Abstract.
Ultrafast lasers in the visible and near-infrared range have emerged as a potential new method for pathogen reduction of blood products and pharmaceuticals. However, the mechanism of enveloped virus inactivation by this method is unknown. We report the inactivation as well as the molecular and structural effects caused by visible (425 nm) femtosecond laser irradiation on murine cytomegalovirus (MCMV), an enveloped, double-stranded DNA virus. Our results show that laser irradiation (1) caused a 5-log reduction in MCMV titer, (2) did not cause significant changes to the global structure of MCMV virions including membrane and capsid, as assessed by electron microscopy, (3) produced no evidence of double-strand breaks or crosslinking in MCMV genomic DNA, and (4) caused selective aggregation of viral capsid and tegument proteins. We propose a model in which ultrafast laser irradiation induces partial unfolding of viral proteins by disrupting hydrogen bonds and/or hydrophobic interactions, leading to aggregation of closely associated viral proteins and inactivation of the virus. These results provide new insight into the inactivation of enveloped viruses by visible femtosecond lasers at the molecular level, and help pave the way for the development of a new ultrafast laser technology for pathogen reduction.
Keywords: ultrafast lasers, pathogen reduction, pathogen inactivation, murine cytomegalovirus
1. Introduction
Pathogen reduction (PR), which aims to proactively eliminate infectious agents from blood products, is an attractive strategy to address the threat of known and emerging pathogens and ensure the continued safety of the blood supply. However, the various PR methods explored to date suffer from limitations that prevent their widespread use and acceptance by the transfusion medicine community. Clinically tested PR techniques for human plasma include solvent-detergent (SD) treatment,1 visible light-activated sensitizers such as methylene blue,2 and ultraviolet (UV) light-activated photochemicals such as riboflavin and amotosalen.3–11 All current techniques involve the introduction of chemicals with risks of unknown or unpredictable side effects. These side effects include immune reactions, carcinogenicity, or loss of coagulation factors in the product, all of which can lead to adverse consequences in patients.12 SD treatment is also limited because it cannot inactivate nonenveloped viruses, and thus is ineffective against many transfusion-transmitted pathogens, such as parvovirus B19 and hepatitis A virus (HAV).12 Furthermore, with all of the above mentioned methods, the introduction and subsequent removal of chemicals is an additional step that adds to the cost of implementing the PR technology.
Short wavelength UV (UVC) radiation has been tested as an alternative, chemical-free PR technology. UVC radiation inactivates pathogens by DNA damage through dimerization of adjacent pyrimidines13 as well as generation of reactive oxygen species.14 UVC treatment has shown effects against certain viruses and bacteria.15–17 However, there is already evidence of resistance to UVC among blood borne pathogens such as HIV.17 Moreover, UVC is strongly absorbed by proteins and has been shown to damage plasma components18 and cause platelet aggregation.19 Thus, there is a need to develop a new chemical-free PR technique with broader pathogen coverage and minimal effects on the blood product.
In this regard, ultrafast lasers in the visible and near-infrared range are a potentially ideal approach for PR. Visible/near-infrared ultrafast laser irradiation does not cause ionization effects that can damage the blood product. It does not introduce potentially toxic or carcinogenic chemicals, and thereby has minimal concern of adverse effects. Our group has recently shown femtosecond laser irradiation to be effective in inactivating (achieving 3 to 5 log reduction of) a broad spectrum of viruses,20 including human immunodeficiency virus (HIV),21,22 human papillomavirus (HPV),22 encephalomyocarditis virus,23 M13 bacteriophage,23–27 and tobacco mosaic virus (TMV).22 More importantly, femtosecond laser irradiation at sufficient power to kill the above mentioned viruses does not kill human cells27 and does not appear to damage either bovine serum albumin (BSA) protein or single stranded DNA.23
Human cytomegalovirus (HCMV) is a widespread pathogen responsible for multiple significant diseases. It is the leading viral cause of congenital diseases in newborns, a common cause of opportunistic infections in acquired immunodeficiency syndrome (AIDS) and transplant patients, and a potential risk factor in certain cardiovascular diseases.28,29 Significant limitations are seen with current antiviral therapeutics,30–33 and there are considerable needs for new treatments of HCMV disease.34 The severity of medical problems associated with HCMV in these vulnerable populations underlies the necessity for ensuring that blood products are safer for this group of patients. Murine cytomegalovirus (MCMV) is now widely used as a surrogate model for HCMV because of its robust replication, its tractable genetic systems, the availability of many reagents for both the virus and host, and access to an animal model for in vivo experimentation which is impossible for HCMV.
We note that the specific effects of femtosecond laser irradiation on viral membranes, capsids, and nucleic acids at the molecular level remain unclear. This information is essential for the optimization, application, and approval of femtosecond lasers for use in PR of therapeutics including pharmaceuticals and blood products. Toward these goals, we report the inactivation of MCMV, a model herpes virus, by using a 425 nm-femtosecond laser and determine the molecular effects of laser irradiation on MCMV virions, viral genomic DNA, and virion-associated proteins. In remarkable contrast to the atomic force microscope images of nonenveloped viruses such as M13 bacteriophage and TMV showing that the capsids were broken by the ultrashort pulsed (USP) laser irradiation presented in the previous work, our TEM images revealed that the USP laser did not break/dissociate the capsid of MCMV. We propose a novel mechanism for the enveloped virus inactivated by visible femtosecond lasers through induction of viral protein aggregation. By correlating viral inactivation with the observed structural and molecular effects, a better insight into the inactivation of enveloped viruses by femtosecond laser irradiation was obtained.
2. Materials and Methods
2.1. Cells and Viruses
Murine embryonic fibroblast 10.1 (MEF 10.1) cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM), supplemented with 10% fetal calf serum, 1 mM sodium pyruvate, and nonessential amino acids. GFP-expressing MCMV virus (hereafter referred to as MCMV) was generated as previously described.35 To produce viral stocks, MEF 10.1 cells were infected with MCMV at a low multiplicity of infection. Cell supernatants were harvested 24 h postinfection after 100% cytopathic effect and cleared of cell debris by centrifugation. Extracellular virions were pelleted by ultracentrifugation with sorbitol cushion and resuspended in phosphate-buffered saline (PBS). Viral titers were determined in quadruplicate using a median tissue culture infectious dose () assay.
2.2. Femtosecond Laser Irradiation
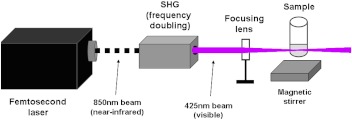
The experimental setup for laser irradiation is shown in Fig. 1. The excitation source employed in this work was a diode-pumped cw mode-locked Ti-sapphire laser. The laser produced a continuous train of 60 fs pulses at a repetition rate of 80 MHz. The output of the second harmonic generation system of the Ti-sapphire laser was used to irradiate the sample. The excitation laser was chosen to operate at a wavelength of and with an average power of approximately 150 mW. It has a pulse width of full width at half maximum. An achromatic lens was used to focus the laser beam into a spot about 100 μm in diameter within the sample volume. Samples of virus suspended in phosphate buffered saline at a concentration of were irradiated for 1.5 h. In order to facilitate the interaction of laser with the virus, a magnetic stirring system was used so that the virus would enter the laser-focused volume as described above and interact with the photons. Controls were similarly stirred. Irradiation was carried out at 22°C and with the single laser beam excitation. After laser irradiation, samples were immediately stored at .
Fig. 1.
Experimental setup for femtosecond laser irradiation. An 850 nm (near-infrared) laser beam was frequency doubled to produce 425 nm (visible) irradiation. Samples were magnetically stirred to expose the virus to the laser-focused volume.
2.3. Assays
assays were performed to determine reduction in viral titers following laser irradiation. MEF 10.1 cells were seeded into 96 well plates at a density of and incubated overnight. Cells were approximately 80% confluent at the time of infection. Laser-treated or control (untreated) virus were serially diluted and added to cells, and cells were incubated for four days. Viral titers were determined on day 4 postinfection by scoring each well for GFP-positive cells using a fluorescent microscope.
2.4. Electron Microscopy
Laser-treated or control (untreated) MCMV virions were allowed to absorb onto formvar/carbon-coated copper grids for 10 min. Grids were washed in distilled water and stained with 1% phosphotungstic acid (Electron Microscopy Sciences, Hatfield, Pennsylvania) for 1 min. Excess liquid was gently wicked off and grids were allowed to air dry. Samples were viewed on a JEOL 1200EX transmission electron microscope (JEOL USA, Peabody, Massachusetts) at an accelerating voltage of 100 kV. Images were acquired with a XR80M-B 8 megapixel CCD camera system (Advanced Microscopy Techniques Corporation, Woburn, Massachusetts).
2.5. Purification of MCMV DNA
Laser-treated or control (untreated) MCMV virions were treated with DNase I for 30 min at 37°C, and then transferred to 75°C to inactivate DNase I. Viral membranes were lysed using a lysis buffer {800 mM NaCl, 20 mM Tris [pH 8.0], 20 mM EDTA, 0.4% sodium dodecyl sulfate (SDS)}, and viral capsids were digested by incubation with proteinase K () at 55°C overnight. DNA was extracted with phenol-chloroform, precipitated with isopropanol, and centrifuged at 13,000×g for 30 min at 4°C. The pellets were washed with 70% ethanol and resuspended in water. For subsequent agarose gel electroporesis, MCMV DNA samples were left intact or digested using EcoRI or HindIII restriction enzymes for 5 h at 37°C prior to gel loading.
2.6. Agarose Gel Electrophoresis
Agarose gels, 0.6% and 5 mm in thickness, were cast in a full-length gel apparatus. The MCMV DNA samples were mixed with loading buffer and then loaded into wells. Electrophoresis was carried out at overnight. The gel was then stained with ethidium bromide for 30 min, destained for 3 h and visualized under UV illumination.
2.7. Protein Gel Electrophoresis
Protein concentration of viral solutions was determined by Bradford assay (colorimetric protein assay kit, Bio-Rad). Solutions of laser-treated or control (untreated) virus containing equivalent quantities of protein were boiled in reducing loading buffer and separated on a 10% SDS-PAGE gel. Protein bands were visualized with Coomassie blue staining (LabSafe Gel Blue, G-Biosciences).
2.8. Mass Spectrometry Analysis
Gel slices were excised manually and submitted to Midwest Bio Services LLC (Overland Park, Kansas) for trypsin digest followed by nano LC-MS/MS analysis and protein identification. For details on the protocol, please refer to http://www.midwestbioservices.com/proteinid.html.
2.9. Dynamic Light Scattering
mAb(04) samples in buffer solution (50 mM sodium acetate, PH 7.0) were from Enzo life Sciences (Farmingdale, New York). BSA samples in buffer solution (50 mM sodium acetate, pH 7.0) were from Thermal Scientific Inc. (Mansfield, Texas). The dynamic light scattering (DLS) experiments were carried out by using a 90Plus Particle Size Analyzer from Brookhaven Instruments Corp. (Holtsville, New York).
2.10. Statistics
Differences between mean titers of control and laser-treated virus were analyzed by Student’s -test. was used as a threshold for statistical significance.
3. Results
3.1. MCMV is Efficiently Inactivated by Femtosecond Laser Irradiation
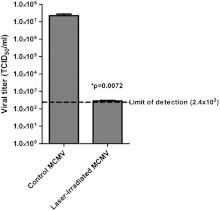
We have previously shown that a variety of enveloped/nonenveloped, single-stranded DNA/RNA viruses can be inactivated by femtosecond laser irradiation.21–27 Therefore, we sought to demonstrate that irradiation with a femtosecond laser at a similar laser power (150 mW) could also inactivate MCMV, an enveloped, double-stranded DNA virus. The laser setup is illustrated in Fig. 1. For all experiments in this report, we used a previously established GFP-expressing MCMV35 for ease of detection of infectious virus by assay. As shown in Fig. 2, irradiation with a 425 nm-femtosecond laser at 150 mW caused a 5-log reduction in MCMV titer relative to the control (nonirradiated) MCMV (). The titers of all laser-treated samples were at or near the limit of detection of the assay. This is consistent with the inactivation efficiency (3 to 5 log) for other viruses using the same laser conditions. Therefore, we conclude that 425 nm-femtosecond laser irradiation is an effective method to inactivate MCMV.
Fig. 2.
Inactivation of MCMV using a femtosecond laser. Femtosecond laser-induced reduction in viral titer was assessed by TCID36 assay. Results are representative of quadruplicate experiments and error bars indicate SEM.
3.2. MCMV Global Virion Structure is Preserved after Femtosecond Laser Irradiation
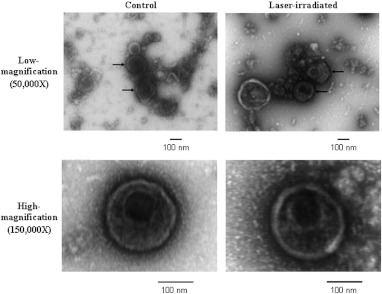
We used negative-stain transmission electron microscopy (TEM) to investigate whether the envelope or capsid structures of MCMV virions were affected by 425 nm-femtosecond laser irradiation at an average laser power of 150 mW. As shown in Fig. 3, no clear differences in the global appearance of the envelope or capsid structure of virions in control (nonirradiated) relative to laser-irradiated groups were observed. At this resolution, we could not find any evidence of MCMV capsid disintegration after laser irradiation. These experimental results suggest that the global envelope and capsid structures of MCMV remained intact after 425 nm-femtosecond laser irradiation.
Fig. 3.
Preservation of MCMV global virion structure after femtosecond laser irradiation. Representative electron microscopy images are shown of control and laser-irradiated virions (150 to 200 nm in diameter) at both and magnification, showing no clear differences in the global appearance of viral envelope and capsid structures after irradiation.
3.3. MCMV Genomic DNA Structure is Preserved after Femtosecond Laser Irradiation
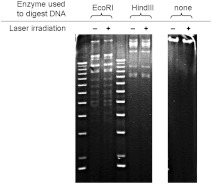
We used gel electrophoresis to assess whether MCMV genomic double-stranded DNA was covalently damaged by 425 nm-femtosecond laser irradiation at an average power of 150 mW. DNA was electrophoresed intact or after digestion with restriction enzymes. If double strand breakage or extensive crosslinking occurred, we would expect to see a change in the banding pattern of laser-irradiated DNA. As shown in Fig. 4, viral genomic DNA from femtosecond laser-irradiated MCMV exhibited identical banding patterns to genomic DNA from control (nonirradiated) MCMV. These experimental findings are consistent with our previous reports that 425 nm-femtosecond laser irradiation does not cause strand breaks in the single-stranded DNA of M13 bacteriophage.23 These data indicate that 425 nm-femtosecond laser irradiation did not cause double strand breaks or crosslinking of the MCMV genome.
Fig. 4.
Preservation of MCMV genomic DNA integrity after femtosecond laser irradiation. Agarose gel analysis was performed on genomic DNA isolated from control or irradiated MCMV, showing essentially identical banding patterns, which suggests a lack of double-strand breaks or crosslinking of viral DNA after femtosecond laser irradiation. DNA was electrophoresed intact or after digestion using restriction enzymes. For clarity, image contrast and sharpness were enhanced uniformly across the entire image using Photoshop. Results are representative of at least two experiments.
3.4. Femtosecond Laser Irradiation Causes Selective Aggregation of MCMV Capsid and Tegument Proteins
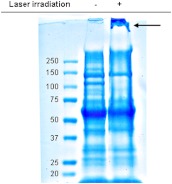
We employed SDS-PAGE to determine the effects of femtosecond laser irradiation on MCMV virion-associated proteins. Interestingly, several protein bands from laser-irradiated virions showed substantially reduced intensities relative to those of control (nonirradiated) virions (Fig. 5). Since our 425-nm laser treatment lacks the energy required to disrupt the covalent bonds commonly found in proteins, fragmentation of viral proteins or the production of covalent cross-linkages were unlikely scenarios. As shown by the arrow in Fig. 5, a high molecular weight protein aggregate was consistently detected in the laser-irradiated group. Based on this observation, we reasoned that laser irradiation may have caused aggregation of viral proteins. Therefore, the aggregate was excised from the gel and submitted for protein identification. The list of MCMV proteins identified is shown in Table 1. The aggregate contained predominantly MCMV virion-associated capsid and tegument proteins. The identified MCMV proteins have previously been detected in MCMV virions.37 In contrast, the MCMV envelope glycoproteins such as glycoprotein B,38 glycoprotein H,39 and glycoprotein M40 were not detected in the aggregate. These data indicate that femtosecond laser irradiation causes selective aggregation of MCMV capsid and tegument proteins, an effect that may hinder viral capsid function (i.e., uncoating) and contribute to inactivation of the virus.
Fig. 5.
Aggregation of MCMV virion proteins after femtosecond laser irradiation. SDS-PAGE analysis was performed on control and laser-irradiated virions. The arrow denotes a high molecular weight aggregate formed by proteins in the laser-treated group. The aggregate was excised for mass spectrometry analysis. For clarity, image contrast and sharpness were enhanced uniformly across the entire image using Photoshop. Results are representative of at least two experiments.
Table 1.
MCMV proteins from the aggregate identified by LC-MS/MS.
| Protein | Accession | Comment | HCMV homologue | -value | No. of peptides |
|---|---|---|---|---|---|
| M25 | gi:190886806 | Tegument protein41 | UL25 | 29 | |
| M32 | gi:190886815 | (HCMV: pp150)42 | UL32 | 18 | |
| M35 | gi:190886819 | UL25 family member, virulence factor43 | UL35 | 4 | |
| M44 | gi:90954727 | DNA binding phosphoprotein44 | UL44 | 4 | |
| M80 | gi:157676178 | Assembly protein-protease45 | UL80 | 4 | |
| M82 | gi:157676179 | Upper matrix phosphoprotein, pp7146 | UL82 | 2 | |
| M83 | gi:1532178 | Lower matrix phosphoprotein, pp6546 | UL83 | 2 | |
| M86 | gi:157676184 | (HCMV: major capsid protein)47 | UL86 | 43 | |
| M94 | gi:157676191 | (HCMV: virion-associated protein)36 | UL94 | 6 |
4. Discussion
In this report we have examined the effects of femtosecond lasers on MCMV structures at the molecular level. The capability of 425 nm-femtosecond laser treatment to inactivate a spectrum of different viruses and bacteria has been well documented.20–27 In these previous studies, it was demonstrated that the nonenveloped viruses such as M13 bacteriophages were inactivated by the USP laser irradiation through breaking/dissociation of their capsids. However, the inactivation mechanism for the enveloped viruses remains unexplored. In this work, we demonstrate that in great contrast to the nonenveloped virus such as M13, whose capsid was broken/dissociated by the USP laser irradiation, our TEM images suggest that the USP laser does not break/dissociate the capsid of the enveloped virus like MCMV. This indicates that the inactivation mechanism for nonenveloped virus is drastically different from that of an enveloped virus. We attribute the inactivation mechanism for MCMV by the USP laser to the aggregation of proteins within the virion.
Interestingly, our experimental results suggest that laser irradiation causes selective aggregation of viral capsid and tegument proteins. It has been suggested that partial unfolding of proteins is required for protein aggregation.48 We posit that femtosecond laser irradiation disrupts hydrogen bonds and/or hydrophobic interactions in viral proteins through the impulsive stimulated Raman scattering process.22,23 Although the reformation time for broken hydrogen bonds/hydrophobic contacts is believed to be short (of the order of 10 picoseconds),49,50 there is a significant chance for the MCMV capsid and tegument proteins to become aggregated since they are confined within a small volume in the virion. This explains the observation of aggregated proteins under femtosecond laser irradiation. As a result, laser-induced aggregation of MCMV capsid and tegument proteins may hinder the function of the viral capsid (i.e., uncoating) and contribute to virus inactivation. The concentration dependence of protein aggregation may thus provide a window for selectively damaging virions while leaving mammalian proteins intact.23
To get better insight into the proposed protein aggregation model for enveloped virus inactivation, we have also performed DLS experiments for a variety of proteins in their buffer solutions: (1) on the concentration dependence of aggregation for monoclonal antibody 04, (2) on the aggregation of BSA proteins, and (3) on the aggregation of the mixture of BSA and mAb04, under the same experimental conditions as MCMV. The integrated area under the primary peak—the monomer, which happens at about 12 nm, 6 nm in diameter for mAb04 and BSA, respectively, divided by the total integrated area is taken as the percentage of the nonaggregated protein. The integrated area under the higher size/mass region divided by the total area is considered as the aggregated percentage.
The results, summarized in Table 2, indicate that (1) the structure of nonaggregated protein is not compromised, (2) the protein aggregation effect by irradiation of the USP laser depends on the type of proteins; apparently, BSA is much more stable than mAb04 and exhibits very little aggregation upon USP laser irradiation, (3) the aggregation effect depends on protein concentration, with higher concentration tending to aggregate more, and (4) mixing the stable protein (BSA) with a less stable one (mAb04) does not help stabilize the less stable protein. This information further supports our proposed model that the USP laser first unfolds the proteins by disrupting their hydrogen bonds/hydrophobic contacts. The unfolded proteins then aggregate before the rapid reformation of these weak bonds. The higher protein concentration means they are closer to each other and as a result have a higher chance of aggregating. We note that under our experimental conditions, the proteins were completely dissolved in their buffer solutions, as evidenced by the almost 99% of monomer (nonaggregated proteins) in the solutions. In other words, our interpretations of protein aggregation by USP laser irradiation were not affected by the problem of solubility of the proteins in the buffer solution. In addition, the two proteins, BSA and mAb04, were chosen for the DLS experiments because neither of them absorb near 425 nm. As a matter of fact, we measured the temperature of the solutions during the laser irradiation experiments with a thermal couple immersed into the solution. The temperature of the solution rose no more than 2°C. Since the denature temperature of both proteins is around 60°C, our results cannot be due to the heating effects.
Table 2.
Dynamic light scattering data for a variety of laser-treated proteins in buffered solution.
| Sample, titer | % of aggregation | % of nonaggregation |
|---|---|---|
| mAb(04), (Control) | ||
| mAb (04), (laser treated) | ||
| mAb (04), (Control) | ||
| mAb (04), (laser treated) | ||
| BSA, (Control) | ||
| BSA, (laser treated) | ||
| mAb(04), , (Control) | (mAb04) (BSA) | |
| mAb(04), , (laser treated) | (mAb04) (BSA) |
We notice that in contrast to the atomic force microscope images of nonenveloped viruses such as M13 bacteriophage and TMV showing that the capsids were broken by the USP laser irradiation presented in the previous work, our TEM images indicate that the USP laser does not break/dissociate the capsid of the enveloped virus like MCMV. Based upon our results from DLS experiments, we propose, for enveloped viruses such as MCMV, that the USP laser partially unfolds both the protein unit of which the capsid is made up without dissociating the capsid and the tegument proteins by breaking some of their hydrogen bonds/hydrophobic contacts. These proteins then form aggregates before the reformation of the weak noncovalent bonds.
In this study, the treated volume is about 100 µL. To scale up our approach for the disinfection of blood products, we suggest the use of a syringe pump-capillary configuration in which the treated solution is forced through a capillary of the order of 1 mm in inner diameter. The laser beam will be adjusted to have the same diameter and passes through the capillary in a perpendicular geometry. A much more powerful commercially available USP laser system with an average power of the order of 10 W can be used for laser irradiation.
The USP laser technology presented here can be readily used for the disinfection of pharmaceuticals, which typically do not contain hemoglobin. The application of this technology to the disinfection of blood products can be done with USP lasers operating at a wavelength of about 700 nm where the absorption of hemoglobin is a minimum and the potential damaging effects can be minimized. In conclusion, we report the first experimental evidence of inactivation of an enveloped virus: MCMV by the USP laser. The molecular and structural effects caused by 425 nm-femtosecond laser irradiation on MCMV are presented and analyzed. In contrast to the atomic force microscope images of nonenveloped viruses such as M13 bacteriophage and TMV showing that the capsids were broken by the USP laser irradiation presented in the previous work, our TEM images revealed that the USP laser did not break/dissociate the capsid of MCMV. A novel mechanism for the inactivation of an enveloped virus by visible femtosecond lasers through induction of viral protein aggregation was proposed. By correlating viral inactivation with the observed structural and molecular effects, a better insight into the inactivation of enveloped viruses by femtosecond laser irradiation was obtained. Furthermore, continued exploration of this laser pathogen inactivation technology is expected to generate applications including sterilization of pharmaceuticals, blood products, and medical equipment.
Acknowledgments
We would like to thank Irina Sorokina (Midwest Bio Services LLC, Overland Park, Kansas) for mass spectrometry analysis. This work was supported in part by the Mallinckrodt Institute of Radiology Development Fund, NIH Grant R33 CA123537, NHLBI Ruth L. Kirschstein NRSA F30 Grant HL116183-01 (Shaw-Wei Tsen), and Public Health Service Grant R01CA120768 (Dong Yu).
References
- 1.Horowitz B., et al. , “Solvent/detergent-treated plasma: a virus-inactivated substitute for fresh frozen plasma,” Blood 79(3), 826–831 (1992). [PubMed] [Google Scholar]
- 2.Lambrecht B., et al. , “Photoinactivation of viruses in human fresh plasma by phenothiazine dyes in combination with visible light,” Vox Sang. 60(4), 207–213 (1991). 10.1111/vox.1991.60.issue-4 [DOI] [PubMed] [Google Scholar]
- 3.Bihm D. J., et al. , “Characterization of plasma protein activity in riboflavin and UV light treated fresh frozen plasma during 2 years of storage at ,” Vox Sang. 98(2), 108–115 (2010). 10.1111/vox.2010.98.issue-2 [DOI] [PubMed] [Google Scholar]
- 4.Cazenave J. P., et al. , “An active hemovigilance program characterizing the safety profile of 7483 transfusions with plasma components prepared with amotosalen and UVA photochemical treatment,” Transfusion 50(6), 1210–1219 (2010). 10.1111/trf.2010.50.issue-6 [DOI] [PubMed] [Google Scholar]
- 5.De Alarcon P., et al. , “Fresh frozen plasma prepared with amotosalen HCl (S 59) photochemical pathogen inactivation: transfusion of patients with congenital coagulation factor deficiencies,” Transfusion 45(8), 1362–1372 (2005). 10.1111/j.1537-2995.2005.00216.x [DOI] [PubMed] [Google Scholar]
- 6.Hambleton J., et al. , “Pharmacokinetic study of FFP photochemically treated with amotosalen (S 59) and UV light compared to FFP in healthy volunteers anticoagulated with warfarin,” Transfusion 42(10), 1302–1307 (2002). 10.1046/j.1537-2995.2002.00220.x [DOI] [PubMed] [Google Scholar]
- 7.Irsch J., et al. , “INTERCEPT plasma: comparability with conventional fresh frozen plasma based on coagulation function–an in vitro analysis,” Vox Sang. 98(1), 47–55 (2010). 10.1111/vox.2009.98.issue-1 [DOI] [PubMed] [Google Scholar]
- 8.Larrea L., et al. , “The influence of riboflavin photochemistry on plasma coagulation factors,” Transfus. Apher. Sci. 41(3), 199–204 (2009). 10.1016/j.transci.2009.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mintz P. D., et al. , “Photochemically treated fresh frozen plasma for transfusion of patients with acquired coagulopathy of liver disease,” Blood 107(9), 3753–3760 (2006). 10.1182/blood-2004-03-0930 [DOI] [PubMed] [Google Scholar]
- 10.Mintz P. D., et al. , “A randomized, controlled Phase III trial of therapeutic plasma exchange with fresh frozen plasma (FFP) prepared with amotosalen and ultraviolet A light compared to untreated FFP in thrombotic thrombocytopenic purpura,” Transfusion 46(10), 1693–1704 (2006). 10.1111/trf.2006.46.issue-10 [DOI] [PubMed] [Google Scholar]
- 11.Smith J., Rock G., “Protein quality in Mirasol pathogen reduction technology–treated, apheresis derived fresh frozen plasma,” Transfusion 50(4), 926–931 (2010). 10.1111/trf.2010.50.issue-4 [DOI] [PubMed] [Google Scholar]
- 12.Webert K. E., et al. , “Proceedings of a Consensus Conference: pathogen inactivation-making decisions about new technologies,” Transfus. Med. Rev. 22(1), 1–34 (2008). 10.1016/j.tmrv.2007.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douki T., Cadet J., “Individual determination of the yield of the main UV-induced dimeric pyrimidine photoproducts in DNA suggests a high mutagenicity of CC photolesions,” Biochemistry 40(8), 2495–2501 (2001). 10.1021/bi0022543 [DOI] [PubMed] [Google Scholar]
- 14.Wei H., et al. , “Singlet oxygen involvement in ultraviolet (254 nm) radiation-induced formation of 8-hydroxy-deoxyguanosine in DNA,” Free Radical Biol. Med. 23(1), 148–154 (1997). 10.1016/S0891-5849(96)00526-6 [DOI] [PubMed] [Google Scholar]
- 15.Mohr H., et al. , “Sterilization of platelet concentrates at production scale by irradiation with short wave ultraviolet light,” Transfusion 49(9), 1956–1963 (2009). 10.1111/j.1537-2995.2009.02228.x [DOI] [PubMed] [Google Scholar]
- 16.Mohr H., “A novel approach to pathogen reduction in platelet concentrates using short wave ultraviolet light,” Transfusion 49(12), 2612–2624 (2009). 10.1111/j.1537-2995.2009.02334.x [DOI] [PubMed] [Google Scholar]
- 17.Terpstra F. G., et al. , “Potential and limitation of UVC irradiation for the inactivation of pathogens in platelet concentrates,” Transfusion 48(2), 304–313 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Chan H. L., et al. , “Proteomic analysis of UVC irradiation-induced damage of plasma proteins: Serum amyloid P component as a major target of photolysis,” FEBS Lett. 580(13), 3229–3236 (2006). 10.1016/j.febslet.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 19.Verhaar R., et al. , “UV-C irradiation disrupts platelet surface disulfide bonds and activates the platelet integrin IIb 3,” Blood 112(13), 4935–4939 (2008). 10.1182/blood-2008-04-151043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsen S., et al. , “Prospects for a novel ultrashort pulsed laser technology for pathogen inactivation,” J. Biomed. Sci. 19, 62 (2012). 10.1186/1423-0127-19-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsen K., et al. , “Selective inactivation of human immunodeficiency virus with subpicosecond near-infrared laser pulses,” J. Phys. Condens. Matter 20(25), 252205 (2008). 10.1088/0953-8984/20/25/252205 [DOI] [Google Scholar]
- 22.Tsen K. T., et al. , “Photonic approach to the selective inactivation of viruses with a near-infrared subpicosecond fiber laser,” J. Biomed. Opt. 14(6), 064042 (2009). 10.1117/1.3275477 [DOI] [PubMed] [Google Scholar]
- 23.Tsen K. T., et al. , “Studies of inactivation of encephalomyocarditis virus, M13 bacteriophage, and Salmonella typhimurium by using a visible femtosecond laser: insight into the possible inactivation mechanisms,” J. Biomed. Opt. 16(7), 078003, (2011). 10.1117/1.3600771 [DOI] [PubMed] [Google Scholar]
- 24.Tsen K., et al. , “Inactivation of viruses with a very low power visible femtosecond laser,” J. Phys. Condens. Matter 19(32), 322102 (2007). 10.1088/0953-8984/19/32/322102 [DOI] [Google Scholar]
- 25.Tsen K. T., et al. , “Inactivation of viruses by laser-driven coherent excitations via impulsive stimulated Raman scattering process,” J. Biomed. Opt. 12(6), 064030 (2007). 10.1117/1.2821713 [DOI] [PubMed] [Google Scholar]
- 26.Tsen K. T., et al. , “Inactivation of viruses by coherent excitations with a low power visible femtosecond laser,” Virol. J. 4, 50 (2007). 10.1186/1743-422X-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsen K. T., et al. , “Selective inactivation of micro-organisms with near-infrared femtosecond laser pulses,” J. Phys. Condens. Matter 19(47), 472201 (2007). 10.1088/0953-8984/19/47/472201 [DOI] [Google Scholar]
- 28.Britt W. J., Alford C. A., Fields Virology, pp. 2493–2523, Lippincott-Raven, New York: (1996). [Google Scholar]
- 29.Mocarski E. S., Shenk T., Pass R. F., Fields Virology, Vol. 2, pp. 2701–2772, Lippincott Williams and Wilkins, Philadelphia: (2007). [Google Scholar]
- 30.Field A. K., Biron K. K., “The end of innocence revisited: resistance of herpesviruses to antiviral drugs,” Clin. Microbiol. Rev. 7(1), 1–13 (1994). 10.1128/CMR.7.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harada K., et al. , “Genetic analysis of a clinical isolate of human cytomegalovirus exhibiting resistance against both ganciclovir and cidofovir,” Arch. Virol. 142(2), 215–225 (1997). 10.1007/s007050050072 [DOI] [PubMed] [Google Scholar]
- 32.Sarasini A., et al. , “Double resistance to ganciclovir and foscarnet of four human cytomegalovirus strains recovered from AIDS patients,” J. Med. Virol. 47(3), 237–244 (1995). 10.1002/(ISSN)1096-9071 [DOI] [PubMed] [Google Scholar]
- 33.Smith I. L., et al. , “Clinical failure of CMV retinitis with intravitreal cidofovir is associated with antiviral resistance,” Arch. Ophthalmol. 116(2), 178–185 (1998). [DOI] [PubMed] [Google Scholar]
- 34.Marty F. M., et al. , “Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial,” Lancet Infect. Dis. 11(4), 284–292 (2011). 10.1016/S1473-3099(11)70024-X [DOI] [PubMed] [Google Scholar]
- 35.Qian Z., et al. , “Murine cytomegalovirus targets transcription factor ATF4 to exploit the unfolded-protein response,” J. Virol. 86(12), 6712–6723 (2012). 10.1128/JVI.00200-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wing B. A., et al. , “The human cytomegalovirus UL94 open reading frame encodes a conserved herpesvirus capsid/tegument-associated virion protein that is expressed with true late kinetics,” J. Virol. 70(6), 3339–3345 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kattenhorn L. M., et al. , “Identification of proteins associated with murine cytomegalovirus virions,” J. Virol. 78(20), 11187–11197 (2004). 10.1128/JVI.78.20.11187-11197.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rapp M., et al. , “Identification of the murine cytomegalovirus glycoprotein B gene and its expression by recombinant vaccinia virus,” J. Virol. 66(7), 4399–4406 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J., et al. , “Identification of the glycoprotein H gene of murine cytomegalovirus,” J. Gen. Virol. 73(Pt 7), 1849–1854 (1992). 10.1099/0022-1317-73-7-1849 [DOI] [PubMed] [Google Scholar]
- 40.Scalzo A. A., et al. , “DNA sequence and transcriptional analysis of the glycoprotein M gene of murine cytomegalovirus,” J. Gen. Virol. 76(Pt 11), 2895–2901 (1995). 10.1099/0022-1317-76-11-2895 [DOI] [PubMed] [Google Scholar]
- 41.Wu C. A., et al. , “The murine cytomegalovirus M25 open reading frame encodes a component of the tegument,” Virology 262(2), 265–276 (1999). 10.1006/viro.1999.9942 [DOI] [PubMed] [Google Scholar]
- 42.Jahn G., et al. , “The two major structural phosphoproteins (pp65 and pp150) of human cytomegalovirus and their antigenic properties,” J. Gen. Virol. 68(Pt 5), 1327–1337 (1987). 10.1099/0022-1317-68-5-1327 [DOI] [PubMed] [Google Scholar]
- 43.Tam A., et al. , “Murine cytomegalovirus with a transposon insertional mutation at open reading frame M35 is defective in growth in vivo,” J. Virol. 77(14), 7746–7755 (2003). 10.1128/JVI.77.14.7746-7755.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loh L. C., et al. , “Sequence analysis and expression of the murine cytomegalovirus phosphoprotein pp50, a homolog of the human cytomegalovirus UL44 gene product,” Virology 200(2), 413–427 (1994). 10.1006/viro.1994.1205 [DOI] [PubMed] [Google Scholar]
- 45.Loutsch J. M., et al. , “Cloning and sequence analysis of murine cytomegalovirus protease and capsid assembly protein genes,” Biochem. Biophys. Res. Commun. 203(1), 472–478 (1994). 10.1006/bbrc.1994.2206 [DOI] [PubMed] [Google Scholar]
- 46.Cranmer L. D., et al. , “Identification, analysis, and evolutionary relationships of the putative murine cytomegalovirus homologs of the human cytomegalovirus UL82 (pp71) and UL83 (pp65) matrix phosphoproteins,” J. Virol. 70(11), 7929–7939 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chee M., et al. , “Identification of the major capsid protein gene of human cytomegalovirus,” J. Virol. 63(3), 1345–1353 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uversky V. N., Fernandez A., Fink A. L., Protein Reviews, Uversky Vladimir N., Fink Anthony L., Eds., Vol. 4, Springer Science+Business Media Inc., New York: (2006). [Google Scholar]
- 49.Gaffney K. J., et al. , “Hydrogen bond dissociation and reformation in methanol oligomers following hydroxyl stretch relaxation,” J. Phys. Chem. A 106(50), 12012–12023 (2002). 10.1021/jp021696g [DOI] [Google Scholar]
- 50.O’Connell C., et al. , “Investigation of the hydrophobic recovery of various polymeric biomaterials after 172 nm UV treatment using contact angle, surface free energy and XPS measurements,” Appl. Surf. Sci. 255(8), 4405–4413 (2009). 10.1016/j.apsusc.2008.11.034 [DOI] [Google Scholar]