Abstract
We previously determined that D1 receptors can endocytose through caveolae, a subset of lipid rafts, in addition to internalization via a clathrin-dependent pathway. In this report, we investigated the potential role that palmitoylation might have on directing D1 receptor internalization through either a clathrin or caveolar-dependent route. Through whole cell binding analysis and sucrose gradient fractionation studies, we demonstrated that although palmitoylation of the D1 receptor was not required for agonist-independent localization to caveolae, agonist induced internalization kinetics of a de-palmitoylated D1 receptor were accelerated ~ 8 fold in comparison to wild-type D1 receptor and were very similar to that observed for clathrin-dependent D1 receptor internalization. Additionally, inhibition of the clathrin mediated pathway led to significant attenuation in the extent of agonist induced internalization of the de-palmitoylated D1 receptor, suggesting the de-palmitoylated D1 receptor was directed to a clathrin-dependent internalization pathway. Taken together, these data suggest that palmitoylation may be involved in directing agonist-dependent D1 receptor internalization through selective endocytic routes.
Keywords: Dopamine D1 Receptor, Palmitoylation, Caveolae, Clathrin, Endocytosis
1. INTRODUCTION
The D1 dopamine receptor belongs to the class A superfamily of G protein-coupled receptors (GPCRs) and activates adenylyl cyclase through the stimulatory G protein subunits Gs and Golf. D1 receptor signaling is a tightly regulated process that is highly dependent on the accessibility of receptors to agonist binding. The acute administration of dopamine agonists has been demonstrated to induce a rapid D1 receptor desensitization response [1] as well as robust internalization of the D1 receptor in both cultured cells and neurons [2, 3] as well as in vivo [4].
Endocytosis of many GPCRs involves agonist-induced phosphorylation of the receptor by G protein-coupled receptor kinases (GRKs), which promotes binding of β-arrestin proteins, followed by uncoupling of the receptor from G-proteins resulting in sequestration into clathrin-coated pits (reviewed by [5]). While this clathrin-coated pit pathway has been extensively characterized, alternative routes for GPCR internalization have been described including a caveolar based mechanism. Caveolae represent a subtype of lipid rafts that exist as morphologically distinct invaginations at the plasma membrane and are rich in glycosphingolipids and cholesterol [6]. These invaginations contain caveolin proteins that are unique to caveolae and they serve a dual role in maintaining the structural integrity of caveolae and by acting as a scaffolding protein that binds to several receptors, signaling molecules and adaptor proteins [7]. Although there are three caveolin isoforms, caveolin-1 is the most abundant in brain [8].
For the D1 receptor, in addition to internalization via a clathrin-dependent pathway [9], we have previously shown that the D1 receptor can endocytose through caveolae, by binding to the scaffolding protein, caveolin-1[10]. This interaction was demonstrated in rat brain by co-immunoprecipitation of the D1 receptor with caveolin-1. However, unlike the relatively rapid clathrin-mediated mechanism of internalization, caveolae-mediated internalization occurred much slower. Although the D1 receptor is capable of internalizing through either the clathrin-coated pit pathway or through the caveolar pathway, the molecular determinants that control which endocytic route is taken remains unclear.
Similar to phosphorylation, the process of palmitoylation is suggested to act as a regulatory mechanism controlling receptor function. Palmitoylation is a reversible post-translational acylation process that occurs though the attachment of palmitate, a long-chain fatty acid, to cysteine via a thioester bond [11]. Most GPCRs have evolved to undergo palmitoylation at one or more cysteine residues in the carboxyl tail near the seventh transmembrane domain [12]. We have previously shown that palmitoylation of the D1 receptor occurs in the carboxyl tail at two cysteines at positions 347 and 351 [13].
There is accumulating evidence that palmitoylation can serve as a targeting signal for proteins into lipid-enriched and detergent insoluble cellular fractions [14]. For example, fusion of the cytosolic protein, GFP, with an acylation consensus sequence was sufficient to target GFP to caveolin-enriched plasma membrane domains [15]. These studies were validated by fluorescence resonance energy transfer showing that GFP-fused acylation consensus sequences were clustered with caveolin-1 at the plasma membrane [16]. Although acylation events, such as palmitoylation, may be required for lipid raft association of proteins, it is not clear whether these requirements are conserved for integral membrane proteins, such as GPCRs. For the endothelin receptor type A, disruption of cholesterol in caveolae by oxidation switched the internalization pathway of this GPCR from caveolae to clathrin [17].
For some GPCRs, receptor palmitoylation has been shown to regulate access to phosphorylation sites in the receptor by various kinases (reviewed by [18]). We previously tested the involvement of phosphorylation by protein kinase A (PKA), since it was responsible for directing the β1-adrenergic receptor through caveolar endocytosis [19], but this was not demonstrated to be involved in D1 receptor caveolar internalization [10]. Since phosphorylation is a requirement for classical arrestin dependent clathrin mediated internalization, we predicted that the manipulation of palmitoylation sites would modulate the kinetics and/or extent of agonist mediated D1 receptor endocytosis. Therefore, the aim of the present study was to explore the potential role that palmitoylation might have on D1 receptor internalization and if the process determined a selective endocytic route (clathrin or caveolar). In this study, we demonstrated that a de-palmitoylated D1 receptor exhibited a significantly greater rate of internalization than wild-type D1 receptor and hypothesized that this may reflect a role for palmitoylation in directing the receptor to the slower caveolae-dependent internalization pathway as opposed to the accelerated clathrin-dependent endocytosis pathway.
2. Materials and Methods
2.1 Chemicals
Concanavalin A was purchased from Calbiochem (La Jolla, CA). Methyl-β-cyclodexytrin and H89 were purchased from Sigma (St. Louis, MO). [3H]SCH-23390 (85 Ci/mmol) was purchased from Perkin Elmer Life Sciences (Boston, MA).
2.2 DNA constructs
We used the full length N-terminal HA-D1 dopamine receptor cDNA that was cloned into the mammalian expression vector, pcDNA3.1 (Invitrogen, Carlsbad, CA) as a template for site-directed mutagenesis studies. The mutant dopamine D1 construct, C347A/C351A, was generated using the Quickchange site-directed mutagenesis kit (Stratagene, La Jolla, CA).
2.3 Cell culture and DNA transfection
COS7 cells were maintained as monolayer cultures at 37 °C in alpha minimum essential medium (University of Toronto) supplemented with 10% fetal bovine serum, antimycotic and antibiotic. Cells were grown to 80% confluence before being transfected using Lipofectamine 2000 (Invitrogen). Transfected cells were grown for 48 hrs before harvesting for all functional assays.
2.4 Radioligand binding
For whole cell binding experiments, COS7 cells expressing either HA-wild-type D1 receptor or HA-D1-C347A/C351A receptor were seeded onto 24-well plates (pre-treated with poly-L-ornithine) at a density of 1.75 × 105 cells/well. Cells were pre-incubated with the appropriate treatments before exposure to 10μM SKF 81297. Cells were washed with ice-cold buffer containing 1 mM EDTA and 50 mM Tris-HCl for 2 min to dissociate agonist before rinsing with 50 mM Tris-HCl for 1 min. Total binding was determined by incubating cells with the D1 receptor antagonist, [3H]SCH-23390 2nM (prepared in antagonist binding buffer) on ice for 3 hrs. Non-specific binding was defined by [3H]SCH-23390 binding in the presence of 1 μM (+) butaclamol. Cells were subsequently washed with ice-cold wash buffer (50 mM Tris-HCl) for 1 min three times before lysing with 0.2 N NaOH for 20 min. Lysates were resuspended in scintillation fluid and radioactivity was detected by a Beckman LS 6500 scintillation counter.
For saturation binding analysis, membranes (prepared as previously described [10]) were used for radioligand binding at [3H]SCH-23390 concentrations ranging from 10 pM to 4 nM. Binding was performed at room temperature for 1.5 hrs before bound ligand was isolated by rapid filtration through a 48-well cell harvester (Brandel, Gaithersburg, MD), using GF/C filters. Radioactivity was detected by a Beckman LS 6500 scintillation counter.
2.5 Detergent-free sucrose gradient fractionation
Caveolae-enriched fractions were prepared by separating whole cell lysates on a discontinuous sucrose gradient column by ultracentrifugation. Each gradient column was prepared with HA-D1-C347A/C351A- transfected COS7 cells from three 100 mm dishes and sucrose gradient fractions were prepared as described previously [10]. An equal volume of each fraction was separated on SDS-PAGE and immunoblotted with an anti-HA-horseradish peroxidase antibody (Roche, Penzburg, Germany). The protein bands were scanned by densitometric analysis and relative intensities were quantified using NIH image software version 1.33.
2.6 Statistical analysis
All pharmacological data were analyzed using Prism (GraphPad Software, San Diego, CA). Saturation binding curves were generated using non-linear least squares regression curve fitting. Statistical analysis between group means was performed using one way ANOVA followed by Tukey’s post hoc test.
3. RESULTS
3.1 Pharmacological Analysis of a Palmitoylation Deficient D1 Receptor (C347A/C351A)
Through PCR-based site-directed mutagenesis methods, we mutated both palmitoylated cysteines (C357, C351) in the carboxyl tail of the D1 receptor to alanine. To verify that this receptor mutant retained a similar pharmacological profile to the wild-type receptor, we performed saturation binding analysis. We found no significant differences in the binding affinity (Kd) between the two receptors (0.427 ± 0.05 nM for wild-type D1 receptor and 0.306 ± 0.05 nM for D1- C347A/C351A receptor), consistent with previous studies [13]. However, the total expression level (Bmax) of the palmitoylation deficient mutant was approximately half that of the wild-type D1 receptor (2.60 ± 0.07 pmol/mg for wild-type D1 receptor and 1.27 ± 0.05 pmol/mg for D1- C347A/C351A receptor (Fig 1)).
Figure 1.
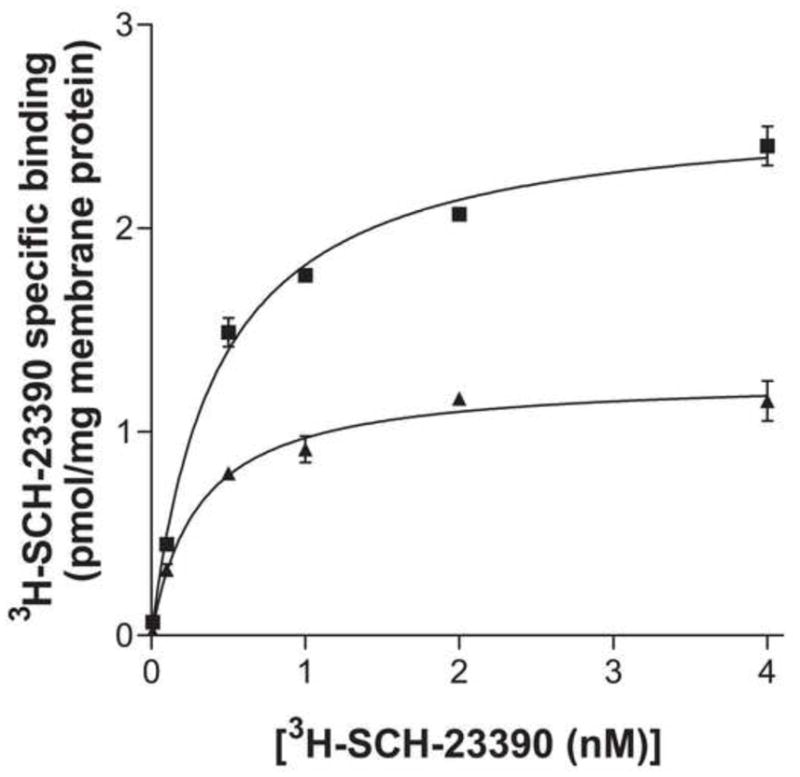
[3H]SCH-23390 saturation binding analysis of membrane preparations from COS7 cells expressing HA-wild-type D1 receptors (■), or HA-D1-C347A/C351A receptors (▲). Results shown are the mean ± S.E.M. of 3 independent experiments.
3.2 Kinetics of D1-C347A/C351A Receptor Internalization
To functionally characterize the palmitoylation-deficient mutant receptor, we investigated if there was a difference in the rate of internalization compared to wild-type D1 receptor. Through whole cell binding, we measured the extent of internalization of the D1-C347A/C351A receptor over a 50 minute time period. Within minutes of receptor stimulation with the agonist, SKF 81297 (10 μM), the cell surface population of D1-C347A/C351A receptor was rapidly internalized (Fig 2). Near maximum internalization was reached at 35 min where approximately 70% of receptors were internalized; the t1/2 for D1-C347A/C351A receptor internalization was 1.7 ± 0.4 min. In contrast, the rate of internalization of wild-type D1 receptor (t1/2 = 12.9 ± 3.5 min) was significantly slower, requiring almost 40 minutes of continuous agonist stimulation for near maximum receptor internalization. The magnitude of maximum internalization of wild-type D1 receptor was also less than D1-C347A/C351A receptor (49.4 ± 12% for wild-type D1 receptor vs. 75.2 ± 6.7% for D1-C347A/C351A receptor) suggesting that palmitoylation negatively modulates the ability of the D1 receptor to undergo agonist induced internalization.
Figure 2.
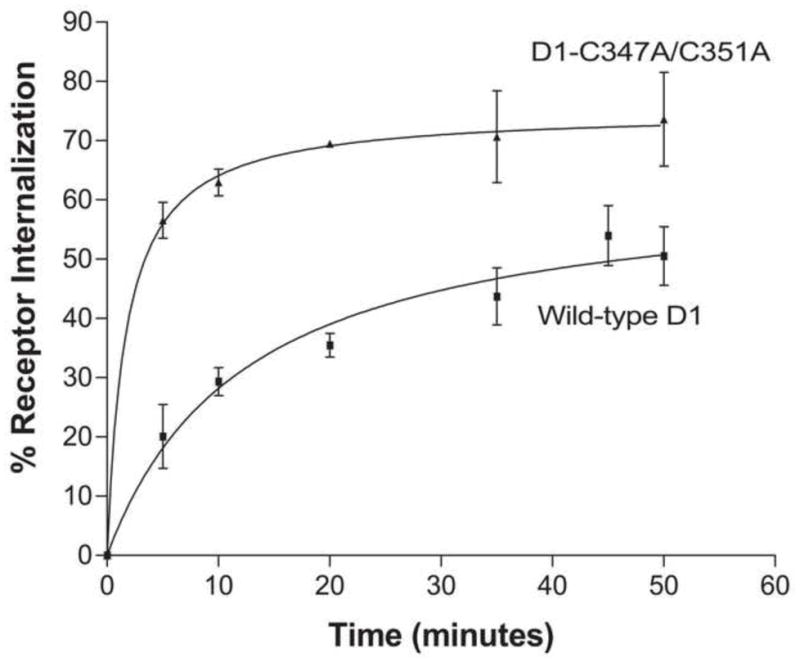
Whole cell radioligand binding analysis with 2 nM [3H]SCH-23390 on HA- wild-type D1 receptor or HA- D1-C347A/C351A receptor-expressing cells exposed to 10 μM SKF 81297 for the indicated time periods. Results are expressed as the mean % internalization ± S.E.M. of 3 independent experiments.
3.3 Effect of Endocytosis Inhibitors on Internalization of the D1-C347A/C351A Receptor
Since the de-palmitoylated D1 receptor internalized much faster than wild-type D1 receptor, we hypothesized that palmitoylation may have a role in acting as a switch between the rapid clathrin-dependent internalization and slower caveolin-dependent internalization. To investigate this possibility, we performed whole cell radioligand binding assays with D1-C347A/C351A receptor transfected COS7 cells to quantify the degree of receptor internalization in the presence of various inhibitors of caveolar and clathrin-mediated endocytic pathways. In whole cell binding assays, D1-C347A/C351A receptor transfected COS7 cells were preincubated with 0.45 M hypertonic sucrose or 0.25mg/ml concanavalin A, both of which are known inhibitors of clathrin-mediated endocytosis, or 2% methyl-β-cyclodexytrin, a cholesterol depleter known to disrupt caveolae structure and function. They were also preincubated with 30 μM of the PKA inhibitor, H89. Whole cell surface binding of [3H] SCH-23390, a selective D1 receptor antagonist, to D1-C347A/C351A receptors was compared before and after 30 minute incubation with 10 μM SKF 81297 in the presence of these compounds (Fig 3). Notably, the magnitude of internalization at this time point (~63%) was significantly higher than that observed with the wild-type D1 receptor (~52%) (Fig 3). Pretreatment of cells with sucrose, concanavalin A, or H89 significantly attenuated the degree of D1-C347/C351A receptor internalization although these treatments did not completely abolish endocytosis. D1-C347/C351A receptor endocytosis was also significantly attenuated (~23% internalization) in the presence of the cholesterol depleter, methyl-β-cyclodexytrin, but was still more resistant to cholesterol depletion than wild-type D1 receptor. Indeed, caveolae disruption almost completely abolished wild-type-D1 receptor endocytosis (~3 % internalization) (Fig 3). Taken together, these results suggest that palmitoylation may play a role in switching the endocytic route of the D1 receptor between clathrin-and caveolin-dependent internalization pathways.
Figure 3.
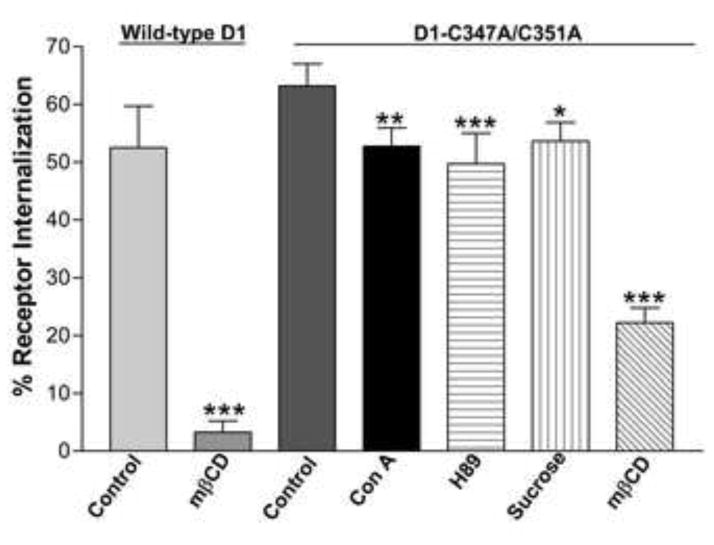
Whole cell radioligand binding analysis with 2 nM [3H]SCH-23390 on HA- wild-type D1 receptor or HA-D1-C347A/C351A receptor-expressing cells exposed to 10 μM SKF 81297. D1-C347A/C351A receptor expressing cells were pre-treated with either vehicle (control) or 0.45 M sucrose, 0.25 mg/ml concanavalin A, 2% methyl-β-cyclodextrin or 30 μM H89 and wild-type D1 receptor expressing cells were pre-treated with either vehicle (control) or 2% methyl-β-cyclodextrin for 30 min prior to agonist stimulation with SKF 81297 (10 μM) for an additional 30 min. The results are expressed as the mean % internalization ± S.E.M. of 3 independent experiments. Significance at p<0.05, p<0.01, and p<0.001 compared to control values is denoted by *, **, and ***, respectively.
3.4 Subcellular Localization of D1- C347A/C351A receptor
Since palmitoylation has been shown to be involved in targeting various proteins to detergent resistant membrane fractions, we investigated if this was an important determinant for caveolar localization of the D1 receptor. Sodium carbonate-based sucrose gradient fractions were prepared from whole cells lysates of HA-D1-C347A/C351A receptor transiently transfected COS7 cells. Analysis of the subcellular distribution of D1-C347A/C351A receptor revealed a substantial fraction of the receptor in the caveolin-enriched fractions (Fig 4, fraction 5) with some recovery in non-caveolin-enriched fractions (Fig 4, fractions 6–8). This was very similar to the pattern of localization for wild type D1 receptor [10] and suggests that palmitoylation does not have a role in the propensity of the D1 receptor to localize in caveolin-enriched domains under basal conditions.
Figure 4.
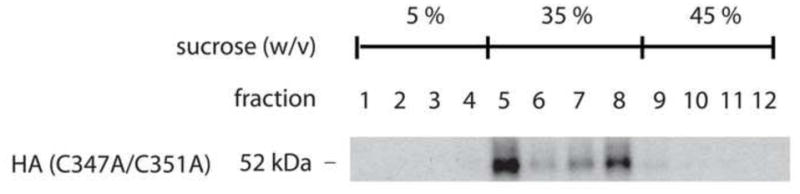
Subcellular localization of HA-D1-C347A/C351A receptors in sucrose gradient fractions. Membranes from HA-D1-C347A/C351A receptor-expressing cells were fractionated by sucrose density gradients, separated on SDS-PAGE, and probed with an anti-HA antibody. Fraction 5 represents localization of D1- C347A/C351A receptors in a caveolin-1 enriched fraction.
5. DISCUSSION
Although palmitoylation of the D1 receptor was not required for agonist-independent localization to caveolae, agonist induced internalization kinetics of the de-palmitoylated D1 receptor, C347A/C351A, was accelerated in comparison to wild-type D1 receptor. Additionally, there was significant attenuation in the extent of D1-C347A/C351A receptor agonist induced internalization after classical inhibition of the clathrin-mediated pathway. Taken together, these results suggest palmitoylation may play a role in modulating the endocytic route of agonist-dependent D1 receptor internalization.
We previously determined that D1 receptor caveolar-mediated receptor internalization was a slow process compared to clathrin-mediated internalization [10]. In this study, the D1-C347A/C351A receptor exhibited a significantly greater rate of internalization than wild-type D1 receptor with a half time that was almost 8-fold faster. We surmised that this might reflect a clear switch in endocytosis pathways as this accelerated rate of internalization was very similar to that observed for clathrin-dependent D1 receptor internalization [9]. It was hypothesized that in the absence of palmitoylation, the D1 receptor would not be able to associate with the lipid raft domains involved in the caveolin-dependent endocytic pathway and hence, switch to the clathrin-dependent endocytic pathway. Although the D1-C347A/C351A receptor was still targeted to the detergent insoluble fractions and agonist-dependent internalization remained sensitive to cholesterol depletion, inhibition of the clathrin-mediated endocytic pathway, with hypertonic sucrose or concanavalin A, did significantly attenuate the extent of D1-C347A/C351A receptor internalization. Additionally, D1-C347A/C351A receptor internalization appeared more resistant to caveolar disruption compared to wild-type D1 receptor as a larger proportion of these receptors were sequestered in response to agonist treatment.
It is conceivable that this apparent resistance was actually due to a switching of the palmitoylation-deficient mutant to a cholesterol-insensitive clathrin-dependent internalization pathway. However, if this is the case, it is unclear why the inhibitory effects of sucrose and concanavalin A were not more marked (though significant) and further, why methyl-β-cyclodexytrin still attenuated D1-C347A/C351A receptor internalization. One possibility is that the components required for clathrin mediated internalization (eg: GRKs, arrestins) may be limiting and therefore the extent to which proteins are sequestered through this pathway is minimal. Therefore, any switching to a clathrin-dependent internalization pathway would be limited and the majority of receptors may still, by default, undergo caveolar internalization. In such an instance, the effects of clathrin inhibition would not appear to be robust. Consistent with this notion, previous studies have shown that COS7 cells endogenously express lower levels of specific kinase and arrestin subtypes than other cell lines such as HEK and CHO cells [20] even though clathrin mediated internalization remains intact [21, 22].
Alternatively, palmitoylation may be required to maintain a specific receptor conformation that prevents the receptor from undergoing dysregulated internalization. For instance, the lack of palmitate moieties in the D1-C347A/C351A receptor might facilitate the exposure of residues to certain kinases that render the receptor more amenable to phosphorylation and rapid internalization. This was shown with the β2-adrenergic receptor in which elimination of a single palmitoylatable cysteine in the carboxyl tail exposed nearby serine residues that were better substrates for PKA phosphorylation [23, 24]. This notion has also been suggested for a number of GPCRs with similar mutations of the same conserved palmitoylatable cysteines [25, 26]. Although there is evidence that PKA phosphorylation is required for caveolar internalization of certain GPCRs [19], this may not be the case for the D1 receptor since it does not have PKA consensus sites nearby the palmitoylated cysteines.
Another site of phosphorylation that might be exposed due to the lack of receptor palmitate moieties is on caveolin-1 itself. A number of kinases including protein kinase C and certain non-receptor tyrosine kinases such as Src have been suggested to regulate caveolar internalization of various molecules [27, 28]. Both palmitoylation sites in the carboxyl-tail of the D1 receptor are in close proximity to the caveolin-1 binding domain that we identified in transmembrane domain 7 of the D1 receptor [10]. Hence, it is conceivable that palmitoylation may sterically hinder the ability of certain kinases to phosphorylate caveolin-1 and that de-palmitoylation permits phosphorylation by these kinases and subsequent receptor internalization.
Taken together, constitutive palmitoylation may serve to stabilize the D1 receptor during agonist-dependent caveolar internalization. The absence of palmitate moieties may allow the receptor to adopt an agonist induced conformational state that permits access of specific kinases to target sites that accelerate D1 receptor internalization.
Research Highlights.
Palmitoylation may target D1 receptors through selective endocytic pathways
The de-palmitoylated D1 receptor internalized faster than wild-type D1 receptor
Inhibition of clathrin endocytoses reduced de-palmitoylated D1 receptor internalization
Palmitoylation was not necessary for basal D1 receptor localization in caveolae
Acknowledgments
The work was supported by a grant from the US National Institute on Drug Abuse. VV was supported by the Canada Graduate Scholarship from the Natural Sciences and Engineering Research Council. SRG is the holder of a Tier 1 Canada Research Chair in Molecular Neuroscience.
ABBREVIATIONS
- GPCRs
G protein-coupled receptors
- GRKs
G protein-coupled receptor kinases
- PKA
protein kinase A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ng GY, Mouillac B, George SR, Caron M, Dennis M, Bouvier M, O’Dowd BF. Desensitization, phosphorylation and palmitoylation of the human dopamine D1 receptor. Eur J Pharmacol. 1994;267:7–19. doi: 10.1016/0922-4106(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 2.Martin-Negrier M, Charron G, Bloch B. Agonist stimulation provokes dendritic and axonal dopamine D(1) receptor redistribution in primary cultures of striatal neurons. Neuroscience. 2000;99:257–266. doi: 10.1016/s0306-4522(00)00187-1. [DOI] [PubMed] [Google Scholar]
- 3.Martin-Negrier ML, Charron G, Bloch B. Receptor recycling mediates plasma membrane recovery of dopamine D1 receptors in dendrites and axons after agonist-induced endocytosis in primary cultures of striatal neurons. Synapse. 2006;60:194–204. doi: 10.1002/syn.20296. [DOI] [PubMed] [Google Scholar]
- 4.Dumartin B, Caille I, Gonon F, Bloch B. Internalization of D1 dopamine receptor in striatal neurons in vivo as evidence of activation by dopamine agonists. J Neurosci. 1998;18:1650–1661. doi: 10.1523/JNEUROSCI.18-05-01650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 6.Rajendran L, Simons K. Lipid rafts and membrane dynamics. J Cell Sci. 2005;118:1099–1102. doi: 10.1242/jcs.01681. [DOI] [PubMed] [Google Scholar]
- 7.Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med. 2004;36:584–595. doi: 10.1080/07853890410018899. [DOI] [PubMed] [Google Scholar]
- 8.Galbiati F, Volonte D, Gil O, Zanazzi G, Salzer JL, Sargiacomo M, Scherer PE, Engelman JA, Schlegel A, Parenti M, Okamoto T, Lisanti MP. Expression of caveolin-1 and -2 in differentiating PC12 cells and dorsal root ganglion neurons: caveolin-2 is up-regulated in response to cell injury. Proc Natl Acad Sci U S A. 1998;95:10257–10262. doi: 10.1073/pnas.95.17.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vickery RG, von Zastrow M. Distinct dynamin-dependent and -independent mechanisms target structurally homologous dopamine receptors to different endocytic membranes. J Cell Biol. 1999;144:31–43. doi: 10.1083/jcb.144.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong MM, Hasbi A, Mattocks M, Fan T, O’Dowd BF, George SR. Regulation of D1 dopamine receptor trafficking and signaling by caveolin-1. Mol Pharmacol. 2007;72:1157–1170. doi: 10.1124/mol.107.034769. [DOI] [PubMed] [Google Scholar]
- 11.el-Husseini AelD, Bredt DS. Protein palmitoylation: a regulator of neuronal development and function. Nat Rev Neurosci. 2002;3:791–802. doi: 10.1038/nrn940. [DOI] [PubMed] [Google Scholar]
- 12.Escriba PV, Wedegaertner PB, Goni FM, Vogler O. Lipid-protein interactions in GPCR-associated signaling. Biochim Biophys Acta. 2007;1768:836–852. doi: 10.1016/j.bbamem.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Jin H, Xie Z, George SR, O’Dowd BF. Palmitoylation occurs at cysteine 347 and cysteine 351 of the dopamine D(1) receptor. Eur J Pharmacol. 1999;386:305–312. doi: 10.1016/s0014-2999(99)00727-x. [DOI] [PubMed] [Google Scholar]
- 14.Chini B, Parenti M. G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J Mol Endocrinol. 2004;32:325–338. doi: 10.1677/jme.0.0320325. [DOI] [PubMed] [Google Scholar]
- 15.Galbiati F, Volonte D, Meani D, Milligan G, Lublin DM, Lisanti MP, Parenti M. The dually acylated NH2-terminal domain of gi1alpha is sufficient to target a green fluorescent protein reporter to caveolin-enriched plasma membrane domains. Palmitoylation of caveolin-1 is required for the recognition of dually acylated g-protein alpha subunits in vivo. J Biol Chem. 1999;274:5843–5850. doi: 10.1074/jbc.274.9.5843. [DOI] [PubMed] [Google Scholar]
- 16.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto Y, Ninomiya H, Miwa S, Masaki T. Cholesterol oxidation switches the internalization pathway of endothelin receptor type A from caveolae to clathrin-coated pits in Chinese hamster ovary cells. J Biol Chem. 2000;275:6439–6446. doi: 10.1074/jbc.275.9.6439. [DOI] [PubMed] [Google Scholar]
- 18.Qanbar R, Bouvier M. Role of palmitoylation/depalmitoylation reactions in G-protein-coupled receptor function. Pharmacol Ther. 2003;97:1–33. doi: 10.1016/s0163-7258(02)00300-5. [DOI] [PubMed] [Google Scholar]
- 19.Rapacciuolo A, Suvarna S, Barki-Harrington L, Luttrell LM, Cong M, Lefkowitz RJ, Rockman HA. Protein kinase A and G protein-coupled receptor kinase phosphorylation mediates beta-1 adrenergic receptor endocytosis through different pathways. J Biol Chem. 2003;278:35403–35411. doi: 10.1074/jbc.M305675200. [DOI] [PubMed] [Google Scholar]
- 20.Menard L, Ferguson SS, Zhang J, Lin FT, Lefkowitz RJ, Caron MG, Barak LS. Synergistic regulation of beta2-adrenergic receptor sequestration: intracellular complement of beta-adrenergic receptor kinase and beta-arrestin determine kinetics of internalization. Mol Pharmacol. 1997;51:800–808. [PubMed] [Google Scholar]
- 21.Gaborik Z, Szaszak M, Szidonya L, Balla B, Paku S, Catt KJ, Clark AJ, Hunyady L. Beta-arrestin- and dynamin-dependent endocytosis of the AT1 angiotensin receptor. Mol Pharmacol. 2001;59:239–247. doi: 10.1124/mol.59.2.239. [DOI] [PubMed] [Google Scholar]
- 22.Vrecl M, Anderson L, Hanyaloglu A, McGregor AM, Groarke AD, Milligan G, Taylor PL, Eidne KA. Agonist-induced endocytosis and recycling of the gonadotropin-releasing hormone receptor: effect of beta-arrestin on internalization kinetics. Mol Endocrinol. 1998;12:1818–1829. doi: 10.1210/mend.12.12.0207. [DOI] [PubMed] [Google Scholar]
- 23.Moffett S, Adam L, Bonin H, Loisel TP, Bouvier M, Mouillac B. Palmitoylated cysteine 341 modulates phosphorylation of the beta2-adrenergic receptor by the cAMP-dependent protein kinase. J Biol Chem. 1996;271:21490–21497. doi: 10.1074/jbc.271.35.21490. [DOI] [PubMed] [Google Scholar]
- 24.Moffett S, Mouillac B, Bonin H, Bouvier M. Altered phosphorylation and desensitization patterns of a human beta 2-adrenergic receptor lacking the palmitoylated Cys341. Embo J. 1993;12:349–356. doi: 10.1002/j.1460-2075.1993.tb05663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munshi UM, Clouser CL, Peegel H, Menon KM. Evidence that palmitoylation of carboxyl terminus cysteine residues of the human luteinizing hormone receptor regulates postendocytic processing. Mol Endocrinol. 2005;19:749–758. doi: 10.1210/me.2004-0335. [DOI] [PubMed] [Google Scholar]
- 26.Ponimaskin E, Dumuis A, Gaven F, Barthet G, Heine M, Glebov K, Richter DW, Oppermann M. Palmitoylation of the 5-hydroxytryptamine4a receptor regulates receptor phosphorylation, desensitization, and beta-arrestin-mediated endocytosis. Mol Pharmacol. 2005;67:1434–1443. doi: 10.1124/mol.104.008748. [DOI] [PubMed] [Google Scholar]
- 27.Dangoria NS, Breau WC, Anderson HA, Cishek DM, Norkin LC. Extracellular simian virus 40 induces an ERK/MAP kinase-independent signalling pathway that activates primary response genes and promotes virus entry. J Gen Virol. 1996;77(Pt 9):2173–2182. doi: 10.1099/0022-1317-77-9-2173. [DOI] [PubMed] [Google Scholar]
- 28.Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]


