Abstract
The objective of this systematic review was to assess whether the orally acting progestagen, dydrogesterone lowers the incidence of miscarriage in women with threatened miscarriage. A computerized search was performed in Medline, Embase, and Ovid Medline for original reports with the product name ‘Duphaston’ or ‘dydrogesterone’, and limited to clinical human data. Twenty-one reports of dydrogesterone treatment were identified with 1380 patients. Five randomized trials were identified, including 660 women who fulfilled the criteria for metaanalysis. The number of subsequent miscarriages or continuing pregnancies per randomized woman was compared in women receiving dydrogesterone compared to standard bed rest or placebo intervention. There was a 13% (44/335) miscarriage rate after dydrogesterone administration compared to 24% in control women [odds ratio for miscarriage 0.47, (CI = 0.31–0.7), 11% absolute reduction in the miscarriage rate]. The adverse and side effects were summarized in all 21 reports, and seemed to be minimal. Although all the predictive and confounding factors could not be controlled for, the results of this systematic review show a significant reduction of 47% in the odds for miscarriage when dydrogesterone is compared to standard care indicating a real treatment effect.
Keywords: Abortion, duphaston, dydrogesterone, miscarriage, progesterone, threatened miscarriage
Introduction
Threatened miscarriage is defined by the National Library of Medicine, Medical Subject Headings (2012 MeSH), as bleeding during the first 20 weeks of pregnancy while the cervix is closed. It is the most common complication in pregnancy, occurring in 20% of all pregnancies. The condition may progress to miscarriage in approximately one-half of cases [1,2], or may resolve. There are problems of definition, as the bleeding may include anything from spots of blood to potentially fatal shock. The treating physician is faced with the question whether any treatment can effectively prevent the pregnancy from being miscarried. Progestational agents have been prescribed since the 1950s in order to prevent miscarriages. There are many theoretical data to support the use of progestagens. Progestagens enhances implantation, affect the cytokine balance, inhibit natural killer cell activity at the feto-maternal interface, inhibit the release of arachidonic acid, prevent myometrial contractility and prevent cervical dilatation. Activation of progesterone receptors on lymphocytes results in the synthesis of a protein known as progesterone-induced blocking factor (PIBF) [3]. PIBF favours the production of asymmetric, pregnancy-protecting antibodies.
Indeed it has often been reported that a progesterone lack may lead to miscarriage [4,5]. Lutectomy prior to 7 weeks causes miscarriage [6]. Mifepristone blocks the progesterone receptor, leading to fetal death and placental separation. Therefore, the question arises whether progesterone supplementation should be used, and if so, which progestagen. Progestagens can be administered orally, vaginally, or intra-muscularly. Oral administration is the easiest route of administration, and generally the most acceptable route for the patient. Vaginal administration results in higher uterine concentrations [7], but is often uncomfortable in the presence of vaginal bleeding, or may be washed out if bleeding is severe. Intramuscular progesterone provides optimal blood levels but can induce abscesses formation and is extremely painful. Of the oral progestagens, progesterone itself has variable plasma concentrations [8], and side effects including such as nausea, headache, and sleepiness. Additionally, a literature search found no trials of the effect of oral progesterone on threatened miscarriage. However, there are a number of studies which have suggested that dydrogesterone can reduce pregnancy loss in women with threatened miscarriage. Dydrogesterone has a good safety and tolerability profile. It is structurally and pharmacologically similar to natural progesterone has good oral bioavailability and few side effects. Dydrogesterone has no androgenic effects on the fetus, and does not inhibit the formation of progesterone in the placenta [9]. The aim of this systematic review was to determine whether dydrogesterone is more effective than conservative management in enabling pregnancy to continue in women with threatened miscarriage.
Material and methods
Sources
A literature search was performed for in September 2010 for all papers available at that time in EMBASE and Ovid MEDLINE® that fulfilled the following criteria: original contributions with product name ‘Duphaston’ or ‘dydrogesterone’, reports limited to clinical human data excluding reviews, case reports and editorials in any language. Case reports were defined as publications describing a single patient. However, case series describing exposure and outcome in multiple patients were included. All articles considered were investigator-initiated trials and published in the scientific literature. As positive results have a better chance of being published the selection of studies used for the metaanalysis may be biased.
Twenty-two publications containing data on dydrogesterone use in threatened miscarriage were retrieved from the literature. They can be classified as follows: 13 controlled studies, including: three double-blind studies [10–12], four open-label randomized studies [13–16] and six open-label non-randomized studies [17–23]. The reports of Kalinka and Szekeres-Bartho [19] and Kalinka and Radwan [20], are based on the same patient populations and are therefore considered as one study. There were three uncontrolled studies [24–26] and there were five case series [27–31].
Study selection
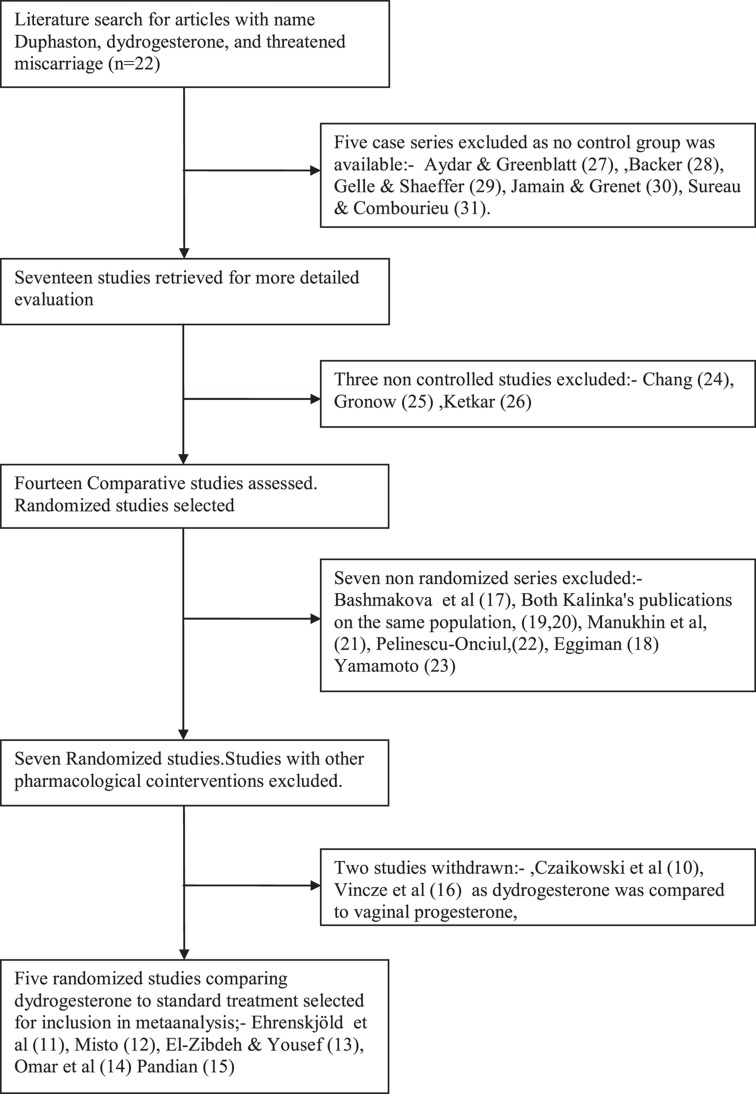
Figure 1 is a flow chart showing how previous series were selected for inclusion in the metaanalysis. The objective of this metaanalysis was to compare dydrogesterone to a control group receiving standard care. Standard care was taken to be included bed rest, vitamin supplementation, placebo and/or bed rest, combined as a single group. Hence, studies comparing dydrogesterone to other progestogens were excluded. It was planned to carry out the metaanalysis on randomized trials only, as they were considered to represent the highest quality. There were six comparative studies comparing dydrogesterone to controls. Ehrenskjöld et al.’s [11] and Mistò’s [12] study were double-blind, and therefore eligible for the inclusion in the metaanalysis. There were three open-label randomized studies [13–15],. Omar et al.’s [14] study claimed to be randomized, but contained no details of the method of randomization. In the El-Zibdeh and Yousef [13] study patients were randomized according to the day of the week on which the patient presented. (which is not the most robust system of randomization). Of the open-labeled studies, only Pandian’s [15] study was truly randomized. Two studies [11,12] were placebo-controlled. All other studies have a mixture of standard supportive care. The metaanalysis included the five reportedly-randomized studies [11–15].
Figure 1. .
Flow chart for inclusion or exclusion of studies.
Summary measures
The main outcome measure chosen for the current metaanalysis was an odds ratio (OR) of miscarriages per patient randomized.
Quantitative data analysis
The data results for each of the studies eligible for metaanalysis were expressed as an OR with 95% confidence intervals (CIs). Study-to-study variation was assessed by using the Chi-square statistic (the hypothesis tested was that the studies are all drawn from the same population – i.e., from a population with the same effect size). The results were combined for metaanalysis using the Mantel–Haenszel model. A fixed effects model was used, because there was no statistically significant heterogeneity between trial results. The metaanalysis was performed with Mix 2.0 software for Office 2007. It was not possible to get access to the original data sets of the literature studies. Therefore, no additional pooled analyses or meta-analyses were performed. However, due to the wide interval of time between the double-blind studies published in 1967 and the three open-label randomized studies (2005–2009) an additional analysis should be performed using only the open-label randomized studies.
The heterogeneity of treatment effects (between-study variation) was assessed through the Cochrane heterogeneity test (significance level at 10%). A fixed effects model was used as the heterogeneity was less than 10%. A DerSimonian & Laird random-effects model would have been used if there were heterogeneity between the studies.
Results
A total of five randomized controlled trials and one non-randomized trial evaluating the efficacy of dydrogesterone in the treatment of threatened miscarriage were identified. Table I shows the inclusion data for each of the trials analyzed, the treatment regimen and sample size of the five studies. The five randomized studies in threatened miscarriage reviewed in this document enrolled a total of 700 pregnant women, of whom 660 (94.28%) were eligible for analyses of pregnancy maintenance. In Omar et al.’s [14] series, 40 patients were lost to follow-up, of the 194 originally recruited. Of these 660 women, 335 received dydrogesterone, whereas 325 received a regimen with standard of care either placebo or bed rest.
Table I. .
Details of included studies.
| Reference | Inclusion Criteria | Exclusion criteria | Sample sizea | Treatment regimen |
|---|---|---|---|---|
| Double-blind placebo-controlled study studies | ||||
| Ehrenskjöld et al. [11] |
|
None reported | 72 | Strict bed rest plus DYD 40 mg in 12 h, then 20 mg t.i.d. until symptoms remit, then 10 mg b.i.d. for 5 days and 5 mg b.i.d. for ≥7 days. Treatment start at Month 2 (n = 17); Month 3 (n = 27); Month 4 (n = 12); or ≥Month 5 (n = 16). |
| 81 | Strict bed rest plus placebo. Treatment start at Month 2 (n = 13); Month 3 (n = 34); Month 4 (n = 14); or ≥Month 5 (n = 20). | |||
| Mistò [12] |
|
None reported | 7 | DYD 20–40 mg/d for 6–15 days |
| 9 | Placebo | |||
| Open-label randomized studies | ||||
| El-Zibdeh and Yousef [13] | Mild or moderate 1st trimester vaginal bleeding |
|
86 | Standard supportive care plus DYD 20 mg/d (10 mg b.i.d.), start when symptoms occurred, continued for 1 week after symptoms stopped. |
| 60 | Standard supportive care alone | |||
| Omar et al. [14] |
|
|
74 | Bed rest, folic acid and DYD 40 mg stat, then 20 mg/d (10 mg b.i.d.) until bleeding stopped or for 1 weekb |
| 80 | Bed rest and folic acid alone | |||
| Pandian [15] |
|
|
96 | DYD 40 mg stat, then 20 mg/d (10 mg b.i.d.), from start of symptoms until Week 16 |
| 95 | Bed rest | |||
Stat, at once; b.i.d., twice daily; t.i.d., three times daily; DYD, dydrogesterone; i.m., intramuscular.
aSample size refers to the number of patients included in the metaanalysis.
bThe duration of treatment in this study is unclear. Both “1 week” and “until bleeding stopped” are mentioned in the publication.
Comparison of trials
Table I shows the regimens used in the six trials. There were slight differences in regimen. The dose of dydrogesterone varied between the trials. The standard dose was 10 mg/ b.i.d. Ehrenskjöld et al. [11], used a loading dose of 40 mg, after 12 h 20 mg was administered t.i.d. until symptoms remit, then the standard dose of 10 mg b.i.d. for 5 days and 5 mg b.i.d. for ≥7 days. Ehrenskjöld et al. [11], combined this treatment with strict bed rest. The other studies did not mention bed rest as a treatment modality for the dydrogesterone group. Similarly there were slight differences in the control group. Both double-blind studies were placebo-controlled. Ehrenskjöld et al. [11], used strict bed rest in addition to placebo, whereas Mistò [12], used placebo alone. Bed rest was also used in the control group in Omar et al.’s [14] study and Pandian’s [15] study
Comparison of efficacy results
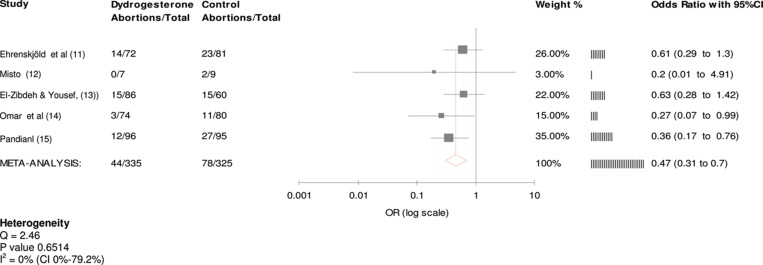
Figure 2 shows the metaanalysis tree for the five trials in the study. The administration of dydrogesterone was associated with a 47% reduction in the odds for miscarrying (OR = 0.47 CI = 0.31–0.7), using a fixed effects model. This figure was statistically significant. The Cochran Q test and I 2 were analyzed and showed homogeneity between the studies (p = 0.6514). Manukhin et al.’s [21] non randomized study showed an OR for miscarriage of 0.05 after dydrogesterone (CI = 0.01–0.25) when compared to standard care. Inclusion of Manukhin et al.’s [21] study would not have altered the results, (Common OR: 0.35, CI = 0.19–0.65). The results of the five randomized studies show that dydrogesterone provides a suitable treatment for the management of threatened miscarriage. None of the patients included in these studies prematurely stopped treatment with dydrogesterone for reasons other than non-compliance or loss to follow-up. Each of the five randomized studies demonstrated that dydrogesterone was associated with a lower trend to miscarriage than standard care. This trend reached statistical significance in two of the studies [14,15]. In Mistò’s [12] trial, the numbers were too small to reach statistical significance.
Figure 2. .
Common Odds Ratio for randomized studies (fixed effects).
Due to the wide time interval between the double-blind studies (1967) and the open-label randomized studies (2005–2009) an additional analysis was performed using only the open-label randomized studies. The metaanalysis showed a 42% significant decrease in the abortion rate in the three more recent open-label randomized studies (OR = 0.42, CI = 0.25-0.69).
Follow-up data
Table II shows the long term follow-up data for the 660 patients in the five studies which were analyzed. However, as side effects may have a relatively low incidence, all of the papers which were obtained from the literature search are included. One-thousand three-hundred and eighty patients were assessed. There were no particular side effects seen. The medication seemed to have no side effects on the mother. Only Pelinescu-Onciul’s [22] paper reported drowsiness. Gelle and Schaeffer [29] reported nausea and vomiting, but in only one patient, and Chang [24], reported nausea and vomiting in two patients. However, nausea and vomiting may be due to early pregnancy itself rather than the medication. Dydrogesterone seemed to be associated with a higher birth weight, higher 1-min Apgar scores, and a lower incidence of growth retardation. However, these differences were not significant. There seemed to be very few birth defects. Many papers specifically reported no congenital anomalies. El-Zibdeh and Yousef [13], reported one neural tube defect in an infant exposed to dydrogesterone and one infant with congenital heart disease, but there was also one neural tube defect in the untreated control group. Eggimann et al. [18], reported Cheilognathourano-schisis, cheilognatho-schisis, hypospadias, duodenal atresia, cryptor-chidism, naevus, pes planus, pes valgus and three infants with hip dislocation. However, in this study, dydrogesterone was part of a complex regimen of bed rest, progesterone, 17α-hydroxy-progesterone caproate, and hexoestrol or buphenine. The effect of dydrogesterone can therefore not be assessed. Additionally, Fallot’s tetralogy, hypoplastic left heart, trisomy 21, bilateral cheilognathourano-schisis, hypoplasia of the right humerus, radius and ulna with three fingers on the right hand, hooked foot and two cases of hypospadias were seen in the control group who received no treatment.
Table II. .
Long term follow-up data.
| Author | Design/Indication | Sample size | Regimen | Outcome | Side Effects/AEs |
|---|---|---|---|---|---|
| Randomized studies | |||||
| Ehrenskjöld et al [11] | Double-blind, placebo-controlled/threatened miscarriage | 72 pts | DYD, | 14 miscarriages or stillbirths, | No birth defects, |
| strict bed rest | 58 live births (3 twins) | no side effects | |||
| 81 pts | Placebo, | 23 miscarriages or stillbirths, | 1 x hip dislocation, | ||
| strict bed rest | 58 live births (1 twin) | 1 x coarctation of the aorta | |||
| Mistò [12] | Double-blind, placebo-controlled/threatened miscarriage | 7 pts | DYD | 7 births | No signs of masculinization |
| 9 pts | placebo | 7 births and 2 abortive interrup-tions of pregnancy | |||
| El-Zibdeh and Yousef [13] | Open-label, randomized/threatened miscarriage, viable fetus | 86 pts | DYD + standard supportive care | 65 deliveries, 6 preterm labors, 15 miscarriages. | No AEs, 1 neural tube defect, 1 heart disease |
| 60 pts | Standard supportive care alone | 40 full-term deliveries, 5 preterm labor, 15 miscarriages (25%). | 1 neural tube defect, 1 unspecified abnormality | ||
| Omar et al. [14] | Open-label, randomized/threatened miscarriage in first trimester, viable fetus | 74 pts | DYD, bed rest, folic acid | 71 pregnancies continued >Week 20, 3 miscarriages. | Safety and tolerability not reported. |
| 80 pts | Bed rest, folic acid | 69 pregnancies continued >Week 20; 11 miscarriages | |||
| Pandian [15] | Open-label, randomized/threatened miscarriage, viable fetus, <Week 16 | 96 pts | DYD | 84 pregnancies continued >Week 20, 12 miscarriages | No intrauterine deaths or congenital abnormalities. 3 (3.1%) low birth weight (<2.500 g) infants. |
| 95 pts | Bed rest | 68 pregnancies continued >Week 20, 27 miscarriages | No intrauterine deaths or congenital abnormalities. 2 (2.1%) low birth weight (<2.500 g) infants | ||
| Czajkowski et al. [10] | Double-blind randomized/threatened miscarriage, <12 weeks, viable embryo | 24 pts | DYD | 2 miscarriages; 8% | Safety and tolerability data not reported. |
| 29 pts | Vaginal progesterone | 4 abortions; 14% | |||
| Vincze et al. [16] | Open-label, randomized/threatened miscarriage, | 86 pts | DYD | 7 abortions | No side effects. No fetal abnormalities in either group. |
| Week 5–13 of gestation | 63 pts | Micronized progesterone (vaginal) | 5 miscarriages | ||
| Open-label studies, non-randomized | |||||
| Bashmakova et al. [17] | Open, cohort/Threatened miscarriage in first trimester. | 275 pts | DYD plus standard therapy | 10% premature births (p < 0.05), 29% healthy (not significant) | DYD: higher birth weight (3,350 ± 62g vs. 3,137 ± 93 g; higher 1 minute Apgar score (7.15 ± 0.98 vs. 6.8 ± 0.18; less hypoxic-ischemic CNS lesions (9% vs. 29%; less requirement for rehabilitation in the pediatric clinic (26% vs. 44%). All p < 0.05. |
| 45 pts | Standard therapy alone | 20% preterm births, 18% healthy | |||
| Kalinka and Szekeres-Bartho [19]; Kalinka and Radwan [20] | Threatened miscarriage | 27 pts | DYD | 3 missed miscarriages; 11.1% (2 deliveries preterm) | No significant differences between groups for mean gestational age or birth weight. |
| 16 normal pregnant women | No treatment | 1 missed miscarriage; 6.3% (no preterm delivery) | |||
| Manukhin, et al. [21] | Open-label/threatened miscarriage | 45 pts | DYD plus standard care | Pregnancy progressed in 43 pts; 95.6% (difference vs. control group statistically significant) | Safety and tolerability data not reported. |
| 41 pts | “Symptomatic treatment” only, | Pregnancy progressed in 22 pts; 53.7% | |||
| Pelinescu-Onciul [22], | Open-label/threatened miscarriage, viable fetus | 100 pts | DYD | Pregnancy progressed in 93 pts. | Only side effect reported with DYD was drowsiness |
| 7 miscarriages (p = 0.002 vs. vaginal progesterone) | |||||
| 125 (from prior study) | Vaginal progesterone | 18.7% miscarriages | |||
| Eggimann [18] | Retrospective/threatened miscariage (including pts with habitual miscarriage or uterine malformations without bleeding) | 238 pts with hexoestrol + DYD; 61 pts with buphenine +DYD | Bed rest, 17β-hydroxy-progesterone caproate, DYD,and hexoestrol/ buphenine | Miscarriage rate 21%, with hexoestrol, 16%, with buphenine. | Cheilognathourano-schisis, cheilognatho-schisis, hypospadias, duodenal atresia, hip dislocation (3 infants), cryptor-chidism, naevus, pes planus, pes valgus |
| 214 full-term babies; | |||||
| 29 (11%) premature births (p < 0.0005) | |||||
| 243 normal pregnant women | No treatment | No miscarriages | Fallot’s tetralogy, hypoplastic left heart, trisomy 21, bilateral cheilognathourano-schisis, hypoplasia of the right humerus, radius and ulna and 3 fingers on right hand, hypospadias (2 cases), hooked foot. | ||
| 228 full-term babies; 15 (5%) premature births | |||||
| Yamamoto [23] | Open-label/threatened miscarriage | 24 pts | DYD | 8 normal deliveries, 7 good (ongoing regnancy), | No side effects |
| 9 miscarriages/D&C | |||||
| 26 pts | 17β-hydroxy-progesterone caproate | 18 deliveries, 1 ongoing pregnancy, | No side effects | ||
| 6 miscarriages, 1 pt not reported | |||||
| Uncontrolled studies | |||||
| Chang [24] | Threatened miscarriage <20 weeks gestation | 7 pts | DYD | 1 term baby, | No abnormalities or side effects noted. No masculinizing effects. |
| 2 ongoing pregnancies 2 miscarriages, 2 premature – not viable | |||||
| 2 pts with vomiting and nausea. | |||||
| Gronow [25] | Threatened miscarriage | 11 IVF pregnancies with bleeding | DYD | 2 miscarriages | Safety and tolerability data not reported. |
| Ketkar [26] | Threatened miscarriage in first trimester | 42 pts | DYD plus folic acid. | 38 pregnancies continued, 25 deliveries, | All infants normal, |
| Apgar scores, 8–10 | |||||
| 13 ongoing pregnancies, 4 missed miscarriages | |||||
| Case series | |||||
| Aydar and Greenblatt [27] | Threatened miscarriage | 7 pts | DYD | 2 miscarriages, 3 term deliveries, | Well accepted. Virtually no undesirable side effects in 192 pts treated for different indications. |
| 2 ongoing pregnancies without problems at time of report. | |||||
| Backer [28] | Threatened miscarriage | 36 pts | DYD | 24 pregnancies continued (16 term deliveries, 2 premtures, 6 ongoing pregnancies at time of report; 12 miscarriages. | No genital abnormalities, |
| no effect on hemato-poietic system, liver or kidney function. | |||||
| No side effects. | |||||
| Gellé and Shaeffer [29] | Threatened miscarriage | 20 pts | DYD | 12 miscarriages | Severe nausea and vomiting in 1 pt who was withdrawn. |
| 8 pregnancies continued (7 delivered at term, 1 in 8th month, all 8 infants normal | |||||
| Jamain and Grenrt [30] | Threatened miscarriage | 19 pts | DYD, | 9 term pregnancies, 5 ongoing pregnancies, | “Perfectly well tolerated” |
| 4 miscarriages, 1 with chromosomal aberration. | |||||
| Sureau and Combourieu [31] | Threatened miscarriage | 23 pts | DYD | 10 term deliveries, 10 ongoing pregnancies, 3 miscarriages. | Well tolerated, all infants healthy |
Aes, adverse events; b.i.d., twice daily; CI, confidence interval; CNS, central nervous system; CON, control; D&C, dilation and curettage; DYD, dydrogesterone; i.m., intramuscular; PROG, progesterone; pt(s), patient(s); t.i.d., three times daily; yrs, years.
Discussion
The results of this metaanalysis of 660 patients show that the effect of dydrogesterone on the risk of miscarriage in women with threatened miscarriage appears to be substantiated. There was a statistically significant reduction in the odds ratio for miscarriage after dydrogesterone compared to standard care of 0.47 (CI = 0.31–0.7) rate. The 24% miscarriage rate in control women (78/325) was reduced to 13% (44/335) after dydrogesterone administration (11% absolute reduction in the miscarriage rate). There is consistency between the results of the five trials in that the confidence limits all overlap (Figure 2). There seem to be no significant side effects.
Only one previous systematic review has been performed [32]. In that analysis, two trials of dydrogesterone were compared to placebo [13,15] and two trials of vaginal progesterone [33,34]. In the women who were treated with vaginal progesterone the treatment was not statistically effective in reducing miscarriage when compared to placebo (RR = 0.47, 95% CI= 0.17–1.30) whereas oral progestogen was effective (RR = 0.54, CI = 0.35–0.84). However, the analysis consisted of two studies only. Therefore the authors concluded, “The analysis was limited by the small number and the poor methodological quality of eligible studies, and the small number of the participants, which limit the power of the metaanalysis and hence of the conclusion”. The present metaanalysis includes five studies with 660 participants which enables a more robust conclusion of efficacy to be drawn.
In order to assess safety, all 22 studies were assessed. The follow-up data on 1380 patients suggest that the side effects including birth defects are minimal. Additionally, a recent review of birth defects associated with dydrogesterone use during pregnancy [35] concluded that clinical experience with dydrogesterone provided no evidence of a causal link between maternal use during pregnancy and birth defects.
The evidence presented in this review derives from research performed as long ago as 1967. At that time, there were fewer diagnostic criteria available than today, and the methodology less strict than today. There are some issues about the methodological quality of some studies included in this metaanalysis and was unclear in some respects, such as the methods of randomization. Ehrenskjöld et al.’s [11] study and Mistò’s [12] study were double-blinded. Omar et al.’s [14] study claimed to be randomized, but contained no details of the method of randomization, or whether there was any allocation concealment. In the El-Zibdeh and Yousef [13] study, patients were randomized according to the day of the week on which the patient presented. Allocation concealment was not described. Therefore, some degree of selection bias is possible. Pandian’s [15] study was randomized, but the lack of blinding raises the question of a placebo effect in the treatment arm.
There is also the possibility of confounding factors affecting the results. None of the studies, controlled for predictive factors for miscarriage, such as ultrasonic detection of a fetal heartbeat, fetal karyotyping, maternal age, body mass index, infertility, assisted conception, high alcohol consumption, low serum hCG levels, previous recurrent miscarriages and psychological stress [36–38],. Several studies included women of different age groups (below and above 35 years of age), at different stages of pregnancy (before and after the first trimester), or with a different history of previous miscarriages, but none of the studies differentiated between the effects per subgroup. The lowest age groups were included in the studies by Ehrenskjöld et al. [11] where 14 of the 153 patients were younger than 19 years, and Pandian [15] where 36 of the 191 patients younger than 20 years of age. However, the age ranges for these studies were not reported, and were not reported in the other studies. Some embryos have abnormalities which are incompatible with life, such as structural malformations or karyotypic aberrations. Administration of dydrogesterone cannot correct these abnormalities, but only affect a normal embryo. As these confounding factors were not taken into account in the metaanalysis, some patients in the treated and control groups with abnormal embryos might have been treated raising the number of miscarriages in both groups. Randomization should however, theoretically cancelled the effect of these confounding factors.
The presence of a fetal heartbeat is predictive for the continuation of pregnancy. After ultrasonic detection of a fetal heartbeat, the incidence of miscarriage has been reported to be 7% in normal pregnancies [39]. In threatened miscarriage, five publications have examined the likelihood of miscarriage after detection of a fetal heartbeat [33,40–43]. There was a 9% chance of miscarrying (Range = 3.4–19.2%). As there was no assessment of the fetal heart in the papers analyzed, some patients with embryonic demise might have been treated after fetal demise. As there was no matching for fetal heart activity at the start of treatment, some patients may have had treatment after fetal viability was assured. Again, randomization should theoretically have distributed these confounding factors equally to treatment and control groups and cancelled their effect. If there is only a 9% chance of miscarrying after detection of a fetal heart beat, and chromosomal aberrations are assumed to account for approximately 50% of those pregnancies terminating in miscarriage, treatment would only be expected to raise the proportion of live births by 4.5%. However, an 11% absolute reduction in the miscarriage rate was observed.
There are few other clinically useful tests to predict fetal outcome. Plasma progesterone levels are problematic as progesterone secretion is pulsatile. Blood may be drawn at a pulse peak or nadir; these may vary 10-fold [44]. Low progesterone levels may indicate failing pregnancy from chromosomal aberrations etc. and may therefore be part of the mechanism rather than cause of miscarriage. Although other markers of luteal defficiency have been sought such as low hCG values [45], low inhibin A levels [46], constant or increasing CA125 levels [47,48], none of these have been shown to be clinically useful. Therefore diagnosis and treatment by dydrogesterone are empirical.
As some of the reports were completed as long ago as 1967, bias could not be assessed. Consequently, newer studies are warranted which use the criteria available today.
Conclusions
Although treatment with progestagens in general and dydrogesterone in particular are somewhat empiric, the results of this systematic review showed that dydrogesterone was associated with a reduction of 47% in the odds for miscarriage, compared to standard care and an absolute decrease in the miscarriage rate of 11%. In many parts of the world, early ultrasound, progesterone and hCG levels are not generally available. Even when available, the patient demands treatment which is risk free and decreases the chance of threatened miscarriage terminating in miscarriage. The evidence presented here suggests that the treating physician should comply with her wishes.
Acknowledgments
Declaration of Interest: This work was supported by, Abbott Products Operations AG, Switzerland Prof. Carp is a member of an advisory committee to Abbott Products.
References
- 1.Weiss JL, Malone FD, Vidaver J, Ball RH, Nyberg DA, Comstock CH, Hankins GD, et al. FASTER Consortium. Threatened abortion: A risk factor for poor pregnancy outcome, a population-based screening study. Am J Obstet Gynecol. 2004;190:745–750. doi: 10.1016/j.ajog.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. BMJ. 1997;315:32–34. doi: 10.1136/bmj.315.7099.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szekeres-Bartho J, Barakonyi A, Par G, Polgar B, Palkovics T, Szereday L. Progesterone as an immunomodulatory molecule. Int Immunopharmacol. 2001;1:1037–1048. doi: 10.1016/s1567-5769(01)00035-2. [DOI] [PubMed] [Google Scholar]
- 4.Stovall TG, Ling FW, Andersen RN, Buster JE. Improved sensitivity and specificity of a single measurement of serum progesterone over serial quantitative beta-human chorionic gonadotrophin in screening for ectopic pregnancy. Hum Reprod. 1992;7:723–725. doi: 10.1093/oxfordjournals.humrep.a137725. [DOI] [PubMed] [Google Scholar]
- 5.al-Sebai MA, Kingsland CR, Diver M, Hipkin L, McFadyen IR. The role of a single progesterone measurement in the diagnosis of early pregnancy failure and the prognosis of fetal viability. Br J Obstet Gynaecol. 1995;102:364–369. doi: 10.1111/j.1471-0528.1995.tb11286.x. [DOI] [PubMed] [Google Scholar]
- 6.Csapo AI, Pulkkinen M. Indispensability of the human corpus luteum in the maintenance of early pregnancy. Luteectomy evidence. Obstet Gynecol Surv. 1978;33:69–81. doi: 10.1097/00006254-197802000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Gibbons WE, Toner JP, Hamacher P, Kolm P. Experience with a novel vaginal progesterone preparation in a donor oocyte program. Fertil Steril. 1998;69:96–101. doi: 10.1016/s0015-0282(97)00457-3. [DOI] [PubMed] [Google Scholar]
- 8.Di Renzo GC, Mattei A, Gojnic M, Gerli S. Progesterone and pregnancy. Curr Opin Obstet Gynecol. 2005;17:598–600. doi: 10.1097/01.gco.0000191899.84567.4d. [DOI] [PubMed] [Google Scholar]
- 9.Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH. Classification and pharmacology of progestins. Maturitas. 2003;46(Suppl 1):S7–S16. doi: 10.1016/j.maturitas.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Czajkowski K, Sienko J, Mogilinski M, Bros M, Szczecina R, Czajkowska A. Uteroplacental circulation in early pregnancy complicated by threatened abortion supplemented with vaginal micronized progesterone or oral dydrogesterone. Fertil Steril. 2007;87:613–618. doi: 10.1016/j.fertnstert.2006.07.1506. [DOI] [PubMed] [Google Scholar]
- 11.Ehrenskjöld ML, Bondo B, Weile F. [Treatment of threatened abortion with dydrogesterone] Ugeskr Laeg. 1967;129:1678–1679. [Article in Danish] [PubMed] [Google Scholar]
- 12.Mistò A. [Experiences with 6-dehydro-retroprogesterone int the treatment of placental insufficiency] Ann Ostet Ginecol Med Perinat. 1967;89:102–112. [Article in Italian] [PubMed] [Google Scholar]
- 13.El-Zibdeh MY, Yousef LT. Dydrogesterone support in threatened miscarriage. Maturitas. 2009;65(Suppl 1):S43–S46. doi: 10.1016/j.maturitas.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Omar MH, Mashita MK, Lim PS, Jamil MA. Dydrogesterone in threatened abortion: pregnancy outcome. J Steroid Biochem Mol Biol. 2005;97:421–425. doi: 10.1016/j.jsbmb.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Pandian RU. Dydrogesterone in threatened miscarriage: a Malaysian experience. Maturitas. 2009;65(Suppl 1):S47–S50. doi: 10.1016/j.maturitas.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Vincze E, Molnár BG, Földesi I, Pál A. Treatment possibilities for threatened abortion using progesterone and progesterone-type drugs. J Hungarian Gynaecol. 2006;69:281–284. [Google Scholar]
- 17.Bashmakova NV, Melkozerova OA, Vinokurova EA, Pepekyeva NA. Health condition of infants and born children to mothers with threatened miscarriages. Reproduction problems. 2004;2:1–3. [Article in Russian] [Google Scholar]
- 18.Eggimann UH, Thürig E, Moser H, Bossi E. [Threatened abortion: patient characteristics, treatment results and consequences for the child] Schweiz Med Wochenschr. 1979;109:288–292. [Article in German] [PubMed] [Google Scholar]
- 19.Kalinka J, Szekeres-Bartho J. The impact of dydrogesterone supplementation on hormonal profile and progesterone-induced blocking factor concentrations in women with threatened abortion. Am J Reprod Immunol. 2005;53:166–171. doi: 10.1111/j.1600-0897.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 20.Kalinka J, Radwan M. The impact of dydrogesterone supplementation on serum cytokine profile in women with threatened abortion. Am J Reprod Immunol. 2006;55:115–121. doi: 10.1111/j.1600-0897.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 21.Manukhin IB, Gevorkyan MA, Minkina GN, Manukhina EI. Efficacy of Duphaston in treating threatened miscarriage in early stages of pregnancy. Reprod Issues. 2004;6:63–64.. [Article in Russian] [Google Scholar]
- 22.Pelinescu-Onciul D. Subchorionic hemorrhage treatment with dydrogesterone. Gynecol Endocrinol. 2007;23(Suppl 1):77–81. doi: 10.1080/09513590701584741. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto K. [Study of 6-dehydro-retro-progesterone; especially in comparison with the effect of 17-alpha-ethynyl-19-nortestosterone] Zasshi Tokyo Ika Daigaku. 1968;26:385–403. [Article in Japanese] [PubMed] [Google Scholar]
- 24.Chang IW. Clinical trial of isopregnenone, a new progestational agent. Preliminary study. Med Ann Dist Columbia. 1962;31:402–405. [PubMed] [Google Scholar]
- 25.Gronow MJ. Ovarian hyperstimulation for successful in vitro fertilization and embryo transfer. Acta Obstet Gynecol Scand Suppl. 1985;131:1–80. doi: 10.3109/00016348509156384. [DOI] [PubMed] [Google Scholar]
- 25.Ketkar S. Role of dydrogesterone in threatened abortion. Obs Gynae Today. 2008;12:197–199. [Google Scholar]
- 27.Aydar CK, Greenblatt RB. 6-Dehydro-retroprogesterone (duphaston) an interesting progesterone-like compound. Int J Fertil. 1964;9:585–595. [PubMed] [Google Scholar]
- 28.Backer MH., Jr Isopregnenone (Duphaston): a new progestational agent. Obstet Gynecol. 1962;19:724–729. [PubMed] [Google Scholar]
- 29.Gellé P, Schaeffer P. [Apropos of the use of dydrogesterone in gynecology and obstetrics; clinical experience] Bull Fed Soc Gynecol Obstet Lang Fr. 1965;17:369–370. [Article in French] [PubMed] [Google Scholar]
- 30.Jamain M, Grenet C. [Utilization of 6-dehydro-retroprogesterone in obstetrics: indications; results] Bull Fed Soc Gynecol Obstet Lang Fr. 1969;21:26–31. [Article in French] [PubMed] [Google Scholar]
- 31.Sureau C, Combourieu P. [Clinical Study Of 6-dehydro-retroprogesterone (dydrogesterone)] Bull Fed Soc Gynecol Obstet Lang Fr. 1964;16:263–269. [Article in French] [PubMed] [Google Scholar]
- 32.Wahabi HA, Fayed AA, Esmaeil SA, Al Zeidan RA. Progestogen for treating threatened miscarriage. Cochrane Database Syst Rev. 2011;12:CD005943. doi: 10.1002/14651858.CD005943.pub4. [DOI] [PubMed] [Google Scholar]
- 33.Gerhard I, Gwinner B, Eggert-Kruse W, Runnebaum B. Double-blind controlled trial of progesterone substitution in threatened abortion. Biol Res Pregnancy Perinatol. 1987;8:26–34. [PubMed] [Google Scholar]
- 34.Palagiano A, Bulletti C, Pace MC, DE Ziegler D, Cicinelli E, Izzo A. Effects of vaginal progesterone on pain and uterine contractility in patients with threatened abortion before twelve weeks of pregnancy. Ann N Y Acad Sci. 2004;1034:200–210. doi: 10.1196/annals.1335.022. [DOI] [PubMed] [Google Scholar]
- 35.Queisser-Luft A. Dydrogesterone use during pregnancy: overview of birth defects reported since 1977. Early Hum Dev. 2009;85:375–377. doi: 10.1016/j.earlhumdev.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Arck PC, Rücke M, Rose M, Szekeres-Bartho J, Douglas AJ, Pritsch M, Blois SM, et al. Early risk factors for miscarriage: a prospective cohort study in pregnant women. Reprod Biomed Online. 2008;17:101–113. doi: 10.1016/s1472-6483(10)60300-8. [DOI] [PubMed] [Google Scholar]
- 37.Maconochie N, Doyle P, Prior S, Simmons R. Risk factors for first trimester miscarriage–results from a UK-population-based case-control study. BJOG. 2007;114:170–186. doi: 10.1111/j.1471-0528.2006.01193.x. [DOI] [PubMed] [Google Scholar]
- 38.Gracia CR, Sammel MD, Chittams J, Hummel AC, Shaunik A, Barnhart KT. Risk factors for spontaneous abortion in early symptomatic first-trimester pregnancies. Obstet Gynecol. 2005;106:993–999. doi: 10.1097/01.AOG.0000183604.09922.e0. [DOI] [PubMed] [Google Scholar]
- 39.Achiron R, Tadmor O, Mashiach S. Heart rate as a predictor of first-trimester spontaneous abortion after ultrasound-proven viability. Obstet Gynecol. 1991;78:330–334. [PubMed] [Google Scholar]
- 40.Tongsong T, Srisomboon J, Wanapirak C, Sirichotiyakul S, Pongsatha S, Polsrisuthikul T. Pregnancy outcome of threatened abortion with demonstrable fetal cardiac activity: a cohort study. J Obstet Gynaecol (Tokyo 1995) 1995;21:331–335. doi: 10.1111/j.1447-0756.1995.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 41.Tannirandorn Y, Sangsawang S, Manotaya S, Uerpairojkit B, Samritpradit P, Charoenvidhya D. Fetal loss in threatened abortion after embryonic/fetal heart activity. Int J Gynaecol Obstet. 2003;81:263–266. doi: 10.1016/s0020-7292(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 42.Falco P, Milano V, Pilu G, David C, Grisolia G, Rizzo N, Bovicelli L. Sonography of pregnancies with first-trimester bleeding and a viable embryo: a study of prognostic indicators by logistic regression analysis. Ultrasound Obstet Gynecol. 1996;7:165–169. doi: 10.1046/j.1469-0705.1996.07030165.x. [DOI] [PubMed] [Google Scholar]
- 43.Bennett GL, Bromley B, Lieberman E, Benacerraf BR. Subchorionic hemorrhage in first-trimester pregnancies: prediction of pregnancy outcome with sonography. Radiology. 1996;200:803–806. doi: 10.1148/radiology.200.3.8756935. [DOI] [PubMed] [Google Scholar]
- 44.Abraham GE, Maroulis GB, Marshall JR. Evaluation of ovulation and corpus luteum function using measurements of plasma progesterone. Obstet Gynecol. 1974;44:522–525. [PubMed] [Google Scholar]
- 45.la Marca A, Morgante G, De Leo V. Human chorionic gonadotropin, thyroid function, and immunological indices in threatened abortion. Obstet Gynecol. 1998;92:206–211. doi: 10.1016/s0029-7844(98)00183-5. [DOI] [PubMed] [Google Scholar]
- 46.Florio P, Luisi S, D’Antona D, Severi FM, Rago G, Petraglia F. Maternal serum inhibin A levels may predict pregnancy outcome in women with threatened abortion. Fertil Steril. 2004;81:468–470. doi: 10.1016/j.fertnstert.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt T, Rein DT, Foth D, Eibach HW, Kurbacher CM, Mallmann P, Römer T. Prognostic value of repeated serum CA 125 measurements in first trimester pregnancy. Eur J Obstet Gynecol Reprod Biol. 2001;97:168–173. doi: 10.1016/s0301-2115(00)00533-9. [DOI] [PubMed] [Google Scholar]
- 48.Fiegler P, Katz M, Kaminski K, Rudol G. Clinical value of a single serum CA-125 level in women with symptoms of imminent abortion during the first trimester of pregnancy. J Reprod Med. 2003;48:982–988. [PubMed] [Google Scholar]