Abstract
Rationale and objectives
Amphetamine-induced sensitization is thought to involve dopamine D1 receptors. Using mice lacking dopamine D1 receptors (D1−/−), we found that they exhibited blunted sensitization to low doses of amphetamine, while others using different treatment and testing regimens reported inconsistent results. We investigated whether experimental variables, alteration in gene expression or cholinergic input played a role in amphetamine-induced responses.
Methods
D1−/− and wild-type (D1+/+) mice pretreated with amphetamine (1 mg/kg, 3–7 days) or various doses of nicotine (chronically but intermittently) were challenged with amphetamine (0.7 and/or 1 mg/kg) after short and long abstinence periods. Expression of brain-derived neurotrophic factor (BDNF) and phosphorylated c-AMP response element binding protein (p-CREB) genes were measured under basal conditions and after acute or repeated amphetamine treatments.
Results
D1−/− mice failed to exhibit amphetamine-induced sensitization following short-term treatments and long abstinence periods, but expressed sensitization following prolonged amphetamine treatment or a shorter abstinence period. Basal expression of p-CREB (but not BDNF) was higher in D1−/− than D1+/+ mice and was reduced after amphetamine treatment. Prolonged nicotine pretreatment augmented locomotor responses to amphetamine in both genotypes and restored sensitization in D1−/− mice.
Conclusions
D1 receptors were necessary for induction, but may not be necessary for expression of amphetamine-induced sensitization at low doses. The manifestation of amphetamine sensitization depended on the duration of treatment and length of the withdrawal period. Cholinergic–nicotinic stimulation restored amphetamine-induced sensitization in D1−/− mice. Enhanced basal expression of p-CREB in D1−/− mice may represent an adaptive mechanism related to lack of D1 receptors.
Keywords: Dopamine D1 receptor, Gene-deleted mice, Amphetamine, Nicotine, Behavioral sensitization, Gene expression, p-CREB, BDNF
Introduction
Repeated administration of amphetamine results in augmentation of its behavioral effects, a phenomenon known as behavioral sensitization (Kalivas and Stewart 1991) and has been implicated in drug self-administration in animals and in human drug addiction (Robinson and Berridge 1993). Increased dopaminergic neurotransmission in the dorsal and ventral striatum has been implicated in locomotor stimulation and sensitization induced by psychostimulants in rodents and humans (Di Chiara and Imperato 1988; White and Kalivas 1998; Vanderschuren and Kalivas 2000; Vezina 2004; Boileau et al. 2006). In rodents, D1- and D2-like receptors have been shown to modulate locomotor stimulation and sensitization induced by amphetamine (Ross et al. 1989; Kuribara 1995; Vezina 1996; O’Neill and Shaw 1999). However, it has been reported that moderate doses of amphetamine activate D1 rather than D2 receptors (Ferger et al. 1994) and that repeated amphetamine administration leads to supersensitivity of the D1 receptor in the nucleus accumbens (Wolf et al. 1994; White and Kalivas 1998). This evidence suggests a prominent role of the D1 receptor in the neurochemical and behavioral adaptation involved in the induction and expression of sensitization by amphetamine (Pierce et al. 1996; Vezina 1996; White and Kalivas 1998). Indeed, amphetamine-induced locomotor sensitization has been associated with enhanced dopamine D1 receptor-mediated signal transduction pathways including activation of several protein kinases (Pierce et al. 1996; Hu et al. 2002; Licata and Pierce 2003), neuroadaptations that involve alterations in the phosphorylation of transcription factors such as CREB (Turgeon et al. 1997), and changes in the expression of neurotrophins, including fibroblast growth factors (Flores et al. 2000) and brain-derived neurotrophic factor (BDNF; Meredith et al. 2002). Furthermore, interactions between the dopaminergic system and other neurotransmitters including the cholinergic system have also been implicated in amphetamine-induced locomotor sensitization. For example, activation of central cholinergic nicotinic receptors increases dopamine neurotransmission in the nucleus accumbens (Di Chiara and Imperato 1988; Vezina et al. 1992; Marshall et al. 1997; Shim et al. 2001) and profoundly enhances nicotine- and amphetamine-induced behavioral sensitization (Birrell and Balfour 1998; Schoffelmeer et al. 2002). In addition, numerous studies have indicated that amphetamine and D1 receptor agonists increase, whereas D2 receptor agonists decrease acetylcholine (ACh) overflow in the striatum (DeBoer and Abercrombie 1996; Acquas et al. 1997) and that augmentation of striatal ACh efflux seen after amphetamine challenge in animals withdrawn from chronic amphetamine was correlated with the expression of behavioral sensitization observed in these animals and hypothesized to be through activation of D1 receptors (Bickerdike and Abercrombie 1997). Other behavioral studies have indicated that ACh is indeed critical for the development of behavioral sensitization, but may not be involved in the expression of this phenomenon (Heidbreder and Shippenberg 1996; Schoffelmeer et al. 2002).
Taken together, these findings suggest that amphetamine-induced sensitization is a complicated process involving the interplay between neurotransmitters, neuropeptides, and trophic factors. However, the direct involvement of the D1 receptor in amphetamine-induced alteration in gene expression and in dopaminergic–cholinergic interactions during acute and repeated amphetamine treatment has not been well established in mice. We, as well as others, have shown that D1−/− mice were able to exhibit locomotor stimulation and develop and express behavioral sensitization to a range of high doses of amphetamine (3–8 mg/kg), though it was less than in control mice in some cases (El-Ghundi et al. 1997; Karper et al. 2002; Xu et al. 2000). However, studies on the responses of D1−/− mice to low doses of amphetamine yielded inconsistent results. Acute exposure to low doses (1 mg/kg) of amphetamine failed to induce locomotor stimulation in both genotypes (Karper et al. 2002; Xu et al. 2000), whereas acute exposure to 2 mg/kg caused locomotor stimulation in D1+/+, but not D1−/− mice (Xu et al. 2000). Another report showed that acute exposure to 2 mg/kg amphetamine did not affect the activity in both genotypes, but repeated exposure for 5 days caused enhanced locomotor responses in D1+/+, but not in D1−/− mice. However, both genotypes expressed sensitization after 3 days of abstinence, although it was less robust in D1−/− mice (Crawford et al. 1997). Another study reported that repeated exposure to amphetamine (1 and 2 mg/kg) for seven consecutive days failed to induce locomotor sensitization in both genotypes, but when challenged with 1 mg/kg amphetamine after 3 and 17 days of abstinence, both genotypes expressed sensitization (Karper et al. 2002). In contrast, we have found that short-term repeated exposure to 1 mg/kg amphetamine caused locomotor stimulation and sensitization in D1+/+ mice, but not in D1−/− mice (El-Ghundi et al. 1997). The apparent discrepancy in these results may, in part, be explained by variable treatment paradigms and differences in genetic background but nevertheless clearly suggests that induction (development) of sensitization during repeated treatment with low doses was abolished in D1−/− mice, whereas the expression of sensitization could occur under certain experimental protocols, as has also been reported for cocaine sensitization (Karlsson et al. 2008). Therefore, we focused on using low doses to investigate whether this impairment could simply be related to the treatment regimen and abstinence period that were appropriate for the induction and expression of sensitized responses in D1+/+ mice, but were inadequate for induction and/or monitoring for detection of any sensitized responses in the D1−/− mice. Alternatively, we investigated whether altered dopamine D1 receptor-mediated gene expression or altered interaction with the cholinergic system plays a role in attenuating amphetamine-induced effects in D1−/− mice.
In the present study, we used congenic D1−/− and D1+/+ mice to address the following issues: (1) role of D1 receptors in the development and expression of sensitization to low doses of amphetamine under variable treatment and testing regimens, (2) whether accompanying neuroadaptive changes in gene expression after acute and repeated amphetamine treatments would correspond with the presence or absence of behavioral sensitization, and (3) whether direct manipulation of ACh release by nicotine receptor activation could enhance amphetamine-induced locomotor sensitization. In the first part of this study, experiments 1–2 were designed to replicate the acute and sensitizing effects of low doses of amphetamine on locomotor activity under variable conditions of treatment and abstinence. Mice from both genotypes were used for the determination of amphetamine-induced gene expression of p-CREB and BDNF following acute and repeated amphetamine treatment. In the second part of this study, we investigated the possible role of cholinergic neurotransmission in amphetamine-induced locomotor activity and behavioral sensitization. We postulated that attenuated induction of amphetamine sensitization in D1−/− mice could relate to reduced D1 receptor-mediated striatal ACh release. Therefore, it was hypothesized that enhancing the cholinergic neurotransmission through direct activation of the nicotinic receptor, and thereby bypassing the direct involvement of the D1 receptor, would restore or enhance amphetamine sensitization in D1−/− and D1+/+ mice, respectively. This hypothesis was tested in experiments designed to study whether impaired induction of sensitization seen in D1−/− mice could be reversed by longterm nicotine pretreatment. A follow-up study was designed to evaluate the effects of nicotine receptor blockade on amphetamine-induced sensitization at least in wild-type mice to verify the involvement of the cholinergic system.
Materials and methods
Animals
Mice with a mixed genetic background (129/Sv×C57BL/6J; Drago et al. 1994) were backcrossed into the C57BL/6J strain for 11 generations to establish a congenic generation of mice lacking the dopamine D1 receptor and wild-type littermates. Genotypes were determined by PCR analyses of genomic DNA extracted from a small piece of each mouse’s ear (Miner et al. 1995). Only male mice, 6–8 months of age, were used in all studies. They were housed in groups of three per cage in a temperature-controlled room (22°C) with free access to food and water. All mice were maintained on a reversed 12-h dark/light cycle. In addition to food pellets, all mice were fed hydrated mouse meal twice per week at weaning age. Animal care and experimental procedures were approved by the Canadian Council for Animal Care.
Habituation procedure
All animals were initially habituated to the new testing environment, activity cages, and injection procedures. This habituation period was intended to reduce the amount of stress-evoked spontaneous activity in reaction to novelty and injection procedure. In all studies, before habituation to activity cages, mice were habituated to the novel environment. They were brought to the experimental room in their original home cages with their cage mates and were gently handled for at least 5 min per day. During the habituation period to the activity cages (1–2 days), mice (all cage mates) were initially put together in the activity cages for 1–2 h, during which time they were gently handled. On the following days, mice chosen for the experiment were brought in individual transporting cages to the experimental room and left individually in the activity cages for 1–2 h daily for 3–5 days. During habituation to injections, mice were injected with saline and left in the activity cages for 1–2 h daily for 2–5 days. As a control experiment for measuring baseline locomotor activities, mice were initially habituated to the activity cages for 1-h daily sessions for 8 days (without injection) then for 10 days following saline injection. Ambulatory activities for all mice were monitored every 5 min during 1- to 2-h sessions that began 5 min after placing the mouse into the cage. After stabilization of locomotor activity, drug-induced locomotor effects were tested. On test days, motor activity was monitored for all mice during 1-h habituation to the activity cages following saline injection, which served as baseline activity, and again after saline or drug treatment. In other protocols, locomotor activity was recorded following saline or drug treatment without saline pretreatment habituation. All sessions were conducted during the dark phase of the dark/light cycle in a room illuminated with a dim red light.
Apparatus
For all experiments, multiple indices of spontaneous locomotor activity were assessed using the same set of eight automated transparent Plexiglas cages (42×20× 20 cm) covered with filter tops and contained corn cob bedding just enough to cover the floors. These cages were enclosed in custom-made horizontal frames equipped with two parallel bottom and two parallel upper arrays of 16 infrared photobeam sensors (1 in. apart). The upper and lower sensors were 2 in. apart, and the bottom and top sensors were 0.75 and 2.75 in. off the cage floor, respectively. All activity cages were connected to a microprocessor counter linked to a computer that recorded photocell beam breaks. Ambulation (expressed as number of beam interruptions) was measured every 5-min intervals.
Drugs
d-Amphetamine sulfate, nicotine hydrogen tartrate, and mecamylamine hydrochloride (Sigma-Aldrich Canada Ltd.) were dissolved in isotonic saline and expressed as free base. Amphetamine and mecamylamine were injected intraperitoneally in a volume of 1 ml/kg, and nicotine was injected subcutaneously in a volume of 5 or 10 ml/kg. All doses were selected for their demonstrated optimal non-stereotypic effects based on our preliminary data and as reported in our previous studies (El-Ghundi et al. 1997) and in several other published data.
Locomotor effects of amphetamine
Because of the discrepancy in the reported responses of D1−/− mice to low doses of amphetamine-induced locomotor stimulation and sensitization, we sought to determine whether methodological variables (i.e., duration and frequency of drug treatment and drug abstinence) played a role in the extent of induction and expression of sensitization to amphetamine. Two independent studies were conducted using multiple experimental approaches.
Experiment 1: Amphetamine sensitization under variable treatment and testing regimen
This experiment was conducted to determine the effect of several experimental parameters (consecutive and intermittent treatments, pretreatment habituation, multiple challenge doses, and cumulative drug abstinence periods) to allow monitoring for detection of sensitization.
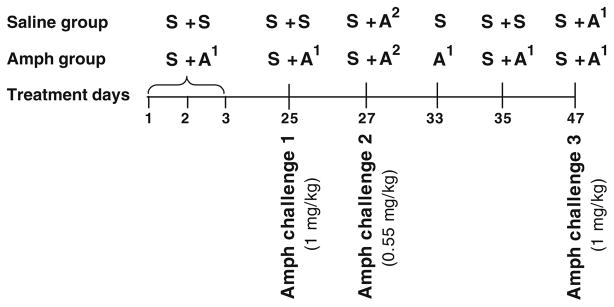
All mice were habituated to activity cages without injection (1 h daily for 3 days) and after saline injections (2 h daily for 5 days). On test days (days 1–3), two groups from each genotype (n=8 per group) were habituated to the activity cages after saline injection followed 1 h later by administration of either saline (S + S, for saline groups) or 1 mg/kg amphetamine (S + A1, for treatment groups; see diagram in Fig. 1). Following injections, each mouse was immediately returned to its activity cage, and its locomotor activity was recorded 5 min after injection for 1 h. On day 25, after 3 weeks of drug-free period in their home cages, all mice were habituated to the activity cages for 1 h following saline injections (baseline activity), then amphetamine pretreated mice were challenged with amphetamine (1 mg/kg), whereas saline-pretreated mice were injected with saline and their activity monitored for 1 h. Since D1−/− mice did not express sensitization to the locomotor stimulant effect induced by amphetamine compared to D1+/+ mice, it was possible that the challenge dose was too high that produced a ceiling effect based on the fact that these mice have elevated basal level of dopamine (El- Ghundi et al. 1998) or that either or both the treatment and abstinence duration were inappropriate for D1−/− mice to develop and express sensitization. Therefore, we investigated whether any one of these possibilities was of outstanding importance in altering responses of D1−/− mice to amphetamine. We addressed these issues in the same experiment by challenging with a lower dose then extending the treatment for two more days spaced by drug-free periods and a final challenge after a shorter period of abstinence. First, we tested whether a low-dose challenge with amphetamine (0.55 mg/kg) given 1 day after the last treatment (day 27) would result in exaggerated locomotor responses in these mice that were pretreated with a higher dose. Then, after 5 days of drug-free period, mice were given two additional saline or amphetamine treatments (days 33 and 35) followed by a final challenge test after 11 days of abstinence (day 47). All sessions were preceded by saline pretreatment habituation except on day 33; mice were treated with either saline or amphetamine (1 mg/kg) directly without the usual saline pretreatment habituation in an effort to test whether pre-habituation period following saline injection played a role in dampening their motor responses to amphetamine. On drug-free days in between treatments, all mice were left undisturbed in their home cages. In a duplicate experiment, mice were treated identically, except that on day 25, all mice were challenged with amphetamine following saline pretreatment and were killed 2 h after the last amphetamine treatment, and their brains were used for Western blot analyses.
Fig. 1.
Diagram outlining treatment schedule for amphetamine experiment 1. All days represent saline (S) and amphetamine 1 mg/kg (A1) or 0.55 mg/kg (A2) treatments during the first and second hours except on day 33; mice were given their respective treatments during the first hour. In between treatment days, mice were left undisturbed in their home cages
Experiment 2: Amphetamine sensitization after short-term treatment (3 days) and 11 days of abstinence
Based on the results of the above experiment, it was not clear whether prolonged intermittent treatment or cumulative periods of abstinence have contributed to expression of sensitized responses in D1−/− mice. It is possible that the treatment regimen (three consecutive days) or abstinence period (3 weeks) that were appropriate for the D1+/+ mice were inadequate for expression of sensitized responses in the D1−/− mice. Therefore, we sought to determine whether sensitized responses to amphetamine could be detected in D1−/− mice after a shorter abstinence period. In this experiment, the treatment duration was kept constant (three consecutive days), but the abstinence period was changed to 11 days instead of 3 weeks. Two groups of D1−/− mice and two groups of D1+/+ mice (n=8 per group) were habituated to activity cages for 2 h daily for 5 days and following saline injections twice daily for 2 days. Following stabilization of activity, one group from each genotype was treated with saline and another group with amphetamine (1 mg/kg) for three consecutive days. After 11 days of abstinence, during which mice were left undisturbed in their home cages, all mice were pretreated with saline (baseline activity) 1 h before they were challenged with amphetamine (1 mg/kg). Locomotor activities were recorded for 1 h following saline and amphetamine treatments.
Amphetamine-induced gene expression: Western blot analysis
As a duplicate of experiment 1, mice from both genotypes (n=8 per group) were treated with either saline or amphetamine (1 mg/kg) for three consecutive days and were challenged with amphetamine after 3 weeks of abstinence, killed 2 h later, and their brains were processed for immunoreactivity to p-CREB and BDNF. Drug-naïve mice (n=12 per genotype), habituated to activity cages for 3 days and to saline injections for 5 days, were also killed following the last saline injection and used as control groups for baseline levels. Striatal tissues were dissected and homogenized in RIPA buffer (Santa Cruz 24948 containing 1× PBS, Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, protease inhibitor cocktail, and phosphatase inhibitor sodium orthovanadate). Homogenates were incubated on ice for 30 min and centrifuged at 14,000×g for 20 min at 4°C. Protein concentration in the supernatant was determined by the Bradford assay. Samples of boiled supernatant protein (30 μg) were loaded on 12% or 16% Tris glycine gel, electrophoresed, and electrotransferred onto nitrocellulose or polyvinylidene fluoride membranes (Pall Canada Limited). Blots were blocked with 5% non-fat dry milk dissolved in Tris-buffered saline with 0.1% Tween 20 (TBS-T) for 1 h at room temperature and then incubated overnight with the respective primary antibodies against either BDNF (1:1,000, Santa Cruz, CA, USA) or p-CREB (1:1,200, Upstate Cell Signalling, NY, USA). Blots were then washed in TBS-T and incubated with horseradish peroxidase (IgG)-conjugated secondary antibody (1:5,000, Chemicon International, Temecula, CA, USA) and visualized by enhanced chemiluminescence detection reagent (Amersham Biosciences, Canada). For loading control, blots were probed with glyceraldehyde 3-phosphate dehydrogenase antibody (anti-GAPDH, 1:20,000; Abcam, Cambridge, MA, USA). Each band intensity was quantified using the MCID digital densitometry system and normalized to its respective GAPDH loading control.
Effect of nicotine pretreatment on locomotor effects of amphetamine
Based on the results from the above two experiments, it was clear that induction of amphetamine-induced sensitization (days 1–3) was impaired in D1−/− mice compared to D1+/+ mice. Therefore, since it is well known that nicotine pretreatment potentiates the behavioral effects of amphetamine (Schoffelmeer et al. 2002) and that ACh is critical for the development of behavioral sensitization (Heidbreder and Shippenberg 1996; Schoffelmeer et al. 2002), we investigated whether nicotine pretreatment could reverse the blunted induction of amphetamine sensitization in D1−/− mice. In a follow-up experiment, we tested the effect of nicotinic receptor blockade by mecamylamine (a central and ganglionic nicotinic receptor antagonist) on the development of behavioral sensitization to verify the involvement of the cholinergic system in the induction of sensitization to amphetamine at least in the D1+/+ mice.
Experiment 1: Prolonged nicotine pretreatment
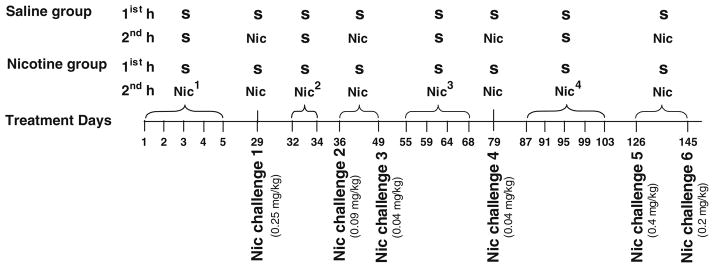
Mice were habituated to activity cages (1 h daily for 5 days) and following saline injections (1 h daily for 5 days). Two groups of D1−/− mice and two groups of D1+/+ mice (n=8 per group) were given either saline or nicotine (0.25 mg/kg) treatment for five consecutive days, and their activity was monitored during each session (see diagram in Fig. 2). All mice were challenged with nicotine (0.25 mg/kg) after 3 weeks of abstinence (day 29). It is important to note that during nicotine treatment and the challenge test, no locomotor stimulation or sensitization was observed in both genotypes, but instead, mice exhibited locomotor depression and signs of sickness; therefore, we gradually reduced the dose of nicotine and prolonged the treatment intermittently to test if mice would develop tolerance to the locomotor depression and exhibit locomotor stimulation. Following 2 days of abstinence from the last challenge test, mice pretreated with nicotine were given additional nicotine treatments (while saline-pretreated mice received saline); these include 0.18 mg/kg daily for 2 days (spaced 1 day apart) followed by two challenge tests for all mice with nicotine 0.09 and 0.04 mg/kg separated by 12 days of abstinence. Then 5 days after the last challenge test, mice in the nicotine group were given four additional doses of nicotine 0.04 mg/kg every third day followed by a challenge test for all mice with 0.04 mg/kg 10 days later. At this stage of the experiment, no locomotor stimulation was observed in mice pretreated chronically with nicotine; therefore, the dose of nicotine was increased. This includes five doses of nicotine (0.4 mg/kg) every third day 1 week after the last nicotine treatment followed 3 weeks later by two challenge tests for all mice with nicotine 0.4 and 0.2 mg/kg separated by 18 days of abstinence. To test whether nicotine pretreatment have sensitized the mice to amphetamine-induced locomotor stimulation, all mice were challenged, for the first time, with amphetamine. We first used a low dose (0.7 mg/kg) because we previously found that it was ineffective in stimulating locomotion in both genotypes (unpublished data) to allow for detection of sensitized responses following nicotine pretreatment. All mice were treated with amphetamine (0.7 mg/kg) 1 week after the last nicotine treatment then 5 days later received amphetamine (1 mg/kg) for three more days separated by 13 and 8 days, respectively. This spaced amphetamine treatment, which usually does not induce sensitization under normal conditions, was chosen to verify whether the mice that exhibited exaggerated responses to a low dose of amphetamine were indeed sensitized by nicotine pretreatment and that this sensitization was long-lasting and not transient. All sessions were preceded by measurement of baseline activity for 1 h following saline injections. In between treatments, mice were left undisturbed in their home cages.
Fig. 2.
Diagram outlining treatment schedule for nicotine experiment 1. All days represent saline (S) and nicotine 0.25 mg/kg (Nic1), 0.18 mg/kg (Nic2), 0.04 mg/kg (Nic3), or 0.4 mg/kg (Nic4) treatments during the first and second hours. In between treatment days, mice were left undisturbed in their home cages
Experiment 2: Effect of nicotine receptor blockade on amphetamine sensitization
Two groups of D1−/− mice and two groups of D1+/+ mice (n=6 per group) were habituated to the activity cages for 3 days and to saline injections for 4 days. On test days, all mice were injected with saline and their baseline activity monitored for 1 h, then one group of mice from each genotype was injected again with saline and the other group was injected with a nicotinic receptor antagonist mecamylamine (3 mg/kg) and immediately placed back in the activity cages. Both saline and mecamylamine injections were followed 10 min later by amphetamine injection (1 mg/kg), and locomotor activity for all mice was monitored again for 1 h. This treatment regimen lasted for three consecutive days (for the sake of comparisons to amphetamine sensitization experiments 1 and 2, see above). All mice were left undisturbed for 3 weeks (drug-free), after which they were first challenged with saline and their baseline activity monitored for 1 h, after which they were challenged with amphetamine (1 mg/kg) and their activity monitored for 1 h.
Data analysis
Activity counts were recorded and averaged (mean ± SEM) for each group. Two-way analysis of variance (ANOVA) for repeated measures was used to examine the overall effect of genotype, drug treatment, and days of treatment. In cases of significant interaction effects, it was followed by one-way ANOVA and post hoc Duncan’s range test to determine statistical significance at P<0.05. Other variables within groups were analyzed by paired Student’s t tests.
Results
Baseline activity
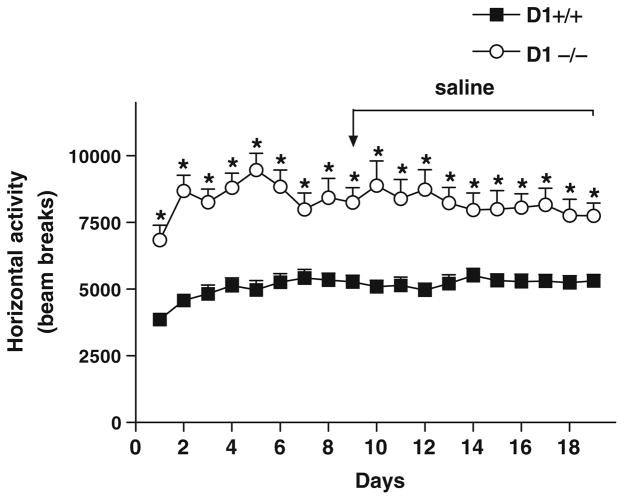
D1−/− mice maintained significantly enhanced basal locomotor activity (P<0.001) than D1+/+ mice (Fig. 3) in this experiment and throughout the course of all experiments. These results are in agreement with other studies reporting hyperactivity in D1−/− mice (Waddington et al. 1995; Karlsson et al. 2008) and further demonstrate that deletion of D1 receptors does not have a negative effect on motor functions.
Fig. 3.
Baseline locomotor activity in D1−/− mice and D1+/+ mice (n=8 per genotype) during 1-h daily sessions. Locomotor activity before and during saline treatment (days 9–19) was significantly higher (*P<0.001–0.0001) in D1−/− mice than in D1+/+ mice throughout the experiment. Values are means + SEM
Locomotor effects of amphetamine
Experiment 1: Amphetamine sensitization under variable treatment and testing regimen
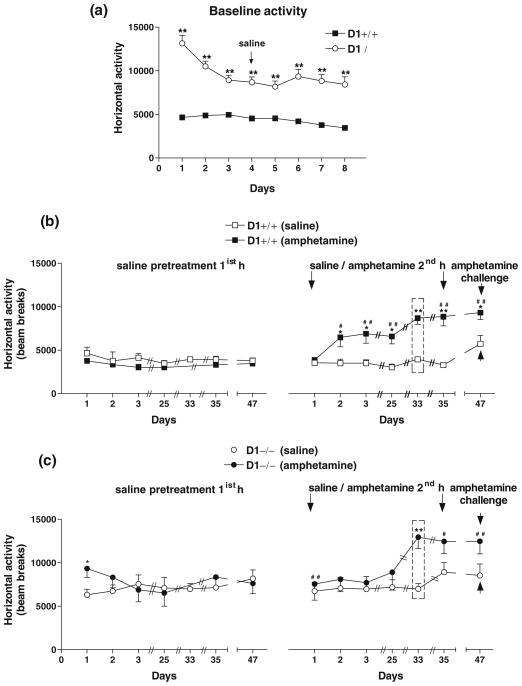
D1−/− mice exhibited significantly enhanced basal locomotor activity than D1+/+ mice (P<0.0001; Fig. 4a). Because of a significant genotype difference in baseline activity, comparisons of locomotor activity were made between saline-treated vs. amphetamine-treated groups for each genotype as shown in Fig. 4b, c.
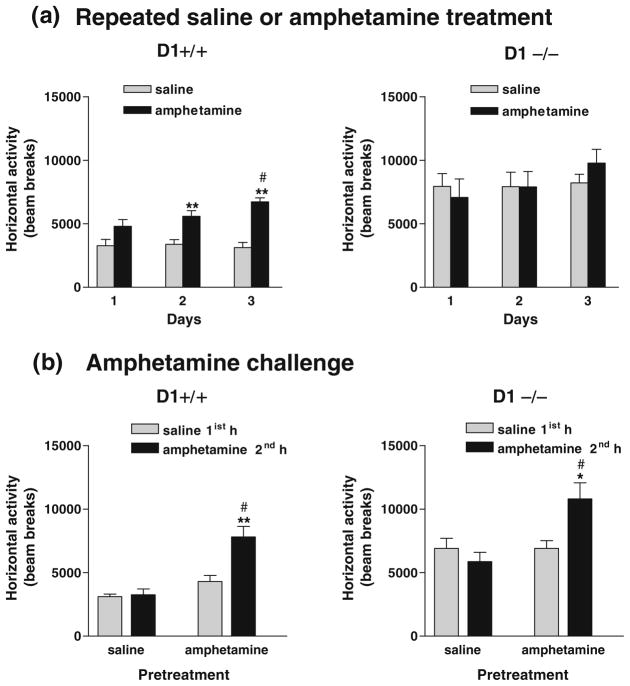
Fig. 4.
Locomotor responses to repeated amphetamine treatment in congenic D1+/+ and D1−/− mice (n=8 per genotype) recorded for 1-h daily sessions. a Baseline locomotor activity in D1−/− mice was significantly higher (**P<0.0001) than in D1+/+ mice. Repeated exposure of mice to amphetamine for three consecutive days and again after 3 weeks of abstinence (day 25) induced locomotor stimulation and sensitization in D1+/+ mice b, but not in D1−/− mice c as compared with saline-pretreated mice and their own responses to saline pretreatment (left panels). Amphetamine-induced locomotor activation was significantly enhanced in amphetamine-pretreated mice from both genotypes on day 33 when all mice were given only 1-h session following saline or amphetamine treatment without saline pre-habituation (as indicated by dashed rectangular boxes). D1+/+ mice maintained sensitized responses to amphetamine on days 35 and 47 compared to their initial response on day 1 and to saline-pretreated controls as well as to their own response to saline (left panel). D1−/− mice failed to show sensitization when compared to their saline-pretreated controls, although their response to amphetamine was significantly different from their own response to saline (left panel). *P<0.01–0.03; **P<0.002–0.0001 compared to saline-pretreated mice. #P<0.01–0.03; ##P<0.002–0.0001 compared to their responses to saline 1 h prior to amphetamine. Values are means + SEM
Acute exposure to amphetamine (1 mg/kg) had no effect on activity in both genotypes. Repeated exposure to amphetamine (1 mg/kg) for three consecutive days (days 1–3) enhanced locomotor activity in D1+/+ mice upon the second exposure (day 2) compared to saline-treated mice [F(1,14)= 7.70, P<0.01] and to their own response to saline 1 h earlier [F(1,14)=6.24, P<0.03]. These mice maintained sensitized responses upon the third exposure (day 3) vs. saline-treated mice [F(1,14)=8.35, P<0.02] and vs. their own response to saline [F(1,14)=11.09, P<0.008] and when challenged 3 weeks later (day 25) [F(1,14)=14.44, P<0.003 vs. saline-treated control mice] and [F(1,14)=16.28, P<0.002 vs. their own response to saline] (Fig. 4b). In contrast, similarly treated D1−/− mice failed to exhibit locomotor stimulation or sensitization over a 3-day period (days 1–3) and after 3 weeks of abstinence (day 25; Fig. 4c). These results indicated that D1−/− mice were either insensitive or oversensitive to the locomotor stimulating and sensitizing effects of 1 mg/kg amphetamine. Therefore, all mice were challenged with a lower dose of amphetamine (0.55 mg/kg) on day 27 (data not shown) to rule out the possibility of a ceiling effect or intense stereotypy. Exposure to 0.55 mg/kg did not produce overall locomotor stimulation in either genotype. Therefore, amphetamine treatment (1 mg/kg) was extended for two more days (days 33 and 35), followed by a challenge test with amphetamine (1 mg/kg) after 11 days of abstinence (day 47). Interestingly, on day 33, when all mice were given only one session to receive their respective treatment without prior saline pre-habituation session, responsivity to amphetamine was enhanced substantially in amphetamine-pretreated D1+/+ mice [F(1,14)=40.11, P<0.0001] and D1−/− mice [F(1,14)=16.50, P<0.002] compared to saline-treated control mice from both genotypes (Fig. 4b, c). Moreover, with extended amphetamine treatment (day 35), amphetamine-pretreated D1+/+ mice maintained enhanced responses to amphetamine compared to saline-treated mice [F(1,14)= 26.39, P<0.0002] and to their own response to saline 1 h prior to amphetamine [F(1,14)=24.21, P<0.0006], whereas D1−/− mice showed no significant difference compared to saline-pretreated mice, but maintained enhanced responses to amphetamine compared to their own response to saline 1 h prior to amphetamine [F(1,14)=6.83, P<0.03]. Amphetamine challenge after 11 days of abstinence had no significant effect on saline-pretreated mice from both genotypes. In contrast, amphetamine-pretreated D1+/+ mice maintained locomotor stimulation and sensitization compared to saline-pretreated mice [F(1,14)=8.4, P<0.01] (Fig. 4b, right panel) or compared to their own response to saline 1 h prior to amphetamine [F(1,14)=45.56, P<0.0001] (Fig. 4b, left panel), whereas amphetamine-pretreated D1−/− mice were able to express locomotor sensitization in response to amphetamine challenge (day 47) when compared to their own response to saline [F(1,14)=6.7, P<0.02] (Fig. 4c, left panel), but not when compared to saline-pretreated D1−/− mice (Fig. 4c, right panel).
Experiment 2: Amphetamine sensitization after short-term treatment (3 days) and 11 days of abstinence
AlthoughD1−/− mice did express sensitization to amphetamine under certain conditions, based on the results from the above experiment, it was not clear whether it was the treatment regimen or the period of abstinence that was important. Therefore, in order to distinguish between these possibilities, the treatment duration was kept constant (three consecutive days) but without saline pretreatment habituation, while the abstinence period was changed to 11 days instead of 3 weeks. Mice were injected with either saline or amphetamine (1 mg/kg) for three consecutive days. Amphetamine initially (day 1) caused a slight increase in horizontal activity that was not significant in D1+/+ mice, but had no effect in D1−/− mice as compared to their saline-treated controls (Fig. 5a). Following repeated amphetamine injections for two more consecutive days, overall locomotor activity was enhanced in D1+/+ mice compared to saline-treated mice as revealed by a significant effect of treatment on day 2 [F(1,14)=14.01, P< 0.0046] and day 3 [F(1,14)=52.07, P<0.0001]. In contrast, repeated amphetamine treatment did not induce locomotor stimulation or sensitization in D1−/− mice. However, when all mice were challenged with amphetamine after 11 days of abstinence, amphetamine induced locomotor stimulation in both amphetamine-pretreated D1+/+ mice [F (1,14)=22.76, P<0.0003] and D1−/− mice [F(1,14)=11.65, P <0.0042] compared to their respective saline-pretreated controls and to their own response to saline given 1 h prior to amphetamine challenge (P<0.005 and P<0.01) for D1+/+ and D1−/− mice, respectively (Fig. 5b).
Fig. 5.
Expression of amphetamine sensitization after a shorter period of abstinence. a Repeated exposure of mice (n=8 per genotype) to amphetamine (1 mg/kg) for three consecutive days induced locomotor stimulation and sensitization in D1+/+ mice, but not in D1−/− mice. **P< 0.005–0.0001 compared to saline-pretreated controls; #P<0.006 compared to day 1. b Amphetamine challenge after 11 days of abstinence had no effect in mice pretreated with saline but induced locomotor sensitization in mice pretreated with amphetamine from both genotypes. **P<0.004; *P<0.01 compared to their own responses to saline treatment, although it was less pronounced in D1−/− mice than D1+/+ mice. #P<0.006–0.0003 compared to saline-pretreated mice challenged with amphetamine. Values are means + SEM
Amphetamine-induced gene expression
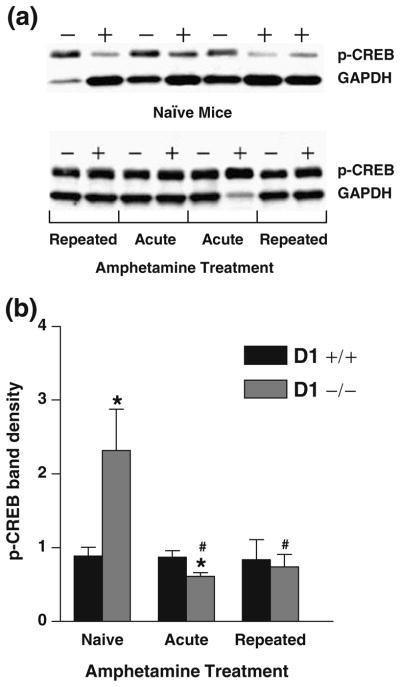
Western blot analyses indicated that in naïve mice, the basal expression level of p-CREB was significantly enhanced in D1−/− mice (P<0.02) compared to D1+/+ mice following saline injection (Fig. 6a, b).
Fig. 6.
Striatal p-CREB immunoreactivity in naïve mice treated with saline (n=12 per group) and mice challenged with amphetamine (n=8 per group). Mice pretreated with saline or amphetamine (1 mg/kg) were challenged with amphetamine after 3 weeks of abstinence and killed 2 h later. a Representative Western blot of p-CREB immunoreactive bands in naïve and amphetamine-challenged D1−/− mice (minus sign) and D1+/+ mice (plus sign). The p-CREB immunoreactive band at ~43 kDa was visualized with greater intensity in naïve D1−/− mice compared to D1+/+ mice. b Relative band intensities were quantified by densitometry and normalized to their respective GAPDH (~37 kDa) loading control and presented as means + SEM. The basal expression level of p-CREB in naïve mice was significantly higher in D1−/− mice (*P<0.02) compared to D1+/+ mice. Expression of p-CREB was significantly lower in amphetamine-treated D1−/− mice pretreated with saline (acute) or amphetamine (repeated) compared to naïve D1−/− mice (#P<0.02–0.01) or similarly treated D1+/+ mice following acute amphetamine treatment (*P<0.045)
Amphetamine challenge in mice pretreated with either saline (acute) or repeated amphetamine failed to alter the striatal expression of p-CREB in D1+/+ mice compared to their drug-naïve control mice. However, following amphetamine challenge, the level of p-CREB gene expression was significantly lowered (P<0.01) in saline-pretreated (acute) D1−/− mice vs. naïve D1−/− mice and vs. similarly treated D1+/+ mice (P<0.045) and in amphetamine-pretreated D1−/− mice (repeated) compared to saline-treated naïve D1−/− mice (P<0.02; Fig. 6b).
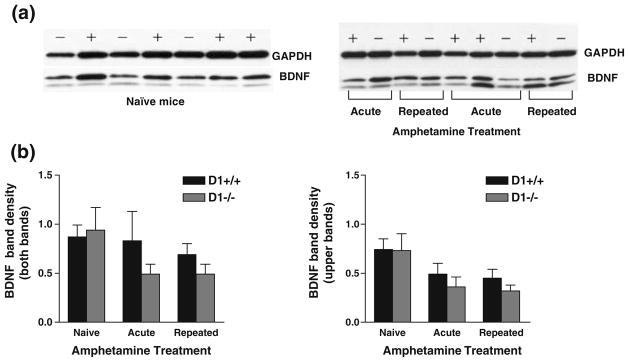
Immunoreactivity to BDNF in the striatum was visualized by Western blot analysis as doublet bands migrating together with a molecular mass of ~14 and 12 kDa (Fig. 7a) and were also observed in other brain regions including the hippocampus, hypothalamus, and frontal cortex. The lower band may correspond to different posttranslational modification of the protein or may represent a degradation product. Since the nature of the lower band is not known, quantification of band density was assessed using the upper band as well as both bands as shown in Fig. 7b.
Fig. 7.
Striatal BDNF immunoreactivity in naïve mice (n=12 per group) and mice challenged with amphetamine (n=8 per group). Mice pretreated with saline or amphetamine (1 mg/kg) were challenged with amphetamine after 3 weeks of abstinence and killed 2 h later. a Representative of Western blot of BDNF immunoreactive bands in naïve (left panel) and amphetamine-challenged (right panel) D1−/− mice - and D1+/+ mice + were visualized as doublets of 14 and 12 kDa. b Bands were quantified by densitometry and normalized to their respective GAPDH (~37 kDa) loading controls and shown as the density of both bands (left panel) or of the upper band only (right panel). No significant genotype difference in BDNF band intensity was detected in naïve mice and after acute or repeated amphetamine treatment. Values are means + SEM
Our results showed no significant genotype difference in the basal expression level of BDNF following saline injection. Also, acute or repeated amphetamine treatment did not significantly alter the expression of BDNF in either genotype; although there was a slight trend toward a lower expression level in D1−/− mice, it did not reach a statistical significance (Fig. 7a, b).
Effect of nicotine pretreatment on locomotor effects of amphetamine
Experiment 1: Prolonged nicotine pretreatment
Although D1−/− mice were able to express locomotor sensitization to amphetamine under certain conditions, they failed to develop sensitization following repeated amphetamine treatment for three consecutive days, a regimen that was sufficient to induce locomotor stimulation and sensitization in D1+/+ mice. Therefore, based on the reported role of nicotine pretreatment in potentiating the behavioral effects of amphetamine and the critical involvement of ACh in the development of behavioral sensitization, we determined whether enhanced cholinergic activity via the nicotinic receptor could enhance the locomotor effects and induction of sensitization to low doses of amphetamine that previously had no effect in D1−/− mice when given acutely or repeatedly (this study and unpublished data). In this experiment, mice from both genotypes were chronically pretreated with nicotine then were challenged with amphetamine. Repeated administration of variable doses of nicotine (ranging from 0.04 to 0.2 mg/kg) for a total of 22 treatment days failed to induce locomotor stimulation in any of the genotypes (Fig. 8), but instead caused a brief (5–10 min) locomotor depression that was apparent in both D1+/+ and D1−/− mice (data not shown).
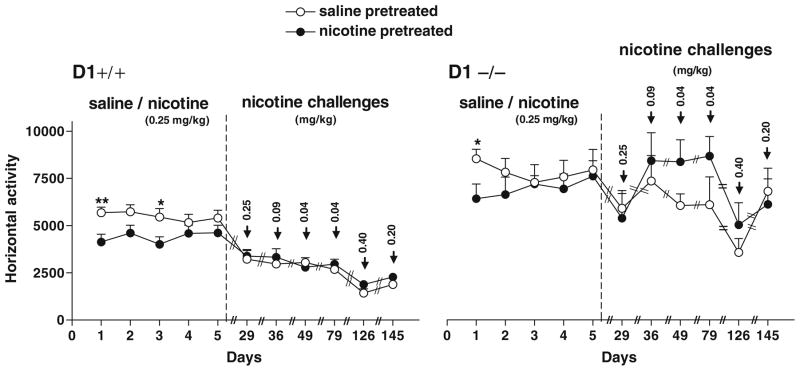
Fig. 8.
Effect of repeated nicotine treatment on locomotor activity during 1-h sessions. Repeated exposure of mice (n=8 per genotype) to either saline (saline-pretreated) or nicotine (0.25 mg/kg; nicotine-pretreated) for five consecutive days did not induce any locomotor stimulation or sensitization in both genotypes after 3 weeks of abstinence (day 29) and following subsequent challenges after additional treatments with variable doses (0.18, 0.09, 0.04, 0.4, and 0.2 mg/kg) of nicotine. *P<0.03–0.04; **P<0.0002 compared to nicotine-pretreated mice. Values are means + SEM
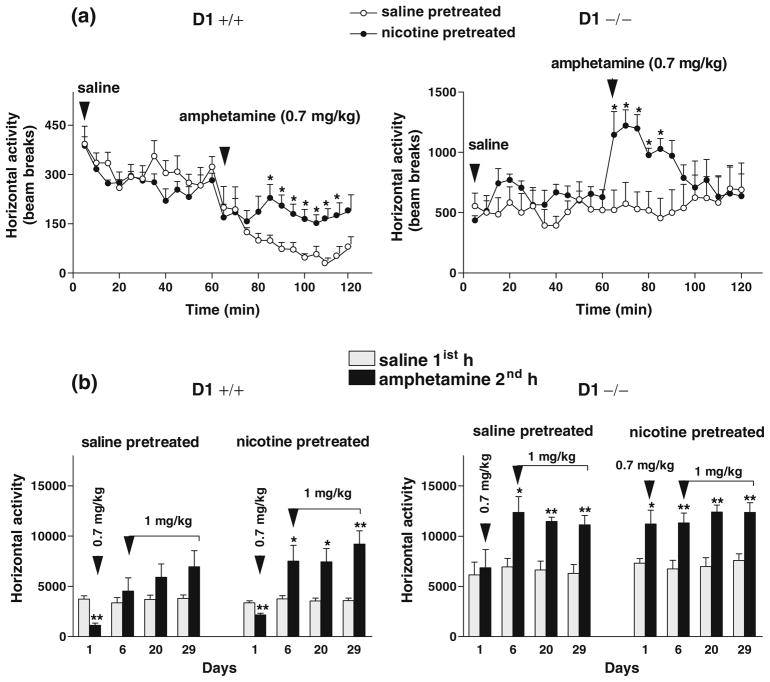
We tested whether nicotine pretreatment could enhance locomotor responses to low doses of amphetamine. Since basal activity of D1−/− mice was significantly higher than that of D1+/+ mice, comparisons were made between salinevs. amphetamine-pretreated groups from each genotype as shown in Fig. 8a, b.
Acute amphetamine (0.7 mg/kg) challenge 1 week after the last nicotine injection caused a brief (30 min) but significant locomotor stimulation in nicotine-pretreated compared to saline-pretreated D1−/− mice or to their own response to saline pretreatment (Fig. 9a). Repeated measures ANOVA detected a significant main effect of pretreatment (saline-pretreated group vs. nicotine-pretreated group) during the first 30 min following amphetamine challenge [F(1,14)=10.70, P<0.01] and a significant effect of challenge drug (saline first hour vs. amphetamine second hour) only in nicotine-pretreated D1−/− mice [F(1,14)=6.23, P< 0.04], but not in saline-pretreated D1−/− control mice. In contrast, the initial exposure to amphetamine (0.7 mg/kg) caused locomotor depression in both saline- and nicotine-pretreated groups of D1+/+ mice compared to their baseline activity following saline administration 1 h prior to amphetamine treatment (Fig. 9a). However, locomotor depression was less pronounced in the nicotine-pretreated D1+/+ mice. ANOVA detected a significant main effect of treatment (saline first hour vs. amphetamine second hour) in both saline-pretreated [F(1,14)=48.40, P=0.00] and nicotine-pretreated [F(1,14)=20.10, P<0.0001] groups of D1+/+ mice as well as a significant main effect of pretreatment (saline-pretreated vs. nicotine-pretreated groups) [F(1,14)=16.10, P<0.002]. In addition, further challenges with amphetamine (1 mg/kg) three more times (days 6–29), spaced by 1–2 weeks of abstinence, caused locomotor stimulation and sensitization to a larger extent in nicotine-pretreated than saline-pretreated D1+/+ mice. ANOVA detected a significant main effect of treatment (saline first hour vs. amphetamine second hour) in nicotine-pretreated D1+/+ mice [F(1,14)=11.13, P<0.006], but not in saline-pretreated D1+/+ mice (Fig. 9b), whereas both saline-and nicotine-pretreated groups of D1−/− mice exhibited (to a similar extent) significantly enhanced responses to repeated amphetamine challenges (days 6–29) vs. their own responses to saline 1 h earlier [F(1,14)=20.15, P<0.002] and [F(1,14)=33.83, P<0.0004], respectively (Fig. 9b). It is important to note that saline-pretreated mice in both genotypes were not naïve to nicotine treatment since they were intermittently challenged six times with variable doses of nicotine (see diagram in Fig. 2).
Fig. 9.
Effect of prolonged nicotine pretreatment on locomotor responses to amphetamine during 1-h sessions. a Acute exposure of mice (n=8 per genotype) to amphetamine (0.7 mg/kg, day 1) 1 week after the last nicotine treatment caused less locomotor depression in nicotine-pretreated than saline-pretreated D1+/+ mice. In contrast, amphetamine caused a brief (30 min) but significant locomotor stimulation in nicotine-pretreated but not saline-pretreated D1−/− mice. *P<0.03–0.002 compared to saline-pretreated mice. b Total activity scores during acute and repeated amphetamine treatment for three non-consecutive days (days 6, 20, and 29) caused locomotor stimulation and sensitization to a larger extent in nicotine-pretreated compared to saline-pretreated D1+/+ mice. However, D1−/− mice pretreated with saline or nicotine similarly exhibited significantly enhanced responses to amphetamine treatment. *P<0.05–0.01; **P< 0.008–0.0008 compared to saline-pretreated mice. Values are means + SEM
Experiment 2: Effect of nicotine receptor blockade on amphetamine sensitization
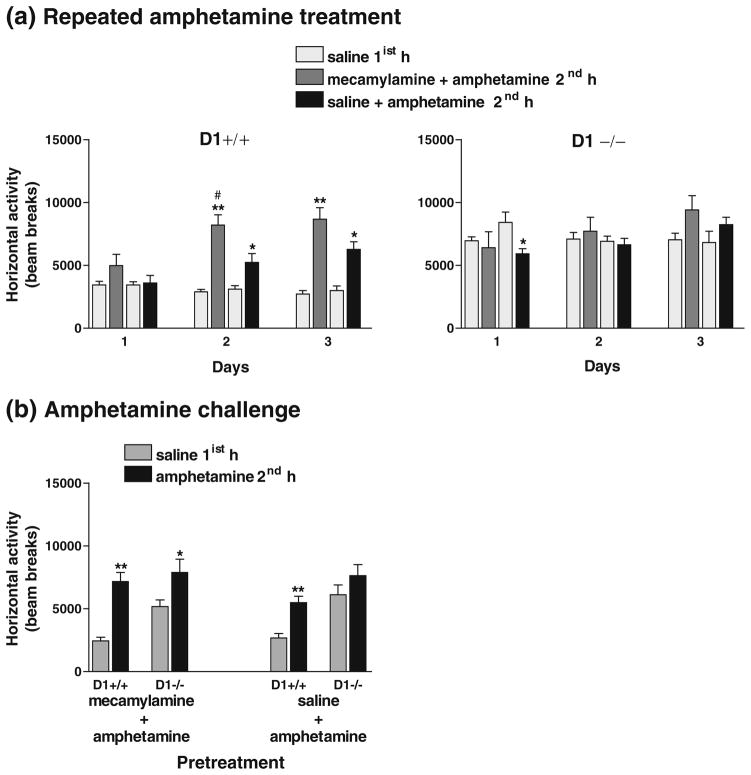
In another effort to further investigate the contribution of the cholinergic system to behavioral sensitization to amphetamine, we tested whether blockade of the nicotinic receptor by mecamylamine during amphetamine treatment would attenuate the induction of sensitization at least in the D1+/+ mice. Following saline treatments for 4 days, D1−/− mice maintained significantly enhanced basal locomotor activity than D1+/+ mice (P<0.0001). On day 1, overall activity in D1+/+ mice was not significantly altered following the co-administration of mecamylamine and amphetamine [although there was a significant increase (P<0.02) in activity during the last 20 min] or co-administration of saline and amphetamine compared to their baseline activity following saline pretreatment 1 h prior to drug treatments (Fig. 10a). In contrast, overall activity of D1−/− mice was not significantly altered following co-administration of mecamylamine and amphetamine, whereas their activity following co-administration of saline and amphetamine was significantly reduced only during the last 20 min [F(1,10)=7.23, P<0.02] compared to their baseline activity following saline pretreatment (Fig. 10a).
Fig. 10.
Effect of co-administration of mecamylamine and amphetamine or saline and amphetamine on locomotor activity in mice (n=6 per group) during 1-h sessions. a Repeated amphetamine treatments for three consecutive days caused greater augmentation of locomotor activity in D1+/+ mice (P<0.0001) when co-administered with mecamylamine than with saline (P<0.02–0.01), whereas D1−/− mice failed to show any response to either treatment during days 1–2 and exhibited a marginal increase in locomotor activity (P=0.08) on day 3 when amphetamine was co-administered with mecamylamine than with saline. b Amphetamine challenge after 3 weeks of abstinence induced locomotor sensitization in both D1+/+ and D1−/− mice pretreated with mecamylamine + amphetamine and only in D1+/+ mice (but not D1−/− mice) pretreated with saline + amphetamine. *P<0.05–0.001; **P<0.0001 compared to their responses to saline 1 h earlier. #P<0.02 compared to saline + amphetamine treatment. Values are means + SEM
Following the second exposure to amphetamine (day 2), D1+/+ mice exhibited augmented locomotor activity following co-administration of either mecamylamine and amphetamine [F(1,10)=39.30, P<0.0001] or saline and amphetamine [F(1,10)=7.94, P<0.02]. ANOVA detected a significant main effect of treatment (mecamylamine + amphetamine vs. saline + amphetamine) on overall activity in D1+/+ mice [F(1,10)=7.52, P<0.02] (Fig. 10a). Post hoc analysis indicated that amphetamine-induced locomotor stimulation was greatly enhanced by its co-administration with mecamylamine than with saline. In contrast, D1−/− mice failed to show any response to amphetamine with any of these treatment combinations (Fig. 10a). On day 3 of repeated amphetamine treatment, D1+/+ mice maintained sensitized locomotor responses following co-administration of mecamylamine and amphetamine [F(1,10)=39.33, P<0.0001] or co-administration of saline and amphetamine [F(1,10)=20.93, P<0.001] compared to their activity following saline pretreatment. Moreover, analysis of horizontal activity every 5 min revealed a significant enhancement during the last 20 min of the session following co-administration of mecamylamine and amphetamine compared to co-administration of saline and amphetamine in D1+/+ mice [F(1,10)=4.85, P<0.05]. In contrast, overall activity of D1−/− mice was not significantly increased following either of the two treatment combinations, although there was a trend towards a higher activity in these mice following co-administration of mecamylamine and amphetamine (Fig. 10a). Moreover, when challenged with amphetamine after 3 weeks of abstinence, both D1+/+ and D1−/− mice pretreated with mecamylamine and amphetamine exhibited significantly enhanced locomotor activity compared to their baseline activity following saline treatment, although it was less pronounced in D1−/− mice [F(1,10)=5.19, P<0.046] than D1+/+ mice [F(1,10)= 35.57, P<0.0001] (Fig. 10b). Sensitized responses to amphetamine was apparent only in D1+/+ mice pretreated with saline and amphetamine [F(1,10) = 21.62, P<0.001], but not in similarly treated D1−/− mice (Fig. 10b).
Discussion
This study highlights two main findings. Firstly, although sensitization to a low dose of amphetamine is severely blunted in D1−/− mice, it can be expressed in these mice under certain conditions depending on the duration of treatment and the abstinence period. Secondly, the inability of D1−/− mice to develop amphetamine sensitization, after short-term repeated treatment, could be reversed by nicotine pretreatment.
We have demonstrated that induction of behavioral sensitization following short-term repeated exposure to amphetamine (1 mg/kg) and its expression after 3 weeks of abstinence was significantly impaired in D1−/− mice. However, the expression of sensitization to amphetamine in these mice was facilitated by several manipulations including prolonged intermittent treatment and/or abstinence periods shorter than 3 weeks. Furthermore, the basal level of expression of p-CREB was significantly upregulated in D1−/− mice compared to D1+/+ mice and was significantly lowered by repeated amphetamine treatment, whereas no significant changes were detected in the expression of BDNF in either of the genotypes under basal or amphetamine treatment conditions. Most importantly, we report for the first time that nicotine pretreatment augmented locomotor responses in D1+/+ mice and reversed the insensitivity of D1−/− mice to low doses of amphetamine.
Locomotor effects of amphetamine
We have shown that short-term (3 days) repeated exposure to amphetamine (1 mg/kg) produced significant locomotor stimulation and sensitization during the induction phase and following 3 weeks of abstinence in D1+/+ mice but not in D1−/− mice. Thus, under these conditions, absence of the dopamine D1 receptor significantly impaired development and expression of sensitized responses to amphetamine in D1−/− mice and suggests that the dopamine D1 receptor contributes to overall locomotor activity induced by amphetamine upon short-term exposure. A similar conclusion was reported in dopamine D2 receptor-deficient mice with reduced stimulatory effects of acute methamphetamine but enhanced sensitization comparable to wild-type mice when treated every 48 h for 4 days and challenged 2 days later (Kelly et al. 2008). It was concluded that the dopamine D2 receptor acts in concert with D1-like receptors in the mechanisms underlying methamphetamine stimulation and sensitization, although the D1 receptor serves a more critical function than the D2 receptor in the acquisition phase. Recently, we have provided evidence that D1 and D2 receptors function together in a hetero-oligomeric complex to generate a novel signaling pathway that elevates intracellular calcium levels when co-activated (Lee et al. 2004; So et al. 2005; Rashid et al. 2007). Therefore, we postulate that sensitization to amphetamine, under conditions of short-term exposure and long abstinence period, may result from activation of the D1–D2 hetero-oligomer, so lacking one constituent receptor could have altered amphetamine-induced locomotor stimulation and sensitization.
Having established that D1−/− mice failed to develop and express sensitization compared to D1+/+ mice in our study (up to day 25), we investigated whether different experimental variables including a lower dose amphetamine challenge, pre-habituation influence on motor responses, additional amphetamine treatments, or shorter abstinence periods would enhance responsiveness of D1−/− mice to amphetamine. Further challenge with a lower dose of amphetamine (0.55 mg/kg) in mice pretreated with amphetamine (1 mg/kg) did not produce any stimulant or sensitizing effect in any of the groups from both genotypes. Others have shown that challenge with amphetamine 0.5 mg/kg produced sensitizing effects only after 28 days (but not 2 and 7 days) of abstinence in animals pretreated with escalating doses of amphetamine (1 to 10 mg/kg; Paulson and Robinson 1995). This evidence indicates that the abstinence period and the challenge dose following amphetamine pretreatment are critical variables that must be considered in studying behavioral sensitization.
Having established that a lower dose challenge was ineffective in both genotypes, we extended the treatment for two more days before a final challenge. At this phase, we also tested whether the pre-habituation period following saline injection played a role in dampening their motor responses to amphetamine especially in D1−/− mice. Interestingly, following a direct treatment with amphetamine without saline pretreatment habituation (day 33, first hour), locomotor responses of both D1+/+ and D1−/− mice to amphetamine were enhanced (compared to saline-treated mice and to their own responses on day 25), although less pronounced in D1−/− mice. Enhanced locomotor responses were maintained in both genotypes when treated with amphetamine 1 day later (day 35) and when challenged after 11 days of abstinence. Therefore, under certain conditions, be it enhanced stress reaction as a result of a direct exposure to amphetamine without saline prehabituation, more intermittent treatment, or shorter abstinence periods, D1−/− mice were able to express sensitized responses to the same dose that previously failed to induce any effect in them after 3 weeks of abstinence. However, at this point, it was not clear which variable(s) had contributed to sensitized responses in these mice. Three explanations could be offered for the enhanced activity in D1−/− mice on day 33 and thereafter. First, it is important to note that D1−/− mice have basal hyperactivity (see Fig. 3) and all mice were tested during the dark period of the light cycle; therefore, it is possible that exposure to amphetamine, without the usual pre-habituation to the environment and injection procedure, might have caused an enhanced stress response that is ultimately expressed as increased locomotor activity, thereby exaggerating responses to amphetamine in amphetamine-pretreated mice from both genotypes. However, based on our results from experiment 2, this possibility seems unlikely since D1−/− mice treated with amphetamine without prehabituation failed to develop locomotor stimulation or sensitization following repeated treatment for three consecutive days. The notion that the impact of the change in treatment regimen (as happened on day 33) vs. implementing it from the start might not be the same warrants further investigation. Second, a previous challenge with 0.55 mg/kg amphetamine on day 27 (see diagram in Fig. 1) might have produced a sensitizing effect that was apparent only after 5 days of abstinence (day 33 but not day 27) in animals pretreated with amphetamine (1 mg/kg), thereby exaggerating the effects of subsequent amphetamine treatment on day 33 in both genotypes. However, the effect of initial exposure to a lower dose (day 1) might not be the same as following amphetamine pretreatment (day 27); therefore, further investigation is needed to directly assess this possibility and to determine if priming with a higher dose of amphetamine followed by a low-dose challenge 5–48 h later facilitates induction of behavioral sensitization in D1−/− mice. Third, the most likely explanation is accumulated shorter periods of abstinence between treatments. Taken together, these results suggest that although induction of sensitization was impaired in D1−/− mice, they were eventually able to express sensitized responses (to a less extent than in D1+/+ mice) only under certain conditions. This conclusion is supported by a recent study showing evidence of a partial sensitization to cocaine in D1−/− mice and suggesting that these mice can exhibit locomotor sensitization under certain conditions (Karlsson et al. 2008). Additional studies will be required to determine if prolonged intermittent treatment during the acquisition phase facilitates induction of behavioral sensitization in D1−/− mice.
Based on the above results, we sought to determine whether the ability of D1−/− mice to express sensitization was due to prolonged intermittent treatment or a shorter abstinence period. By maintaining short-term treatment and changing the abstinence period to 11 days instead of 3 weeks, we have shown that although D1−/− mice failed to develop sensitization to amphetamine during three consecutive days of treatment, they eventually expressed locomotor sensitization when challenged after 11 days of abstinence. This suggests that three consecutive days of amphetamine treatment was enough to induce some sort of adaptation that was short-lived and maintained after 11 days (but not 3 weeks) of abstinence. Since D1−/− mice were shown to express sensitization after a short abstinence period, it suggests that the D1 receptor system is not the only system that mediates locomotor responses to amphetamine. Indeed, it has been shown that the VTA is the primary anatomical site involved in initiation of sensitization, and many studies have reported that psychostimulant-induced adaptations in the VTA are transient (Wolf 1998; Vanderschuren and Kalivas 2000; Licata and Pierce 2003) and are evident at short withdrawal times, whereas those occurring in the nucleus accumbens are more persistent and in some cases are only detectable after longer withdrawals (Wolf et al. 1993, 1994). Thus, our data may suggest that the neuroadaptation occurring after a short withdrawal period could be transient and may involve different neuronal system(s) other than the dopamine D1 receptor system. This is supported by studies that have found a significant increase in D2L mRNA in the VTA and dorsal striatum of C57BL/6J mice pretreated with amphetamine that positively correlated with their sensitized locomotor responses following 14 days of abstinence (Giordano et al. 2006).
Taken together, the findings from the above two experiments suggest that experimental variables related to frequency and duration of treatment and most importantly the length of the abstinence period were determining factors in the expression of amphetamine-induced sensitization in D1−/− mice. Nevertheless, taken at face value, the data suggest that in the absence of D1 receptor-mediated effects, any of these variables was sufficient to induce limited neuroadaptation, possibly via the D2-like receptor or other neurotransmitter systems. However, there are two scenarios that could be proposed based on our data. First, induction of sensitization to low doses of amphetamine is critically dependent on D1 receptor-mediated neuroadaptation, so lacking the D1 receptor would impact the development of sensitization. Second, expression of sensitization could involve either D1- or D2-like receptors or both, as well as other systems depending on the treatment regimen and duration of abstinence. We infer that the D1 receptor signaling pathways, particularly in the nucleus accumbens, may play a prominent and persistent role in the acquisition and expression of amphetamine-induced sensitization following short-term amphetamine treatment (three consecutive days) and after a long drug withdrawal period (3 weeks). Sensitization following prolonged intermittent treatment or a short abstinence period may involve other mechanisms acting in concert with the D1 receptor so that absence of this receptor creates a shift towards dependence on these other mechanisms.
Amphetamine-induced gene expression
Prolonged exposure to amphetamine and cocaine has been shown to regulate the expression of several genes via striatal D1 receptor mechanisms including transcription factors such as p-CREB (Konradi et al. 1994). An important role of D1 receptor-mediated CREB phosphorylation appears to mediate adaptation to psychostimulant drugs in rat striatum (Konradi et al. 1994) and long-term neuroadaptive changes associated with drug dependence. In this study, having established that D1−/− mice exhibited attenuated responses to amphetamine during the acquisition phase, we investigated whether this deficit is caused by alteration in specific D1 receptor-mediated expression of these genes. In naïve mice, the striatal basal expression level of p-CREB was significantly higher in D1−/− mice compared to D1+/+ mice. This could represent a developmental adaptation to the genetic ablation of D1 receptors in these mice. Indeed, we have shown that drug-naïve D1−/− mice had higher basal levels of dopamine and its metabolite DOPAC in the midbrain region than their D1+/+ controls (El-Ghundi et al. 1998). A recent study showed evidence consistent with increased glutamatergic neurotransmission in D1−/− mice (Rodrigues et al. 2007). Therefore, higher basal pCREB in the striatum of D1−/− mice may correlate with increased glutamatergic or dopaminergic activity and hence increased phosphorylation of CREB. The fact that D1−/− mice have enhanced basal locomotor activity in our study could also support this conclusion. Furthermore, following amphetamine challenge, striatal p-CREB expression was downregulated in D1−/− mice. This could be explained by changes mediated by D2-like receptor mechanisms or other neurotransmitter systems in the absence of the D1-mediated influence. No significant changes in pCREB levels were detected after amphetamine challenge in D1+/+ mice pretreated with saline or amphetamine. Previous studies in rats indicated enhancement of striatal pCREB expression following acute amphetamine (Konradi et al. 1994; Cole et al. 1995; Choe et al. 2002; Choe and Wang 2002) or amphetamine challenge in sensitized rats pretreated with a high-dose but not low-dose amphetamine (McPherson et al. 2007). Based on these findings, our results may suggest either that the pretreatment dose of amphetamine was not high enough to enhance the pCREB gene expression in D1+/+ mice or that there may be a differential time course of CREB activation and that it had partly recovered 2 h following treatment. Alternatively, it may be that expression of sensitization is less dependent upon striatal pCREB levels than during the development of sensitization.
Several neurotrophic factors have also been shown to modulate the induction and expression of psychostimulant-induced sensitization. In this study, we found that both genotypes had similar basal levels of BDNF expression in the striatum and amphetamine did not alter its expression in these mice. These results suggest that striatal BDNF level did not appear to contribute to amphetamine-induced locomotor effects nor was it impacted by dopamine D1 receptor deletion. However, differences in regional expression of BDNF could have occurred but may have not been detected in this study.
Effect of nicotine pretreatment and nicotine receptor blockade on locomotor effects of amphetamine
The contribution of the cholinergic system to amphetamine-induced locomotor sensitization was of a particular interest since numerous studies in rats have shown that ACh is critical for the development of behavioral sensitization (Heidbreder and Shippenberg 1996; Schoffelmeer et al. 2002) and that D1 receptor agonists and amphetamine increase, whereas D2 agonists decrease ACh overflow in the striatum (DeBoer and Abercrombie 1996; Acquas et al. 1997; Keys and Mark 1998). Therefore, based on our results providing evidence of lack of amphetamine-induced locomotor stimulation and sensitization in D1−/− mice during the acquisition phase (days 1–3), we postulated that the reduced responsiveness to amphetamine in D1−/− mice in our study could relate to reduced D1 receptor-mediated striatal ACh release. To test this hypothesis, we sought to directly activate cholinergic neurotransmission and circumvent the absence of the D1 receptor. Here, we report for the first time that prolonged nicotine pretreatment enhanced and therefore reversed the blunted locomotor responses of D1−/− mice to low doses of amphetamine. In this paradigm, both D1+/+ and D1−/− mice that were pretreated with variable doses of nicotine did not show nicotine-induced locomotor stimulation over the course of treatment or when challenged with nicotine after 3 weeks of abstinence, but instead exhibited brief locomotor depression. Our results are in contrast with the reported locomotor stimulation and sensitization following repeated nicotine treatment in rats (Schoffelmeer et al. 2002) and therefore might suggest species differences or that extended daily treatment with higher doses is needed for adaptive changes to occur in these mice. Nicotine-induced locomotor depression observed in this study could be due to excessive ACh release or, alternatively, the result of nicotine stimulating GABAergic receptors or nicotinic cholinergic receptors located on the presynaptic dopaminergic terminals to transiently increase extracellular dopamine, thereby stimulating D2 autoreceptors to result in subsequent inhibition of dopamine release. Although repeated nicotine pretreatment failed to produce locomotor sensitization in both genotypes, it augmented the acute locomotor effects of a low dose (0.7 mg/kg) of amphetamine in nicotine-pretreated mice from both genotypes relative to their saline-pretreated controls. However, these effects were more pronounced in D1−/− mice. This was demonstrated by the fact that in mice pretreated with saline, the initial exposure to amphetamine (0.7 mg/kg) produced either locomotor depression in D1+/+ mice or no effect in D1−/− mice. However, in nicotine-pretreated mice, this dose of amphetamine caused locomotor stimulation in D1−/− mice that lasted for about 30 min and attenuated the locomotor depression in D1+/+ mice compared to their corresponding control groups. To validate that the enhancing effect of nicotine pretreatment on amphetamine was real and not an artifact, we then intentionally employed a regimen of amphetamine treatment that does not normally induce significant sensitization since the sessions were spaced weeks apart. Surprisingly, exposure to a second challenging dose of amphetamine (1 mg/kg) 5 days later enhanced locomotor activity in both saline- and nicotine-pretreated D1−/− mice to a similar extent, whereas the extent of locomotor stimulation was higher in nicotine-pretreated than in saline-pretreated D1+/+ mice. Therefore, these results suggest that nicotine pretreatment had indeed enhanced the responses to amphetamine in both genotypes, although to a greater extent in the D1−/− mice. Furthermore, these sensitized responses were maintained in both genotypes during subsequent intermittent amphetamine treatments. It is important to note that saline-pretreated groups were not naïve to nicotine since they were also intermittently challenged (six times) with nicotine over the course of the experiment (see diagram in Fig. 2). However, enhanced locomotor responses to amphetamine in D1−/− mice, but not D1+/+ mice, pretreated with saline but intermittently tested with nicotine could reflect different cellular adaptations to nicotine exposure in the presence and absence of D1 receptors.
These results suggest that either intermittent or prolonged nicotine administration was sufficient to cause some long-lasting neuroadaptive changes in D1−/− mice that enhanced their responses to subsequent amphetamine treatment, whereas only prolonged nicotine pretreatment enhanced amphetamine-induced locomotor stimulation in D1+/+ mice. However, it seems intriguing that despite the lack of nicotine-induced locomotor sensitization, pretreatment with this drug was sufficient to enhance the locomotor stimulant effects of a low dose of amphetamine in both D1+/+ and D1−/− mice. Previous studies reported that psychostimulant-induced locomotor sensitization is associated with an increase in ACh release from rat nucleus accumbens slices (Vanderschuren et al. 1999) and upregulation of the endogenous activation of nicotinic receptors (de Rover et al. 2004). Moreover, nicotine may stimulate the release of several neurotransmitters in various brain regions including ACh, dopamine, serotonin, GABA, glutamate, and norepinephrine (Gray et al. 1996; Wonnacott 1997; Fedele et al. 1998; Lu et al. 1998; Forster and Blaha 2000; Wu et al. 2000). One possible mechanism by which nicotine may influence the locomotor effects of amphetamine is through modulating glutamate neurotransmission. For example, acute stimulation of central nicotinic ACh receptors has been shown to enhance the release of glutamate and dopamine in the nucleus accumbens (McGhee et al. 1995; Reid et al. 2000) and that NMDA and D1 receptor antagonists prevent the development of sensitization to psychostimulants and opiates (Vezina 1996; Wolf 1998; Vanderschuren and Kalivas 2000). Therefore, in the absence of D1 receptor, one can postulate that prolonged direct manipulation of nicotinic–cholinergic activity could have caused a long-lasting neuroadaptation in glutamatergic neurotransmission that eventually enhanced locomotor effects of amphetamine, thereby bypassing the direct involvement of the D1 receptor in behavioral sensitization to amphetamine.
It has been shown that amphetamine-induced behavioral sensitization in rats can be blocked by the nicotinic antagonist mecamylamine (Karler et al. 1996; Schoffelmeer et al. 2002). Therefore, we hypothesized that if nicotinic ACh receptors were involved in the induction of sensitization to amphetamines, then concomitant administration of the nicotinic receptor antagonist mecamylamine with amphetamine would attenuate amphetamine-induced sensitization. However, we found that co-administration of mecamylamine and amphetamine actually enhanced amphetamine-induced sensitization during repeated treatment only in D1+/+ mice and after 3 weeks of abstinence in both genotypes. This seemed paradoxical to our conclusion that nicotine pretreatment had led to enhanced sensitivity to amphetamine in both genotypes; however, several explanations could be offered. Since we found that nicotine caused locomotor depression in mice, this suggests that nicotine is exerting an inhibitory effect on locomotion either directly through increased ACh release or indirectly through GABAergic activation (Lu et al. 1998; Forster and Blaha 2000). Therefore, blockade of the nicotinic receptor would relieve the inhibition and hence augment the behavioral effects of amphetamine. In support of this hypothesis, mecamylamine has been found to prevent nicotine-induced locomotor depression in mice (Tritto et al. 2004) and rats (Clarke and Kumar 1983; Stolerman et al. 1995). In a preliminary experiment, we found that when nicotine is co-administered with amphetamine, it actually reduces the overall effect of amphetamine initially, and when a lower dose of mecamylamine (1 mg/kg) was given in combination with amphetamine, it enhanced the overall effect of amphetamine up to 70%, even though mecamylamine had no significant effect on activity when administered alone (data not shown). These data are supported by previous studies (Kuribara 1999) reporting that nicotine (0.03–1 mg/kg) dose-dependently reduced the progressive locomotor stimulation of methamphetamine. Further literature review revealed that there were both stimulant and depressant components to the effect of mecamylamine that are dose-dependent. For example, smaller doses of mecamylamine alone could significantly stimulate certain behaviors in rats to the same degree as nicotine (Driscoll and Bättig 1973a), whereas a depressant effect has been demonstrated with higher doses (Oliverio 1966; Driscoll and Bättig 1973b; Rodgers 1979). In addition, peripheral effects of mecamylamine have been shown to mimic the actions of nicotine (Stolerman et al. 1973; Driscoll 1976).
In summary, we have provided clear evidence that induction of locomotor sensitization to amphetamine was attenuated by the deletion of the dopamine D1 receptor, whereas expression of amphetamine sensitization could be elicited under certain experimental conditions. This suggests that D1 receptors were critical for the development of behavioral sensitization, but may not be necessary for the expression of this phenomenon. The manifestation of amphetamine sensitization depended on the frequency of treatment and length of the withdrawal period. Nicotine pretreatment enhanced acute locomotor stimulant and sensitizing effects of amphetamine in both genotypes. Therefore, blunted responsiveness to amphetamine in D1−/− mice during the acquisition phase could be reversed by prolonged or intermittent nicotine pretreatment. We further showed that lack of dopamine D1 receptors had led to an adaptive increase in basal expression of pCREB in striatum. The present series of studies have highlighted the importance of intermittent vs. continuous drug exposure regimens and the effectiveness of shorter vs. longer periods of drug abstinence in the expression of behavioral sensitization in D1−/− mice. Although these experimental manipulations have contributed to the augmentation of locomotor responses to amphetamine in D1−/− mice, the consequences of direct modulation of the cholinergic system via nicotinic receptor activation on amphetamine-induced sensitization represent a novel and major finding that has great significance in addressing and understanding not only the neurochemical mechanisms underlying behavioral sensitization to amphetamine but also the possible relationship between the dopamine D1 receptor and the cholinergic system in modulating the development of sensitization to amphetamine. These findings and their implications may provide key insights into the role of dopamine and dopamine D1 receptors in the potential for psychostimulant drug abuse.
Acknowledgments
The authors wish to thank Dr. John Drago, Dr. David Sibley, and Dr. Heiner Westphal for generously providing the D1 receptor knockout mice. This work was supported by the Canadian Institutes of Health Research and the National Institute on Drug Abuse. SRG is the recipient of a Canada Research Chair in Molecular Neuroscience from the CIHR.
Contributor Information
Mufida B. El-Ghundi, Department of Pharmacology, University of Toronto, Medical Sciences Building, 1 King’s College Circle, Toronto, ON, Canada M5S 1A8
Theresa Fan, Department of Pharmacology, University of Toronto, Medical Sciences Building, 1 King’s College Circle, Toronto, ON, Canada M5S 1A8.
Joanna M. Karasinska, Department of Pharmacology, University of Toronto, Medical Sciences Building, 1 King’s College Circle, Toronto, ON, Canada M5S 1A8
John Yeung, Department of Pharmacology, University of Toronto, Medical Sciences Building, 1 King’s College Circle, Toronto, ON, Canada M5S 1A8.
Millee Zhou, Department of Pharmacology, University of Toronto, Medical Sciences Building, 1 King’s College Circle, Toronto, ON, Canada M5S 1A8.
Brian F. O’Dowd, Department of Pharmacology, University of Toronto, Medical Sciences Building, 1 King’s College Circle, Toronto, ON, Canada M5S 1A8. Centre for Addiction and Mental Health, Toronto, ON, Canada M5T 1R8
Susan R. George, Email: george@utoronto.ca, Department of Pharmacology, University of Toronto, Medical Sciences Building, 1 King’s College Circle, Toronto, ON, Canada M5S 1A8. Department of Medicine, University of Toronto, Medical Sciences Building, 1 King’s College Circle, Toronto, ON, Canada M5S 1A8. Centre for Addiction and Mental Health, Toronto, ON, Canada M5T 1R8. Department of Pharmacology and Medicine, University of Toronto, Medical Sciences Building, Room 4358, 1 Kings College Circle, Toronto, ON, Canada M5S 1A8
References
- Acquas E, Wilson C, Fibiger HC. Nonstriatal dopamine D1 receptors regulate striatal acetylcholine release in vivo. J Pharmacol Exp Ther. 1997;281:360–368. [PubMed] [Google Scholar]
- Bickerdike MJ, Abercrombie ED. Striatal acetylcholine release correlates with behavioral sensitization in rats withdrawn from chronic amphetamine. J Pharmacol Exp Ther. 1997;282:818–826. [PubMed] [Google Scholar]
- Birrell CE, Balfour DJ. The influence of nicotine pretreatment on mesoaccumbens dopamine overflow and locomotor responses to D-amphetamine. Psychopharmacology. 1998;140:142–149. doi: 10.1007/s002130050751. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: an [11C] raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Choe ES, Wang JQ. CaMKII regulates amphetamine-induced ERK1/2 phosphorylation in striatal neurons. NeuroReport. 2002;13:1013–1016. doi: 10.1097/00001756-200206120-00006. [DOI] [PubMed] [Google Scholar]
- Choe ES, Chung KT, Mao L, Wang JQ. Amphetamine increases phosphorylation of extracellular signal-regulated kinase and transcription factors in the rat striatum via group I metabotropic glutamate receptors. Neuropsychopharmacology. 2002;27:565–575. doi: 10.1016/S0893-133X(02)00341-X. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Kumar R. The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. B J Pharmacol. 1983;78:329–337. doi: 10.1111/j.1476-5381.1983.tb09398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RL, Konradi C, Douglass J, Hyman SE. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14:813–823. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CA, Drago J, Watson JB, Levine MS. Effects of repeated amphetamine treatment on the locomotor activity of the dopamine D1A-deficient mouse. NeuroReport. 1997;8:2523–2527. doi: 10.1097/00001756-199707280-00021. [DOI] [PubMed] [Google Scholar]
- DeBoer P, Abercrombie ED. Physiological release of striatal acetylcholine in vivo: modulation by D1 and D2 dopamine receptor subtypes. J Pharmacol Exp Ther. 1996;277:775–783. [PubMed] [Google Scholar]
- de Rover M, Mansvelder HD, Lodder JC, Wardeh G, Schoffelmeer ANM, Brussaard A. Long-lasting nicotinic modulation of GABAergic synaptic transmission in the rat nucleus accumbens associated with behavioral sensitization to amphetamine. Eur J NeuroSci. 2004;19:2859–2870. doi: 10.1111/j.0953-816X.2004.03370.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago J, Gerfen CR, Lachowicz JE, Steiner H, Hollon TR, Love PE, Ooi GT, Grinberg A, Lee EJ, Huang SP, Bartlettii PF, Jose PA, Sibley DR, Westphal H. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci USA. 1994;91:12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll P. Nicotine-like behavioral effect after small doses of mecamylamine in Roman high-avoidance rats. Psychopharmacologia. 1976;46:119–121. doi: 10.1007/BF00421559. [DOI] [PubMed] [Google Scholar]
- Driscoll P, Bättig K. Cigarette smoke and behavior: some recent developments. Rev Environ Health. 1973a;1:113–133. [Google Scholar]
- Driscoll P, Bättig K. The effect of small doses of mecamylamine on shuttlebox behavior in the guinea-pig. Experientia. 1973b;29:991–993. doi: 10.1007/BF01930423. [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, Fletcher PJ, Drago J, Ashby P, O’Dowd BF, George SR. Dopamine D1 knockout mice are less sensitive to the locomotor effect of alcohol and amphetamine. Society for Neuroscience Abstract 165.15 1997 [Google Scholar]
- El-Ghundi M, George SR, Drago J, Fletcher PJ, Fan T, Nguyen LC, Sibley DR, Westphal H, O’Dowd BF. Disruption of dopamine D1 receptor gene expression attenuates alcohol-seeking behavior. Eur J Pharmacol. 1998;353:149–158. doi: 10.1016/s0014-2999(98)00414-2. [DOI] [PubMed] [Google Scholar]
- Fedele E, Varnier G, Ansaldo MA, Raiteri M. Nicotine administration stimulates the in vivo N-methyl-D-aspartate receptor/nitric oxide/cyclic GMP pathway in rat hippocampus through glutamate release. Br J Pharmacol. 1998;125:1042–1048. doi: 10.1038/sj.bjp.0702130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferger B, Kropf W, Kuschinsky K. Studies on electroencephalogram (EEG) in rats suggest that moderate doses of cocaine or d-amphetamine activate dopamine D1 rather than D2 receptors. Psychopharmacology. 1994;114:297–308. doi: 10.1007/BF02244852. [DOI] [PubMed] [Google Scholar]
- Flores C, Samaha AN, Stewart J. Requirement of endogenous basic fibroblast growth factor for sensitization to amphetamine. J Neurosci. 2000;20:R55. doi: 10.1523/JNEUROSCI.20-02-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J NeuroSci. 2000;12:3596–3604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- Giordano TP, III, Satpute SS, Striessnig J, Kosofsky BE, Rajadhyaksha AM. Up-regulation of dopamine D(2)L mRNA levels in the ventral tegmental area and dorsal striatum of amphetamine-sensitized C57BL/6 mice: role of Ca(v)1.3L-type Ca(2+) channels. J Neurochem. 2006;99:1197–1206. doi: 10.1111/j.1471-4159.2006.04186.x. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Shippenberg TS. Evidence for an involvement of muscarinic cholinergic systems in the induction but not expression of behavioral sensitization to cocaine. Synapse. 1996;24:182–192. doi: 10.1002/(SICI)1098-2396(199610)24:2<182::AID-SYN10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Hu XT, Koeltzow TE, Cooper DC, Robertson GS, White FJ, Vezina P. Repeated ventral tegmental area amphetamine administration alters dopamine D1 receptor signaling in the nucleus accumbens. Synapse. 2002;45:159–170. doi: 10.1002/syn.10095. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Karler R, Calder LD, Bedingfield JB. A novel nicotinic-cholinergic role in behavioral sensitization to amphetamine-induced stereotypy in mice. Brain Res. 1996;725:192–198. doi: 10.1016/0006-8993(96)00248-x. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Hefner KR, Sibley DR, Holmes A. Comparison of dopamine D1 and D5 receptor knockout mice for cocaine locomotor sensitization. Psychopharmacology. 2008;200:117–127. doi: 10.1007/s00213-008-1165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karper PE, De La Rosa H, Newman ER, Krall CM, Nazarian A, McDougall SA, Crawford CA. Role of D1-like receptors in amphetamine-induced behavioral sensitization: a study using D1A receptor knockout mice. Psychopharmacology. 2002;159:407–414. doi: 10.1007/s00213-001-0936-7. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Low MJ, Rubinstein M, Phillips TJ. Role of dopamine D1-like receptors in methamphetamine locomotor responses of D2 receptor knockout mice. Genes Brain Behav. 2008;7:568–577. doi: 10.1111/j.1601-183X.2008.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys AS, Mark GP. D1 and D2 dopamine receptor mediation of amphetamine-induced acetylcholine release in nucleus accumbens. Neuroscience. 1998;86:521–531. doi: 10.1016/s0306-4522(98)00018-9. [DOI] [PubMed] [Google Scholar]
- Konradi C, Cole RL, Heckers S, Hyman SE. Amphetamine regulates gene expression in rat striatum via transcription factor CREB. J Neurosci. 1994;14:5623–5634. doi: 10.1523/JNEUROSCI.14-09-05623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribara H. Inhibition of methamphetamine sensitization by post-methamphetamine treatment with SCH 23390 or haloperidol. Psychopharmacology. 1995;119:34–38. doi: 10.1007/BF02246051. [DOI] [PubMed] [Google Scholar]
- Kuribara H. Does nicotine modify the psychotoxic effect of methamphetamine? Assessment in terms of locomotor sensitization in mice. J Toxicol Sci. 1999;24:55–62. doi: 10.2131/jts.24.55. [DOI] [PubMed] [Google Scholar]
- Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lanca AJ, O’Dowd BF, George SR. Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–3568. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- Licata SC, Pierce RC. The roles of calcium/calmodulin-dependent and Ras/mitogen-activated protein kinases in the development of psychostimulant-induced behavioral sensitization. J Neurochem. 2003;85:14–22. doi: 10.1046/j.1471-4159.2003.01662.x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Grady S, Marks MJ, Picciotto M, Changeux JP, Collins AC. Pharmacological characterization of nicotinic receptor-stimulated GABA release from mouse brain synaptosomes. J Pharmacol Exp Ther. 1998;287:648–657. [PubMed] [Google Scholar]
- Marshall DL, Redfern PH, Wonnacott S. Presynaptic nicotinic modulation of dopamine release in the three descending pathways studied by in vivo microdialysis: comparisons of naïve and chronic nicotine-treated rats. J Neurochem. 1997;68:1511–1519. doi: 10.1046/j.1471-4159.1997.68041511.x. [DOI] [PubMed] [Google Scholar]
- McGhee DS, Heath MJS, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by pre-synaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- McPherson CS, Featherby T, Krstew E, Andrew JL. Quantification of phosphorylated cAMP-response element-binding protein expression throughout the brain of amphetamine-sensitized rats: activation of hypothalamic orexin A-containing neurons. J Pharmacol Exp Ther. 2007;323:805–812. doi: 10.1124/jpet.107.125732. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Callen S, Scheuer DA. Brain-derived neurotrophic factor expression is increased in the rat amygdala, piriform cortex and hypothalamus following repeated amphetamine administration. Brain Res. 2002;949:218–227. doi: 10.1016/s0006-8993(02)03160-8. [DOI] [PubMed] [Google Scholar]
- Miner LL, Drago J, Ghamberlain PM, Donovan D, Uhl GR. Retained cocaine conditioned place preference in D1 receptor deficient mice. NeuroReport. 1995;6:2314–2316. doi: 10.1097/00001756-199511270-00011. [DOI] [PubMed] [Google Scholar]
- O’Neill MF, Shaw G. Comparison of dopamine receptor antagonists on hyperlocomotion induced by cocaine, amphetamine, MK-801 and the dopamine D1 agonist C-APB in mice. Psychopharmacology. 1999;145:237–250. doi: 10.1007/s002130051055. [DOI] [PubMed] [Google Scholar]
- Oliverio A. Effects of mecamylamine on avoidance conditioning and maze learning of mice. J Pharmacol Exp Ther. 1966;154:350–356. [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Born B, Adams M, Kalivas PW. Repeated intra-ventral tegmental area administration of SKF-38393 induces behavioral and neurochemical sensitization to a subsequent cocaine challenge. J Pharmacol Exp Ther. 1996;278:384–392. [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O’Dowd BF, George SR. D1–D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci USA. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Fox L, Ho LB, Berger SP. Nicotine stimulation of extracellular glutamate levels in the nucleus accumbens: neuropharmacological characterization. Synapse. 2000;35:129–136. doi: 10.1002/(SICI)1098-2396(200002)35:2<129::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ. Effects of nicotine, mecamylamine, and hexamethonium on shock-induced fighting, pain reactivity, and locomotor behaviour in rats. Psychopharmacology. 1979;66:93–98. doi: 10.1007/BF00431996. [DOI] [PubMed] [Google Scholar]
- Rodrigues TB, Granado N, Ortiz O, Cerdán S, Moratalla R. Metabolic interactions between glutamatergic and dopaminergic neurotransmitter systems are mediated through D(1) dopamine receptors. J Neurosci Res. 2007;85:3284–3293. doi: 10.1002/jnr.21302. [DOI] [PubMed] [Google Scholar]
- Ross SB, Jackson DM, Edwards SR. The involvement of dopamine D1 and D2 receptors in the locomotor stimulation produced by (+)-amphetamine in naive and dopamine-depleted mice. Pharmacol Toxicol. 1989;64:72–77. doi: 10.1111/j.1600-0773.1989.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer ANM, De Vries TJ, Wardeh G, van de Ven HWM, Vanderschuren LJMJ. Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J Neurosci. 2002;22:3269–3276. doi: 10.1523/JNEUROSCI.22-08-03269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim I, Javaid JI, Wirtshafter D, Jang SY, Shim KH, Lee HJ, Chung YC, Chun BG. Nicotine-induced behavioral sensitization is associated with extracellular dopamine release and expression of c-fos in the striatum and nucleus accumbens of the rat. Behav Brain Res. 2001;121:137–147. doi: 10.1016/s0166-4328(01)00161-9. [DOI] [PubMed] [Google Scholar]
- So CH, Varghese G, Curley KJ, Kong MM, Alijaniaram M, Ji X, Nguyen T, O’dowd BF, George SR. D1 and D2 dopamine receptors form heterooligomers and co-internalize after selective activation of either receptor. Mol Pharmacol. 2005;68:568–578. doi: 10.1124/mol.105.012229. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Fink R, Jarvik ME. Acute and chronic tolerance to nicotine measured by activity in rats. Psychopharmacologia. 1973;30:329–342. doi: 10.1007/BF00429192. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Mirza NR. Dissociations between the locomotor stimulant and depressant effects of nicotinic agonists in rats. Psychopharmacology. 1995;117:430–437. doi: 10.1007/BF02246215. [DOI] [PubMed] [Google Scholar]
- Tritto T, McCallum SE, Waddle SA, Hutton SR, Paylor R, Collins AC, Marks MJ. Null mutant analysis of responses to nicotine: deletion of beta2 nicotinic acetylcholine receptor subunit but not alpha7 subunit reduces sensitivity to nicotine-induced locomotor depression and hypothermia. Nicotine Tob Res. 2004;6:145–158. doi: 10.1080/14622200310001656966. [DOI] [PubMed] [Google Scholar]
- Turgeon SM, Pollack AE, Fink JS. Enhanced CREB phosphorylation and changes in c-Fos and FRA expression in striatum accompany amphetamine sensitization. Brain Res. 1997;749:120–126. doi: 10.1016/s0006-8993(96)01316-9. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Schmidt ED, De Vries TJ, Van Moorsel CAP, Tilders FJH, Schoffelmeer ANM. A single exposure to amphetamine is sufficient to induce long-term behavioral, neuroendocrine and neurochemical sensitization in rats. J Neurosci. 1999;19:9579–9586. doi: 10.1523/JNEUROSCI.19-21-09579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. D1 dopamine receptor activation is necessary for the induction of sensitization by amphetamine in the ventral tegmental area. J Neurosci. 1996;16:2411–2420. doi: 10.1523/JNEUROSCI.16-07-02411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vezina P, Blanc G, Glowinski J, Tassin JP. Nicotine and morphine differentially activate brain dopamine in prefrontocortical and subcortical terminal fields: effects of acute and repeated injections. J Pharmacol Exp Ther. 1992;261:484–490. [PubMed] [Google Scholar]
- Waddington JL, Daly SA, Downes RP, Deveney AM, McCauley PG, O’Boyle KM. Behavioural pharmacology of ‘D-1-like’ dopamine receptors: further subtyping, new pharmacological probes and interactions with ‘D-2-like’ receptors. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:811–131. doi: 10.1016/0278-5846(95)00130-n. [DOI] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptation involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wolf ME, White FJ, Nassar R, Brooderson RJ, Khansa MR. Differential development of autoreceptor subsensitivity and enhanced dopamine release during amphetamine sensitization. J Pharmacol Exp Ther. 1993;264:249–255. [PubMed] [Google Scholar]
- Wolf ME, White FJ, Hu XT. MK-801 prevents alterations in the mesoaccumbens dopamine system associated with behavioral sensitization to amphetamine. J Neurosci. 1994;14:1735–1745. doi: 10.1523/JNEUROSCI.14-03-01735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Wu M, Shanabrough M, Leranth C, Alreja M. Cholinergic excitation of septohippocampal GABA but not cholinergic neurons: implications for learning and memory. J Neurosci. 2000;20:3900–3908. doi: 10.1523/JNEUROSCI.20-10-03900.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Guo Y, Vorhees CV, Zhang J. Behavioral responses to cocaine and amphetamine administration in mice lacking the dopamine D1 receptor. Brain Res. 2000;852:198–207. doi: 10.1016/s0006-8993(99)02258-1. [DOI] [PubMed] [Google Scholar]