Abstract
Objective
To investigate the impact of checkpoint kinase 2 (CHK2)-small interfering RNA (CHK2-siRNA) on the enhancement of radiosensitivity by CpG oligodeoxynucleotide (ODN) 7909 in lung cancer A549 cells.
Methods
The A549 cells were randomly divided into five groups: control, CpG, X-ray, CpG + X-ray, and CHK2-siRNA + CpG + X-ray. Cell colonization was observed using inverted microscopy. Cell cycle and apoptosis were analyzed by flow cytometry. CHK2 expression was detected by Western blot. CHK2-siRNA was adopted to silence the expression of CHK2.
Results
The level of CHK2 phosphorylation was higher in the CpG + X-ray group than in the X-ray group. Increases in G2/mitotic (M) phase arrest and apoptosis and a decrease of cell survival rate in the CpG + X-ray group were statistically significant (P < 0.05) when compared with the CHK2-siRNA + CpG + X-ray group in which the expression of CHK2 was obviously inhibited. The combination of CpG ODN7909 and X-ray irradiation was found to enhance the mitotic death of A549 cells. The sensitization enhancement ratio of mean death dose (D0) was 1.42 in the CpG + X-ray group, which was higher than that of the CHK2-siRNA + CpG + X-ray group, in which D0 was 1.05.
Conclusion
To a certain extent, the impact of a combination of CpG ODN7909 and X-ray on G2/M phase arrest, apoptosis, and rate of cell survival was attenuated by CHK2-siRNA in human lung adenocarcinoma A549 cells, indicating that increased phosphorylation of CHK2 might be a radiosensitive pathway.
Keywords: oligodeoxynucleotide, checkpoint kinase 2, mitotic death, apoptosis, X-ray
Background
Radiotherapy plays a pivotal role in achieving local control of tumors and in the relief of symptoms resulting from metastatic lesions. However, therapeutic efficacy is compromised when cancer cells develop resistance to X-rays and long-term survival in patients with lung cancer remains terribly low. Studies have showed that ionizing radiation (IR) kills tumor cells via inducing an array of lesions in DNA, including base damage, intra- and inter-strand cross-linking and single- or double-strand breaks (DSBs).1 Of these types of DNA damage, DSBs represent a particularly dangerous form of damage, which causes cell-cycle arrest and/or cell death.2 It has been well demonstrated that radiation sensitivity is associated with cell cycle distribution and that checkpoint kinase 2 (CHK2) plays a crucial role in the DNA damage responses (DDRs) that arrest the cell cycle, induce apoptosis, and, in some cases, promote DNA repair.3–6 As is well understood, cells arrested at the G2/mitotic (M) phase are the most sensitive to IR, while apoptosis eliminates cells harboring abnormal DNA. It is also widely believed that these DDRs are required for the maintenance of genomic stability and prevention of tumor development.7
CHK2, mutated in patients with Li–Fraumeni syndrome, is a homolog of the Rad53 gene in budding yeast and of the Cds1 gene in fission yeast. In response to IR-induced DSBs, the CHK2 protein becomes rapidly hyperphosphorylated at the Thr-68 site by several kinases, such as the ataxia-telangiectasia-mutated protein, which is critical in the cellular response to DNA damage because it regulates the G1, synthesis (S), and G2/M cell-cycle checkpoints and phosphorylates an array of protein substrates.8,9 Once phosphorylated, activated CHK2 phosphorylates multiple downstream molecules, which are thought to inhibit the activation of cyclin-dependent kinases and induce apoptosis.6
Previous work on synthetic oligodeoxynucleotides containing unmethylated CpG motifs (CpG ODNs) has shown that CpG ODNs may induce antitumor immune responses in a therapeutic adjuvant strategy through functioning as Th-1 adjuvants and activating B lymphocytes and dendritic cells.10,11 However, some studies have suggested that CpG ODNs may enhance the sensitivity of tumor cells to chemotherapy by increasing chemotherapy-induced tumor cell apoptosis and inhibiting tumor cell proliferation.12,13 As CpG ODN7909, a type-B ODN, has a fully phosphorothioate-modified backbone that resists nuclease attack and, in vivo, increases the stability of the ODNs by extending their half-life from a few minutes to about 2 days, it can initiate downstream-signaling cascades involved in regulating transcription by acting as a specific ligand of toll-like receptor 9 (TLR9), which is also expressed in human lung carcinoma A549 cells.14 Most importantly, our previous studies have shown that CpG ODN7909 potentiates X-ray-induced inhibition to proliferate human non-small cell lung cancer A549 cells.15
The purpose of this study was to further explore the relationship between the impact of checkpoint kinase 2-small interfering RNA (CHK2-siRNA) on A549 cell-cycle arrest and apoptosis induced by CpG ODN7909 plus X-rays and CHK2, providing a new theoretical basis for enhancement of radiosensitivity by CpG ODN7909 in lung cancer A549 cells.
Materials and methods
Antibodies and reagents
The human lung adenocarcinoma cell line A549 was obtained from Chinese Academy of Science (Shanghai, China). Roswell Park Memorial Institute (RPMI)-1640 medium and fetal bovine serum was purchased from BioWest (Loire Valley, France). CpG ODN7909 (5′-TCGTCGTTTTGTCG TTTTGTCGTT-3′) was purchased from Shanghai Sangon Biological Engineering Technology and Services (Shanghai, China), dissolved in deionized water, and stored at 4°C. An annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit, a bicinchoninic acid protein assay kit, and rabbit anti-mouse secondary antibodies were purchased from the Beyotime Institute of Biotechnology (Jiangsu, China). Primary antibodies against β-actin, CHK2, and phosphor-CHK2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). CHK2-siRNA and LipofectamineTM 2000 were purchased from Cell Signaling Technology (Danvers, MA).
Cell culture
The human lung adenocarcinoma A549 cells were maintained in RPMI-1640 medium supplemented with 100 units/mL of penicillin, 100 μg/mL of streptomycin and 10% heat-inactivated fetal bovine serum (Gibco, Carlsbad, CA) at 37°C in a humidified air containing 5% carbon dioxide. The medium was replaced every 2 or 3 days. The cells in the logarithmic growth phase were used to perform the experiments described as follows.
Transfection of siRNA
For transfection, the A549 cells in the logarithmic growth phase were seeded in six-well culture plates. When the cells grew to reach 50% confluence, 100 nM of CHK2-siRNA was transfected with 5 μL of Lipofectamine 2000 plus 1.5 mL of serum-free RPMI-1640 medium without antibiotics under the conditions described by the manufacturer. After incubation for 6 hours, the medium was replaced with the standard culture medium already described. After another incubation of 18 hours, the cells were used in the following tests.
Irradiation treatment
The A549 cells were irradiated with X-rays at 6 MV at room temperature using a linear accelerator (Elekta Precise, Stockholm, Sweden) under the source-to-skin distance (the distance from the radiation source to the central surface of the six-well plate – 100 cm), and the dose rate was 2.0 Gy/min. In colony-formation experiments, A549 cells were randomly placed into either the X-ray group or CpG + X-ray group and irradiated with doses of 0, 2, 4, 6, 8, and 10 Gy X-rays. Based on our previous studies, A549 cells were pre-treated with CpG ODN7909 (10 μg/mL) 24 hours before irradiation.15 In other experiments, A549 cells seeded in six-well plates were randomly divided into five groups: control, CpG, X-ray, CpG + X-ray, and CHK2- siRNA + CpG + X-ray. The X-ray group and CpG + X-ray group were irradiated with X-rays 24 hours after the CpG group, the CpG + X-ray group was treated with 10 μg/mL of CpG ODN7909, and the control and X-ray groups were treated with a corresponding volume of sterile distilled water. The CHK2-siRNA + CpG + X-ray group and CpG + X-ray group were similarly treated except that the former was transfected with CHK2-siRNA 24 hours before administration of CpG ODN7909.
Clonogenic survival assay
Inverted phase contrast microscopy was used in the course of observation. After irradiation, the cells were immediately trypsinized and suspended. The cells were then seeded in triplicate in 60 mm petri dishes at a density whereby the cells could form colonies of 50 to approximately 200 cells, as determined in pre-experiments. After incubation for 10 days, the cells were washed twice with phosphate-buffered saline, fixed in methanol, and then stained with Giemsa stain. A “colony” was defined as a cluster of at least 50 cells. The number of colonies was counted manually using a microscope. Cell survival rates in different dose groups were counted using the colony-formation rate. The 0 Gy group was a control group. Clonogenic survival fraction (SF) was calculated as: (irradiated cell colony numbers/unirradiated cell colony numbers) × 100%.
Cell-cycle distribution
The cell-cycle phases were analyzed by measuring the DNA fragments stained with propidium iodide (PI; Sigma-Aldrich, St Louis, MO), used as described by the manufacturer. A549 cells grown in six-well plates were harvested and centrifuged at 24 hours after irradiation. Cell pellets were counted and washed twice with pre-cool phosphate-buffered saline. Then the cells were fixed and permeabilized overnight by adding 1 mL of 70% pre-cooled ethanol to each tube at 4°C. After centrifugation, the fixatives were decanted and the cell pellets were resuspended in 0.5 mL of staining solution containing 200 μL each of DNAse-free RNAse (Sigma-Aldrich) and PI and incubated for 30 minutes at room temperature in the dark. Then the cells were analyzed immediately by flow cytometry with a FACScanTM system using CellQuestTM software (version 3.3) (BD Biosciences, San Jose, CA).
Cell apoptosis
A549 cells grown in six-well plates were harvested and counted at 24 hours after irradiation. The tests were performed using the annexin V-FITC apoptosis detection kit. The cell pellets were resuspended in 195 μL of binding buffer and stained with 5 μL each of annexin V-FITC and PI staining solution for 10 minutes at room temperature in the dark. Flow cytometry was performed with the FACScan system using CellQuest software. Cell apoptosis rate was calculated as: (the number of cell apoptosis in each group/the total number of cells in each group) × 100%.
Expression and phosphorylation of ataxia-telangiectasia-mutated kinase
Western blot analysis was used to determine the expression and phosphorylation of CHK2 in A549 cells after irradiation. The treatment schedule in Western blot analysis was the same as for cell-cycle assay. At 1 hour and 3 hours after irradiation, cells in each group were lysed in 100 μL of radio-immuno-precipitation assay protein lysis buffer (Beyotime Institute of Biotechnology) supplemented with 1 nM phenylmethylsulfonyl fluoride and 1 nM sodium orthovanadate. Protein was extracted on ice for at least 30 minutes. The protein concentrations of the lysates were measured using the bicinchoninic acid protein assay kit. Lysate protein (50 μg) was fractionated on 12% gradient sodium dodecyl sulfate polyacrylamide gel electrophoresis under reducing conditions. After electrophoresis, proteins were transferred onto polyvinylidene difluoride membranes (Thermo Fisher Scientific, Waltham, MA) and blocked for 1 hour in Tris-buffered saline containing 5% bovine serum albumin and 0.05% polysorbate 20 at room temperature. Blots were probed with the appropriate primary antibodies and peroxidase-conjugated goat anti-rabbit or goat anti-mouse secondary antibodies (Santa Cruz Biotechnology). Specific signals were detected with an enhanced chemiluminescence kit (Beyotime Institute of Biotechnology). The images were analyzed using Adobe Photoshop CS3 software (Adobe Systems, San Jose, CA).
Statistical analysis
A multi-target single-hitting model was used to fit cells to a survival curve. Statistics analysis and mapping were performed with SPSS software (v 16.0; IBM, Armonk, NY). Measurement data were expressed as mean ± standard deviation and a probability of <0.05 was considered statistically significant between groups by Student’s t-test.
Results
Impact of CHK2-siRNA on CHK2 expression and phosphorylation in A549 cells treated with CpG ODN plus X-rays
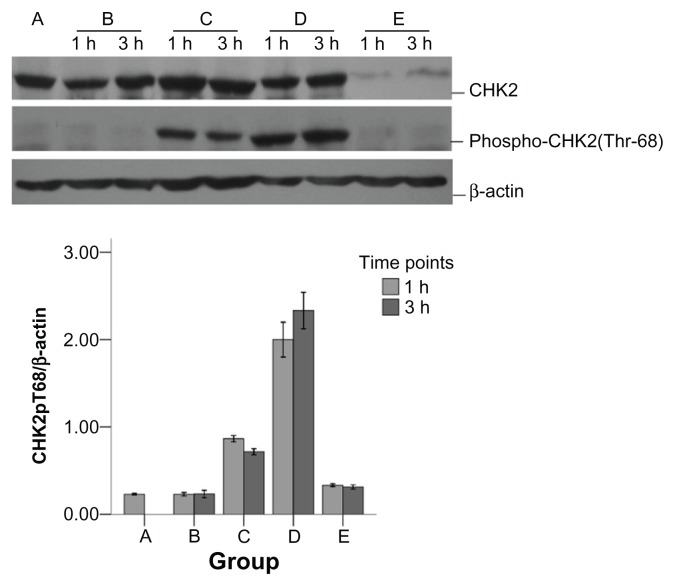
As shown in Figure 1, compared with the control group, there was no obvious difference in CHK2 expression between the CpG, X-ray, and CpG + X-ray groups. The level of CHK2 phosphorylation was increased in the X-ray group, with the level much higher than in the control group. The level of CHK2 phosphorylation increased more in the CpG + X-ray group than in the X-ray group, and the difference between the two groups was statistically significant (t = 12.35, P < 0.01). However, CHK2 expression and phosphorylation were decreased in the CHK2- siRNA + CpG + X-ray group.
Figure 1.
Impact of checkpoint kinase 2-small interfering RNA (CHK2-siRNA) on CHK2 in A549 cells treated with CpG oligodeoxynucleotide (ODN) plus X-rays.
Notes: A, control group; B, CpG group; C, X-ray group; D, CpG + X-ray group; E, CHK2-siRNA + CpG + X-ray group. Total extract from A549 cells was immunoblotted against CHK2, phospho-CHK2 (Thr68), and β-actin at the indicated time points following irradiation. CHK2 and phospho-CHK2 status were assessed by Western blot using CHK2 and phospho-CHK2 antibody, respectively, and equal gel loading was verified using an anti-actin antibody. Quantitative analysis showed that the phospho-CHK2 difference between the X-ray group and CpG + X-ray group was significant at the 0.05 level (t = 12.35, P < 0.01).
Impact of CHK2-siRNA on G2/M phase arrest in A549 cells treated with CpG ODN plus X-rays
As shown in Table 1, there was no significant difference between the number of cells found in the G2/M phase in the CpG group and control group (t = 1.63, P = 0.178). The number of cells in the G2/M phase was found increased in both the X-ray group and CpG + X-ray group – especially in the latter – and there was a significant difference between the two groups (t = 17.32, P < 0.01). However, there were fewer cells in the G2/M phase in the CHK2-siRNA + CpG + X-ray group than in the CpG + X-ray group and there was a significant difference between the two groups (t = 26.84, P < 0.01).
Table 1.
Impact of checkpoint kinase 2-small interfering RNA (CHK2-siRNA) on G2/mitotic (M) phase arrest in A549 cells treated with CpG oligodeoxynucleotide plus X-rays
| Group | G2/M | G1 | S |
|---|---|---|---|
| Control | 13.12 ± 0.56 | 65.43 ± 0.87 | 21.45 ± 0.37 |
| CpG | 14.23 ± 0.65 | 66.18 ± 0.54 | 19.59 ± 0.41 |
| X-ray | 23.54 ± 0.67* | 67.89 ± 0.78 | 8.57 ± 0.35 |
| CpG + X-ray | 34.56 ± 0.19** | 57.83 ± 0.25 | 7.61 ± 0.26 |
| CHK2-siRNA + CpG + X-ray | 10.09 ± 0.23 | 55.38 ± 0.73 | 34.53 ± 0.14 |
Notes:
The G2/M phase difference between the X-ray group and CpG + X-ray group was significant at the 0.05 level (t = −17.32, P < 0.01).
The G2/M phase difference between the CpG + X-ray group and CHK2-siRNA + CpG + X-ray group was significant at the 0.05 level (t = −26.84, P < 0.01).
Abbreviation: S, synthesis phase.
Impact of CHK2-siRNA on apoptosis in A549 cells treated with CpG ODN plus X-rays
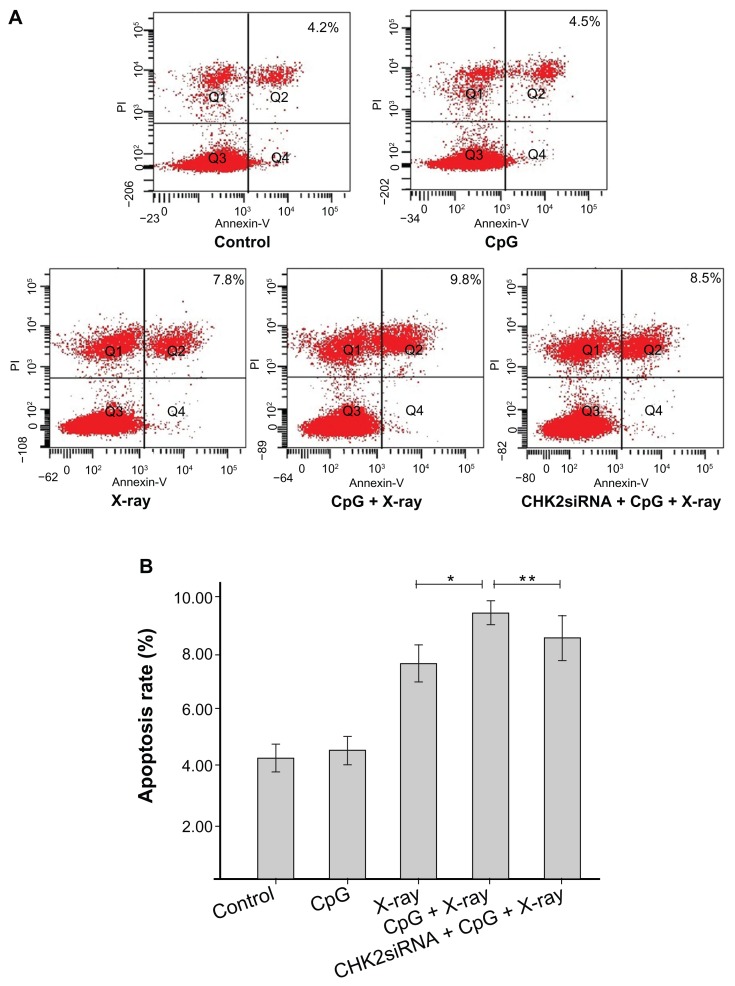
As shown in Figure 2, there was no significant difference in the apoptosis rate between the CpG and control groups (t = 1.24, P = 0.28). Increase in rate of apoptosis was observed in the X-ray and CpG + X-ray groups, particularly in the latter, and there was a significant difference between the two groups (t = 7.71, P < 0.01). Compared with the CpG + X-ray group, a decrease in rate of apoptosis was observed in the CHK2-siRNA + CpG + X-ray group and there was a significant difference between the two groups (t = 2.98, P = 0.041).
Figure 2.
Impact of checkpoint kinase 2-small interfering RNA (CHK2-siRNA) on apoptosis in A549 cells treated with CpG oligodeoxynucleotide plus X-rays.
Notes: (A) After treatment, A549 cells were stained with Annexin V/propidium iodide(PI) and measured by flow cytometry. Bottom right quadrant, cells stained mainly by Annexin V (early apoptotic cells); top right quadrant, cells stained by both PI and Annexin V (late apopototic cells); top left quadrant, cells stained mainly by PI (necrotic cells); bottom left quadrant, cells negative for both Annexin V and PI. (B) The columns illustrate the flow cytometric results. *The difference between the X-ray group and CpG + X-ray group is significant at the 0.05 level (t = −7.71, P < 0.01); **the difference between the CpG + X-ray group and CHK2-siRNA + CpG + X-ray group is significant at the 0.05 level (t = −2.98, P < 0.041).
Abbreviation: PI, propidium iodide.
Impact of CHK2-siRNA on colony formation in A549 cells treated with CpG ODN plus X-rays
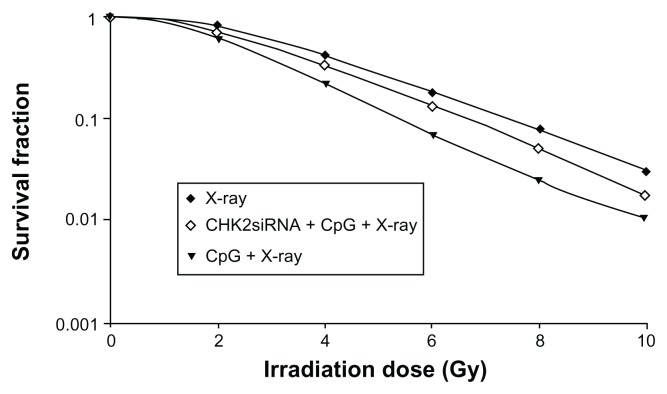
Dose–survival curves fitted using a multi-target single-hitting model are shown in Figure 3. In terms of radiobiological parameters, D0 equaled 3.84 Gy and the sensitization enhancement ratio, calculated by D0 value, equaled 1.46 in the CpG + X-ray group and 1.05 in the CHK2-siRNA + CpG + X-ray group.
Figure 3.
Dose–survival curves fitted using a multi-target single-hitting model showing the impact of checkpoint kinase 2-small interfering RNA (CHK2-siRNA) in A549 cells treated with CpG oligodeoxynucleotide plus X-rays.
Notes: The sensitization enhancement ratio was higher in the CpG + X-ray group (1.46) than in the CHK2-siRNA + CpG + X-ray group (1.05).
Discussion
Some in-depth studies of CpG ODNs as immunoadjuvants in combination with other therapies such as immunotherapy, cryotherapy, and chemotherapy for the treatment of malignant tumors have been performed.16–18 However, in recent years, there has been more focus on the direct impact of CpG ODNs on some malignant tumors. For example, Wu et al16 found that CpG ODN2216 inhibit the invasion and migration of pancreas cancer cells in experiments and Mason et al17 showed that CpG ODN1826 improve the killing effect of docetaxel on breast cancer cells. Our previous studies have showed that CpG ODN1826 increases the reaction of Lewis mouse lung cancers to X-rays and that CpG ODN7909 might enhance the radiosensitivity of A549 cells by increasing X-ray-induced apoptosis and G2/M phase arrest.15,18 However, methods of directing the sensitizing effect of CpG ODNs onto lung cancer cells have been unclear until now.
As is well known, cell-cycle distribution is associated with the sensitivity of cells to radiation. It is an effective approach to enhance the radiosensitivity of tumors and improve efficacy via promoting the tumor cell cycle to enter and then arrest the G2/M phase. Regulation of cell cycle is closely related to DDR, in which checkpoint kinase 1 and CHK2 are vital regulators. Studies have shown that CHK2 phosphorylation is induced by X-ray-induced DNA damage and that this is followed by CHK2 activation.19,20 Activated CHK2 eventually induces cell arrest at the G2/M phase by initiating a downstream cascade reaction. In the present experiments, we found that CpG ODN7909 also enhanced the X-ray-induced cell G2/M phase arrest and increased the X-ray-induced phosphorylation of CHK2, although CpG ODN7909 alone did not induce CHK2 phosphorylation. To further explore the relationship between CHK2 phosphorylation and the impact of CpG ODN7909 on G2/M phase arrest in irradiated A549 cells, we applied CHK2- siRNA. The combination of CpG ODN7909 and X-rays did not obviously induce G2/M phase arrest in A549 cells, whereas the expression of CHK2 was inhibited by the use of CHK2-siRNA. These results suggest that CHK2 phosphorylation might play a role in the enhancement of X-ray-induced G2/M phase arrest by CpG ODN7909 in A549 cells.
Apoptosis is important in the use of radiation to kill tumor cells, and it is now widely recognized that radiation-induced apoptosis may be used to measure the sensitivity of cells to radiation, with an increased rate of apoptosis meaning that the cells have a higher sensitivity to radiation.21 Adams et al22 found that the CHK2/p53 signal pathway plays a vital role in X-ray-induced apoptosis. Through in vivo retina experiments in newborn mice, Borges et al23 found that the pro-apoptotic activation of the p53 protein was phosphorylated by CHK2 in a radiation dose-dependent manner. To the best of our knowledge, our study has demonstrated that CpG ODN7909 not only increases the level of CHK2 phosphorylation induced by X-rays, but also increases apoptosis in A549 cells. However, the impact of the combination of CpG ODN7909 and X-rays on apoptosis was subdued after the expression of CHK2 was inhibited in advance by CHK2-siRNA in A549 cells. These results suggest that CHK2 phosphorylation might also play a role in the enhancement of X-ray-induced apoptosis by CpG ODN7909 in A549 cells.
Previously, it was thought that TLR9, described as the receptor of CpG ODNs, only expressed in immune cells such as dendritic cells and B lymphocytes. However, recently, there has been increasing evidence of TLR9 expression in human tumor cells and some studies have found that the direct impact of CpG ODNs on tumor cells is related to TLR9 expression in tumor cells.13 Our previous experiments have shown that the TLR9 gene is expressed in lung adenocarcinoma A549 cells.15 However, the regulation of CHK2 phosphorylation is complex and contentious. The precise mechanisms by which CHK2 is phosphorylated after X-ray irradiation are as yet unclear. Thus, it is worth exploring further whether CpG ODN7909 has a radiosensitizing effect when CHK2 phosphorylation is induced via activation of the TLR9 signal pathway in A549 cells.
Acknowledgments
This research was supported by a grant from the Science and Technology Council of the Jinshan District, Shanghai, China (2010-3-16). For providing critical assistance, we thank Professor Guoxiong Xu for reading the manuscript, Dr Chengli Qiu for performing FACScan analysis, and Dr Xiao-fang Jia and Dr Yanming Wan for their excellent guidance.
Footnotes
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck – a systematic review and meta-analysis. Radiother Oncol. 2011;100(1):22–32. doi: 10.1016/j.radonc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Willers H, Feng Z, et al. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Mol Cell Biol. 2004;24(2):708–718. doi: 10.1128/MCB.24.2.708-718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(4):928–942. doi: 10.1016/j.ijrobp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Yan T, Desai AB, Jacobberger JW, Sramkoski RM, Loh T, Kinsella TJ. CHK1 and CHK2 are differentially involved in mismatch repair-mediated 6-thioguanine-induced cell cycle checkpoint responses. Mol Cancer Ther. 2004;3(9):1147–1157. [PubMed] [Google Scholar]
- 5.Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408(6811):433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 6.Hirao A, Cheung A, Duncan G, et al. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)- dependent and an ATM-independent manner. Mol Cell Biol. 2002;22(18):6521–6532. doi: 10.1128/MCB.22.18.6521-6532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Stern DF. DNA damage regulates Chk2 association with chromatin. J Biol Chem. 2005;280(45):37948–37956. doi: 10.1074/jbc.M509299200. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Wiltshire T, Wang Y, et al. ATM-dependent CHK2 activation induced by anticancer agent, irofulven. J Biol Chem. 2004;279(38):39584–39592. doi: 10.1074/jbc.M400015200. [DOI] [PubMed] [Google Scholar]
- 9.Lavin MF, Kozlov S. ATM activation and DNA damage response. Cell Cycle. 2007;6(8):931–942. doi: 10.4161/cc.6.8.4180. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto M, Sato M. Toll-like receptor signaling in anti-cancer immunity. J Med Invest. 2003;50(1–2):9–24. [PubMed] [Google Scholar]
- 11.Shi R, Hong L, Wu D, et al. Enhanced immune response to gastric cancer specific antigen Peptide by coencapsulation with CpG oligodeoxynucleotides in nanoemulsion. Cancer Biol Ther. 2005;4(2):218–224. doi: 10.4161/cbt.4.2.1472. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Rayburn ER, Wang W, Kandimalla ER, Agrawal S, Zhang R. Chemotherapy and chemosensitization of non-small cell lung cancer with a novel immunomodulatory oligonucleotide targeting Toll-like receptor 9. Mol Cancer Ther. 2006;5(6):1585–1592. doi: 10.1158/1535-7163.MCT-06-0094. [DOI] [PubMed] [Google Scholar]
- 13.Rayburn ER, Wang W, Zhang R, Wang H. Experimental therapy for colon cancer: anti-cancer effects of TLR9 agonism, combination with other therapeutic modalities, and dependence upon p53. Int J Oncol. 2007;30(6):1511–1519. [PubMed] [Google Scholar]
- 14.Kumagai Y, Takeuchi O, Akira S. TLR9 as a key receptor for the recognition of DNA. Adv Drug Deliv Rev. 2008;60(7):795–804. doi: 10.1016/j.addr.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Zha L, Qiao T, Yuan S, Lei L. Enhancement of radiosensitivity by CpG-oligodeoxyribonucleotide- 7909 in human non-small cell lung cancer A549 cells. Cancer Biother Radiopharm. 2010;25(2):165–170. doi: 10.1089/cbr.2009.0686. [DOI] [PubMed] [Google Scholar]
- 16.Weigel BJ, Rodeberg DA, Krieg AM, et al. CpG oligodeoxynucleotides potentiate the antitumor effects of chemotherapy or tumor resection in an orthotopic murine model of rhabdomyosarcoma. Clin Cancer Res. 2003;9(8):3105–14. [PubMed] [Google Scholar]
- 17.Milas L, Mason KA, Ariga H, et al. CpG oligodeoxynucleotide enhances tumor response to radiation. Cancer Res. 2004;64(15):5074–7. doi: 10.1158/0008-5472.CAN-04-0926. [DOI] [PubMed] [Google Scholar]
- 18.Jahrsdörfer B, Weiner GJ. CpG oligodeoxynucleotides as immunotherapy in cancer. Update Cancer Ther. 2008;3(1):27–32. doi: 10.1016/j.uct.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu HQ, Wang B, Zhu SK, Tian Y, Zhang JH, Wu HS. Effects of CPG ODN on biological behavior of PANC-1 and expression of TLR9 in pancreatic cancer. World J Gastroenterol. 2011;17(8):996–1003. doi: 10.3748/wjg.v17.i8.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mason KA, Neal R, Hunter N, Ariga H, Ang K, Milas L. CpG oligodeoxynucleotides are potent enhancers of radio- and chemoresponses of murine tumors. Radiother Oncol. 2006;80(2):192–198. doi: 10.1016/j.radonc.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Yuan S, Qiao T, Chen W. CpG oligodeoxynucleotide 1826 enhances the Lewis lung cancer response to radiotherapy in murine tumor. Cancer Biother Radiopharm. 2011;26(2):203–208. doi: 10.1089/cbr.2010.0871. [DOI] [PubMed] [Google Scholar]
- 22.Smith J, Tho LM, Xu N, Gillespie DA. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res. 2010;108:73–112. doi: 10.1016/B978-0-12-380888-2.00003-0. [DOI] [PubMed] [Google Scholar]
- 23.Stolz A, Ertych N, Bastians H. Tumor suppressor CHK2: regulator of DNA damage response and mediator of chromosomal stability. Clin Cancer Res. 2011;17(3):401–405. doi: 10.1158/1078-0432.CCR-10-1215. [DOI] [PubMed] [Google Scholar]
- 24.Vellanki SH, Grabrucker A, Liebau S, et al. Small-molecule XIAP inhibitors enhance gamma-irradiation-induced apoptosis in glioblastoma. Neoplasia. 2009;11(8):743–752. doi: 10.1593/neo.09436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams CJ, Graham AL, Jansson M, et al. ATM and Chk2 kinase target the p53 cofactor Strap. EMBO Rep. 2008;9(12):1222–1229. doi: 10.1038/embor.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borges HL, Chao C, Xu Y, Linden R, Wang JY. Radiation-induced apoptosis in developing mouse retina exhibits dose-dependent requirement for ATM phosphorylation of p53. Cell Death Differ. 2004;11(5):494–502. doi: 10.1038/sj.cdd.4401366. [DOI] [PubMed] [Google Scholar]