Abstract
Introduction:
Leishmaniasis is a parasitic disease transmitted by phlebotomine sandflies. Between 700,000 and 1.2 million cases of cutaneous leishmaniasis and between 200,000 and 400,000 cases of visceral leishmaniasis (VL), which is fatal if left untreated, occur annually worldwide. Liposomal amphotericin B (LAMB), alone or in combination with other drugs, has been extensively studied as VL treatment, but data on routine field use are limited, and several challenges to patients' access to this life-saving drug remain.
Areas covered:
This article provides a review of clinical studies on LAMB for VL and other forms of leishmaniasis. The current development of generic versions of LAMB and related challenges are also discussed.
Expert opinion:
LAMB proved to be highly efficacious and safe in over 8000 VL patients treated by MÉdecins Sans Frontières in South Asia, and its use was feasible even at primary healthcare level. Despite requiring higher doses, LAMB is the drug of choice to treat vulnerable groups (e.g., pregnant or HIV positive) and relapsing VL patients in East Africa. LAMB should be included in national VL guidelines and registered in all VL endemic countries. Its cost should be further reduced and regulatory pathways to prove bioequivalence for generic LAMB products should be implemented.
Keywords: drug access, generics, leishmaniasis, liposomal amphotericin B, review, treatment
1. Background
Leishmaniasis is a parasitic and vector-borne disease transmitted by the phlebotomine sandfly. It occurs in several forms in humans, the two most common of which are visceral leishmaniasis (VL) and cutaneous leishmaniasis (CL). Among parasitic diseases, leishmaniasis is responsible for the second-highest burden of disease after malaria: 2,357,000 disability adjusted life years (DALYs). There are 350 million people in 98 countries at risk of contracting the disease [1]; between 700,000 and 1.2 million cases of CL, and between 200,000 and 400,000 cases of VL occur annually worldwide. A lack of effective surveillance systems, however, makes it difficult to ascertain the true burden of disease [2].
VL, also known as kala azar, occurs mainly in poor, remote areas in 70 countries across South Asia, East Africa, Latin America and the Mediterranean region. The five most affected countries are: India, Sudan, Bangladesh, South Sudan and Ethiopia [2]. The disease is characterized by prolonged fever, enlarged spleen and liver, substantial weight loss and progressive anemia. It is fatal if not treated. VL is mainly caused by two species of the Leishmania parasite: L. donovani, prevalent in South Asia and East Africa, and L. infantum, prevalent in the Mediterranean region and in Latin America.
Cutaneous leishmaniasis has a wider geographical distribution. The five most affected countries are Afghanistan, Algeria, Brazil, Iran and Syria [2]. The disease is characterized by lesions on the skin, either self-healing or chronic. Some forms of CL, particularly diffuse CL and mucosal leishmaniasis (ML) can be severely disfiguring and extremely debilitating, causing significant morbidity and, in some cases, death. There are numerous causative species of CL, which differ in geographical distribution, clinical presentation, the associated risk of complications (e.g., ML) and response to treatment.
Finally, post-kala azar dermal leishmaniasis (PKDL), a cutaneous complication of VL, can occur months or years after initial successful treatment. PKDL is a potential parasitic reservoir for VL, thus contributing to transmission of the disease.
HIV co-infection, particularly with VL, is a growing problem: co-infection often results in severe forms of the disease, and is frequently refractory to treatment, even when patients have started antiretroviral therapy.
This expert opinion publication will review the use of liposomal amphotericin B (LAMB) for the treatment of leishmaniasis, with a particular focus on VL, where its use has been largely applied.
2. Medical need
Treatment objectives vary with the form of leishmaniasis. In VL, the main objective of treatment is to save the patient's life, as the disease is fatal if untreated. Patients with severe VL (e.g., severely anemic patients) are at increased risk of death in the days following admission in the treatment center, due to progression of the illness or drug-induced toxicity. Drugs against VL therefore need to be rapidly effective and safe. High drug efficacy is also essential to prevent relapses. In South Asia and East Africa, where transmission of VL remains anthroponotic (i.e., humans are the main reservoir of the parasite), treatment can also play a pivotal part in disease control. In CL, the primary objective of treatment is to reduce the duration and to heal the cutaneous lesion and, for a limited number of species (e.g., L. braziliensis), prevent the occurrence of mucous lesions.
2.1. Existing treatments
There are numerous drugs available for the treatment of VL (and CL). A summary of these treatments is presented in Table 1. Pentavalent antimonials – sodium stibogluconate (SSG) and meglumine antimoniate (MA) – have been the mainstay of treatment against VL for over five decades. Drug resistance to antimonials, specifically in India, has been a growing problem since the 1980s [3]. In addition, pentavalent antimonials are associated with important toxicity and, in the case of VL, with significantly high death rate, especially in some sub-groups of patients: severely ill, > 45 years, HIV co-infected. For these reasons, development of new drugs for leishmaniasis became a priority and several new drugs have been introduced. Conventional amphotericin B (deoxycholate) and later its lipid formulations, including liposomal formulations (LAMB), were tested for use against VL and introduced for this indication. This was followed by the introduction of miltefosine (MF), the first oral treatment against leishmaniasis, in 2002. Paromomycin (PM), a disused aminoglycoside antibiotic, was also developed against VL and was registered in 2006, initially in India.
Table 1.
Summary of currently available monotherapies for VL*.
| Drugs | SSG/MA | Amphotericin B deoxycholate | LAMB | MF | PM |
|---|---|---|---|---|---|
| Drug class | Pentavalent antimonial | Polyene antibiotic | Polyene antibiotic | Alkyllysophospholipid | Aminoglycoside |
| Regimen | 20 mg/kg daily for 20 – 30 days (depending on geographic area) |
1 mg/kg every other day for 30 days (15 mg/kg total dose) |
10 – 30 mg/kg total dose in 1 – 6 doses over 1 – 10 days |
1.5 – 2.5 mg/kg/day over 28 days (South Asia only) |
11 mg/kg (= 15 mg/kg sulfate) for 21 days (in monotherapy) |
| Marketing authorization holder |
Albert David (generic SSG) GSK (Pentostam™) Sanofi-Aventis (Glucantime™) |
Bristol-Myers Squibb (Fungizone™) | Gilead (AmBisome™) | Paladin (Impavido™) | Gland Pharma |
| Administration | i.v. or i.m. | Slow i.v. | Slow i.v. | Oral | i.m. |
| Clinical efficacy (cure at 6 months post-treatment) | 35 – 95% (depending on geographic area) |
> 90% all regions | 95% in South Asia (dose of ≥ 10 mg/kg) ∼ 90% in other regions (doses of 20 – 30 mg/kg) |
> 90% in South Asia not clearly established in other regions |
> 90% in monotherapy in South Asia and in combination with SSG for 17 days in Africa |
| Resistance | Failure rate as high as 60% (Bihar state, India) | Not documented | Not documented | Laboratory isolates | Laboratory isolates |
| Toxicity | +++ Cardiac toxicity Pancreatitis Nephrotoxicity Hepatotoxicity |
+++ Nephrotoxicity Hypokalemia Rigors and chills during infusion |
+/- Rigors and chills during infusion Hypokalemia and renal failure are rare |
+ Gastrointestinal effects (in 20 – 55% of patients, usually mild) Nephrotoxicity Hepatotoxicity Possible teratogenicity |
+ Nephrotoxicity Ototoxicity Hepatotoxicity Pain at injection site |
| Approximate cost of drugs per course USD [70] |
SSG ∼ US$55 Glucantime ∼ US$60 |
Generic versions price ∼ US$20 | WHO negotiated price: US $126 – 378 |
WHO negotiated price: ∼ US$65 – 150 | ∼ US$15 |
| Issues | Quality control Availability Length of treatment Painful injection (i.m.) Toxicity Resistance in India |
Slow i.v. infusion Dose-limiting Nephrotoxicity Heat stability |
High price Slow i.v. infusion Heat stability (storage < 25°C) Efficacy variable between regions |
Relatively high price Possible teratogenicity Potential for resistance Patient compliance |
Efficacy variable between and within regions |
*Based on data from the WHO expert committee report 2010.
i.m.: Intramuscular; i.v.: Intravenous; LAMB: Liposomal amphotericin B; MA: Meglumine antimoniate; MF: Miltefosine; PM: Paromomycin; SSG: Sodium stibogluconate.
Over the last 10 years, considerable efforts have been made to develop either short courses (specifically for LAMB) or combinations (co-administrations) of current drugs. A 17-day regimen of SSG and PM has been shown to be as safe and effective as the standard 30-day treatment of SSG in East Africa [4]. This regimen was recommended in 2010 as a first-line treatment for the region by a WHO expert committee [1]. Phase III trials were completed in 2010 for three double-drug combinations (MF and PM, LAMB and MF, LAMB and PM) and single-dose LAMB (10 mg/kg dose) in India, all of which showed efficacy of above 95% [5,6]. However, particularly for treatments in South Asia and East Africa, little is known about the feasibility and field effectiveness of these new treatments in a wide population.
Despite these developments, in the large majority of cases, treatment of VL across the world still relies on pentavalent antimonials or conventional amphotericin B, despite their inherent toxicities or complex administration [7]. MF has been increasingly used in India, Bangladesh and Nepal in the last years, but there is recent evidence of its declining efficacy in monotherapy [8]; and concerns remain about its teratogenicity. Implementation of PM has been limited to use in combination with SSG in East Africa, where its uptake continues to increase. Use in monotherapy has been avoided due to risk of resistance and its variable efficacy between and within geographical regions [9]. Its use in South Asia has been restricted to clinical studies. Large scale use of LAMB will be discussed later, but has largely been limited to a few key sites with the adequate resources to use the drug.
3. Market review
Uncertainty on the disease's true burden of VL in South Asia and East Africa prevents a correct estimation of the current and future market size. This uncertainty directly impacts on drug demand forecasting, and subsequently on production and pricing policies by pharmaceutical companies.
The demand for VL drugs is reliant on the treatment recommendations adopted by high-burden countries. As India alone accounts for 73% of global cases, the future update of treatment guidelines in India will be critical to determine the potential market for LAMB and other drugs for VL.
With the vast majority of the 200,000 – 400,000 VL cases annually worldwide occurring in disadvantaged populations in low- and middle-income countries, access to treatment mostly relies on resources mobilized by donors, with the market for VL drugs being widely shaped by donors' policies. Full financing of VL treatment programs by donors and governments from endemic countries is important to increase the attractiveness of the VL drug market to private companies.
4. Current research goals
Current research is now shifting away from developing optimized regimens of existing drugs toward demonstrating their implementation is feasible in the field. A joint project by the Indian Council of Medical research (RMRIMS/ICMR), the Indian National Vector Borne Disease Control Programme (NVBDCP), the Bihar State Health Society (BSHS), the Drugs for Neglected Diseases initiative (DNDi), the WHO special program for tropical disease research (TDR), OneWorld Health (OWH) and Médecins Sans Frontières (MSF) is ongoing in India to implement a pilot program and feasibility study on both combinations and single-dose LAMB. In Bangladesh, TDR and ICDDR have implemented a proof-of-concept feasibility study at the primary healthcare level using a single 10 mg/kg dose of LAMB. This will provide valuable information on wide-scale use in the field, as well as facilitate access to LAMB-based treatments.
The other major priority now is to develop new chemical entities to treat leishmaniasis that will circumvent the key constraints of the current drugs, that is, toxicity, lack of feasibility of use and/or high cost. DNDi, a product development partnership dedicated to the development of new treatments for a number of diseases, including leishmaniasis, has developed a target product profile (TPP) for drug research and development for the treatment of VL (Table 2) [10,11]. Current drugs are a far cry from even a minimal target profile developed for a new chemical entity (NCE). Developing an NCE with an optimal TPP is expected to allow greater feasibility of use in the field, greater roll out and access to treatment and sustainability of control and elimination efforts against VL. Such a tool could also be used for CL, PKDL and for HIV co-infected VL patients. Strategies for developing new medicines for leishmaniasis include high-throughput screening and sourcing of high quality leads to source NCEs, as well as therapeutic switching and reformulating existing drugs (e.g., oral formulations of amphotericin B). However, it is unlikely that an NCE will be registered for use against VL before 2018.
Table 2.
TPP for new clinical entities against VL (as monotherapy)*.
| Optimal target profile | Minimal target profile | |
|---|---|---|
| Target label | VL and PKDL | VL |
| Species | All species | Leishmania donovani |
| Distribution | All areas | Either India or Africa |
| Target population | Immunocompetent and immunosuppressed | Immunocompetent |
| Clinical efficacy | > 95% | > 90% |
| Resistance | Active against resistant strains | |
| Safety and tolerability | No AEs requiring clinical monitoring | One clinical monitoring visit in mid/end point |
| Contraindications | None | Pregnancy/lactation |
| Interactions | None – compatible for combination therapy | None for malaria, TB and HIV concomitant therapies |
| Formulation | Oral/i.m. depot | Oral/i.m. depot |
| Treatment regimen | 1/day for 10 days p.o./3 shots over 10 days‡ | b.i.d. for < 10 days p.o.; or > 3 shots over 10 days |
| Stability | 3 years in zone 4 | Stable under conditions that can be reasonably achieved in the target region (> 2 years) |
| Cost | < US$10/course | < US$125/course |
*Developed by and reproduced with permission by DNDi.
‡For primary VL only. PKDL, HIV co-infection and relapse case treatments may require longer treatment durations.
DNDi: Drugs for Neglected Diseases initiative; PKDL: Post-kala azar dermal leishmaniasis; TPP: Target product profile; VL: Visceral leishmaniasis.
5. Review of LAMB use against leishmaniasis
This review will focus on the use of LAMB to treat VL. Table 3 summarizes regimens involving LAMB for the treatment of VL, some of which have only been developed in the last 5 – 6 years. Data on the use of LAMB for other forms of leishmaniasis (CL, MCL and PKDL) are scarce and summarized in the Box 1.
Table 3.
Potential regimens of LAMB that have been developed for use against VL*.
| Regimen | Cost (USD) [70] | Efficacy (phase of trial done) | Comments |
|---|---|---|---|
| LAMB i.v. 10 mg/kg single dose | 126 | 95% (P3) | South Asia only, poor efficacy in East Africa |
| LAMB 20 mg/kg over 4 doses | 252 | 98% (P4) | South Asia only and possibly Europe and Latin America, poor efficacy in East Africa |
| LAMB 5 mg/kg + MF 100 mg/kg/day for 8 days | 88 – 109 | 97.5% (P3) | South Asia only; teratogenicity of MF may hinder uptake |
| LAMB 5 mg/kg + PM 15 mg/kg/day for 11 days | 79 | 97.5% (P3) | South Asia only; use of daily PM injections may hinder uptake |
| LAMB 30 mg/kg over 6 – 10 doses | 378 | 90% (observational field data only) | East Africa only; no clinical trial data available at this dose |
*Based on data from the WHO expert committee report 2010.
LAMB: Liposomal amphotericin B (all trials here have used AmBisome™); MF: Miltefosine; PM: Paromomycin.
Box 1.
Review of LAMB treatment for cutaneous, mucosal and post-kala azar leishmaniasis as leishmaniasis also refer back to cutaneous and mucosal.
| CL |
| Geographical distribution of CL, its clinical features, potential to spread (e.g., to mucosae), as well as recommended treatment (e.g., local vs systemic) and response to anti-leishmanial drugs vary with the Leishmania species [71]. Published data on LAMB use for CL are limited to individual case reports or small case series. Out of 19 patients with CL caused by various Leishmania species, treatment with LAMB (median total dose: 21 mg/kg) was curative in 16 (84%), while 3 patients required a second course of LAMB [72]. In Israel, 11/13 (83%) patients with L. tropica and 29/34 (85%) travelers with L. braziliensis CL (88% returning from Bolivia) were cured with LAMB (total dose: 18 mg/kg) [73,74]. In the L. braziliensis CL study, LAMB was more effective and better tolerated than SSG administered for 3 weeks. However, in Brazil, only 50% of 16 patients with CL caused by various Leishmania species who received a lower dose of LAMB (total dose: 7.5 mg/kg) were cured [75] |
| ML |
| In contrast to CL, patients with ML do not self-heal and require systemic treatment. Pentavalent antimonials and conventional amphotericin B have been the mainstay of ML treatment during the last decades with 51 – 88% reported cure rates [76]. There are very scarce published data on LAMB use for ML. In Brazil, 5/6 (83%) patients unresponsive to antimonials were cured after receiving 2 – 3 mg/kg/day for a minimum of 20 days [77]. The largest case series includes eight patients who were cured after a mean total dose of 35 mg/kg of LAMB [78], which is lower than the total dose recommended in the most recent WHO technical report (40 – 60 mg/kg) |
| PKDL |
| Anti-leishmanial therapy is indicated in severe or prolonged PKDL in East Africa and in all forms of PKDL in South Asia. Treatment still relies on prolonged – sometimes up to several months – regimens of pentavalent antimonials, which are cumbersome, painful (daily intramuscular injections) and sometimes toxic. LAMB (2.5 mg/kg/day for 20 days) was successful in 10/12 (83%) patients with SSG-unresponsive PKDL in Sudanese patients [79]. Apart from rare isolated case reports, there are no other published case series of LAMB use in PKDL. In Bangladesh, a large cohort (n = 1303) of PKDL patients were recently treated with LAMB (5 mg/kg twice a week for 3 weeks; 30 mg/kg total dose) with encouraging results (K. Ritmeijer, personal communication) |
CL: Cutaneous leishmaniasis; LAMB: Liposomal amphotericin B; ML: Mucosal leishmaniasis; PKDL: Post-kala azar dermal leishmaniasis; SSG: Sodium stibogluconate.
A review of the literature was undertaken on PubMed/MEDLINE using the search terms ‘liposomal amphotericin B' and ‘visceral leishmaniasis' (search performed on 10 July 2012). Selection criteria were used to include the following: publications in English only, all clinical trials, observational studies (including cohort studies and case series) including over 10 patients. Of the 263 articles revealed in the original search, 27 studies and clinical trials were included. These studies are summarized in descending chronological order in Table 4 [5,6,12-36]. The design and quality of the studies varied, and included two Phase III, nine dosing/Phase II and four other clinical trials, as well as eight retrospective and four prospective observational studies. Of the 27 studies, 25 were performed using the AmBisome™ formulation, and two using the Fungisome™ formulation (a generic LAMB formulation manufactured by Lifecare in India).
Table 4.
Summary of studies and clinical trials on LAMB use for VL.
| Country/Author/Ref. | Study design | Regimen used | Indication | Sample size | Efficacy/effectiveness | Comments |
|---|---|---|---|---|---|---|
| Ethiopia/Ritmeijer et al.; 2011 [24] | Retrospective cohort analysis | LAMB 30 mg/kg split over 6 doses* | A: Severely ill B: HIV/VL +/- relapse |
A: N = 94 B: N = 195 (79 relapses) |
A: IC = 93% B: IC = 60% |
IC among HIV+ relapse patients = 38% |
| India/Sinha et al.; 2011 [28] | Retrospective cohort analysis | LAMB 20 – 25 mg/kg split over 4 – 15 days* | HIV/VL +/- relapse | N = 55 (27 relapses) | Survival at 2 years = 85.5% | Relapse at 1 and 2 years = 8% and 26.5% |
| India/Sundar et al.; 2011 [5] | Phase III non-inferiority RCT | A: ampho B B: LAMB and MF* C: LAMB and PM* D: MF and PM |
Primary uncomplicated VL (e.g., no HIV or severely ill) | A: N = 157 B: N = 160 C: N = 158 D: N = 159 |
A: ITT DC = 93% B: ITT DC = 97.5% C: ITT DC = 97.5% D: ITT DC = 98.7% |
LAMB regimens were single LAMB doses + MF/PM (7 – 10 days) |
| India/Sundar et al.; 2011 [34] | Non-comparative clinical trial | LAMB and MF*
(single dose + 14-day MF) |
Primary uncomplicated VL | N = 135 | ITT DC = 91.9% | Some GI side effects noted with MF use |
| India/Sinha et al.; 2010 [27] | Prospective cohort study | LAMB 20 mg/kg split over 4 doses* | Primary and relapse cases in routine care | N = 251 | DC = 98.8% (lost to follow-up = 17.5%) |
1% of cases experienced lip swelling |
| India/Mondal et al.; 2010 [20] | Open-label dose finding (Phase II) clinical trial | A: LAMB 5 mg/kg B: LAMB 7.5 mg/kg C: LAMB 10 mg/kg (total doses) |
Uncomplicated VL | A: N = 10 B: N = 10 C: N = 10 |
A: ITT DC = 60% (5 people excluded) B: ITT DC = 50% C: ITT DC = 90% |
Fungisome™ (Lifecare) formulation used |
| India/Sundar et al.; 2010 [6] | Phase III non-inferiority RCT | A: ampho B B: LAMB 10 mg/kg single dose* |
Uncomplicated VL | A: N = 108 B: N = 306 |
A: ITT DC = 96.3% B: ITT DC = 95.7% |
Only 1% SAEs (nephro/hepatotoxicity) per group |
| India/Sundar et al.; 2008 [33] | Phase II RCT | A: LAMB 5 mg/kg* B: LAMB 5 mg/kg and MF 10 days* C: LAMB 5 mg/kg and MF 14 days* D: LAMB 3.75 mg/kg and MF 14 days* E: LAMB 5 mg/kg and MF 7 days* |
Uncomplicated VL | A: N = 45 B: N = 46 C: N = 45 D: N = 45 E: N = 45 |
A: ITT = 91% B: ITT = 98% C: ITT = 96% D: ITT = 96% E: ITT = 98% cure rates are at 9 months follow-up for all groups |
All treatments were tolerated to completion, but hepatotoxicity noted especially in group C |
| Spain/Molina et al.; 2007 [19] | Retrospective cohort analysis | LAMB 40 mg/kg total initial Rx + 5 mg/kg secondary prophylaxis* | HIV co-infected relapses cases | N = 17 | Free of relapse: 6 months = 89.7% 12 months = 79.1% 36 months = 55.9% |
Significant increase seen in CD4 count in the non-relapses |
| Sudan/Mueller et al.; 2007 [22] | Retrospective cohort analysis | LAMB 15 – 49 mg/kg given over 6 doses* | 52 relapses and 12 complicated cases | N = 64 | IC = 55% only | TB, HIV, initial parasite density linked with failure |
| Sudan/Mueller et al.; 2006 [21] | Retrospective cohort analysis | A: SSG alone B: SSG and LAMB* C: LAMB alone* |
VL in pregnant women | A: N = 23 B: N = 4 C: N = 12 |
IC = 100% all groups; 13 abortions noted in group A, none in B and C |
LAMB appears safer than SSG in pregnant women with VL |
| India/Sanath et al.; 2005 [25] | Post-marketing cohort study | LAMB 1 – 3 mg/kg/day for 7 – 76 days | Mixed population in routine care | 91 out of 144 assessed for cure | IC = 73.6%, 8.7% had no response |
Fungisome™ (Lifecare) used, no drug-related SAEs noted |
| Greece/Kafetzis et al.; 2005 [18] | Retrospective cohort analysis | A: MA (20 mg/kg for 21 days) B: LAMB varying doses* |
Children under the age of 15 | A: N = 10 B: N = 19 |
All patients initially cured, no relapses noted after | Shorter median hospitalization with LAMB (19 vs 7 days) |
| Italy/Cascio et al.; 2004 [14] | Retrospective cohort analysis | LAMB 3 mg/kg in 6 doses over 10 days* | HIV negative children under the age of 15 | N = 164 | IC = 100% 4.3% relapsed 3 – 15 months after |
All relapses cured with LAMB (total dose 30 mg/kg) |
| India/Sundar et al.; 2004 [32] | Open-label RCT | A: ampho B B: LAMB 2 mg/kg/5 days* |
Uncomplicated VL | A: N = 51 B: N = 51 |
A: ITT DC = 96% B: ITT DC = 96% |
2 patients in group A died, 2 failures in group B |
| India/Sundar et al.; 2003 [30] | Non-comparative clinical trial | LAMB 7.5 mg/kg single dose* | Uncomplicated VL | N = 203 | ITT IC = 96% ITT DC = 90% |
Few AEs noted, mainly infusion reactions |
| Italy/Pagliano et al.; 2003 [23] | Retrospective cohort analysis | A: MA (20 mg/kg for 21 days) B: LAMB 3 mg/kg in 6 doses over 10 days* |
HIV negative adults | A: N = 24 B: N = 40 |
After 2 years post-treatment: A: 12% failures B: 5% failures |
Faster recovery time noted with LAMB |
| Greece/Syriopoulou et al.; 2003 [35] | Prospective study | LAMB 10 mg/kg over 2 days* | HIV negative children | N = 41 | DC = 98% | Fast recovery time, mild infusion related reactions in < 10% of patients |
| India/Sundar et al.; 2002 [31] | Double-blind Phase II RCT | A: LAMB 3.75 mg/kg over 5 days* B: LAMB 7.5 mg/kg over 5 days* A: LAMB 15 mg/kg over 5 days* |
Refractory cases to antimonials | A: N = 28 B: N = 28 C: N = 28 |
A: ITT DC = 89% B: ITT DC = 93% C: ITT DC = 97% |
Mild/moderate infusion-related reactions were common |
| India/Sundar et al.; 2001 [29] | Open-label Phase II RCT | A: LAMB 5 mg/kg single dose* B: LAMB 5 mg/kg over 5 days* |
Uncomplicated VL | A: N = 46 B: N = 45 |
A: ITT DC = 91% B: ITT DC = 93% |
Mild infusion-related reactions were common |
| India/Thakur; 2001 [36] | Open-label RCT | A: LAMB 15 mg/kg single dose* B: ampho B (1 mg/kg × 20 days) |
Uncomplicated VL | A: N = 17 B: N = 17 |
A: ITT DC = 100% B: ITT DC = 100% |
Fewer adverse events noted in the LAMB group |
| India/Bodhe et al.; 1999 [13] | Dosing study (Phase II) clinical trial |
Various doses of LAMB ranging from 1 mg/kg for 21 days to 3 mg/kg for 7 days | Primary and relapse cases | N = 63 | Cure rates varied from 90 to 100% depending on dose | Used L-AMP-LRC 1 (liposome pharmacology center) |
| India, Kenya, Brazil/ Berman et al.; 1998 [12] |
Phase II dose ranging clinical trial | A: LAMB 14 mg/kg total dose* B: LAMB 10 mg/kg total dose* C: LAMB 6 mg/kg total dose* D: LAMB 20 mg/kg total dose (Brazil only)* |
Groups were done sequentially in descending doses; parallel studies done in India, Kenya and Brazil |
A: N = 10 (India, Kenya) N = 13 (Brazil) B: N = 10 (India, Kenya) C: N = 10 (India, Kenya) D: N = 15 (Brazil only) |
India: 100% DC rates in A, B, C Kenya: 100%, 80% and 20% DC in A, B, C Brazil: 62% and 83% DC in groups A and D, respectively |
Differential efficacy seen across 3 continents with India > Kenya ≥ Brazil |
| Italy/di Martino et al.; 1997 [17] | Prospective study | LAMB various doses* | Pediatric population | N = 106 | Up to 100% | 18 mg/kg total identified as the optimum dose |
| Italy, Brazil, UK/ Davidson et al.; 1996 [15] |
Dose decreasing (Phase II) clinical trial | A: LAMB 24 mg/kg total dose* B: LAMB 18 mg/kg total dose* C: LAMB 15 mg/kg total dose* D: LAMB 12 mg/kg total dose* (split into 6 doses) |
Adult and pediatric population Uncomplicated VL |
A: N = 13 B: N = 42 C: N = 32 D: N = 1 |
Cure at 1 year = A: 10/13 (3 LTFU were IC) B: 41/42 (1 relapse) C: 29/32 (1 failure, 2 relapse) D: 1/1 |
Treatment well tolerated with no cessation of treatment; and well tolerated in children |
| Sudan/Seaman et al.; 1995 [26] | Open label, dosing (Phase II) clinical trial | A: LAMB 3 – 5 mg/kg × 3 doses* B: LAMB 3 – 5 mg/kg × 6 doses* C: LAMB 4 – 5 mg/kg × 4 doses* |
Relapse and complicated (severely ill) cases included | A: N = 16 B: N = 16 |
A: 50% cured B: 88% cured C: 64% cured |
Long-term follow-up not done |
| Europe/Davidson et al.; 1994 [16] | Open label, dosing (Phase II) clinical trial | A: LAMB 1 – 1.38 mg/kg × 21 doses* B: LAMB 3 mg/kg × 10 doses* C: LAMB 1.38 – 1.85 mg/kg × 21 doses* |
Gp A and B were immune-competent Gp C was immune-compromised |
A: N = 10 B: N = 10 C: N = 11 |
Cure rates at over 12 months: A: 100% B: 100% C: 27% (100% were IC) |
Groups A and B were largely made up of children; Group C included adults only |
Country denotes where patients were recruited.
*Denotes when AmBisome™ (currently Gilead, previously Vestar) was used.
AE: Adverse event; ampho B: Conventional amphotericin B 15 mg/kg total dose; DC: Definitive cure at 6 months; indication is for type of VL (primary, relapse, HIV co-infected); IC: Initial cure; ITT: Intention to treat; LAMB: Liposomal amphotericin B; MA: Meglumine antimoniate; MF: Miltefosine; PM: Paromomycin; RCT: Randomised controlled trial; SAE: Serious adverse event; VL: Visceral leishmaniasis.
Of note is that studies using non-liposomal lipid formulations of amphotericin B are not included in Table 4. Comparative studies report that different types of lipid amphotericin B formulations have proved to be superior to conventional amphotericin B formulation with higher drug concentrations in the liver and spleen and lower concentrations in kidneys and lungs, thus enhancing its efficacy and decreasing its toxicity [37]. Nevertheless, LAMB has a better safety profile compared with lipid formulations [1,38].
Over the last 20 years, studies have focused on several areas of development. Initial studies (those published between 1994 and 1998) were early pivotal studies. Some of them were used for a New Drug Application (NDA) to the United States Food and Drug Administration (US FDA) [12,16]. This led to the registration of AmBisome in 1997 for treatment of VL, as well as for use as empirical therapy for presumed fungal infections in febrile neutropenic patients, and treatment of patients with systemic fungal infections refractory to conventional amphotericin B [39]. A critical finding in these early studies was that there appeared to be different dose requirements for LAMB in different endemic regions, with South Asia (India) requiring lower doses than Europe (Mediterranean), Latin America (Brazil) or East Africa (Sudan, Kenya) [40].
The next group of studies, those published between 1998 and 2005, focused on Phase II dose optimization studies, mainly in the Mediterranean region of Europe and India. The studies in Europe included separate groups of children (the traditional patient population of L. infantum) and immunocompromised/HIV+ adults. These observational studies confirmed earlier studies showing that total doses of 18 – 20 mg/kg appeared effective, at least in non-immunocompromised individuals [14,18,23,35]. However, longer-term outcomes of HIV co-infected individuals appeared to be poor. Recent use in high-dose monotherapy in HIV-positive VL patients in East Africa has shown similar poor outcomes [24]. The second group of studies, conducted mainly in South Asia, were driven by the considerable interest in developing low-dose or single-dose regimens of LAMB, given the profile of the drug formulation: on the one hand safe and highly efficacious, and on the other, relatively complex to administer and extremely expensive (the WHO-negotiated price of AmBisome for treatment of VL was as high as US$50 per vial at that time) [41]. These studies confirmed the results of the early Phase II clinical trial by Berman et al. and demonstrated high efficacy (over 90%) with a dose as low as 5 mg/kg, as well as the feasibility and safety of use in high single doses [29,30,36].
Since 2005, two interesting strategies have been investigated, the first of which relied on developing combination treatments using LAMB (specifically AmBisome) together with other anti-leishmanial drugs. In India, trials were supported by both TDR and DNDi. Phase II and III trials demonstrated extremely high efficacy of low-dose, short-course regimens involving only a single dose of LAMB [5,33,34]. This led to the evaluation of LAMB combination regimens in both Africa and Latin America, where trials are still ongoing (in co-administration with MF and MA, respectively) [42,43].
The second strategy looked into the use of single-dose LAMB, specifically for the South Asia focus. A pivotal Phase III trial published in 2010 by Sundar et al. demonstrated high efficacy (> 95%) and safety of a single 10 mg/kg dose of LAMB [6]. This prompted the WHO expert committee on the control of leishmaniasis to recommend the regimen as first-line treatment for VL in South Asia that same year [1]. Of additional interest, a trial in East Africa (Sudan and Ethiopia) involving single doses of LAMB (AmBisome) has recently been terminated [44]. Results are due to be published soon; however, other recent data confirmed early findings that high doses of LAMB were required to achieve definitive cure (6 months) rates of even 90% [22,26].
It is difficult to predict if use of LAMB in single dose may increase the risk of drug resistance, as has occurred with SSG in India (where short course treatments of the drug were used). The mode of action of amphotericin B on membrane ergosterol is such that an organism would have to undergo significant changes in order to become resistant. Moreover, it is likely that the LAMB single-dose regimen will improve compliance and therefore reduce the risk of underdosing. There is evidence that resistance may not be easily generated in practice as was shown in a small study of 10 HIV-VL co-infected patients who were exposed to long-term treatment of LAMB, with no changes in drug susceptibility of the patient parasite isolates [45]. By contrast, an earlier study has demonstrated a reduction in amphotericin B susceptibility (IC90 and IC50) in promastigote and intracellular amastigote forms of Leishmania sp. in immunocompromised patients treated with a lipidic emulsion of amphotericin B [46]. In addition, the recent exploration and in vitro elaboration of mechanisms of L. donovani resistance to amphotericin B suggest that there are indeed pathways to resistance [47].
6. Competitive environment
AmBisome, the liposomal formulation of amphotericin B produced by Gilead, is one of the very few liposomal formulations to have been registered by the US FDA and other stringent national drug authorities since the potential of liposomes as a drug delivery system was discovered in the 1970s (Table 5).
Table 5.
Injectable liposomal products approved by the US FDA.
| Trade name | Drug formulation | Year of US FDA approval |
|---|---|---|
| Doxil™ | Pegylated liposomal doxorubicin injection | 1995 |
| Daunoxome™ | Liposomal daunorubicin injection | 1996 |
| Ambisome™ | LAMB injection | 1997 |
| Depocyt™ | Liposomal cytarabine injection | 1999 |
| Visudyne™ | Liposomal verteporfin injection | 2000 |
| Definity™ | Liposomal perflutren injection | 2001 |
| Depodur™ | Liposomal morphine sulfate injection | 2004 |
| Exparel™ | Liposomal bupivacaine injection | 2011 |
LAMB: Liposomal amphotericin B; US FDA: United States Food and Drug Administration.
A liposome is a microvesicle composed of a bilayer of lipid amphipathic molecules enclosing an aqueous compartment that can entrap hydrophilic, as well as lipophilic drugs. The liposome acts as a masking system for the encapsulated drug: as long as the liposome is intact, the drug is isolated from the environment and protected from metabolism and inactivation in the plasma. The versatility of a liposome's design (composition, morphology, size and surface characteristics) enables more targeted distribution and consequent improvement in the therapeutic index.
Despite research and development and investment in liposomal formulations, guidance on the regulatory pathways for the registration of generic versions of liposomal formulations is still lacking, even in stringent regulatory authorities where the expiry of patents and data exclusivity should open up the market to generic competition for AmBisome and other medicines with liposomal drug delivery systems (e.g., pegylated liposomal doxorubicin). As a result, in 2012 MSF began to collect data on generic versions of LAMB and on the regulatory challenges associated with their evaluation.
In 2002, the US FDA published draft guidance on liposomal drug products intended for the industry [48]. This is currently under review. In 2010, the FDA also published product-specific draft guidance for determining the bioequivalence of doxorubicin pegylated liposomal injectable formulations [49]. The European Medicines Agency (EMA) published a ‘draft reflection paper' in 2011 to assist applicants in the generation of relevant data (quality, clinical and non-clinical) to support a marketing authorization for intravenous liposomal products developed with reference to an innovator liposomal product [50]. All these drafts contain non-binding recommendations. No other National Drug Regulatory Authority (NDRA) belonging to the International Conference on Harmonisation, and therefore considered stringent, has published standards for evaluating generic liposomal formulations and/or assessing bioequivalence. Moreover, no NDRA from VL-endemic countries or from countries manufacturing generic versions have such standards. A few NDRAs refer to the US FDA and EMA draft guidance. In this vacuum, WHO has not set any standards for liposomal formulations to support countries in the evaluation of liposomal products. The debate on how to prove bioequivalence and allow the registration of generic versions has been brought into the public domain. Contributions to the debate have come from NDRAs, academic experts and also from studies funded by innovator pharmaceutical companies [51-57]. According to a group of academic experts, the classical bioequivalence approach could be applicable, if properly adapted [57].
The uncertainty of the regulatory pathway is one of the elements impeding the entry of generic competitors to stringently regulated markets [53]. This vacuum in guidance also has an impact on access to generic versions of LAMB produced and marketed in other countries. Through the survey, the authors found that a number of generic LAMB formulations have already been marketed in a few countries or are under development as reported in Table 6. The lack of guidance and the absence of an internationally recognized system for evaluation of generic products, such as the WHO prequalification system set for vaccines, HIV, TB and Malaria medicines, are major obstacles impairing the search for alternative quality-assured options to the innovator product.
Table 6.
Overview of LAMB formulations introduced in the market or under development*.
| Company | Country | Brand name | Year of marketing approval in the country of origin | Storage conditions | Presentation (special warnings before use) |
|---|---|---|---|---|---|
| Gilead | USA | AmBisome™ | 1997 | Below 25°C | Lyophilized (reconstitution, filtration and dilution before use) |
| Lifecare | India | Fungisome™ | 2003 | 2 – 8°C | Liquid (sonication 45 min before use) |
| Cipla | India | Phosome™ | 2008 | 2 – 8°C | Lyophilized (reconstitution, filtration and dilution before use) |
| Sun Pharma | India | Lambin™ | 2009 | 2 – 8°C | Lyophilized (reconstitution, filtration and dilution before use) |
| Lyka Labs | India | Lipholyn™ | 2010 | 2 – 8°C | Lyophilized (reconstitution, filtration and dilution before use) |
| Celon Labs | India | Under development | 2 – 8°C | Lyophilized (reconstitution, filtration and dilution before use) | |
| Laboratorios Richmond | Argentina | Withdrawn, under further development (Anfogen) | Lyophilized (reconstitution, filtration and dilution before use) | ||
| Genex Pharma | India | Under development | |||
| Claris Lifescience | India | Under development | |||
| TTY Pharma | Taiwan | Under development | |||
*This table is not exhaustive as some producers from India and Taiwan have chosen not to disclose information about their products under development.
LAMB: Liposomal amphotericin B.
Currently, the main incentive behind the development of generic LAMB is to compete with AmBisome for more lucrative conditions and markets than VL, as most of the US$330 million annual revenue that comes from AmBisome relates to sales in high-income countries. The innovator company Gilead now offers AmBisome at the WHO-negotiated price of US$18 per vial for VL in developing countries (‘access price'). Outside developing countries, and for conditions other than VL in developing countries, the ‘commercial price' is about 10 times higher: the price of AmBisome in the UK and South Africa is US$150 and US$230 per vial, respectively [58,59].
The generic manufacturers contacted during the MSF survey appeared open to discuss prices and volumes for VL treatment with stakeholders like WHO and MSF. A number of manufacturers were developing their products with a view to entering the US and European markets. However, if the market size of LAMB for VL expanded – for example, through large-scale public procurement mechanisms – those competitors could also enter the less profitable, but still attractive, VL market. The increased competition would drive the price of LAMB down and would be beneficial for patients with VL as well as patients who need LAMB for other conditions [41].
Finding alternative sources of LAMB is even more essential given that a single plant is currently responsible for the worldwide production of AmBisome. As declared by Gilead: ‘in the event of a disaster, including an earthquake, equipment failure or other difficulty, we may be unable to replace this manufacturing capacity in a timely manner and may be unable to manufacture AmBisome to meet market needs' [60].
Finally, the inferior profile of existing generic LAMB formulations in terms of thermostability should not deter the search for and investigation of alternative sources of LAMB. A cold chain with a narrow temperature range (2 – 8°C) was also required for AmBisome when it was first registered and marketed; further stability data later led to an extension of the upper storage temperature range to 25°C [61]. It is not excluded that generics under development may reach a thermostability profile similar to that of AmBisome.
7. Conclusion
LAMB is a very safe and highly effective treatment for primary VL in L. infantum endemic areas and in the L. donovani South Asian focus (India, Bangladesh, Nepal), where it was recently recommended as first-line treatment by the WHO expert committee on the control of leishmaniasis [1]. In East Africa, the optimal total dose of LAMB to cure primary VL remains to be determined, but will be higher than in other endemic areas. Therefore, LAMB cannot be recommended as first-line treatment for VL in this region, except for patients at increased risk of death with pentavalent antimonials-based treatments. Additional research is needed to better define the indication of LAMB for other forms of leishmaniasis (CL, MCL, PKDL) and to design optimal (combination) regimens for HIV co-infected patients.
Several generic LAMB formulations already exist and many others are under development. Their introduction in the market is however happening without clear standards to which companies can refer to and abide by. Stringent regulatory authorities have not been issuing definitive guidance on the evaluation of generic liposomal formulations.
The recommendation to use LAMB as first-line treatment for VL in most regions needs to be coupled with a sustainable plan for its use in endemic countries. In the short term, further reductions of the ‘access price' should be negotiated with Gilead, while in the longer term the pool of suppliers to national VL control programs should be expanded. Large public procurement schemes for LAMB as a VL treatment could also represent an attractive market to manufacturers who currently target only niche markets for the treatment of fungal infections for countries or patients who can afford to pay. Major stakeholders in control of leishmaniases, such as WHO and donors, should jointly invest in ambitious plans to support access to generic affordable quality-assured formulations of LAMB for VL.
8. Expert opinion
In this section, the authors will describe the practical experience acquired by MSF teams and partners on the use of AmBisome for treating VL in rural areas of South Asia and East Africa. They will then discuss the remaining barriers preventing a large-scale use of LAMB for VL and suggest some ways to remove them.
8.1. MSF experience in India and Bangladesh
In mid-2007, MSF began reinforcing existing VL diagnostic and treatment services in the highly endemic Bihar district of Vaishali in India, with support from the State Health Society, the Rajendra Memorial Research Institute (RMRI) and the National Vector Borne Disease Control Programme. LAMB 20 mg/kg (4 doses of 5 mg/kg over 5 – 10 days, depending on clinical condition) was introduced as first-line treatment, both at district hospital and Primary Health Care (PHC) levels (Figure 1). It was felt critical to demonstrate feasibility of LAMB use at the PHC level, where the majority of patients in rural India currently access healthcare.
Figure 1.
Young female patient with visceral leishmaniasis receiving liposomal amphotericin B infusion at Vaishali District Hospital, Bihar State, India.
Up until June 2012, 6766 patients were treated at the district level inpatient facility, while 1440 were treated at the PHC level, and only 4% were treated at a tertiary referral center. Of all the patients diagnosed at the PHC level, just over a quarter (26.3%) required referral to inpatient facilities for VL treatment. Initial clinical cure (i.e., improved symptoms, cessation of fever and recession of spleen following the last dose of LAMB) was achieved in 98.1% of patients, 1.4% defaulted before or during treatment and 0.4% patients died before, during or immediately after treatment (Figure 2). In addition, LAMB was found to have a very low rate of adverse reactions: approximately 8% of patients reported adverse events – mainly minor – during treatment, with five patients with hypersensitivity requiring cessation of LAMB and switching to an alternative treatment. Only 1.2% of immunocompetent patients were readmitted with VL relapse after an average post-treatment period of 12.4 (7.4) months. The rate of relapse was higher (13.7%) in the 167 HIV co-infected patients.
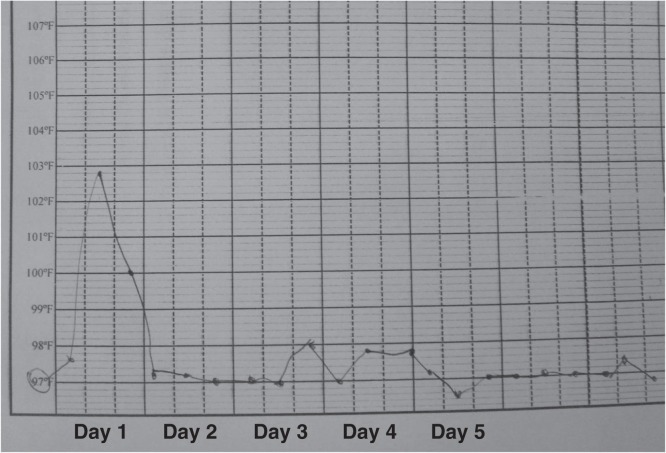
Figure 2.
Nursing chart showing rapid clearance of fever after initiation of liposomal amphotericin B treatment in a patient with visceral leishmaniasis at Vaishali District Hospital, Bihar State, India.
Although typically considered feasible at the hospital level, the major challenge at the PHC level was to maintain a reliable cool chain to store AmBisome between 2 and 8°C until 2011, as was required by the Indian drug authorities, and below 25°C since then. After starting with a complex and logistically heavy passive cold chain requiring regular changes of ice packs, a simpler solution was found when the state authorities provided dedicated Ice Lined Refrigerators (ILRs) for use to store AmBisome at both PHC and hospital levels. Since the start of the program, over 17,000 vials have been successfully used in PHCs. Since the change of temperature requirements (up to 25°C), it is now possible to store AmBisome in air-conditioned rooms, ensuring a back-up generator can guarantee no power cuts.
The second major constraint was the perception that such a ‘complex' treatment could not be safely and effectively administered at the PHC level for lack of skilled human resources. After being trained, health workers in PHC centers proved to be able to independently prepare and administer the appropriate dose of LAMB on an ambulatory basis, and identify patients with more severe disease for referral to the district level inpatient treatment facility. Finally, another major issue faced was the complexity of importing AmBisome into India, where it is registered but not produced.
In Bangladesh, since May 2010, MSF provides VL treatment services in PHC settings in the VL hyper-endemic sub-district of Fulbaria, in the Mymensingh district, using an ambulatory treatment regimen of 15 mg/kg LAMB (three doses of 5 mg/kg on day 0, 1 and 5). As of July 2012, 1439 immunocompetent primary VL patients had been treated, with an initial cure rate of 99.6%, 4 deaths (0.3%) and 1 (0.1%) defaulter. Active follow-up of patients at 1, 6 and 12 months post-treatment showed that 39 patients (2.7%) had relapsed, the majority of which (90%) after 6 months post-treatment. These data confirm that very favorable outcomes can be achieved in PHC settings with a 15 mg/kg dose of LAMB.
In summary, LAMB 15 – 20 mg/kg total dose has shown very high cure rates and a very low rate of adverse reactions and relapses under field conditions in India and Bangladesh. Although the use of LAMB in hospital settings is more widely recognized, with appropriate training and commitment, its use is feasible, safe and effective at the PHC level, utilizing logistic and human resources that already exist within the public health system. The ongoing proof-of-concept studies in India and Bangladesh will show whether similar outcomes can be achieved even with a single 10 mg/kg dose.
8.2. MSF experience in East Africa
In East Africa, where pentavalent antimonials still remain the mainstay of VL treatment, LAMB was introduced in MSF programs in the 1990s for the treatment of severe and complicated VL [26]. Patients with severe or complicated VL have a poor tolerability for antimonials, resulting in a significantly increased mortality risk [62]. Because of its more favorable safety profile, LAMB is the first-line treatment in pregnancy, due to the high risk of spontaneous abortion with antimonials [21], as well as in elderly VL patients, because of high antimonial toxicity-related mortality in patients over 45 years of age [63]. Because of the poor tolerability and high mortality associated with pentavalent antimonials in HIV co-infected VL patients, LAMB is the recommended drug of choice in this patient group, even though its efficacy is limited [24].
The total dose of LAMB needed to establish cure in East Africa was found to be much higher than that required in South Asia. Initially, a regimen was used with a total dose of 20 mg/kg LAMB, divided over 6 doses on alternate day (Figure 2). However, over the years, there has been growing evidence of significant treatment failure rates with this dose [22], especially in patients with complicated VL and concomitant infections, such as TB and HIV. Therefore, the recommended total dose of LAMB in East Africa for non-HIV-infected VL has been increased to 30 mg/kg.
The use of AmBisome in East Africa was initially limited, for several reasons: its prohibitively high price, its intravenous administration, the lack of trained health staff and the temperature requirements to transport and store it. It meant that LAMB could only be used in referral hospital settings. Successive negotiations resulting in a decrease of the ‘access price' and the gradual expansion of cold chain capacity and trained health staff to peripheral health centers in remote and extremely resource-poor endemic areas in East Africa have increased access to LAMB for patients from specific vulnerable groups with poor tolerability for pentavalent antimonials. This has contributed to the gradual decrease in overall VL mortality in MSF programs, from above 10% in the 1990s to well under 5% in recent years [64,65].
8.3. Access to LAMB: bottlenecks that prevent large-scale implementation
VL, like other neglected tropical diseases, predominantly affects poor populations. In South Asia, it is linked to poor housing and unhealthy living environments [66]. Economic and social exclusion are strong underlying determinants of poor access to VL treatment, as a recent study of low-caste Indians with the disease indicates [67]. Access to timely diagnosis and prompt, effective treatment is hindered by geographical, financial and social obstacles. Lack of knowledge about the disease and treatment options among both patients and physicians results in delays in seeking treatment. For people in East Africa, physical distance from health facilities is a major problem. The private market, traditional healers and quacks can also pose barriers to quality care.
8.3.1. Storage and drug administration
There are also very specific drawbacks preventing access to LAMB for VL. The temperature requirement for the transport and storage of AmBisome, which is now up to 25°C, is a significant challenge in the countries where the disease is most endemic, such as India and Sudan, where temperatures can exceed 45°C. Adding a cold chain monitor to vials (similar to those used with vaccines) would help guarantee that the drug can be used safely. In addition, LAMB is administered intravenously, and therefore requires trained staff. These obstacles to the uptake of this treatment can be overcome but only with adequate resources, as shown by MSF's experience in East Africa and South Asia.
8.3.2. Implementation of guidelines
While the recommendations of the WHO expert committee on leishmaniasis have been implemented in most endemic East African countries (SSG and PM as first-line treatment), they have not yet been properly translated in South Asian countries. The WHO committee considered LAMB single dose (10 mg/kg) or LAMB short course (15 mg/kg) as the preferred option for first-line VL treatment in the Indian subcontinent, and combination therapies (LAMB and MF, LAMB and PM, MF and PM) as other possible first-line options [1]. In Bihar, the Indian state with the highest VL burden, MF monotherapy is recommended by the national program as the first-line treatment. Also in Bangladesh, MF monotherapy is still recommended by the Ministry of Health as first-line therapy. Integrating the latest evidence-based treatment recommendations into national guidelines is a first step toward facilitating access to LAMB. Adherence to the WHO recommendations is likely to improve when the above-mentioned field feasibility studies concerning LAMB in monotherapy or in combination are available.
8.3.3. Need for registration
For a drug to be easily imported into a country, it needs to be registered by the national drug authority. The AmBisome Access label product is now registered in most developing countries with a high VL burden (Bangladesh, Ethiopia, Kenya and Sudan), but not yet in India. Although it has not been registered in India, MSF has been able to treat patients in the country with AmBisome for the last 5 years, initially through an agreement with the RMRI, and more recently through its own import license. All high-burden countries should include LAMB in their national treatment guidelines and in their Essential Medicines List, while Gilead should be encouraged to deploy more efforts to register access label AmBisome in all VL-endemic countries.
8.3.4. Bringing down prices
The WHO-negotiated prices of AmBisome (US$20 per vial in 2006, then US$18 per vial in 2008) have increased access to LAMB in developing countries, notably in MSF projects, where it is now routinely used. This ‘access price' is, however, still too high for many health systems in developing countries: a full course of treatment for an average VL patient (weight: 35 kg) is estimated to cost between US$126 and US$378, depending on the necessary dosage. A study published in 2010 compared the cost-effectiveness ratios of 10 treatment strategies in the Indian subcontinent. The MF and PM combination was found to have the best cost-effectiveness ratio [68]. According to the authors, ‘if the price of a LAMB vial is decreased (…) to less than US$ 9.8, then [single-dose LAMB -10 mg/kg] becomes the most cost-effective strategy'. Based on these findings, MSF has been asking Gilead to reduce the price of AmBisome to US$10 per vial.
In December 2011, Gilead agreed to donate 445,000 vials of AmBisome, under the supervision of the WHO, for the treatment of more than 50,000 VL patients over the next 5 years. The scope of this donation is very limited. Fewer than 5% of patients with VL will benefit from the donation, and India, the country with the highest VL burden, is not a recipient [69]. This donation also reduces the size of the market for VL treatment, and thereby helps discourage other companies from targeting the market with alternative or generic LAMB formulations. Extending the donation would only be a solution if it sustainably covered the needs of all VL patients in developing countries who need LAMB.
The market for LAMB is now dual and segmented: there is a WHO-negotiated ‘access price' for VL in developing countries, and a much higher and lucrative price in wealthy markets. Gilead's tiered pricing policy, which was not pushed by a strong competitive environment, but rather by negotiations with WHO, has not achieved the price levels that are necessary for full uptake in developing countries [41]. Competition with generic producers, if promoted by an enabling environment, would be likely to bring prices down further, but making generic LAMB formulations available at a lower price than the current AmBisome ‘access price' is unlikely to be feasible without a clearer and stronger demand. Generics producers are more interested in lucrative potential sales for the other indications of LAMB than in occasional small-scale public procurement for national leishmaniasis programs. Securing high-volume public calls for tender will be critical to the creation of a more predictable and larger market for LAMB for VL. Finally, bringing down prices through enhanced competition with generics suppliers would also help to facilitate access to LAMB in developing countries for other conditions, notably cryptococcal meningitis.
8.3.5. Implementation of regulatory pathways to prove bioequivalence for generic liposomal products
As discussed previously, even stringent regulatory authorities have not been able to provide guidance to manufacturers wishing to enter the market in high-income countries. The lucrative markets for LAMB will be open to Gilead's competitors within the next 2 – 5 years. AmBisome is protected by two patents (US 5874104 and US 5965156) in the USA until 2016 and by one patent (CA 1339008) in Canada until 2014. The de facto monopolistic situation of Gilead on the LAMB market is likely to continue beyond these dates. Indeed, scientific, regulatory and manufacturing hurdles have now been recognized as clear opportunities for extending market exclusivity beyond patent protection for therapeutic nanoparticles like LAMB [53].
Price is a key barrier to access to LAMB in endemic countries, but it is certainly not the only issue. Attention needs to be paid to the quality of generic versions, in order to ensure that patients benefit from the efficacy and low toxicity of LAMB, as opposed to conventional amphotericin B and other lipidic formulations. Both demand and supply of LAMB need to be improved. This requires a plan to expand the pool of LAMB suppliers, which involves the WHO, governments of endemic countries and donors. Within the London Declaration, a global coalition for neglected tropical diseases, new stakeholders, including the UK Department for International Development, have pledged more support to VL-control programs. Better access to quality-assured affordable LAMB for VL treatment should be a priority objective within this new initiative.
Acknowledgement
The authors would like to thank H Bilak (DNDi) for editing the manuscript.
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.WHO Control of the Leishmaniasis. Report of a meeting of the WHO expert committee on the control of Leishmaniasis; 22 – 26 March 2010; Geneva. [Google Scholar]; • Important document summarizing the epidemiology and latest diagnostic and treatment recommendations for all forms of leishmaniasis, per region.
- 2.Alvar J, Velez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sundar S. Drug resistance in Indian visceral leishmaniasis. Trop Med Int Health. 2001;6(11):849–54. doi: 10.1046/j.1365-3156.2001.00778.x. [DOI] [PubMed] [Google Scholar]
- 4.Musa A, Khalil E, Hailu A, et al. Sodium stibogluconate (SSG) & paromomycin combination compared to SSG for visceral leishmaniasis in East Africa: a randomised controlled trial. PLoS Negl Trop Dis. 2012;6(6):e1674. doi: 10.1371/journal.pntd.0001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sundar S, Sinha PK, Rai M, et al. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet. 2011;377(9764):477–86. doi: 10.1016/S0140-6736(10)62050-8. [DOI] [PubMed] [Google Scholar]; •• Pivotal Phase III trial demonstrating good efficacy and safety of short-course LAMB-based combination treatments against VL in India.
- 6.Sundar S, Chakravarty J, Agarwal D, et al. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med. 2010;362(6):504–12. doi: 10.1056/NEJMoa0903627. [DOI] [PubMed] [Google Scholar]; •• Pivotal Phase III trial demonstrating good efficacy and safety of single-dose LAMB treatment against VL in India.
- 7.Rijal S, Chappuis F, Singh R, et al. Sodium stibogluconate cardiotoxicity and safety of generics. Trans R Soc Trop Med Hyg. 2003;97(5):597–8. doi: 10.1016/s0035-9203(03)80043-3. [DOI] [PubMed] [Google Scholar]
- 8.Sundar S, Singh A, Rai M, et al. Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin Infect Dis. 2012;55(4):543–50. doi: 10.1093/cid/cis474. [DOI] [PubMed] [Google Scholar]
- 9.Hailu A, Musa A, Wasunna M, et al. Geographical variation in the response of visceral leishmaniasis to paromomycin in East Africa: a multicentre, open-label, randomized trial. PLoS Negl Trop Dis. 2010;4(10):e709. doi: 10.1371/journal.pntd.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.http://www.dndi.org/diseases/vl.html
- 11.DNDi Target Product Profile. http://dndi.org/diseases/vl/target-product-profile.html Available from:
- 12.Berman JD, Badaro R, Thakur CP, et al. Efficacy and safety of liposomal amphotericin B (AmBisome) for visceral leishmaniasis in endemic developing countries. Bull World Health Organ. 1998;76(1):25–32. [PMC free article] [PubMed] [Google Scholar]; •• Multiregional Phase II study demonstrating the efficacy of LAMB against VL; the study also indicated that efficacy could vary across different regions.
- 13.Bodhe PV, Kotwani RN, Kirodian BG, et al. Dose-ranging studies on liposomal amphotericin B (L-AMP-LRC-1) in the treatment of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1999;93(3):314–18. doi: 10.1016/s0035-9203(99)90036-6. [DOI] [PubMed] [Google Scholar]
- 14.Cascio A, di Martino L, Occorsio P, et al. A 6 day course of liposomal amphotericin B in the treatment of infantile visceral leishmaniasis: the Italian experience. J Antimicrob Chemother. 2004;54(1):217–20. doi: 10.1093/jac/dkh279. [DOI] [PubMed] [Google Scholar]
- 15.Davidson RN, di Martino L, Gradoni L, et al. Short-course treatment of visceral leishmaniasis with liposomal amphotericin B (AmBisome) Clin Infect Dis. 1996;22(6):938–43. doi: 10.1093/clinids/22.6.938. [DOI] [PubMed] [Google Scholar]
- 16.Davidson RN, Di Martino L, Gradoni L, et al. Liposomal amphotericin B (AmBisome) in Mediterranean visceral leishmaniasis: a multi-centre trial. Q J Med. 1994;87(2):75–81. [PubMed] [Google Scholar]
- 17.di Martino L, Davidson RN, Giacchino R, et al. Treatment of visceral leishmaniasis in children with liposomal amphotericin B. J Pediatr. 1997;131(2):271–7. doi: 10.1016/s0022-3476(97)70165-3. [DOI] [PubMed] [Google Scholar]
- 18.Kafetzis DA, Velissariou IM, Stabouli S, et al. Treatment of paediatric visceral leishmaniasis: amphotericin B or pentavalent antimony compounds? Int J Antimicrob Agents. 2005;25(1):26–30. doi: 10.1016/j.ijantimicag.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Molina I, Falco V, Crespo M, et al. Efficacy of liposomal amphotericin B for secondary prophylaxis of visceral leishmaniasis in HIV-infected patients. J Antimicrob Chemother. 2007;60(4):837–42. doi: 10.1093/jac/dkm294. [DOI] [PubMed] [Google Scholar]
- 20.Mondal S, Bhattacharya P, Rahaman M, et al. A curative immune profile one week after treatment of Indian kala-azar patients predicts success with a short-course liposomal amphotericin B therapy. PLoS Negl Trop Med. 2010;4(7):e764. doi: 10.1371/journal.pntd.0000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller M, Balasegaram M, Koummuki Y, et al. A comparison of liposomal amphotericin B with sodium stibogluconate for the treatment of visceral leishmaniasis in pregnancy in Sudan. J Antimicrob Chemother. 2006;58(4):811–15. doi: 10.1093/jac/dkl342. [DOI] [PubMed] [Google Scholar]
- 22.Mueller M, Ritmeijer K, Balasegaram M, et al. Unresponsiveness to AmBisome in some Sudanese patients with kala-azar. Trans R Soc Trop Med Hyg. 2007;101(1):19–24. doi: 10.1016/j.trstmh.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Pagliano P, Rossi M, Rescigno C, et al. Mediterranean visceral leishmaniasis in HIV-negative adults: a retrospective analysis of 64 consecutive cases (1995-2001) J Antimicrob Chemother. 2003;52(2):264–8. doi: 10.1093/jac/dkg340. [DOI] [PubMed] [Google Scholar]
- 24.Ritmeijer K, ter Horst R, Chane S, et al. Limited effectiveness of high-dose liposomal amphotericin B (AmBisome) for treatment of visceral leishmaniasis in an Ethiopian population with high HIV prevalence. Clin Infect Dis. 2011;53(12):e152–8. doi: 10.1093/cid/cir674. [DOI] [PubMed] [Google Scholar]
- 25.Sanath SS, Gogtay NJ, Kshirsagar NA. Post-marketing study to assess the safety, tolerability and effectiveness of Fungisome: an Indian liposomal amphotericin B preparation. J Postgrad Med. 2005;51(Suppl 1):S58–63. [PubMed] [Google Scholar]
- 26.Seaman J, Boer C, Wilkinson R, et al. Liposomal amphotericin B (AmBisome) in the treatment of complicated kala-azar under field conditions. Clin Infect Dis. 1995;21(1):188–93. doi: 10.1093/clinids/21.1.188. [DOI] [PubMed] [Google Scholar]
- 27.Sinha PK, Roddy P, Palma PP, et al. Effectiveness and safety of liposomal amphotericin B for visceral leishmaniasis under routine program conditions in Bihar, India. Am J Trop Med Hyg. 2010;83(2):357–64. doi: 10.4269/ajtmh.2010.10-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study demonstrated the feasibility of using LAMB under programmatic conditions in the most important endemic foci.
- 28.Sinha PK, van Griensven J, Pandey K, et al. Liposomal amphotericin B for visceral leishmaniasis in human immunodeficiency virus-coinfected patients: 2-year treatment outcomes in Bihar, India. Clin Infect Dis. 2011;53(7):e91–8. doi: 10.1093/cid/cir521. [DOI] [PubMed] [Google Scholar]
- 29.Sundar S, Agrawal G, Rai M, et al. Treatment of Indian visceral leishmaniasis with single or daily infusions of low dose liposomal amphotericin B: randomised trial. BMJ. 2001;323(7310):419–22. doi: 10.1136/bmj.323.7310.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundar S, Jha TK, Thakur CP, et al. Single-dose liposomal amphotericin B in the treatment of visceral leishmaniasis in India: a multicenter study. Clin Infect Dis. 2003;37(6):800–4. doi: 10.1086/377542. [DOI] [PubMed] [Google Scholar]
- 31.Sundar S, Jha TK, Thakur CP, et al. Low-dose liposomal amphotericin B in refractory Indian visceral leishmaniasis: a multicenter study. Am J Trop Med Hyg. 2002;66(2):143–6. doi: 10.4269/ajtmh.2002.66.143. [DOI] [PubMed] [Google Scholar]
- 32.Sundar S, Mehta H, Suresh AV, et al. Amphotericin B treatment for Indian visceral leishmaniasis: conventional versus lipid formulations. Clin Infect Dis. 2004;38(3):377–83. doi: 10.1086/380971. [DOI] [PubMed] [Google Scholar]
- 33.Sundar S, Rai M, Chakravarty J, et al. New treatment approach in Indian visceral leishmaniasis: single-dose liposomal amphotericin B followed by short-course oral miltefosine. Clin Infect Dis. 2008;47(8):1000–6. doi: 10.1086/591972. [DOI] [PubMed] [Google Scholar]
- 34.Sundar S, Sinha PK, Verma DK, et al. Ambisome plus miltefosine for Indian patients with kala-azar. Trans R Soc Trop Med Hyg. 2011;105(2):115–17. doi: 10.1016/j.trstmh.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Syriopoulou V, Daikos GL, Theodoridou M, et al. Two doses of a lipid formulation of amphotericin B for the treatment of Mediterranean visceral leishmaniasis. Clin Infect Dis. 2003;36(5):560–6. doi: 10.1086/367843. [DOI] [PubMed] [Google Scholar]
- 36.Thakur CP. A single high dose treatment of kala-azar with Ambisome (amphotericin B lipid complex): a pilot study. Int J Antimicrob Agents. 2001;17(1):67–70. doi: 10.1016/s0924-8579(00)00312-5. [DOI] [PubMed] [Google Scholar]
- 37.Torrado JJ, Espada R, Ballesteros MP, Torrado-Santiago S. Amphotericin B formulations and drug targeting. J Pharm Sci. 2008;97(7):2405–25. doi: 10.1002/jps.21179. [DOI] [PubMed] [Google Scholar]
- 38.Dupont B. Overview of the lipid formulations of amphotericin B. J Antimicrob Chemother. 2002;49(Suppl 1):31–6. doi: 10.1093/jac/49.suppl_1.31. [DOI] [PubMed] [Google Scholar]
- 39.Approval letter. FDA http://www.accessdata.fda.gov/drugsatfda_docs/nda/97/050740a_081797-1.pdf Available from:
- 40.Berman JD. U.S Food and Drug Administration approval of AmBisome (liposomal amphotericin B) for treatment of visceral leishmaniasis. Clin Infect Dis. 1999;28(1):49–51. doi: 10.1086/515086. [DOI] [PubMed] [Google Scholar]
- 41.Moon S, Jambert E, Childs M, von Schoen-Angerer T. A win-win solution?: a critical analysis of tiered pricing to improve access to medicines in developing countries. Global Health. 2011;7(1):39. doi: 10.1186/1744-8603-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clinical Trial to Assess the Safety and Efficacy of Sodium Stibogluconate (SSG) and AmBisome® Combination, Miltefosine and AmBisome® and Miltefosine Alone for the Treatment Visceral Leishmaniasis in Eastern Africa ClinicalTrials.gov. http://clinicaltrials.gov/ct2/show/NCT01067443?term=ambisome+AND+miltefosine&rank=2 Available from:
- 43.Efficacy and Safety Study of Drugs for Treatment of Visceral Leishmaniasis in Brazil (LVBrasil) ClinicalTrials.gov. http://clinicaltrials.gov/ct2/show/NCT01310738?term=ambisome%2C+meglumine+antimoniate&rank=2 Available from:
- 44.Open-Label, Sequential Step, Safety and Efficacy Study to Determine the Optimal Single Dose of Ambisome for Patients With Visceral Leishmaniasis ClinicalTrials.gov. http://clinicaltrials.gov/ct2/show/NCT00832208?term=ambisome+AND+visceral+leishmaniasis&rank=10 Available from:
- 45.Lachaud L, Bourgeois N, Plourde M, et al. Parasite susceptibility to amphotericin B in failures of treatment for visceral leishmaniasis in patients coinfected with HIV type 1 and Leishmania infantum. Clin Infect Dis. 2009;48(2):e16–22. doi: 10.1086/595710. [DOI] [PubMed] [Google Scholar]
- 46.Di Giorgio C, Faraut-Gambarelli F, Imbert A, et al. Flow cytometric assessment of amphotericin B susceptibility in Leishmania infantum isolates from patients with visceral leishmaniasis. J Antimicrob Chemother. 1999;44(1):71–6. doi: 10.1093/jac/44.1.71. [DOI] [PubMed] [Google Scholar]
- 47.Purkait B, Kumar A, Nandi N, et al. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob Agents Chemother. 2012;56(2):1031–41. doi: 10.1128/AAC.00030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Draft Guidance for Industry on Liposome Drug Products. US FDA http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070570.pdf 2002 Available from:
- 49.Draft Guidance on Doxorubicin Hydrochloride. US FDA http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM199635.pdf 2010 Available from:
- 50.Reflection paper on the data requirements for intravenous liposomal products developed with reference to an innovator liposomal product. EMA http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/07/WC500109479.pdf 2011 Available from:
- 51.Burgess DJ, Crommelin DJ, Hussain AS, et al. Assuring quality and performance of sustained and controlled release parenterals: EUFEPS workshop report. AAPS PharmSci. 2004;6(1):E11. doi: 10.1208/ps060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burgess DJ, Hussain AS, Ingallinera TS, Chen ML. Assuring quality and performance of sustained and controlled release parenterals: workshop report. AAPS PharmSci. 2002;4(2):E7. doi: 10.1208/ps040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burgess P, Hutt PB, Farokhzad OC, et al. On firm ground: IP protection of therapeutic nanoparticles. Nat Biotechnol. 2010;28(12):1267–70. doi: 10.1038/nbt.1725. [DOI] [PubMed] [Google Scholar]; •• This article explains why the development of generic therapeutic nanoparticles faces a combination of scientific and regulatory challenges in the US market.
- 54.Lionberger RA. FDA critical path initiatives: opportunities for generic drug development. AAPS J. 2008;10(1):103–9. doi: 10.1208/s12248-008-9010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mamidi RN, Weng S, Stellar S, et al. Pharmacokinetics, efficacy and toxicity of different pegylated liposomal doxorubicin formulations in preclinical models: is a conventional bioequivalence approach sufficient to ensure therapeutic equivalence of pegylated liposomal doxorubicin products? Cancer Chemother Pharmacol. 2010;66(6):1173–84. doi: 10.1007/s00280-010-1406-x. [DOI] [PubMed] [Google Scholar]
- 56.Olson JA, Adler-Moore JP, Jensen GM, et al. Comparison of the physicochemical, antifungal, and toxic properties of two liposomal amphotericin B products. Antimicrob Agents Chemother. 2008;52(1):259–68. doi: 10.1128/AAC.00870-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schellekens H, Klinger E, Muhlebach S, et al. The therapeutic equivalence of complex drugs. Regul Toxicol Pharmacol. 2011;59(1):176–83. doi: 10.1016/j.yrtph.2010.09.021. [DOI] [PubMed] [Google Scholar]; •• This article describes the point of view of academic experts on regulatory challenges in the US and Europe, and a possible way forward for the evaluation of generic liposomal formulations.
- 58.British National Formulary http://www.bnf.org/bnf/index.htm 2012 Available from:
- 59.Registry of the South African Medicine Price http://www.mpr.gov.za/PublishedDocuments.aspx#DocCatId=21 2012 Available from:
- 60.Gilead, Annual Report 2011 http://www.gilead.com/AR2011/GileadSciences_10K_20120223.pdf 2012 Filed in February. Available from:
- 61.Approved draft labelling AmBisome. US FDA http://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/50-740S001_AmBisome_prntlbl.pdf 1997 Available from:
- 62.Collin S, Davidson R, Ritmeijer K, et al. Conflict and kala-azar: determinants of adverse outcomes of kala-azar among patients in southern Sudan. Clin Infect Dis. 2004;38(5):612–19. doi: 10.1086/381203. [DOI] [PubMed] [Google Scholar]
- 63.Chappuis F, Alirol E, Worku DT, et al. High mortality among older patients treated with pentavalent antimonials for visceral leishmaniasis in East Africa and rationale for switch to liposomal amphotericin B. Antimicrob Agents Chemother. 2011;55(1):455–6. doi: 10.1128/AAC.01298-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gorski S, Collin SM, Ritmeijer K, et al. Visceral leishmaniasis relapse in Southern Sudan (1999 – 2007): a retrospective study of risk factors and trends. PLoS Negl Trop Med. 2010;4(6):e705. doi: 10.1371/journal.pntd.0000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seaman J, Mercer AJ, Sondorp HE, Herwaldt BL. Epidemic visceral leishmaniasis in southern Sudan: treatment of severely debilitated patients under wartime conditions and with limited resources. Ann Intern Med. 1996;124(7):664–72. doi: 10.7326/0003-4819-124-7-199604010-00007. [DOI] [PubMed] [Google Scholar]
- 66.Boelaert M, Meheus F, Sanchez A, et al. The poorest of the poor: a poverty appraisal of households affected by visceral leishmaniasis in Bihar, India. Trop Med Int Health. 2009;14(6):639–44. doi: 10.1111/j.1365-3156.2009.02279.x. [DOI] [PubMed] [Google Scholar]
- 67.Pascual Martinez F, Picado A, Roddy P, Palma P. Low castes have poor access to visceral leishmaniasis treatment in Bihar, India. Trop Med Int Health. 2012;17(5):666–73. doi: 10.1111/j.1365-3156.2012.02960.x. [DOI] [PubMed] [Google Scholar]
- 68.Meheus F, Balasegaram M, Olliaro P, et al. Cost-effectiveness analysis of combination therapies for visceral leishmaniasis in the Indian subcontinent. PLoS Negl Trop Med. 2010;4(9):e818. doi: 10.1371/journal.pntd.0000818. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A cost-effectiveness analysis suggesting that single-dose LAMB would be the most cost-effective treatment for VL in South Asia if the price of LAMB was reduced to US$9.80 per vial.
- 69.Burki T. Drug donated for treatment of visceral leishmaniasis. Lancet Infect Dis. 2012;12(2):106–7. doi: 10.1016/s1473-3099(12)70024-5. [DOI] [PubMed] [Google Scholar]
- 70.WHO 2010. Technical Report Series (TRS), Annex 6 "Costs of medicines in current use for the treatment of leishmaniasis".
- 71.Reithinger R, Dujardin JC, Louzir H, et al. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7(9):581–96. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 72.Wortmann G, Zapor M, Ressner R, et al. Liposomal amphotericin B for treatment of cutaneous leishmaniasis. Am J Trop Med Hyg. 2010;83(5):1028–33. doi: 10.4269/ajtmh.2010.10-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Solomon M, Pavlotsky F, Leshem E, et al. Liposomal amphotericin B treatment of cutaneous leishmaniasis due to Leishmania tropica. JEADV. 2011;25(8):973–7. doi: 10.1111/j.1468-3083.2010.03908.x. [DOI] [PubMed] [Google Scholar]
- 74.Solomon M, Pavlotsky F, Barzilai A, et al. Liposomal amphotericin B in comparison to sodium stibogluconate for Leishmania braziliensis cutaneous leishmaniasis in travelers. J Am Acad Dermatol. 2012 doi: 10.1016/j.jaad.2012.06.014. In press. [DOI] [PubMed] [Google Scholar]
- 75.Motta JO, Sampaio RNR. A pilot study comparing low-dose liposomal amphotericin B with N-methyl glucamine for the treatment of American cutaneous leishmaniasis. J Eur Acad Dermatol Venereol. 2012;26:331–5. doi: 10.1111/j.1468-3083.2011.04070.x. [DOI] [PubMed] [Google Scholar]
- 76.Amato VS, Tuon FF, Siqueira AM, et al. Treatment of mucosal leishmaniasis in Latin America: systematic review. Am J Trop Med Hyg. 2007;77(2):266–74. [PubMed] [Google Scholar]
- 77.Sampaio RN, Marsden PD. Treatment of the mucosal form of leishmaniasis without response to glucantime, with liposomal amphotericin B. Rev Soc Bras Med Trop. 1997;30(2):125–8. doi: 10.1590/s0037-86821997000200007. [DOI] [PubMed] [Google Scholar]
- 78.Amato VS, Tuon FF, Camargo RA, et al. Can we use a lower dose of liposomal amphotericin B for the treatment of mucosal American leishmaniasis? Am J Trop Med Hyg. 2011;85(5):818–19. doi: 10.4269/ajtmh.2011.11-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Musa AM, Khalil EA, Mahgoub FA, et al. Efficacy of liposomal amphotericin B (AmBisome) in the treatment of persistent post-kala-azar dermal leishmaniasis (PKDL) Ann Trop Med Parasitol. 2005;99(6):563–9. doi: 10.1179/136485905X514127. [DOI] [PubMed] [Google Scholar]