SUMMARY
The Mre11/Rad50/NBS1 (MRN) complex is thought to be a critical sensor that detects damaged DNA and recruits ATM to DNA foci for activation. However, it remains to be established how the MRN complex regulates ATM recruitment to the DNA foci during DNA double-strand breaks (DSBs). Here we show that Skp2 E3 ligase is a key component for the MRN complex-mediated ATM activation in response to DSBs. Skp2 interacts with NBS1 and triggers K63-linked ubiquitination of NBS1 upon DSBs, which is critical for the interaction of NBS1 with ATM, thereby facilitating ATM recruitment to the DNA foci for activation. Finally, we show that Skp2 deficiency exhibits a defect in homologous recombination (HR) repair, thereby increasing IR sensitivity. Our results provide molecular insights into how Skp2 and the MRN complex coordinate to activate ATM, and identify Skp2-mediatetd NBS1 ubiquitination as a vital event for ATM activation in response to DNA damage.
INTRODUCTION
Ataxia-telangiectasia mutation (ATM) kinase is a keystone in controlling genomic stability, and loss of ATM function leads to defects in DNA damage response and homologous recombination (HR) repair (Kastan and Lim, 2000; Lavin, 2008; Lavin and Kozlov, 2007). Mutations of the ATM gene often lead to ATM protein instability and are attributed to ataxia-telangiectasia, a genetic disorder characterized by defective cell-cycle check-point, immunodeficiency, radiosensitivity, progressive cerebellar ataxia, and cancer susceptibility (Kastan and Lim, 2000; Lavin, 2008; Lavin and Kozlov, 2007). ATM kinase is activated upon genotoxic stress, such as γ-irradiation (IR), which causes DNA double-strand breaks (DSBs) through a mechanism that is currently not well understood. Nevertheless, it has been proposed that upon DSBs, MRN complex, which consists of Mre11, Rad50, and NBS1, is recruited to the DNA repair foci and in turn facilitates recruitment of ATM to the DNA repair foci for activation (Lavin, 2007; Lavin and Kozlov, 2007; Lee and Paull, 2007).
Interestingly, mutations on NBS1 or Mre11 genes are linked to Nijmegen breakage syndrome (NBS) and ataxia-telangiectasia-like disorder (ATLD), accompanied by ATM activation defect (Carney et al., 1998; Digweed and Sperling, 2004; Taylor et al., 2004). Although MRN complex can activate ATM kinase activity directly in an in vitro kinase assay and is critical for ATM activation in vivo upon DSBs (Lee and Paull, 2004, 2005), the mechanism of how MRN complex recruits ATM to the DNA repair foci for its activation upon genotoxic stress remains elusive. Therefore, in this study, we aim to determine the molecular mechanism by which MRN complex regulates the recruitment of ATM to sites of DNA breaks for its subsequent activation.
Skp2 is an F box protein, which is a component of the Skp2 SCF E3 ligase complex that regulates cell-cycle progression by targeting cell-cycle inhibitors such as p27 and p21 for degradation (Frescas and Pagano, 2008; Lin et al., 2009; Nakayama and Nakayama, 2005). Overexpression of Skp2 is found in a variety of human cancers (Frescas and Pagano, 2008; Lin et al., 2009; Nakayama and Nakayama, 2005). Prominently, we have recently demonstrated that Skp2 overexpression promotes cancer progression, invasion, and metastasis, while its deficiency inhibits these processes (Chan et al., 2010; Lin et al., 2009, 2010), suggesting that Skp2 overexpression may contribute to the development of human cancers. Interestingly, Skp2 deficiency also causes polyploidy and micronuclei formation (Nakayama et al., 2000), indicating that Skp2 may be required for the maintenance of genomic stability.
In this study, we examine the role of Skp2 in DNA damage response and DNA repair. We show that Skp2 promotes K63-linked ubiquitination of NBS1, in turn regulating activation and recruitment of ATM to DNA damage foci.
RESULTS
Skp2 Is Required for ATM Activation upon Genotoxic Stress
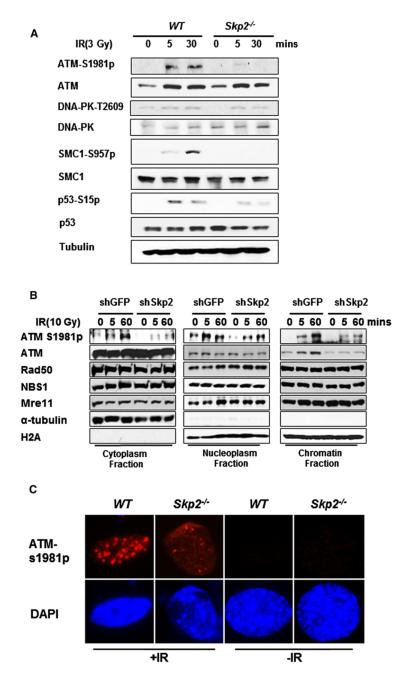
Given that Skp2 deficiency causes polyploidy and micronuclei formation (Nakayama et al., 2000), it is conceivable that Skp2 may be required for the maintenance of genomic stability. Since the DNA repair machinery also critically maintains the integrity of genomic stability, we postulated that Skp2 may be involved in DNA damage response. As ATM kinase is a key player in the execution of DNA damage response, we assessed the role of Skp2 in ATM activation upon DSBs. To this end, we compared the phosphorylated ATM (p-ATM) level in western blot, which represents the activated form of ATM, in wild-type (WT) and Skp2–/– primary mouse embryonic fibroblasts (MEFs) upon genotoxic stress. Similar to ATM inhibitor treatment, Skp2 deficiency in MEFs largely impaired ATM phosphorylation upon IR treatment (Figure 1A and see Figure S1A online). The reduction of ATM phosphorylation in Skp2–/– MEFs upon IR correlated with a reduction in SMC1 phosphorylation (Figure 1A), a well-known substrate for ATM (Kim et al., 2002; Yazdi et al., 2002), suggesting that the activation of ATM upon DSBs is impaired in Skp2–/– MEFs. The phosphorylation of p53, another well-established ATM substrate, was also reduced but not completely diminished in Skp2–/– MEFs upon IR (Figure 1A). In contrast, Skp2–/– MEFs displayed comparable DNA-PK activation, as determined by DNA-PK phosphorylation, in response to IR treatment compared to WT MEFs (Figure 1A) (Kastan and Lim, 2000; Lavin and Kozlov, 2007). To eliminate the possibility that Skp2 has an indirect effect on ATM activation through its role in cell-cycle regulation, we synchronized WT and Skp2–/– MEFs in prometaphase using nocodazole and found that ATM phosphorylation upon IR treatment was still reduced in Skp2–/– MEFs compared to WT MEFs (Figure S1C), suggesting that Skp2 directly regulates ATM activation. To further exclude the possibility that Skp2 regulates ATM activation indirectly through its effect on p27 degradation, we examined whether p27 overexpression can cause a defect in ATM activation upon IR treatment, similar to that of Skp2 deficiency. While Skp2 knockdown readily impaired ATM activation in response to IR treatment, p27 overexpression failed to do so (Figure S1D), indicating that p27 stability is most unlikely to be involved in Skp2-mediated ATM activation in response to IR treatment.
Figure 1. Skp2 Is Required for ATM Activation upon Genotoxic Stress.
(A) WT and Skp2–/– MEFs were treated with IR (3 Gy) and harvested for western blot analysis at various time points.
(B) 293 cells with GFP or Skp2 knockdown were treated with or without IR (10 Gy) and harvested at various time points for the isolation of cytoplasm, nucleoplasm, and chromatin fraction, followed by western blot analysis.
(C) WT and Skp2–/– MEFs were treated with IR (3 Gy) and fixed for IF analysis 5 min after IR treatment.
Notably, the recruitment of ATM to the chromatin-enriched fraction was reduced in Skp2 knockdown cells (Figure 1B). Furthermore, similar to ATM inhibitor treatment, Skp2 deficiency caused a drastic reduction in pATM foci (Figure 1C and Figure S1B). These results suggest that Skp2 is required for the recruitment of ATM to the DNA repair foci upon genotoxic stress. Collectively, our results reflect once again that Skp2 has essential roles in ATM kinase activation and DNA damage response upon DSBs.
Skp2 Forms a Complex with ATM, and Its E3 Ligase Activity Is Required for Activation and Recruitment of ATM to DNA Damage Foci
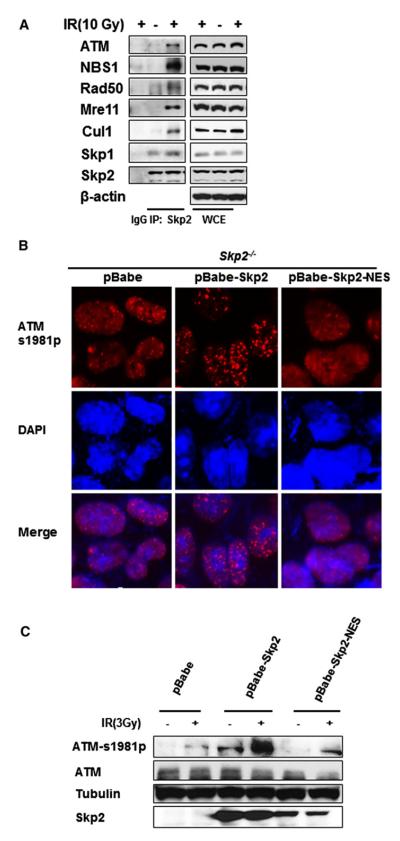
Having shown that Skp2 is required for ATM activation upon genotoxic stress, we next sought to determine the mechanism by which Skp2 regulates ATM activation. Using coimmunoprecipitation assay to examine the interaction between Skp2 and ATM, we found that Skp2 interacted with ATM endogenously 5 min after IR treatment (Figure 2A). Immunofluorescence assay (IF) also revealed that Skp2 exhibited a punctate structure and was partially colocalized with pATM foci upon IR treatment (Figures S2A–S2D). Since Skp2 is a component of the Skp2 SCF E3 ligase complex, (Chan et al., 2010; Lin et al., 2009), we next evaluated whether this E3 ligase activity is required for ATM activation and pATM foci formation upon DNA damage. To this end, we restored the WT Skp2 and catalytic inactive mutant Skp2-NES, which cannot form Skp2 SCF complex (Lin et al., 2009), into Skp2–/– MEFs and analyzed ATM activation and pATM foci upon genotoxic stress. While WT Skp2 readily rescued the defect in ATM phosphorylation and pATM foci formation upon IR in Skp2–/– MEFs, Skp2-NES mutant failed to do so (Figure 2B). Consistently, the same phenomenon was also observed in Skp2 knockdown 293 cells restored with WT Skp2 or Skp2-NES (Figure S3A), suggesting that Skp2 E3 ligase activity is crucial for ATM activation and pATM foci formation upon DSBs.
Figure 2. Skp2 Interacts with ATM and MRN Complex, and Its E3 Ligase Activity Is Required for the Recruitment of ATM to DNA Damage Foci.
(A) 293 cells with GFP or Skp2 knockdown were treated with or without IR (10 Gy) and harvested for coimmmunoprecipitation assay 5 min after IR treatment.
(B) Skp2–/– MEFs restored with mock, Skp2, and Skp2-NES were treated with IR (3 Gy) and harvested for IF analysis 5 min after IR treatment.
(C) Skp2–/– MEFs restored with mock, Skp2, and Skp2-NES were treated with IR (3 Gy) and harvested for western blot analysis 5 min after IR treatment.
To gain further insight into how Skp2 regulates ATM activation upon DSBs, we assessed whether Skp2 can trigger ATM ubiquitination. Although Skp2 interacted with ATM, it did not induce ATM ubiquitination, regardless of IR stimulation (Figure S3B). These results propose that Skp2 regulates ATM activation independently of ATM ubiquitination status.
Skp2 Interacts with the MRN Complex and Is Required for the Interaction between ATM and the MRN Complex
As aforementioned, although the MRN complex is relevant for recruiting ATM to the DNA repair foci for its subsequent activation, the underlying mechanism has not been well established. Since Skp2 regulates the activation and recruitment of ATM to the DNA damage foci, which resembles the function of MRN complex in ATM activation, we hypothesized that there exists a crosstalk between Skp2 and MRN complex in DNA damage response. To test this hypothesis, we examined the possible interactions between Skp2 and the MRN complex components. Coimmunoprecipitation assay revealed that Skp2 readily interacted with the MRN complex components, including NBS1, Mre11, and Rad50, 5 min after IR treatment, but not in the cells without IR treatment (Figure 2A). Moreover, we found that the components of the Skp2 SCF complex, such as Skp1 and Cul-1, were also detected in this Skp2 immunocomplex. While it is interesting that Cul-1 binding was further enhanced upon IR (Figure 2A), the Skp2-Skp1 and Skp2-Smad4, a known Skp2 substrate (Liang et al., 2004), were not IR dependent (Figure 2A and Figure S3C), confirming the IR-induced Skp2-MRN complex binding is a specific event. To further determine their association, we performed IF assay to examine whether Skp2 colocalizes with MRN complex upon IR treatment. Expectedly, some Skp2 immunostaining colocalized with NBS1 foci (Figure S2D). Since Skp2 signal does not completely colocalize with NBS1 foci, it reflects the fact that Skp2 is involved in regulating many events other than DNA damage response and thus may not be solely present in the NBS1 foci. Nonetheless, we cannot exclude the possibility that the Skp2-NBS1 interaction is transient and/or also happened even before NBS1 foci formation.
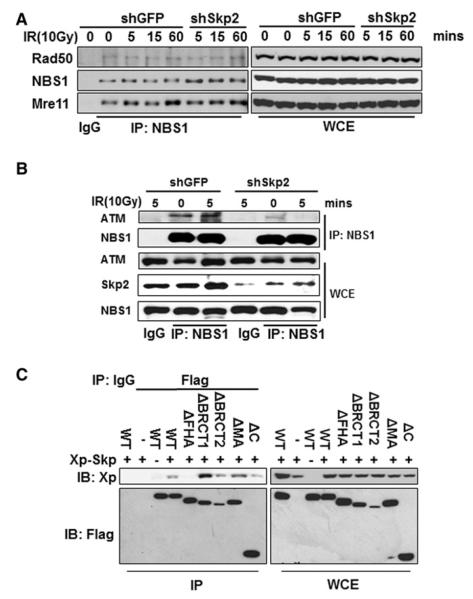
Since Skp2 forms a complex with the MRN complex upon IR, we next strived to determine whether Skp2 regulates the formation of MRN complex. We found that the formation of the MRN complex was comparable between control and Skp2 knockdown, irrespectively of IR (Figure 3A). Similarly, Skp2 knockdown did not affect the localization of the MRN complex in the chromatin fraction (Figure 1B and Figure S3D). These results indicate that Skp2 is dispensable for the formation of MRN complex and the localization of these proteins in the DNA damage foci.
Figure 3. Skp2 Is Required for the Interaction of NBS1 with ATM upon DNA Damage.
(A) 293 cells with GFP or Skp2 knockdown were treated with IR (10 Gy) and harvested for coimmunoprecipitation assay at various time points, followed by western blot analysis.
(B) 293 cells with GFP or Skp2 knockdown were treated with or without IR (10 Gy) and harvested for coimmmunoprecipitation assay at various time points.
(C) 293T cells were transfected with Xp-Skp2 and various Flag-NBS1 truncation mutants as indicated, and cells were harvested for coimmmunoprecipitation assay, followed by western blot analysis.
NBS1 has been shown to interact with and recruit ATM to the sites of DNA breaks upon DSBs (Falck et al., 2005; Lavin, 2007; Lavin and Kozlov, 2007; Lee and Paull, 2007). To better understand how Skp2 regulates ATM activation upon DSBs, we examined whether Skp2 regulates the interaction between ATM and NBS1. Strikingly, the interaction of NBS1 with ATM was impaired in Skp2 knockdown cells compared to the control cells (Figure 3B), a result which correlated with the defects in the recruitment of ATM to the DNA damage foci and to the chromatin-enriched fraction (Figures 1B and 1C). These results therefore highlight the critical role of Skp2 in regulating the interaction between ATM and NBS1, thereby providing a molecular insight into how Skp2 regulates ATM activation.
As Skp2 forms a complex with NBS1 and ATM upon DSBs, we next investigated whether NBS1 is required for the interaction between Skp2 and ATM. To this end, we examined the interaction between ATM and Skp2 in control and NBS1 knockdown cells and found that NBS1 is indeed required for ATM and Skp2 interaction (Figure S3E). Having shown that Skp2 interacts with NBS1, we next sought to map the Skp2-binding region on NBS1 by generating serial truncation mutants of NBS1. While Skp2 could interact with most NBS1 mutants, it failed to interact with the NBS1 mutant with FHA domain deletion, suggesting that NBS1 interacts with Skp2 through its FHA domain (Figure 3C and Figure S4A). Since Skp2 usually binds to its protein substrates through its C-terminal leucine-rich repeat (LRR) domain (Frescas and Pagano, 2008; Lin et al., 2009; Nakayama and Nakayama, 2005), we next determined whether Skp2 LRR mediates Skp2 and NBS1 interaction. To our surprise, while WT Skp2 and Skp2 fragment (aa 1–200) readily interacted with NBS1, Skp2 LRR failed to do so (Figure S4B).
We then asked the question of how IR triggers the formation of Skp2 and NBS1 complex. Since our result showed that Skp2 binds to the FHA domain of NBS1, which is thought to interact with phosphorylated proteins, it is highly possible that NBS1 may bind to phosphorylated Skp2 upon IR treatment. We therefore hypothesized that Skp2 may be phosphorylated by IR, and this phosphorylation may mediate the NBS1 and Skp2 interaction upon IR. To test this notion, we examined whether Skp2 is phosphorylated upon IR. Notably, we found that Skp2 phosphorylation at serine/threonine residue(s), but not tyrosine residue(s), was induced upon IR treatment (Figure S4C). To understand whether Skp2 phosphorylation regulates Skp2 and NBS1 interaction upon IR, we treated the Skp2 IP (immunoprecipitated) products with phosphatases such as CIAP and λ-phosphatases to induce Skp2 dephosphorylation in vitro (Figure S4C) and found that the interaction between Skp2 and NBS1 was profoundly reduced by the treatment of either one of these two phosphatases (Figure S4D). Thus, Skp2 phosphorylation likely mediates NBS1 and Skp2 interaction in response to IR treatment. Since ATM and ATR kinases recognize a consensus SQ/TQ phosphorylation site, and Skp2 harbors two of these sites, we used a SQ/TQ phosphorylation antibody to detect whether Skp2 is phosphorylated on SQ/TQ sites upon IR treatment. While p53 was readily phosphorylated at SQ/TQ sites by IR, Skp2 phosphorylation was not detected (Figure S4E), suggesting that ATM and ATR may not be required for Skp2 phosphorylation upon IR.
Skp2 SCF Complex Is an E3 Ligase for NBS1 Ubiquitination
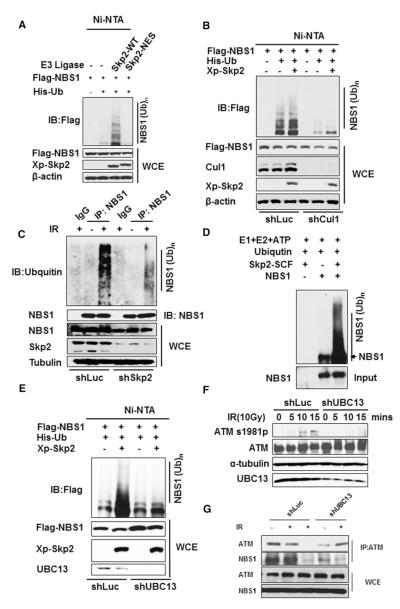
Although ubiquitination is commonly known to target protein for proteasome-dependent degradation, recent studies suggest that ubiquitination also plays nonproteolytic functions in kinase activation, protein trafficking, and DNA repair (Bhoj and Chen, 2009; Hoeller and Dikic, 2009; Yang et al., 2009, 2010). To gain further insight into how Skp2 regulates the interaction between NBS1 and ATM to facilitate ATM activation and pATM foci formation, we studied whether Skp2 regulates the ubiquitination of NBS1. Indeed, we found that Skp2 remarkably triggered the in vivo ubiquitination of NBS1, while Skp2-NES mutant compromised this effect (Figure 4A). The inability of Skp2-NES to promote NBS1 ubiquitination was not due to its defect in NBS1 binding, as it interacted with NBS1 as efficiently as WT Skp2 did (Figure S4B). These results indicate that the Skp2 SCF E3 ligase activity is required to ubiquitinate NBS1. In further support of this notion, we found that the knockdown of Cul-1, an essential component of the Skp2 SCF complex, significantly inhibited Skp2-mediated NBS1 ubiquitination (Figure 4B). Notably, we found that endogenous NBS1 ubiquitination was induced by IR, and Skp2 knockdown compromised this effect (Figure 4C), suggesting that Skp2 is required for IR-induced NBS1 ubiquitination. We also performed the in vitro ubiquitination assay and found that NBS1 ubiquitination could be induced by the addition of the Skp2 SCF complex, suggesting that the Skp2 SCF complex is a direct E3 ligase for NBS1 (Figure 4D).
Figure 4. Skp2 SCF Complex Triggers NBS1 Ubiquitination.
(A) 293T cells were transfected with the indicated plasmids and harvested for in vivo ubiquitination assay.
(B) 293 cells with Cul-1 and luciferase control knockdown were transfected with indicated plasmids and harvested for in vivo ubiquitination assay (see the Experimental Procedures for details).
(C) 293 cells with GFP (left three lanes) or Skp2 knockdown (right three lanes) were treated with or without IR (3 Gy) and harvested for immunoprecipitation assay with IgG or anti-NBS1 antibody 5 min after IR treatment, followed by western blot analysis.
(D) E1, E2, ATP, and ubiquitin were incubated with Flag-NBS1 proteins in the presence or absence of Skp2 SCF complex for in vitro ubiquitination analyzed by western blot analysis (see the Experimental Procedures for details).
(E) 293 cells with luciferase or UBC13 knockdown were transfected with indicated plasmids and harvested for in vivo ubiquitination assay.
(F) 293 cells with luciferase or UBC13 knockdown were treated with or without IR (10 Gy) and harvested at various time points for western blot analysis.
(G) 293 cells with luciferase or UBC13 knockdown were treated with or without IR (10 Gy) and harvested at various time points for immunoprecipitation assay with IgG or anti-ATM antibody 5 min after IR treatment, followed by western blot analysis.
Although Skp2 induced NBS1 ubiquitination, it did not promote NBS1 degradation (Figure 4A), indicating that Skp2 likely induces lysine (K) 63-linked ubiquitination of NBS1. Indeed, Skp2 promoted K63-linked ubiquitination of NBS1 but only slightly induced K48-linked ubiquitination of NBS1 (Figure S5A). Since K63-linked ubiquitination is known to provide a docking site for protein-protein interaction (Bhoj and Chen, 2009; Yang et al., 2010), it is very likely that the K63-linked ubiquitination of NBS1 induced by Skp2 may enable ATM-NBS1 interaction, in turn facilitating activation and recruitment of ATM to DNA damage foci.
Since UBC13 is a major E2 (ubiquitin-conjugating enzyme) that triggers K63-linked ubiquitination (Chen and Sun, 2009; Yang et al., 2010), we next determined if Skp2 mediates NBS1 ubiquitination through UBC13. As expected, UBC13 knockdown not only reduced the basal ubiquitination of NBS1 but also inhibited Skp2-mediated NBS1 ubiquitination in vivo (Figure 4E). Thus, UBC13 is an important E2 required for Skp2-mediated NBS1 ubiquitination. Having shown that UBC13 is required for NBS1 ubiquitination, we next investigated whether UBC13 regulates ATM activation upon DSBs as Skp2 does. Consistent with the result in Figure 1A, UBC13 knockdown also reduced ATM activation upon IR, which correlated well with the reduction in NBS1 ubiquitination (Figures 4E and 4F). Moreover, UBC13 knockdown attenuated NBS1 and ATM interaction (Figure 4G). Thus, K63-linked ubiquitination of NBS1 may serve as a key event for the interaction of NBS1 with ATM and subsequent ATM activation.
Skp2-Mediated NBS1 Ubiquitination Regulates the Interaction of NBS1 with ATM and ATM Activation
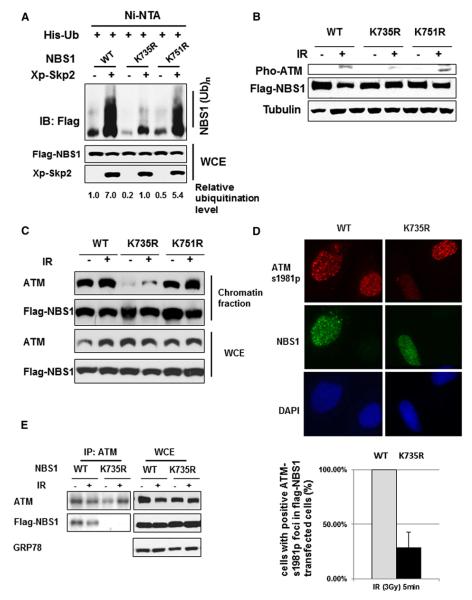
To address the direct role of NBS1 ubiquitination in ATM activation, we strived to identify the NBS1 ubiquitination sites for Skp2. To accomplish this goal, we first identified a NBS1 fragment (aa 735–754), which is located within the putative ATM-binding region (Falck et al., 2005; Lee and Paull, 2007), as the minimal region responsible for Skp2-mediated NBS1 ubiquitination (Figure S5B). We further mutated the lysine (K) residue to arginine (R) within this minimal region and the FHA domain of NBS1. The mutations on the FHA domain did not affect Skp2-mediated NBS1 in vivo ubiquitination (Figure S5C), as expected. However, only K735R mutant markedly inhibited Skp2-mediated in vivo NBS1 ubiquitination (around 85% inhibition, Figure 5A) and in vitro NBS1 ubiquitination (around 90% inhibition, Figure S5D). These results suggest that Skp2 triggers NBS1 ubiquitination on K735 residue located within the putative ATM-binding region (Lee and Paull, 2007).
Figure 5. NBS1 Ubiquitination Is Critical for ATM Activation and Recruitment.
(A) 293T cells were transfected with indicated plasmids and harvested for in vivo ubiquitination assay.
(B) 293 cells transfected with Flag-NBS1 WT, K735R, and K751R respectively were treated with IR (3 Gy) and harvested for western blot analysis 5 min after IR treatment.
(C) 293 cells were transfected with Flag-NBS1 WT, K735R, and K751R, respectively, treated with or without IR (10 Gy), and harvested for the isolation of chromatin fraction 5 min after IR, followed by western blot analysis.
(D) HeLa cells transfected with WT-NBS1 and NBS1-K735R were treated with IR (3 Gy) and harvested for IF analysis 5 min after IR treatment. Percentages of cells with positive ATM-s1981p foci were quantified from cells with positive Flag-NBS1 transfection. Results are presented as mean values ± standard deviation (SD) from three different experiments.
(E) 293 cells were transfected with Flag-NBS1 WT and K735R, respectively, treated with IR (3 Gy), and harvested for coimmunoprecipitation assay at various time points, followed by western blot analysis.
We next determined whether Skp2-mediated NBS1 ubiquitination regulates ATM activation upon DSBs. While overexpression of WT NBS1 and NBS1-K751R did not affect ATM phosphorylation and its recruitment to damaged DNA upon IR, overexpression of NBS1-K735R inhibited ATM phosphorylation and the recruitment of ATM to DNA damage foci (Figures 5B–5D). Accordingly, these results strongly suggest that Skp2-mediated NBS1 ubiquitination is a prerequisite for ATM activation and recruitment.
Earlier study reveals an ATM-interacting motif (AIM) of NBS1, which mediates NBS1-ATM interaction and is required for ATM activation (Falck et al., 2005). Given that the NBS1 ubiquitination driven by Skp2 SCF complex is mainly located within the AIM and is required for activation and recruitment of ATM to DNA damage foci, it is highly possible that NBS1 ubiquitination may regulate NBS1 and ATM interaction, in turn regulating ATM activation. Strikingly, while WT NBS1 readily interacted with ATM, NBS1-K735R failed to do so (Figure 5E). Although K735 residue is located in the AIM, it is not identified as an essential amino acid for the AIM structure (Falck et al., 2005), suggesting that K735R mutation affects NBS1-ATM interaction primarily by reducing the NBS1 ubiquitination, rather than affecting the AIM structure. Taken together, these results suggest that Skp2-mediated NBS1 ubiquitination induced by Skp2 SCF complex plays a critical role in NBS1 and ATM interaction.
Having established that the interaction of ATM with NBS1 requires Skp2-mediated NBS1 ubiquitination, it is likely that ATM may possess a ubiquitin-binding property. In accordance with this notion, we found that ATM readily interacted with K63-linked tetraubiquitin in vitro, but not with K48-linked tetraubiquitin (Figure S5E). We further found that one ATM fragment (aa 1439–1770) could predominantly interact with K63-linked tetraubiquitins, but not with K48-linked tetraubiquitins (Figure S5F). We noticed that one additional ATM fragments (aa 1245–1435) could also interact with K63-linked tetraubiquitins, albeit to a lesser extent (Figure S5F). We further showed that both ATM fragments were able to interact with NBS1 (Figure S5G). Thus, ATM interacts with K63-linked polyubiquitin chains and contains ubiquitin-binding regions, which may be responsible for the interaction between ATM and ubiquitinated NBS1. This notion was further supported by the fact that ATM could interact with ubiquitinated NBS1 upon DNA damage (Figure S3E).
Skp2 Regulates Homologous Recombination Repair and IR Sensitivity
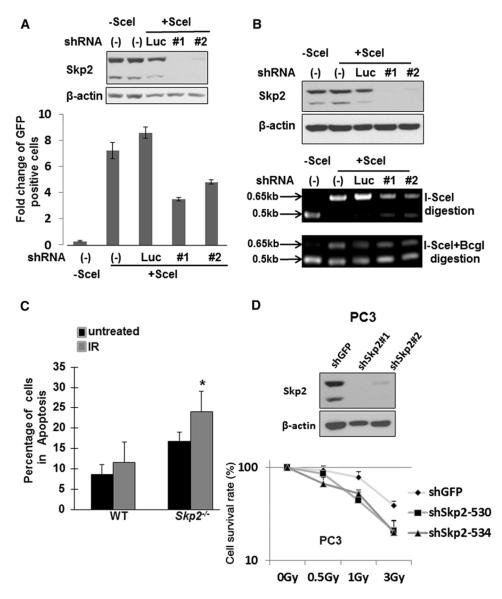
As Skp2 participates in ATM activation and DNA damage response by ubiquitinating NBS1 upon DSBs, we next aimed to establish the role of Skp2 in DNA repair. There are two major DNA repair pathways for DSBs: HR and nonhomologous endjoining (NHEJ) repairs. We took advantage of a well-established HR repair system to examine the role of Skp2 in DNA repair (see the Experimental Procedures and Figure S6A) (Peng et al., 2009). We found that Skp2 knockdown showed a significant decrease in HR-repaired GFP-positive cells, similar to that of the ATM knockdown cells, indicating a defect in HR repair (Figure 6A and Figure S6C). We next examined whether Skp2 also regulates NHEJ repair. To accomplish this, we used a PCR-based method to investigate both HR and NHEJ repair (Figure S6B) (Nakanishi et al., 2005; Peng et al., 2009). Using this approach, we found that Skp2 knockdown, once again, reduced the HR repair ability (Figure 6B, middle panel). In contrast, there was no difference in the NHEJ repair competence between control and Skp2 knockdown cells (Figure 6B, lower panel). Accordingly, our results suggest that Skp2 plays a critical role in regulating HR repair, but not NHEJ repair.
Figure 6. Skp2 Regulates HR Repair and DNA Damage Response.
(A) DR-GFP-integrated U2OS cells silenced with control or Skp2 were transfected with mock or I-Scel along with MSCV-GFP as a transfection efficiency control. Two days after IR treatment, cells were collected to analyze HR-repaired GFP-positive cells by flow cytometry assay. Results are presented as mean values ±SD, n = 3.
(B) DR-GFP-integrated U2OS cells silenced with control or Skp2 were transfected with mock or I-Scel. Forty-eight hours after transfection, genomic DNA was extracted for PCR amplification, followed by I-SceI or I-SceI plus BcgI digestion, and PCR products were subjected to gel electrophoresis. This result indicates that Skp2 knockdown impairs HR repair, but not NHEJ repair.
(C) WT and Skp2–/– MEFs were treated with or without IR (6 Gy). Seven hours after IR treatment, cells were harvested for apoptosis assay. Results are presented as mean values ±SD. *p < 0.05, using Student’s t test, n = 3.
(D) PC3 cells silenced with control or Skp2 were treated with various doses of IR, and the survival rate of these cells was determined by colony formation assay. Results are presented as mean values ±SD. *p < 0.05, using Student’s t test, n = 3.
Having identified the function of Skp2 in ATM activation and HR repair, we next examined whether Skp2 orchestrates the sensitivity of cells to IR. To this end, we treated WT and Skp2–/– MEFs with or without IR, followed by the apoptosis assay using the Annexin V staining and flow cytometry. As expected, we found that Skp2–/– MEFs displayed much higher sensitivity to IR than WT MEFs (Figure 6C). Similarly, Skp2 knockdown in various cancer cells lines exhibited heightened IR sensitivity, as determined by colony formation assay (Figure 6D and Figure S6D). Hence, these results indicate that Skp2 regulates HR repair and IR sensitivity.
DISCUSSION
Ubiquitination has important functions in many aspects of biological activities. Although ubiquitination is traditionally thought to only target proteins for degradation, recent studies suggest additional roles of ubiquitination in nonproteolytic functions involved in protein kinase activation (Chen and Sun, 2009; Yang et al., 2009, 2010). In this study, we show that NBS1 undergoes K63-linked ubiquitination upon genotoxic stress, which does not trigger NBS1 degradation. Instead, NBS1 ubiquitination serves as a scaffold for the recruitment of ATM to DNA damage foci for its subsequent activation. Notably, overexpression of the NBS1 ubiquitinationdeficient mutant inhibits ATM activation upon genotoxic stress. Thus, NBS1 ubiquitination is a key event in ATM activation and DNA damage response in response to genotoxic stress.
ATM plays a critical role in governing DNA damage response, and loss of its function is associated with human diseases and cancers (Lavin, 2008; Negrini et al., 2010). Although extensive effort on studying ATM regulation has been made in the last decade, exactly how ATM is activated during DNA damage remains to be established. Recent studies suggest that the MRN complex is required for ATM activation upon DNA damage; however, how the MRN complex regulates and activates ATM activation remains elusive. It has been proposed that the MRN complex is responsible for the initial recognition of DSBs upon genotoxic stress and recruits ATM to DNA damage foci for its subsequent activation (Lee and Paull, 2005; You et al., 2005). NBS1 is a key component of the MRN complex, which serves as a bridging factor for the interaction of Mre11 with ATM (Williams et al., 2007). Thus, NBS1 plays a central role in empowering the MRN complex to activate ATM.
In this study, we provide further molecular insight into how MRN complex regulates ATM activation. We show that Skp2 E3 ligase is a critical regulator required for the recruitment of ATM by the MRN complex to DNA damage foci and subsequent ATM activation and retention upon genotoxic stress. Skp2 promotes cell-cycle progression and tumorigenesis by triggering ubiquitination and degradation of cell-cycle inhibitors p27 and p21 (Bornstein et al., 2003; Carrano et al., 1999; Lin et al., 2009, 2010). So far, all Skp2 substrates identified in the last decade are known to undergo ubiquitin-dependent proteasome degradation (Chan et al., 2011), suggesting that the majority of the Skp2 function is attributed to its role in protein ubiquitination and degradation. However, in this study we identify a nonproteolytic function for Skp2 in DNA damage response. We show that Skp2 triggers K63-linked ubiquitination of NBS1, which is critical for its interaction with ATM, in turn facilitating activation and recruitment of ATM to DNA damage foci. Our findings suggest that ATM may possess a ubiquitin-binding property. In support of this notion, we found that ATM could directly interact with K63-linked polyubiquitins, but not K48-linked polyubiquitins (Figure S5E), through a region within aa 1439–1770. Thus, ATM consists of a ubiquitin-binding property that may mediate its interaction with K63-linked ubiquitination of NBS1.
An earlier study demonstrates that Skp2-deficient MEFs display centrosome overduplication, micronuclei formation, and polyploidy, suggesting that Skp2 may be a key player in maintaining genomic integrity (Nakayama et al., 2000). Our study supports this notion by showing that Skp2 deficiency displayed impairment in ATM activation and HR repair, recapitulating the phenotypes observed in ATM inactivation. However, Skp2 deficiency does not affect the NHEJ repair. Thus, our finding reveals a previously unrecognized function for Skp2 in regulating the HR repair.
UBC13 is involved in RNF8-mediated K63-linked polyubiquitination of H2A and H2AX and is critical for the recruitment of BRCA1/RAP80 complex and 53BP1 to DNA damage foci (Kolas et al., 2007; Mailand et al., 2007; Wang and Elledge, 2007). In this study, we identify another mechanism by which UBC13 regulates DNA damage response. We have demonstrated that UBC13 is also required for Skp2-mediated NBS1 ubiquitination and ATM activation, suggesting that UBC13 and Skp2 SCF complex coordinate to promote NBS1 ubiquitination to activate ATM. Thus, UBC13 may be engaged in the recruitment of BRCA1/RAP80 complex and 53BP1 to DNA foci by regulating NBS1 ubiquitination and ATM activation.
Our study reveals that Skp2 serine/threonine phosphorylation is induced upon IR treatment, and that this phosphorylation mediates the interaction between Skp2 and NBS1. We further show that ATM/ATR is not involved in IR-induced Skp2 phosphorylation. To identify the upstream serine/threonine kinase responsible for this phosphorylation, we used the Scansite and NetPhos 2.0 websites and found that CKI and CKII may be involved. To validate the potential of CKI and CKII as kinases involved in Skp2 phosphorylation, we used their specific inhibitors (D4476 and TBBz, respectively) to examine whether Skp2 phosphorylation and Skp2-NBS1 interaction upon IR were disrupted (Figure S7A). We found that CKII inhibition, but not CKI and ATM inhibition, abrogated Skp2 phosphorylation and the interaction of Skp2 with NBS1 upon IR. Thus, CKII is an upstream kinase required for Skp2 phosphorylation and Skp2-NBS1 interaction upon IR treatment. Future study will be important to identify potential Skp2 phosphorylation sites responsible for Skp2-NBS1 interaction.
Our findings have important clinical implications given that Skp2 overexpression is associated with human cancers and its level is shown to be critical for the sensitivity of cancer cells to radiotherapy (Figure 6D and Figure S6D). Interestingly, examining the cancer samples from patients under radiotherapy reveals that Skp2 overexpression is a poor prognosis factor and reduces overall survival of cancer patients (Harada et al., 2005), consistent with our data showing that Skp2 deficiency induced IR hypersensitivity in cancer cells. Moreover, we found that ectopic expression of WT Skp2, but not of Skp2 NES, promoted ATM phosphorylation and cell proliferation, and ATM inhibition reduced the cell proliferation effect of Skp2 (Figure S7B). However, ATM inhibition did not affect cell proliferation upon vector or Skp2-NES overexpression (Figure S7C), suggesting that Skp2-mediated ATM activation may contribute to Skp2 oncogenic activity. Furthermore, we found there was a positive correlation between Skp2 and ATM phosphorylation in human cancer lines (Figure S7D). Our study therefore suggests that Skp2 targeting can be a potential approach to improve the efficacy of radiotherapy and chemotherapy for cancer treatment.
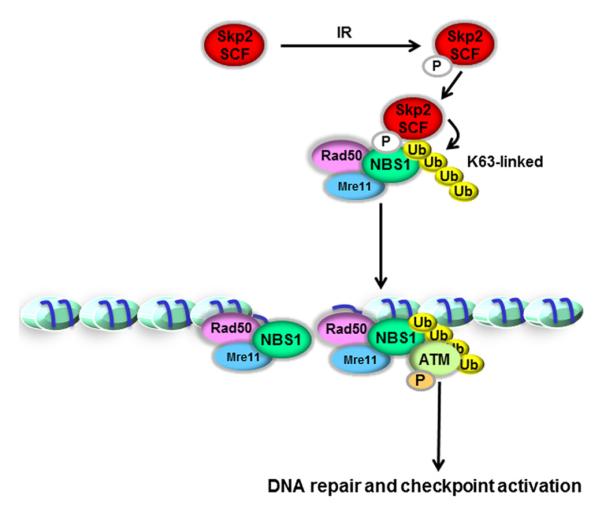
In summary, our study reveals that Skp2 has critical functions for allowing the MRN complex to recruit ATM to the DNA repair foci and to activate ATM. Based on our findings, we propose a working model to demonstrate the mechanism of how ATM is activated during DSBs. Upon DSBs, Skp2 is phosphorylated in a CKII-dependent manner, interacts with NBS1, and induces K63-linked ubiquitination of NBS1, in turn recruiting ATM to the DNA damage foci for its activation by the MRN complex (Figure 7). Our finding reveals a molecular insight into how ATM kinase activity is regulated, in turn providing innovative paradigms for DNA damage response and therapeutic applications for cancer treatment.
Figure 7. The Working Model for the Role of Skp2 in DNA Damage Response.
Upon DSBs, Skp2 is phosphorylated in a CKII-dependent manner. Skp2 SCF complex then interacts with the MRN complex and triggers K63-linked ubiquitination of NBS1, which is critical for the recruitment of ATM to DNA foci for its activation by the MRN complex, in turn regulating DNA damage response and HR repair.
EXPERIMENTAL PROCEDURES
In Vivo and In Vitro Ubiquitination Assays
In vivo ubiquitination assay was performed as described (Yang et al., 2009). In brief, 293T cells were transfected with the indicated plasmids for 48 hr and harvested by denatured buffer (6 M guanidine-HCl, 0.1 M Na2HPO4/NaH2PO4, 10 mM imidazole). The cell extracts were then incubated with nickel beads (Ni-NTA) for 3 hr, washed, and subjected to western blot analysis. In vitro ubiquitination assay was performed as described (Yang et al., 2009). Briefly, Flag-NBS1 proteins and Flag-Skp2-SCF complex isolated from 293T cell lysates were incubated with 0.5 μg E1 (Boston Biochem), 1.5 μg Ubiquitin (Boston Biochem), 1 μg UBC13/UEV1A (Boston Biochem), 0.85 μg UBCH5c (Boston Biochem), and 2.5 mM ATP in a final 30 μl reaction buffer (1.5 mM MgCl2, 5 mM KCl, 1 mM DTT, 20 mM HEPES [pH 7.4]) at 30°C for 2 hr. NBS1 ubiquitination was detected by western blot analysis.
Chromatin Fractionation
Chromatin fractionation was performed as described (Lukas et al., 2004). Briefly, cells were first lysed with buffer A (50 mM HEPES [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 10% glycerol, 1 mM DTT, protease inhibitor cocktail [Roche], 0.1% Triton X-100) on ice for 15 min. After centrifuge, pellets including the nucleus were further lysed with buffer B (3 mM EDTA, 0.2 mM EGTA, 1 mM DTT, protease inhibitor cocktail [Roche]). After centrifuge, pellets containing the chromatin were washed and sonicated in RIPA lysis buffer for western blot analysis.
Cell Apoptosis Assay
WT and Skp2–/– MEFs cultured in 10% FBS were treated with or without IR (10 Gy) for 2 days, harvested, and labeled with Annexin V-FITC, followed by flow cytometry analysis.
Cell-Cycle Analysis by Phosphoinisitle Propidium Iodide Staining
WT and Skp2–/– MEFs cultured in 10% FBS were treated with or without IR (10 Gy) for 7 hr, fixed in 75% ethanol, and stained with propidium iodide (PI), followed by flow cytometry analysis.
HR Repair Analysis
U2OS cells with a single copy of the HR repair reporter substrate DR-GFP in a random locus were kindly provided by Dr. Shiaw-Yih Lin. These U2OS cells were infected with lentiviral shRNAs against luciferase or Skp2 to generate U2OS cells with luciferase and Skp2 knockdown. These cells were then transfected with mock or the I-Scel plasmid along with MSCV-GFP as a control for transfection efficiency for 36 hr, treated with sodium butyrate (5 mM) to induce chromatin relaxation for 16 hr, and subjected to flow cytometry assay to analyze HR-repaired GFP-positive cells.
PCR for NHEJ Repair Analysis
Genomic DNA was extracted from mock- or I-Scel-transfected U2OS cells. PCR was then performed with primers as follows: DRGFP-F, CTGCTAAC CATGTTCATGCC; DRGFP-R, AAGTCGTGCTGCTTCATGTG. PCR products were subsequently digested with I-SceI or I-SceI + BcgI and subjected to gel electrophoresis using 1% agarose gel.
Colony Formation Assay
PC3, HeLa, or MDA-MB-231 cells silenced with GFP or Skp2 were treated with various doses of IR and split into 12-well plates (3,000 cells per well). Seven days after IR treatment, the viable cells were fixed and stained with crystal violet. The number of colonies (more than 50 cells for each colony) was calculated.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Keiichi I. Nakayama for Skp2–/– mice, and Drs. Junjie Chen, Dean Tang, Shiaw-Yih Lin, and Li Ma for cells and reagents. We extend special thanks to Dr. Zheng-Bo Han and Su Zhang for their technical support. This study was supported by MD Anderson Research Scholar Fund, National Institutes of Health (NIH) RO1 grants, a Cancer Prevention Research Institute of Texas (CPRIT) grant, a Department of Defense (DOD) grant to H.-K.L., and China Scholarship Council fund to J. Wu.
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information includes seven figures, Supplemental Experimental Procedures, and Supplemental References and can be found with this article online at doi:10.1016/j.molcel.2012.02.018.
REFERENCES
- Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J. Biol. Chem. 2003;278:25752–25757. doi: 10.1074/jbc.M301774200. [DOI] [PubMed] [Google Scholar]
- Carney JP, Maser RS, Olivares H, Davis EM, Le Beau M, Yates JR, 3rd, Hays L, Morgan WF, Petrini JH. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell. 1998;93:477–486. doi: 10.1016/s0092-8674(00)81175-7. [DOI] [PubMed] [Google Scholar]
- Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- Chan CH, Lee SW, Li CF, Wang J, Yang WL, Wu CY, Wu J, Nakayama KI, Kang HY, Huang HY, et al. Deciphering the transcriptional complex critical for RhoA gene expression and cancer metastasis. Nat. Cell Biol. 2010;12:457–467. doi: 10.1038/ncb2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CH, Lee SW, Wang J, Lin HK. Regulation of Skp2 expression and activity and its role in cancer progression. ScientificWorldJournal. 2011;10:1001–1015. doi: 10.1100/tsw.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol. Cell. 2009;33:275–286. doi: 10.1016/j.molcel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Digweed M, Sperling K. Nijmegen breakage syndrome: clinical manifestation of defective response to DNA double-strand breaks. DNA Repair (Amst.) 2004;3:1207–1217. doi: 10.1016/j.dnarep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434:605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat. Rev. Cancer. 2008;8:438–449. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K, Supriatno, Kawaguchi S, Kawashima Y, Itashiki Y, Yoshida H, Sato M. High expression of S-phase kinase-associated protein 2 (Skp2) is a strong prognostic marker in oral squamous cell carcinoma patients treated by UFT in combination with radiation. Anticancer Res. 2005;25:2471–2475. [PubMed] [Google Scholar]
- Hoeller D, Dikic I. Targeting the ubiquitin system in cancer therapy. Nature. 2009;458:438–444. doi: 10.1038/nature07960. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Lim DS. The many substrates and functions of ATM. Nat. Rev. Mol. Cell Biol. 2000;1:179–186. doi: 10.1038/35043058. [DOI] [PubMed] [Google Scholar]
- Kim ST, Xu B, Kastan MB. Involvement of the cohesin protein, Smc1, in Atm-dependent and independent responses to DNA damage. Genes Dev. 2002;16:560–570. doi: 10.1101/gad.970602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolas NK, Chapman JR, Nakada S, Ylanko J, Chahwan R, Sweeney FD, Panier S, Mendez M, Wildenhain J, Thomson TM, et al. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science. 2007;318:1637–1640. doi: 10.1126/science.1150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin MF. ATM and the Mre11 complex combine to recognize and signal DNA double-strand breaks. Oncogene. 2007;26:7749–7758. doi: 10.1038/sj.onc.1210880. [DOI] [PubMed] [Google Scholar]
- Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat. Rev. Mol. Cell Biol. 2008;9:759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- Lavin MF, Kozlov S. ATM activation and DNA damage response. Cell Cycle. 2007;6:931–942. doi: 10.4161/cc.6.8.4180. [DOI] [PubMed] [Google Scholar]
- Lee JH, Paull TT. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science. 2004;304:93–96. doi: 10.1126/science.1091496. [DOI] [PubMed] [Google Scholar]
- Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- Lee JH, Paull TT. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene. 2007;26:7741–7748. doi: 10.1038/sj.onc.1210872. [DOI] [PubMed] [Google Scholar]
- Liang M, Liang YY, Wrighton K, Ungermannova D, Wang XP, Brunicardi FC, Liu X, Feng XH, Lin X. Ubiquitination and proteolysis of cancer-derived Smad4 mutants by SCFSkp2. Mol. Cell. Biol. 2004;24:7524–7537. doi: 10.1128/MCB.24.17.7524-7537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HK, Wang G, Chen Z, Teruya-Feldstein J, Liu Y, Chan CH, Yang WL, Erdjument-Bromage H, Nakayama KI, Nimer S, et al. Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nat. Cell Biol. 2009;11:420–432. doi: 10.1038/ncb1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HK, Chen Z, Wang G, Nardella C, Lee SW, Chan CH, Yang WL, Wang J, Egia A, Nakayama KI, et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464:374–379. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas C, Melander F, Stucki M, Falck J, Bekker-Jensen S, Goldberg M, Lerenthal Y, Jackson SP, Bartek J, Lukas J. Mdc1 couples DNA double-strand break recognition by Nbs1 with its H2AX-dependent chromatin retention. EMBO J. 2004;23:2674–2683. doi: 10.1038/sj.emboj.7600269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailand N, Bekker-Jensen S, Faustrup H, Melander F, Bartek J, Lukas C, Lukas J. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell. 2007;131:887–900. doi: 10.1016/j.cell.2007.09.040. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Yang YG, Pierce AJ, Taniguchi T, Digweed M, D’Andrea AD, Wang ZQ, Jasin M. Human Fanconi anemia monoubiquitination pathway promotes homologous DNA repair. Proc. Natl. Acad. Sci. USA. 2005;102:1110–1115. doi: 10.1073/pnas.0407796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama KI, Nakayama K. Regulation of the cell cycle by SCF-type ubiquitin ligases. Semin. Cell Dev. Biol. 2005;16:323–333. doi: 10.1016/j.semcdb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability–an evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- Peng G, Yim EK, Dai H, Jackson AP, Burgt I, Pan MR, Hu R, Li K, Lin SY. BRIT1/MCPH1 links chromatin remodelling to DNA damage response. Nat. Cell Biol. 2009;11:865–872. doi: 10.1038/ncb1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Groom A, Byrd PJ. Ataxia-telangiectasia-like disorder (ATLD)–its clinical presentation and molecular basis. DNA Repair (Amst.) 2004;3:1219–1225. doi: 10.1016/j.dnarep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Wang B, Elledge SJ. Ubc13/Rnf8 ubiquitin ligases control foci formation of the Rap80/Abraxas/Brca1/Brcc36 complex in response to DNA damage. Proc. Natl. Acad. Sci. USA. 2007;104:20759–20763. doi: 10.1073/pnas.0710061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RS, Williams JS, Tainer JA. Mre11-Rad50-Nbs1 is a keystone complex connecting DNA repair machinery, double-strand break signaling, and the chromatin template. Biochem. Cell Biol. 2007;85:509–520. doi: 10.1139/O07-069. [DOI] [PubMed] [Google Scholar]
- Yang WL, Wang J, Chan CH, Lee SW, Campos AD, Lamothe B, Hur L, Grabiner BC, Lin X, Darnay BG, Lin HK. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science. 2009;325:1134–1138. doi: 10.1126/science.1175065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WL, Wu CY, Wu J, Lin HK. Regulation of Akt signaling activation by ubiquitination. Cell Cycle. 2010;9:486–497. doi: 10.4161/cc.9.3.10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi PT, Wang Y, Zhao S, Patel N, Lee EY, Qin J. SMC1 is a downstream effector in the ATM/NBS1 branch of the human S-phase checkpoint. Genes Dev. 2002;16:571–582. doi: 10.1101/gad.970702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z, Chahwan C, Bailis J, Hunter T, Russell P. ATM activation and its recruitment to damaged DNA require binding to the C terminus of Nbs1. Mol. Cell. Biol. 2005;25:5363–5379. doi: 10.1128/MCB.25.13.5363-5379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.