Abstract
Background
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by the presence of pathogenic autoantibodies. Recent studies suggest that microRNAs (miRNAs) play an essential role in immunoregulation and may be involved in the pathogenesis of SLE. Therefore, it was of interest to investigate the potential therapeutic application of miRNAs in SLE, a concept that has not been thoroughly investigated thus far. Virus-like particles (VLPs) are a type of recombinant nanoparticle enveloped by certain proteins derived from the outer coat of a virus. Herein, we describe a novel miRNA-delivery approach via bacteriophage MS2 VLPs and investigate the therapeutic effects of miR-146a, a well-studied and SLE-related miRNA, in BXSB lupus-prone mice.
Methods
VLPs containing miR-146a, and the control VLPs, were prepared using an Escherichia coli expression system and then administered to lupus-prone mice over a 12-day period. We performed an enzyme-linked immunosorbent assay to evaluate the anti-dsDNA antibody, autoantibody to nuclear antigen (ANA), total IgG and total IgM levels in serum. The expression of miR-146a was analyzed by qRT-PCR. SLE-related cytokines as well as some toll-like receptor signaling pathway molecules were also measured.
Results
Treatment with MS2-miR146a VLP showed profound effects on lupus-prone BXSB mice, including an increased level of mature miR-146a, which led to a significant reduction in the expression of autoantibodies and total IgG. Remarkably, these mice also exhibited reduced levels of proinflammatorycytokines, including IFN-Interferon-α (IFN-α), Interleukin-1β (Il-1β) and Interleukin-6 (Il-6). Moreover, we showed that the toll-like receptor pathway was involved in this regulation.
Conclusion
Restoring the loss of miR-146a was effective in eliminating the production of autoantibodies and ameliorating SLE progression in lupus-prone mice. Thus, the induction of dysregulated miRNAs by an MS2 VLP-based delivery system may lead to novel therapies.
Keywords: systemic lupus erythematosus, anti-dsDNA antibody, autoantibody to nuclear antigen, Toll-like receptor, BXSB mice, gene therapy
Introduction
Systemic lupus erythematosus (SLE) is a chronic, complex, and debilitating systemic autoimmune disease characterized by variable involvement of different organ systems. Aberrant activation of T and B lymphocytes and their subsequent production of inflammatory cytokines and autoantibodies has been shown to be one of the main disease signs in SLE patients.1,2 The current treatment approaches include antimalarial drugs, anti-inflammatory agents, and immunosuppressive drugs. Unfortunately, these treatments still have some limitations, such as nonresponse in those with active refractory disease, and known side effects, which continue to pose a substantial challenge.3 Hence, new treatment approaches and a correspondingly better understanding of disease pathogenesis would be of great value.
To develop a new therapy for SLE, we focused on microRNA (miRNA), a type of 20–24 nucleotide noncoding RNA that can bind to the 3′-untranslated region (3′UTR) of target mRNA, resulting in its degradation and/or translational supression.4 The powerful gene-regulatory role of miRNAs is now well recognized, and some miRNA-based therapeutic strategies have been recently introduced that have shown effectiveness in tumor models. However, the study of miRNA in autoimmunity is still at an early stage. Since 2007, several studies have identified some SLE-related miRNAs, such as miR-21, miR-125, and miR-146a.5,6 Using miR-21 -specific Locked Nucleic Acid (LNA) silencing, Garchow et al7 observed an amelioration in lupus-prone mice. But our knowledge of the precise role of these miRNAs in pathogenesis, and more importantly, their possible application as therapeutic agents, is incomplete. Among these SLE-related dysregulated miRNAs, miR-146a, an miRNA important in the negative regulation of acute responses during activation of innate immunity, acts as a significant inhibitor of autoimmunity, myeloproliferation, and cancer.8–10 Reduced expression of miR-146a has been reported in peripheral blood mononuclear cells (PBMCs) from SLE patients,9 and ablation of the miR-146a gene in mice resulted in several severe immune-related phenotypes, which led to a premature death.10 Therefore, there is great interest in whether miR-146a-based therapy can improve SLE status in either animal models or human patients.
A major obstacle in miRNA-related therapy is the availability of an effective delivery system, owing to the instability and anionic charge of miRNA.11,12 A number of approaches for miRNA delivery have been used in reported therapies, but some deficiencies, such as limited transduction efficiency, cytotoxicity, and integration-induced tumorigenesis, remain a concern.13
Virus-like particles (VLPs) are biological constructs that are enveloped with certain proteins derived from the outer coat of a virus, but which do not contain any genetic material from the virus, and thus cannot cause infections. Considerable efforts have been invested in the development of VLPs, making them attractive as potential nanocarriers.14–16 Our previous study17 constructed a nanocarrier based on RNA bacteriophage MS2, the VLPs of which self-assemble from 180 copies of a single coat protein into a monodisperse, 27.5 nm icosahedral capsid. The self-assembly of the MS2 bacteriophage capsids has been used to package mouse pre-miR146a RNA, and these particles were then conjugated to a human immunodeficiency virus-1 (HIV-1) Tat47-57 cell-penetrating peptide. We have shown that the conjugated MS2 VLPs could effectively transfer the packaged pre-miR146a RNA into various cells and tissues, leading to overexpression of mature miR-146a with low toxicity. Thus, this strategy may be used as a novel tool in miRNA therapy. In the present study, we used this delivery approach to transfer miR-146a into lupus-prone mice to explore the possibility that miR-146a may act therapeutically, suppressing the production of autoantibodies. In order to clarify the possible mechanism involved, we also investigated the expression of proinflammatory cytokines and some essential intracellular molecules in response to this treatment.
Materials and methods
Preparation of MS2 VLPs containing pre-miRNAs
The MS2 VLPs containing pre-miR146a were prepared as described previously.17 In brief, the precursor of miR-146a (pre-miR146a) or a nonsense oligonucleotide (mutated pre-miR-146a RNA, CUGCAGAAGGUCAC CCAGGGUAACGUUGACCUUGGUGUUGCUCUAG CAGCGGCCAGGUCGACAGC), which was used as the control, were inserted into a prokaryotic expression vector (pACY-CDuet- 1, Novagen, Gibbstown, NJ, USA) and coexpressed with the capsid protein of bacteriophage MS2 using the BL21 (DE3) prokaryotic expression system. The recombinant MS2 VLPs were produced, purified, and designated MS2-miR146a VLPs and MS2-miRNC VLPs, respectively. These 2 VLPs were then conjugated to HIV Tat47-57 peptides (C-47YGRKKRRQRRR-57) using sulfosuccinimidyl 4-(p-maleimidophenyl) butyrate (Sulfo-SMPB, Thermo, Rocksford, IL, USA) according to the manufacturer’s instructions. Transmission electron microscopy, denaturing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and reverse transcription-PCR (RT-PCR) were used to evaluate the production and conjugation of Tatmodified MS2-miR146a and MS2-miRNC VLPs.
Mice and experimental design
Eight-week-old male BXSB mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA), and C57BL/6 male mice of the same age were purchased from Vital River Laboratories, Inc (Beijing, China). All mice were provided with sterilized food and water and housed in a barrier facility under a 12-hour:12-hour light:dark cycle. The animal experiments were performed in accordance with currently prescribed guidelines and under a protocol approved by the National Center for Clinical Laboratories Animal Care and Use Committee.
On the basis of our previous study, BXSB and C57BL/6 mice were treated with 100 μg (5 mg/kg) Tat-conjugated MS2- miR146a VLPs, 100 μg Tat-conjugated MS2-miRNC VLPs, or vehicle solution alone (phosphate buffer), intravenously via the tail vein route at the age of 16 weeks. The treatments were administered four times, 3 days apart, over 12 days. The mice were sacrificed 24 hours after the last injection, and the blood and organs were collected immediately. In order to evaluate the activation of particular intracellular molecules, PBMCs were purified using Ficoll-Hypaque density gradient centrifugation. Homologous male C57BL/6 mice were used as controls.
Quantitative real-time PCR
The expression levels of miR-146a, monocyte chemotactic protein 1 (Mcp1), Nuclear Factor-κB (NF-κB) p65 subunit, and NF-κB p50 subunit were determined by quantitative real-time polymerase chain reaction (qRT-PCR). Total RNA, including miRNA, was extracted by Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. After extraction, the RNA was treated with RNase-free DNase (Promega, Madison, WI, USA), and its integrity was checked by agarose gel electrophoresis. The recovery of RNA was quantified by absorbance at 260 nm. The gene expression levels were quantified by RT-PCR using a PrimeScript RT reagent Kit and an SYBR Premix Ex Taq II Kit (both from Takara, Otsu, Shiga, Japan). Briefly, approximately 100 ng of total RNA from each sample was reverse-transcribed to cDNA with miR-146a stem-loop RT primer, Mcp1, NF-κB p65, NF-κB p50, or U6 reverse primer. Subsequently, RT-PCR was performed using a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). After PCR amplification, melting curve analysis was performed to verify the specificity of the test. All primers used in this study are listed in Table 1. In this study, U6 RNA was used as an internal control. The relative expression levels were calculated using the 2−ΔΔCt method,18 and the differences in miRNA and RNA concentrations between the treated and control group were expressed as fold changes.
Table 1.
PCR primers used in the study
| Primer | Sequence (5′–3′) |
|---|---|
| U6 forward primer | GCTTCGGCAGCA |
| CATATACTAAAAT | |
| U6 reverse primer | CGCTTCACGAATTT |
| GCGTGTCAT | |
| miR146a reverse primer | GTGCAGGGTCCGA |
| GGT | |
| miR146a forward primer | GGCG TGAGAACTG |
| AATTCCA | |
| miR146a stem-loop RT primer | GTCGTATCCAGTGCAG |
| GGTCCGAGGTATTCGCA | |
| CTGGATACGACAACCCA | |
| Mcp1 forward primer | CTGGATCGGAACCAAATGAG |
| Mcp1 reverse primer | CGGGTCAACTTCACATTCAA |
| NF-κB p50 forward primer | ATAGGCAAGGTCAGAATGCA |
| CCAGAAGTCC | |
| NF-κB p50 reverse primer | AAATGTGTCAGTTTCATAGCC |
| TGAAGAACG | |
| NF-κB p65 forward primer | GGGCCTTGCTTGGCAACA |
| GCACA | |
| NF-κB p65 reverse primer | CGCAATGGAGGAGAAGTCT |
| TCATCTCC |
Abbreviations: Mcp1, monocyte chemotactic protein 1; NF-κB, Nuclear Factor-κB.
ELISA assay
The plasma anti-dsDNA antibody and autoantibody to nuclear antigen (ANA) levels in BXSB and C57BL/6 mice were determined by enzyme-linked immunosorbent assay (ELISA). To conduct the assay, plasma was diluted 200-fold and added to a dsDNA or cell nuclear antigen-coated ELISA plate (BD, Franklin Lakes, NJ, USA). After thermal incubation, the captured antibodies were detected by a horseradish peroxidase (HRP)-conjugated goat antimouse IgG (Sigma, St Louis, MO, USA). Finally, the color was developed by tetramethylbenzidine (Sigma) and then measured using an ELISA plate reader (450/620 nm, BioRad, Richmond, CA, USA). Murine ANA and anti-dsDNA antibodies of known concentration (Univ-bio, Shanghai, China) were diluted two-fold in series, and used as standards. The titers of ANA and anti-dsDNA antibodies in plasma were analyzed in the same way. Concentrations of total IgM antibody, total IgG antibody, Interferon-α (IFN-α), Tumor Necrosis Factor-α (TNF-α), Interleukin-1β (Il-1β), and Interleukin-6 (Il-6) in plasma were measured using commercial ELISA kits (eBioscience, San Diego, CA, USA for total IgG and IgM; Invitrogen for IFN-α; Dakewei, Beijing, China for TNF-α, IL-1β, and IL-6), according to the manufacturer’s instructions. All samples were measured in triplicate.
ANA immunofluorescence
Commercially available slides coated with fixed Hep-2 cells (Euroimmune, Luebeck, Germany) were incubated with diluted mouse plasma (1:200 dilution) at room temperature. ANA was detected following incubation with fluorescein isothiocyanate (FITC)-labeled goat antimouse IgG Fc (Santa Cruz, CA, USA). Slides were then read under a fluorescence microscope (Eclipse 50i, Nikon, Tokyo, Japan). A digital image was acquired using NIS-Elements software (Nikon) and quantified using the NIH ImageJ software package (National Institutes of Health, Bethesda, MA, USA).
Luciferase assay
Luciferase reporter vectors pGL4.32(luc2P/NF-κB-RE/Hygro) and pRL-TK were purchased from Promega. PBMCs isolated from treated mice were plated the day before transfection at 105 cells/well in 24-well plates in RPMI 1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% FBS (Gibco). The following day, 50 ng of pGL4.32(luc2P/NF-κB-RE/Hygro) or pRL-TK vector were cotransfected into PBMCs using Lipofectamine 2000 (Invitrogen). PBMCs from C57BL/6 mice were treated with 20 ng/mL TNF-α or PBS prior to transfection, and used as positive and negative controls, respectively. Twelve hours after transfection, cells were collected and counted, and then luciferase assays were performed using Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s protocol. Each experiment was performed in triplicate, and the luciferase activity was normalized to the cell number of the negative control.
Western blot analysis
PBMCs were lysed in SDS sample buffer. Cell pellets were then homogenized by brief sonication, and protein concentration was determined using the Bradford method. Forty micrograms of total protein were separated onto 20% polyacrylamide gels and transferred onto a 0.45 μm polyvinylidene fluoride (PVDF) membrane in a buffer containing 25 mM Tris-HCl (pH 8.3), 192 mM glycine, 20% methanol, and blocked with 5% fat-free dry milk in PBS for 1 hour. These membranes were incubated with specific primary antibodies and detected by HRP-labeled secondary antibodies. The IL-1 receptor-associated kinase-1 (IRAK-1), TNF receptor-associated factor-6 (TRAF-6) and β-actin monoclonal antibodies were obtained from Santa Cruz, and the HRP-linked secondary antibody was purchased from Univ-bio. β-actin protein was chosen as an internal control, and the signal was detected using the Quantity One imaging system (BioRad).
Statistical analysis
Data were expressed as mean ± SEM. Prism 5 software (GraphPad, La Jolla, CA, USA) was used for all statistical analyses. Testing between two groups was performed using the Mann– Whitney U test for antibodies and proinflammatory cytokines, and the unpaired two-tailed Student’s t-test for miRNA/RNA concentrations. A P-value less than 0.05 was considered statistically significant.
Results
Expression of the MS2 VLP-based miR-146a delivery vehicle
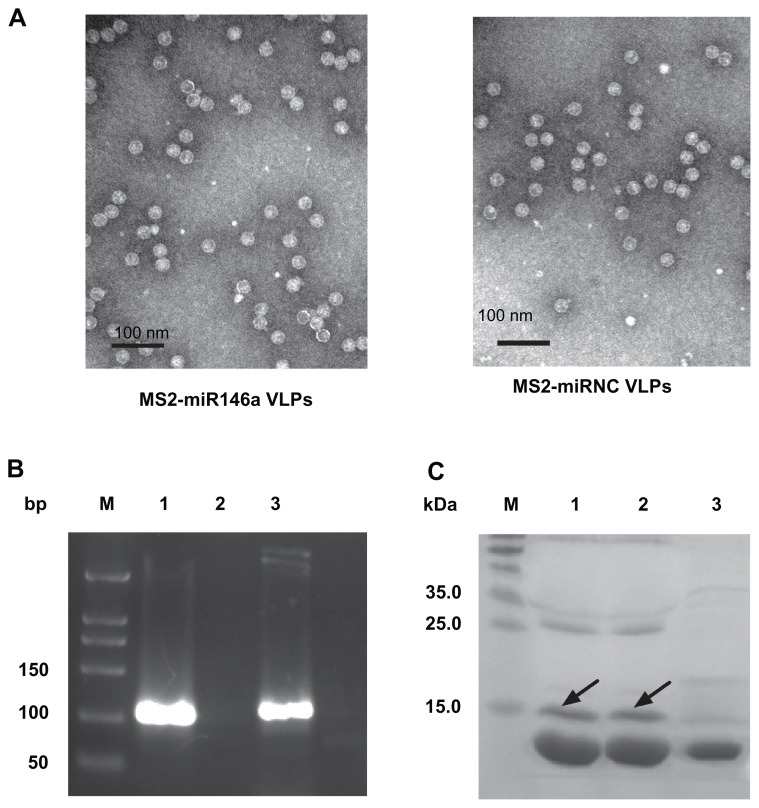
We succeeded in producing Tat-conjugated MS2-miR146a VLPs and Tat-conjugated MS2-miRNC control VLPs. The unmodified MS2 VLPs were observed by transmission electron microscopy (97,000×, Figure 1A). To achieve effective cell penetration, the MS2-miR146a and MS2-miRNC VLPs were conjugated to HIV Tat47-57. RT-PCR was used to validate the packaged RNAs in Tat-conjugated MS2-miR146a VLPs (Figure 1B). The 20% denaturing PAGE showed that the molecular weight of MS2-miR146a and MS2-miRNC VLPs was approximately 14 kDa, while the Tat47-57 conjugated VLPs exhibited retarded mobility (Figure 1C).19 The denaturing PAGE also indicated that about 24% and 26% of total MS2-miR146a VLPs and MS2-miRNC VLPs, respectively, were conjugated to the Tat peptide.
Figure 1.
Synthesis of MS2 VLPs containing pre-miR146a. The coding sequence of pre-miR146a, mutated pre-miR146a, and that of bacteriophage MS2 capsid protein were constructed into the recombinant plasmid. These MS2 VLPs were expressed in Escherichia coli, purified by density gradient centrifugation, and conjugated to the HIV protein transduction domain (Tat47–57 peptide) by sulfosuccinimidyl 4-[p-maleimidophenyl] butyrate (Sulfo-SMPB). (A) Identification of MS2-miR146a VLPs (left) and MS2-miRNC VLPs (right) by transmission electron microscopy (97,000×) before the conjugation reaction. (B) The Tat-MS2-miR146a VLPs were extracted and amplified by RT-PCR. Lane M: DL500 DNA marker; Lane 1: RNA derived from MS2-miR146a VLPs; Lane 2: negative control; Lane 3: positive control (PCR amplification of pre-miR146a cDNA from the recombinant plasmid). (C) The Tat-conjugated MS2-miR146a and Tat-conjugated MS2-miRNC VLPs were analyzed by SDS/PAGE. The Tat-MS2 VLPs exhibited slower mobility (~15 kDa, marked by the arrow) compared with the unmodified MS2 VLPs (~14 kDa). The extent of modification was evaluated through the density of SDS PAGE using the Quantity One imaging system. Lane M, molecular weight marker; Lane 1, Tat-MS2-miR146a VLPs; Lane 2, Tat-MS2-miRNC VLPs; Lane 3, unmodified MS2-miR146a VLPs.
Abbreviations: bp, base pair, VLPs, virus like particles.
miR-146a expression in BXSB mice
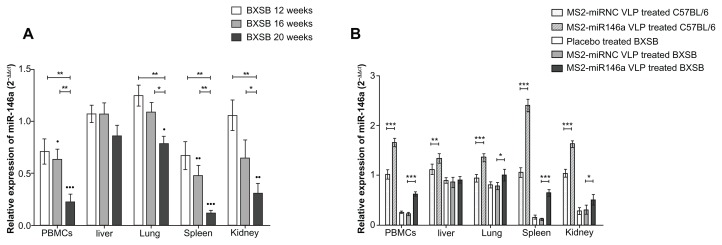
In order to evaluate miR-146a expression in lupus-prone BXSB mice, we performed miRNA qRT-PCR using multiple tissue samples isolated from BXSB and corresponding control mice at 12, 16, and 20 weeks of age. U6 RNA was used as an internal control during this analysis. As indicated in Figure 2A, 20-week-old BXSB mice showed lower miR-146a expression in PBMCs, lung, spleen, and kidney tissues than did age-matched C57BL/6 mice (P < 0.001, P < 0.05, P < 0.001, and P < 0.005, respectively). The miR-146a expression differences ranged from 79% (20-week-old BXSB vs age-matched C57BL/6 mice, in lung tissue) to 12% (20-week-old BXSB vs age-matched C57BL/6 mice, in spleen tissue). Notably, compared to the C57BL/6 controls, the miR-146a in spleen and in the PBMCs of the BXSB mice was constitutively downregulated; further, its expression was significantly reduced at 20 weeks of age, to a level approximately equal to that of BXSB mice in a severely diseased state.20
Figure 2.
The miR-146a expression in BXSB and C57BL/6 mice. (A) Total RNA was extracted from PBMCs, liver, lung, spleen, and kidney tissue of BXSB mice (n = 5 per group) at 12, 16 and 20 weeks of age (equating to a mild, moderate, or severe disease state, respectively) and control C57BL/6 mice (n = 5 per group) at 20 weeks of age. The miRNA expression profile was determined by qRT-PCR assay, normalized by U6 RNA, and then compared with that of C57BL/6 mice. *P < 0.05 and **P < 0.01, respectively, with regard to comparisons between the miR-146a expression levels of BXSB mice at different ages. •P < 0.05; ••P < 0.01 and •••P < 0.001, respectively, with regard to comparisons between the miR-146a expression levels in BXSB mice and age-matched C57BL/6 mice. (B) After administration of MS2-miR146a VLP over 12 days (at approximately 18 weeks of age, n = 5 per group), miR-146a levels in PBMCs, liver, lung, spleen, and kidney tissue were measured by qRT-PCR as described above.
Notes: “*,” “**,” and “***” indicate P < 0.05, P < 0.01 and P < 0.001, respectively.
Abbreviations: PBMC, peripheral blood mononuclear cell, VLPs, virus like particles.
MS2 VLPs induced miR-146a expression in BXSB mice
In our previous studies, we showed that the MS2 VLP-based miRNA delivery system worked effectively both in vitro and in vivo.17 In the current study, we proved that this system also showed its impact on mature miR-146a expression. After the administration of MS2-miR146a VLPs, high levels of miR-146a were detected in PBMCs, lung, spleen, and kidney tissues from both BXSB and C57BL/6 mice (Figure 2B). Conversely, this upregulation was not observed in MS2- miRNC VLP-treated mice. It should also be noted that the miR-146a expression in BXSB mice was close to the normal level, with the help of MS2-miR146a VLPs.
MS2-miR146a VLP-induced elimination of autoantibody
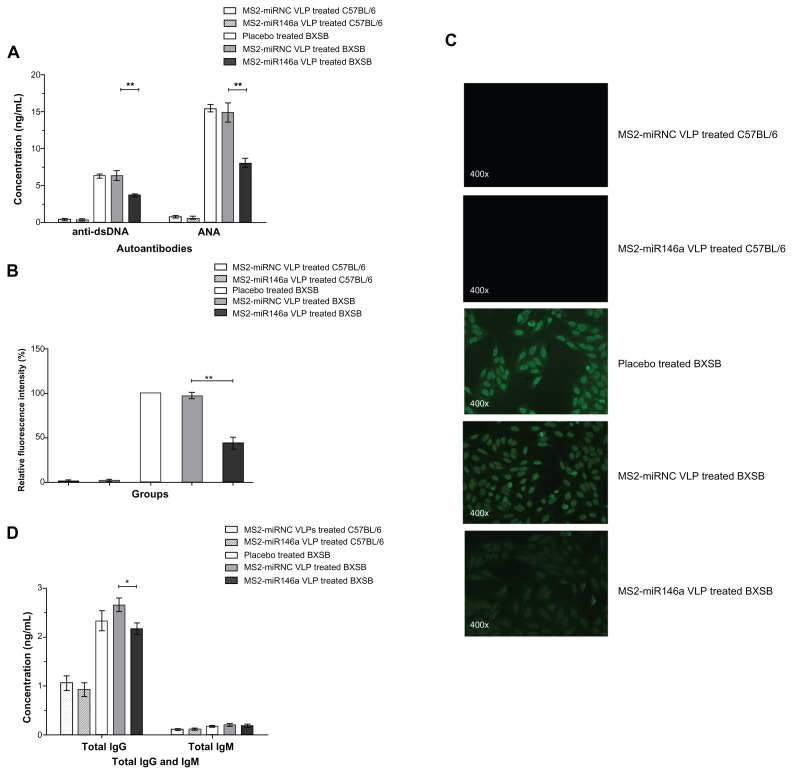
Since anti-dsDNA and ANA are two of the most widely used indicators in the diagnosis of SLE, and their levels reflect the status of autoantibody production, we tested for the presence of anti-dsDNA antibodies and ANA in mice injected with MS2-miR146a VLPs and in control mice, using ELISA and immunofluorescence assay. The MS2-miR146a VLP treatment groups had significantly lower plasma anti-dsDNA levels than did MS2-miRNC VLP-treated animals (3.72 ± 0.57 ng/mL vs 6.40 ± 1.61 ng/mL, P < 0.01) (Figure 3A). Moreover, the ANA concentration was 8.07 ± 1.38 ng/mL in MS2-miR146a VLP-treated BXSB mice and 14.94 ± 3.01 ng/ml in MS2- miRNC VLP-treated controls (P < 0.01). Consistent with the ELISA results, the intensity of ANA fluorescence was reduced by 45.29% (P < 0.01) in the MS2-miR146a VLP-treated group, as determined using indirect immunofluorescence (Figure 3B and C); the ANA titer was 1:1,600 in MS2-miRNC VLP-treated mice, and 1:200 in MS2-miR146a VLP-treated mice, suggesting that MS2-miR146a VLPs induced effective inhibition of ANA production. Further, MS2-miR146a VLPs also led to a significant decrease in total IgG (2.66 ± 0.31 ng/mL vs 2.17 ± 0.27 ng/mL, P < 0.05), while there was no significant difference in IgM levels between the MS2-miR146a VLP-treated mice and the MS2-miRNC VLP-treated control groups (0.22 ± 0.06 ng/mL vs 0.19 ± 0.08 ng/mL, P = 0.548) (Figure 3D).
Figure 3.
Decreased autoantibody production after MS2-miR146a VLP administration. (A) Anti-dsDNA and ANA levels in MS2-miR146a VLP-treated BXSB mice and their respective control mice (approximately 18 weeks of age, n = 5 per group) were evaluated by ELISA. (B and C) Immunofluorescence assay was used to verify the reduced expression of ANA in samples from MS2-miR146a VLP-treated BXSB mice (diluted 200-fold). Representative photomicrographs of ANA fluorescence in treated and respective control groups are shown. Fluorescence patterns were detected by fluorescence microscopy at 400× magnification. The fluorescence intensity of ANA in samples from placebo-treated BXSB mice was designated as 100%. (D) Total IgG and total IgM concentrations in MS2-miR146a VLP-treated BXSB mice and their respective control mice (approximately 18 weeks of age, n = 5 per group) were analyzed by ELISA.
Notes: *P < 0.05 and **P < 0.01, respectively.
Abbreviations: ANA, autoantibody to Nuclear Antigen, ELISA, enzyme-linked immunosorbent assay, VLPs, virus like particles.
MS2-miR146a VLPs inhibit the expression of SLE-related cytokine
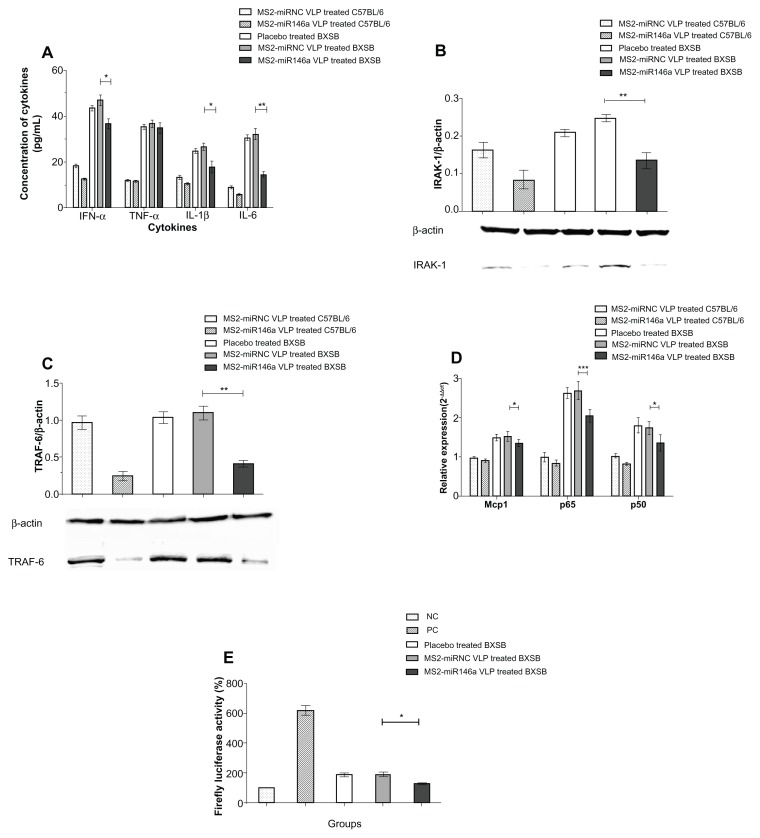
Four SLE-related proinflammatory cytokines, IFN-α, TNF-α, IL-1β, and IL-6, were measured to evaluate the severity of SLE and the potential mechanism by which MS2-miR146a VLPs exert an effect in BXSB mice. In plasma, IFN-α, IL-1β, and IL-6 concentrations were significantly reduced in MS2 miR146a VLP-treated BXSB mice, compared to controls (P < 0.001, P < 0.05, and P < 0.001, respectively). Conversely, plasma TNF-α levels differed only mildly between these two groups, and this difference was not significant (P = 0.532) (Figure 4A).
Figure 4.
MS2-miR146a VLPs inhibit plasma cytokine production and impair the activation of NF-κB. (A) Plasma levels of IFN-α, TNF-α, IL-1β, and IL-6 were measured by competitive ELISA assay. (B and C) After administration of MS2-miR146a or control VLPs over 12 days (approximately 18 weeks of age, n = 5 per group), the expression of IRAK-1 and TRAF-6 in PBMCs was analyzed by Western blot. The relative concentration was normalized against that of β-actin. Photomicrographs are representative of results obtained from each group. (D) Total RNA was isolated from PBMCs and the mRNA transcript levels of NF-κB-responsive genes (Mcp1, NF-κB p65, and NF-κB p50) were determined by qRT-PCR using U6 RNA as an internal control. (E) NF-κB activity analysis using a luciferase assay. Luciferase activity was defined as the ratio of the reporter firefly luciferase activity to the control Renilla luciferase activity. The value obtained from PBMCs of PBS-treated C57BL/6 mice was designated as 100%.
Notes: *P < 0.05; **P < 0.01 and ***P < 0.001, respectively.
Abbreviations: ELISA, enzyme-linked immunosorbent assay; IFN-α, Interferon-α; Il-1β, Interleukin-1β; Il-6, Interleukin-6; IRAK-1, Il-1 receptor-associated kinase; Mcp1, monocyte chemotactic protein 1; NF-κB, nuclear factor-κB; PBMCs, peripheral blood mononuclear cell; TNF-α, tumor necrosis factor-α; TRAF-6, TNF receptor-associated factor-6; VLPs, virus like particles; NC, Negative control; PC, positive control.
MS2-miR146a VLPs participated in a tolllike receptor (TLR)-mediated immune response in lupus-prone mice
To further evaluate the therapeutic efficacy of MS2-miR146a VLPs and explore the possible mechanism involved, we measured miR-146a target gene expression levels, as well as levels of expression of the mRNA transcripts of some additional TLR-related genes – Mcp1, NF-κB p65, and NF-κB p50 – by Western blot or qRT-PCR.21 According to previous studies, IRAK-1 and TRAF-6 are two major targets of miR-146a.22 In our study, MS2-miR146a VLPs effectively suppressed both IRAK-1 and TRAF-6 in the PBMCs of lupus-prone mice, while no such changes were observed in control groups (Figure 4B and C). We also observed that Mcp1, NF-κB p65, and NF-κB p50 mRNA levels were significantly reduced in MS2-miR146a VLP-treated BXSB mice, compared to age-matched MS2-miRNC VLP-treated control mice (P < 0.05, P < 0.001, and P < 0.05, respectively, Figure 4D), suggesting that the TLR pathway was compromised. At the same time, the luciferase reporter assay showed that NF-κB activity was substantially reduced after MS2-miR146a VLP administration, which also suggests suppression of TLR pathway activity (P < 0.05, Figure 4E).
Discussion
In this study, we first evaluated the miR-146a expression profile in lupus-prone BXSB mice. As one of the most widely used spontaneous genetically lupus-prone murine models,20 this phenotype exhibits significantly reduced miR-146a in PBMCs, lung, spleen, and kidney tissues. Subsequently, we analyzed the miR-146a expression at 12, 16, and 20 weeks of age in BXSB mice. The level of miR-146a in BXSB mice tended to constitutively decrease with age in PBMCs, lung, spleen, and kidney tissues, and this reduction in expression was correlated with disease progression. However, the reported expression patterns of miR-146a in tissue samples from both human SLE patients and some lupus-prone murine models are not fully consistent. For example, Tang et al observed downregulation of miR-146a in PBMCs in SLE patients,9 while this has not been observed in other studies.23,24 Differences are also apparent when comparing human SLE with murine lupus. Dai et al25 reported that miR-146a was not upregulated in purified splenic B cells, but was significantly upregulated in T cells from lupus-prone MRL-lpr mice, while universally low-level expression of miR-146a was evident in our study. Many factors, including differences in disease stage, treatment history, types of samples, and detection methods, are possible causes for the discrepancies between reported data from different studies, though the most important difference is likely to be the genetic background of SLE patients or animal models. While miR-146a is a well-recognized inhibitor of innate immunity and inflammatory responses,10 its expression pattern and mechanism may differ between humans and mice because of interspecies genetic variation, including the genetic polymorphism in miRNA genes and the 3′UTR sequences of its target genes, gene translocation, gene amplification, and gene deletion in the host genome.26,27
A particularly novel aspect of our study is the administration of miRNAs to lupus-prone mice for therapeutic purposes. Compared with other SLE molecular therapies, such as B cell depletion (BCD) and anticytokine therapy, miRNA-based therapy shows several attractive characteristics. Since physiological gene expression networks have evolved extensively to accommodate the regulation of endogenous miRNAs, miRNA-based therapy seems to be more mild and moderate than that based on exogenous RNA interfere (RNAi). In addition, this type of treatment may be more effective because the regulation of a single miRNA involves hundreds of targets in multiple pathways rather than one or two transcripts.28 Therefore, the miRNA-based approach is a promising strategy for SLE treatment.
In the present study, MS2 VLP-based miR-146a treatment first significantly increased the expression of miR-146a, and then showed its impact on lupus-prone mice via the inhibition of pathogenic autoantibody production. After receiving four doses over 12 days, an upregulation of miR-146a could be observed in PBMCs, lung, spleen, and kidney tissues in BXSB mice. Since SLE is mainly characterized by the aberrant activation of T and B lymphocytes for the production of inflammatory cytokines and autoantibodies,2 the induced miR-146a expression in PBMCs may play an essential role in immune regulation. And as expected, the MS2-miR146a VLP-treated lupus-prone mice subsequently showed a remarkable decrease in anti-dsDNA, ANA, and total IgG antibody burden, and a significant improvement in SLE-related cytokines. None of these effects were observed in the control VLP-injected groups. Notably, since 16-week-old BXSB mice were used in this study, the MS2-miR146a VLP treatment was initiated after SLE symptoms had manifested.20,29 It is possible that this artificial miR-146a interference may have a similar positive influence with regard to the prevention of SLE in these mice.
A question of interest is how miR-146a inhibits the progression of murine lupus. One possible mechanism is that a high concentration of miR-146a downregulates the TLR-mediated immune response, and moderates the production of several SLE-related cytokines, thus inhibiting the overexpression of autoantibodies. It has been demonstrated that TLR7 and TLR9 signaling are required for optimal production of pathogenic autoantibodies targeting RNA-associated or DNA-associated antigens in SLE patients and lupus-prone mice.30–32 Nickerson et al33 showed that suppression of TLR7 and TLR9 could lead to a significant decrease in ANA and anti-dsDNA autoantibody in MRL/lpr mice. Notably, IRAK1 and TRAF6, the major targets of miR-146a, are two crucial signaling factors involved in the TLR7 and TLR9 pathway. Thus, their overexpression in mice treated with MS2 VLPs containing miR-146a could result in suppressed activation of downstream signal molecules such as NF-κB, and suppress the expression of NF-κB-targeting proinflammatory cytokines, including IL-6, IL-1b, TNF-α, and type-I IFN, which are involved in SLE incidence or progression.10,34,35 It has been shown that the increased expression of type-I IFNs, termed the IFN-signature, plays a central role in SLE pathogenesis, resulting in autoantibody production and disease activity.36 It has also been shown that B cell hyperactivity and autoantibody production can be reduced by neutralizing IL-6, and restored by adding exogenous IL-6 in vitro.37,38 In order to explore the therapeutic effects of MS2-miR146a VLPs on lupus-prone mice and the related mechanisms, we selectively quantified, by multiple methods, IRAK1, TRAF6, certain NF-κB responsive genes, and several SLE-related cytokines, including IFN-α, IL-1β, IL-6, and TNF-α. Significantly low levels of IRAK1 and TRAF6, accompanied by lower NF-κB activity and decreases in IFNα, IL-1β, and IL-6, were observed in MS2-miR146a VLP-treated mice. These results suggested possible correlation between miRNAs and TLR-mediated immune regulation and thus supported our hypothesis that the therapeutic effect of MS2-miR146a VLPs was achieved via a TLR signaling pathway. Several published reports have suggested that increased levels of TNF-α parallel the increasing organ inflammation in MRL/lpr and NZB/W mice.39,40 Interestingly, the expression of TNF-α was relatively constant during the administration of MS2-miR146a VLPs in our study, even though the levels of autoantibodies dropped dramatically. Furthermore, the cause of the difference from either genetic background or other key factors will need to be studied extensively.
Off-target effects have always been a concern of RNAi-based therapy.41 Off-target effects include specific effects, which are caused by the limited degree of complementarity to nontargeted mRNAs, and nonspecific off-target effects, which are due to immune and toxicity-related responses derived from the RNAi construct itself or the delivery vehicle. 42 However, since the induced miR-146a expression in BXSB mice did not exceed the normal range among healthy controls, the specific off-target effects of MS2-miR146a VLP administration were limited. Also, only a slight immune response was observed in MS2-miRNC VLP-treated mice; the acute toxicity of this approach was determined previously. 17 Thus, the MS2 VLP-based miR-146a therapy is not only convenient and effective, but also has a relatively low off-target effect.
Our experiments addressed whether MS2 VLP-based miR-146a delivery is an effective therapeutic option for murine lupus. Because of the rapid progression of murine lupus after 16 weeks of age in BXSB mice (50% mortality for males by approximately 5 months of age16), it is difficult to administer a long-term treatment examining the decrease of autoantibodies at different time points. However, since the TLR-involved therapeutic approach has been verified in this study, we anticipate a similar benefit from a study involving long-term administration of MS2 VLPs.
Conclusion
In conclusion, this is the first report of the administration of miR-146a for the treatment of SLE. Our data provide strong evidence that delivering miR-146a systemically via MS2 VLPs is an effective therapeutic approach for reducing the levels of antibodies in lupus-prone mice. This study provides us with a better understanding of the role of miR-146a as an important inflammatory inhibitor in SLE, and suggests that the TLR signal pathway is involved in this regulation.
Acknowledgments/disclosure
The authors thank Professor DS Peabody (University of New Mexico School of Medicine) for supplying the MS2 capsid protein gene. We are also grateful to Dr Shipeng Sun (Guang’anmen Hospital) for his helpful advice. This study was supported by the National High Technology Research and Development Program of China (863 program No 2011AA02A116). The authors report no conflicts of interest in this work.
References
- 1.Sherer Y, Gorstein A, Fritzler MJ, Shoenfeld Y. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum. 2004;34(2):501–537. doi: 10.1016/j.semarthrit.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358(9):929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 3.Yildirim-Toruner C, Diamond B. Current and novel therapeutics in the treatment of systemic lupus erythematosus. J Allergy Clin Immunol. 2011;127(2):303–314. doi: 10.1016/j.jaci.2010.12.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagga S, Bracht J, Hunter S, et al. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122(4):553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Dai R, Ahmed SA. MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Transl Res. 2011;157(4):163–179. doi: 10.1016/j.trsl.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceribelli A, Yao B, Dominguez-Gutierrez PR, Nahid MA, Satoh M, Chan EK. MicroRNAs in systemic rheumatic diseases. Arthritis Res Ther. 2011;13(4):229. doi: 10.1186/ar3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garchow BG, Encinas OB, Leung YT, et al. Silencing of microR6-21 in vivo ameliorates autoimmune splenomegaly in lupus mice. EMBO Mol Med. 2011;3(10):605–615. doi: 10.1002/emmm.201100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Chen XP, Li YJ. MicroRNA-146a and human disease. Scand J Immunol. 2010;71(4):227–231. doi: 10.1111/j.1365-3083.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- 9.Tang Y, Luo X, Cui H, et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009;60(4):1065–1075. doi: 10.1002/art.24436. [DOI] [PubMed] [Google Scholar]
- 10.Boldin MP, Taganov KD, Rao DS, et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011;208(6):1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson BL, Jr, McCray PB. Current prospects for RNA interference-based therapies. Nat Rev Genet. 2011;12(5):329–340. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Lu Z, Wientjes MG, Au JL. Delivery of siRNA therapeutics: barriers and carriers. AAPS J. 2010;12(4):492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J. Efficient gene knockdowns in mouse embryonic stem cells using microRNA-based shRNAs. Methods Mol Biol. 2010;650:241–256. doi: 10.1007/978-1-60761-769-3_18. [DOI] [PubMed] [Google Scholar]
- 14.Carrico ZM, Romanini DW, Mehl RA, Francis M. Oxidative coupling of peptides to a virus capsid containing unnatural amino acids. Chem Commun (Camb) 2008;14(10):1205–1207. doi: 10.1039/b717826c. [DOI] [PubMed] [Google Scholar]
- 15.Kovacs EW, Hooker JM, Romanini DW, Holder PG, Berry KE, Francis MB. Dual-surface-modified bacteriophage MS2 as an ideal scaffold for a viral capsid-based drug delivery system. Bioconjugate Chem. 2007;18(4):1140–1147. doi: 10.1021/bc070006e. [DOI] [PubMed] [Google Scholar]
- 16.Wu M, Sherwin T, Brown WL, Stockley PG. Delivery of antisense oligonucleotides to leukemia cells by RNA bacteriophage capsids. Nanomedicine. 2005;1(1):67–76. doi: 10.1016/j.nano.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Pan Y, Zhang Y, Jia T, Zhang K, Li J, Wang L. Development of a microRNA delivery system based on bacteriophage MS2 virus-like particles. FEBS J. 2012;279(7):1198–1208. doi: 10.1111/j.1742-4658.2012.08512.x. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Wei B, Wei Y, Zhang K, et al. Development of an antisense RNA delivery system using conjugates of the MS2 bacteriophage capsids and HIV-1 TAT cell-penetrating peptide. Biomed Pharmacother. 2009;64(3):313–318. doi: 10.1016/j.biopha.2008.07.086. [DOI] [PubMed] [Google Scholar]
- 20.Izui S, Ibnou-Zekri N, Fossati-Jimack L, Iwamoto M. Lessons from BXSB and related mouse models. Int Rev Immunol. 2000;19(4–5):447–472. doi: 10.3109/08830180009055507. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Zutter MM, Santoro SA, Clark RA. A three-dimensional collagen lattice activates NF-kappaB in human fibroblasts: role in integrin alpha2 gene expression and tissue remodeling. J Cell Biol. 1998;140(3):709–719. doi: 10.1083/jcb.140.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nahid MA, Pauley KM, Satoh M, Chan EK. MiR-146a is critical for endotoxin-induced tolerance: IMPLICATION IN INNATE IMMUNITY. J Biol Chem. 2009;284(50):34590–34599. doi: 10.1074/jbc.M109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai Y, Sui W, Lan H, Yan Q, Huang H, Huang Y. Comprehensive analysis of microRNA expression patterns in renal biopsies of lupus nephritis patients. Rheumatol Int. 2009;29(7):749–754. doi: 10.1007/s00296-008-0758-6. [DOI] [PubMed] [Google Scholar]
- 24.Te JL, Dozmorov IM, Guthridge JM, et al. Identification of unique microRNA signature associated with lupus nephritis. PLoS One. 2010;5(5):10344. doi: 10.1371/journal.pone.0010344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai R, Zhang Y, Khan D, et al. Identification of a common lupus disease-associated microRNA expression pattern in three different murine models of lupus. PLoS One. 2010;5(12):14302. doi: 10.1371/journal.pone.0014302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sethupathy P, Collins FS. MicroRNA target site polymorphisms and human disease. Trends Genet. 2008;24(10):489–497. doi: 10.1016/j.tig.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Huppi K, Volfovsky N, Mackiewicz M, et al. MicroRNAs and genomic instability. Semin Cancer Biol. 2007;17(1):65–73. doi: 10.1016/j.semcancer.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455(7209):58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 29.Perry D, Sang A, Yin Y, Zheng YY, Morel L. Murine models of systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:271694. doi: 10.1155/2011/271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kono DH, Haraldsson MK, Lawson BR, et al. Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus. Proc Natl Acad Sci U S A. 2009;106(29):12061–12066. doi: 10.1073/pnas.0905441106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416(6881):603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 32.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6(11):823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nickerson KM, Christensen SR, Shupe J, et al. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184(4):1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Campisi J, Benz CC. Expression of microRNA-146 suppresses NF-kappaB activity with reduction of metastatic potential in breast cancer cells. Oncogene. 2008;27(42):5643–5647. doi: 10.1038/onc.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pauley KM, Satoh M, Chan AL, Bubb MR, Reeves WH, Chan EK. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res Ther. 2008;10(4):101. doi: 10.1186/ar2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25(3):383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Grondal G, Gunnarsson I, Ronnelid J, Rogberg S, Klareskog L, Lundberg I. Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 2000;18(5):565–570. [PubMed] [Google Scholar]
- 38.Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004;13(5):339–343. doi: 10.1191/0961203304lu1023oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yokoyama H, Kreft B, Kelley VR. Biphasic increase in circulating and renal TNF-alpha in MRL-lpr mice with differing regulatory mechanisms. Kidney Int. 1995;47(1):122–130. doi: 10.1038/ki.1995.14. [DOI] [PubMed] [Google Scholar]
- 40.Brennan DC, Yui MA, Wuthrich RP, Kelley VE. Tumor necrosis factor and IL-1 in New Zealand Black/White mice. Enhanced gene expression and acceleration of renal injury. J Immunol. 1989;143(11):3470–3475. [PubMed] [Google Scholar]
- 41.Rao DD, Senzer N, Cleary MA, Nemunaitis J. Comparative assessment of siRNA and shRNA off target effects: what is slowing clinical development. Cancer Gene Ther. 2009;16(11):807–809. doi: 10.1038/cgt.2009.53. [DOI] [PubMed] [Google Scholar]
- 42.Singh S, Narang AS, Mahato RI. Subcellular fate and off-target effects of siRNA, shRNA, and miRNA. Pharm Res. 2011;28(12):2996–3015. doi: 10.1007/s11095-011-0608-1. [DOI] [PubMed] [Google Scholar]