallin et al. 2005). Thus, the observed differences in responses of the domestic fowl are likely to be solely due to the presence or absence of eyespots.
Large butterfly eyespots have been shown to intimidate a number of small passerine birds (Blest 1957; Vallin et al. 2005; Kodandaramaiah et al. 2009; Merilaita et al. 2011), but there is yet no consensus as to why this is so, and whether the reason is that eyespots deceive the predator that it is confronted by one of its own predators ( “Eye-mimicry hypothesis”), or whether the conspicuousness of eyespots is a sufficient reason explaining the reaction of the predator (“Conspicuous signal hypothesis”) (Stevens 2005; Stevens et al. 2008a , 2008b; Brilot et al. 2009). To our knowledge, this study is the first to show that butterfly eyespots can also intimidate a considerably larger bird species (cf. Kodandaramaiah 2011). Given that a warning behavior of a prey is effective only with respect to a given size range of prospective predator (Tinbergen 1958), it is somewhat surprising that the peacock’s eyespot display was so effective in also intimidating such a large bird as domestic fowl that is 50 times heavier (ca 0.5–1kg) than a blue tit (weighing on average between 9–12.5g).
One explanation for the strong response of the domestic fowl when confronting peacock butterflies is that, being poorer flyers, they are particularly alert and vigilant when foraging on the ground because they are more subject to attacks from terrestrial predators than are passerines. Previous experiments with another galliform species, Japanese quail, Coturnix coturnix japonica, demonstrated that these were strongly intimidated by aposematic butterflies, the European swallowtail Papilio machaon and the monarch Danaus plexippus, and exhibited signs of fear when encountering adult butterflies of both species, which they were hesitant to attack (Wiklund and Sillén-Tullberg 1985). An additional reason why galliforms may be particularly responsive is that they are social birds and with limited dispersal (Collias et al. 1966; Collias and Collias 1996; Løvlie and Pizzari 2007) resulting in increased relatedness between group-members. Such a social organization is typically associated with improved defense against predators, by promoting among other factors vigilance and the evolution of a broad alarm call repertoire (Alcock 2009).
It has been convincingly demonstrated that domestic fowl elicit unambiguous and detailed responses to aerial and terrestrial predators or predator models (Gyger et al. 1987; Bayly and Evans 2003) and show differentiated responses dependent on, for example, the shape and speed of the predator models (Evans et al. 1993). The birds in this study explicitly uttered alarm calls that have been attributed to ground predator warning (Collias and Joos 1953). We argue that alarm calling in the fowl in this experiment provides direct evidence that birds conceive large eyespots as threatening, compared with customarily deployed measures such as latency to attack or prey survival (e.g., Vallin et al. 2005; Stevens et al. 2008a; Kodandaramaiah et al. 2009; Merilaita et al. 2011) and substantiate that cognitive mechanisms are instrumental in explaining why eyespots are intimidating to birds. Nevertheless, further investigation is needed to establish whether production of an appropriate alarm call really signifies that the bird perceives the eyespots as belonging to an attacking predator, which would support the “Eye-mimicry hypothesis.” In this experiment, the domestic fowl were not only more likely to utter alarm calls but also displayed increased vigilance (i.e., ceased foraging) and took longer to return to butterflies that had their eyespots visible, which suggests that eyespots elicit fear. Although the distribution of red jungle fowl and peacock butterflies is largely allopatric, we contend that the results of this study are relevant. No matter if large eyespots are intimidating because of eye-mimicry (Blest 1957) or due to their mere conspicuousness (Stevens 2005), we would expect similar responses from a wide range of predators (including nonsyntopic ones) that share similar lifestyles. That is, predators which face strong predation pressure and use “eyes” as a proxy for potential danger are expected to generalize among patterns that could be real eyes of a predator (cf. Janzen et al. 2010). Similarly, we would also expect nonsyntopic predators that feed on similar prey to have evolved mechanisms to avoid eating what could be noxious prey.
Merilaita and colleagues (2011) showed that aversion against large butterfly eyespots (in I. io) is an innate character in birds (pied flycatcher, Ficedula hypoleuca)—our results are in agreement with that conclusion. Furthermore, in an attempt to distinguish the “Eye-mimicry hypothesis” from the “Conspicuous signal hypothesis,” Merilaita and colleagues (2011) presented mounted wings from peacock butterflies (with a meal worm as bait), which had either 1) both pairs of eyespots painted over, 2) one pair of eyespots painted over, or 3) all eyespots visible. Interestingly, two pair of eyespots (i.e., a stronger conspicuous signal) did not increase attack latency compared with when the birds encountered prey models with only one pair of eyespots (i.e., a weaker conspicuous signal) and by that the authors rejected the generality of the “Conspicuous signal hypothesis.” Although this study, as well as the study of Merilaita and colleagues (2011), does not provide conclusive evidence that birds are intimidated by some butterflies’ eyespots because they mimic true eyes of predators, we contend that responses involving strong fear reactions, such as alarm calls in this study, have evolved in the context of avoiding being attacked by predators, or prey capable of actually harming the attacking predator physically, rather than in the context of preventing predators from attacking novel, potentially noxious, prey as inherently suggested by the “Conspicuous signal hypothesis” (cf. Stevens 2005). Ruxton (2005) discussed why predators seem to be unable to cease responding to bluff displays that are performed by palatable prey species such as the peacock. To us, the most reasonable inference is that learning to ignore large eyespots in order to get access to prey would be a dangerous strategy for a mesopredator (cf. Ruxton 2005; Olofsson et al. 2011), as it has been pointed out that even a single mistake (i.e., not responding to real eyes of a predator) would be fatal to the predator (cf. Janzen et al. 2010).
Lepidopteran eyespots vary greatly in appearance and often include dislocated pattern elements that may make the eyespots look three dimensional (i.e., attaining a closer resemblance to real eyes) (Kodandaramaiah 2011). For example, some species in the genus Caligo have large eyespots on their ventral wing surface with a crescent-shaped highlight (or “sparkle”) in the “pupil,” and there is experimental evidence that both the presence and position of such features are important in enhancing the protective function of eyespots (Blut et al. 2012).
We suggest that future studies attempting to address the issue why birds are intimidated by large lepidopteran eyespots should make use of the fact that some predators employ distinct interspecific and intraspecific signaling, such as alarm calling in the domestic fowl. A fundamental question worthwhile investigating would be to what extent alarm calls that are associated with the presence of a predator would be elicited when the predator perceives circular patterns of the same contrast but with varying resemblance to vertebrate eyes. Unfortunately, such manipulations are difficult to perform on real lepidopteran wings. However, artificial “eyespots,” such as those used by Blut and colleagues (2012) and Stevens and colleagues (2008a), may function as good proxies. Nevertheless, further experiments are clearly required to distinguish between the competing hypotheses attempting to explain why eyespots elicit fear in birds, to further improve our understanding of the evolution of eyespots specifically, and predator–prey interactions more generally.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at http://www.beheco.oxfordjournals.org/
FUNDING
This study was financed by the Swedish Research Council (C. W.). Grant number: 621-2010-5579.
Supplementary Material
Acknowledgments
We thank Sami Merilaita and one anonymous reviewer for comments that substantially improved this paper.
References
- Alcock J. Animal behavior. 9th ed. Sunderland (MA):: Sinauer Associates, Inc.; 2009. [Google Scholar]
- Bayly KL, Evans CS. 2003. Dynamic changes in alarm call structure: a strategy for reducing conspicuousness to avian predators? Behaviour 140 353–369 [Google Scholar]
- Blest AD. 1957. The function of eyespot patterns in the Lepidoptera. Behaviour 11 209–255 [Google Scholar]
- Blut C, Wilbrandt J, Fels D, Girgel EI, Lunau K. 2012. The ‘sparkle’ in fake eyes - the protective effect of mimic eyespots in leipdoptera. Entomol Exp Appl. 143 231–244 [Google Scholar]
- Brilot BO, Normandale CL, Parkin A, Bateson M. 2009. Can we use starlings’ aversion to eyespots as the basis for a novel ‘cognitive bias’ task? Appl Anim Behav Sci. 118 182–190 [Google Scholar]
- Collias N, Joos M. 1953. The spectrographic analysis of sound signals of the domestic fowl. Behaviour 5 175–188 [Google Scholar]
- Collias NE. 1987. The vocal repertoire of the red junglefowl: a spectrographic classification and the code of communication. Condor. 89 510–524 [Google Scholar]
- Collias NE, Collias EC. 1996. Social organization of a red junglefowl, Gallus gallus, population related to evolution theory. Anim Behav. 51 1337–1354 [Google Scholar]
- Collias NE, Collias EC, Hunsaker D, Minning L. 1966. Locality fixation, mobility and social organization within an unconfined population of red jungle fowl. Anim Behav 14 550–559 [DOI] [PubMed] [Google Scholar]
- Cott HB. Adaptive coloration in animals. London:: Methuen & Co.; 1940. [Google Scholar]
- Edmunds M. Defence in animals: a survey of anti-predator defences. New York: Longman Ltd; 1974. [Google Scholar]
- Evans CS, Evans L, Marler P. 1993. On the meaning of alarm calls: functional reference in an avian vocal system. Anim Behav. 46 23–38 [Google Scholar]
- Gyger M, Marler P, Pickert R. 1987. Semantics of an avian alarm call system - the male domestic-fowl, Gallus domesticus. Behaviour 102 15–40 [Google Scholar]
- Janzen DH, Hallwachs W, Burns JM. 2010. A tropical horde of counterfeit predator eyes. Proc Natl Acad Sci USA 107 11659–11665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsgard PA. The pheasants of the world: biology and natural history. Washington (DC): Smithsonian Institute Press; 1999. [Google Scholar]
- Kodandaramaiah U. 2011. The evolutionary significance of butterfly eyespots. Behav Ecol. 22 1264–1271 [Google Scholar]
- Kodandaramaiah U, Vallin A, Wiklund C. 2009. Fixed eyespot display in a butterfly thwarts attacking birds. Anim Behav 77: 1415–1419 [Google Scholar]
- Løvlie H, Pizzari T. 2007. Sex in the morning or in the evening? Females adjust daily mating patterns to the intensity of sexual harassment. Am Nat 170 E1–13 [DOI] [PubMed] [Google Scholar]
- Mappes J, Marples N, Endler JA. 2005. The complex business of survival by aposematism. Trends Ecol Evol 20 598–603 [DOI] [PubMed] [Google Scholar]
- Merilaita S, Vallin A, Kodandaramaiah U, Dimitrova M, Ruuskanen S, Laaksonen T. 2011. Number of eyespots and their intimidating effect on naïve predators in the peacock butterfly. Behav Ecol 22 1326–1331 [Google Scholar]
- Mohl B, Miller LA. 1976.. Ultrasonic clicks produced by peacock butterfly - possible bat-repellent mechanism. J Exp Biol. 64 639–644 [Google Scholar]
- Olofsson M, Jakobsson S, Wiklund C. 2012. Auditory defence in the peacock butterfly (Inachis io) against mice (Apodemus flavicollis and A. sylvaticus). Behav Ecol Sociobiol. 66 209–215 [Google Scholar]
- Olofsson M, Vallin A, Jakobsson S, Wiklund C. 2011. Winter predation on two species of hibernating butterflies: monitoring rodent attacks with infrared cameras. Anim Behav. 81 529–534 [Google Scholar]
- Pizzari T, Birkhead TR. 2001. For whom does the hen cackle? The function of postoviposition cackling. Anim Behav. 61 601–607 [Google Scholar]
- R Development Core Team. 2009. R: a language and environment for statistical computing. Vienna (Austria) R Foundation for Statistical Computing.ISBN 3-900051-07-0. Available from: http://www.R-project.org [Google Scholar]
- Ruxton GD. 2005. Intimidating butterflies. Trends Ecol Evol 20 276–278 [DOI] [PubMed] [Google Scholar]
- Ruxton GD, Sherratt TN, Speed MP. Avoiding attack. Oxford: Oxford University Press; 2004. [Google Scholar]
- Schütz KE, Jensen P. 2001. Effects of resource allocation on behavioural strategies: A comparison of red junglefowl (Gallus gallus) and two domesticated breeds of poultry. Ethology. 107 753–765 [Google Scholar]
- Stevens M. 2005. The role of eyespots as anti-predator mechanisms, principally demonstrated in the Lepidoptera. Biol Rev Camb Philos Soc 80 573–588 [DOI] [PubMed] [Google Scholar]
- Stevens M, Hardman CJ, Stubbins CL. 2008a. Conspicuousness, not eye mimicry, makes “eyespots” effective antipredator signals. Behav Ecol. 19 525–531 [Google Scholar]
- Stevens M, Stubbins CL, Hardman CJ. 2008b. The anti-predator function of ‘eyespots’ on camouflaged and conspicuous prey. Behav Ecol Sociobiol. 62: 1787–1793 [Google Scholar]
- Tinbergen N. Curious naturalists. London: Country Life; 1958. [Google Scholar]
- Vallin A, Jakobsson S, Lind J, Wiklund C. 2005. Prey survival by predator intimidation: an experimental study of peacock butterfly defence against blue tits. Proc Biol Sci 272 1203–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallin A, Jakobsson S, Lind J, Wiklund C. 2006. Crypsis versus intimidation - anti-predation defence in three closely related butterflies. Behav Ecol Sociobiol. 59 455–459 [Google Scholar]
- Vallin A, Jakobsson S, Wiklund C. 2010. Constant eyespot display as a primary defence - survival of male and female emperor moths attacked by blue tits. . J Res Lepidoptera 43 9–17 [Google Scholar]
- Wiklund C, Sillén-Tullberg B. 1985. Why distasteful butterflies have aposematic larvae and adults, but cryptic pupae - evidence from predation experiments on the monarch and the european swallowtail. Evolution 39 1155–1158 [DOI] [PubMed] [Google Scholar]
- Wiklund C, Vallin A, Friberg M, Jakobsson S. 2008. Rodent predation on hibernating peacock and small tortoiseshell butterflies. Behav Ecol Sociobiol. 62 379–389 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.