Abstract
Objectives. We used the fundamental cause hypothesis as a framework for understanding the creation of health disparities in colorectal cancer mortality in the United States from 1968 to 2005.
Methods. We used negative binomial regression to analyze trends in county-level gender-, race-, and age-adjusted colorectal cancer mortality rates among individuals aged 35 years or older.
Results. Prior to 1980, there was a stable gradient in colorectal cancer mortality, with people living in counties of higher socioeconomic status (SES) being at greater risk than people living in lower SES counties. Beginning in 1980, this gradient began to narrow and then reversed as people living in higher SES counties experienced greater reductions in colorectal cancer mortality than those in lower SES counties.
Conclusions. Our findings support the fundamental cause hypothesis: once knowledge about prevention and treatment of colorectal cancer became available, social and economic resources became increasingly important in influencing mortality rates.
Colorectal cancer is the third leading cause of cancer-related deaths among men and women in the United States.1 In 2010 an estimated 142 570 people in the United States were diagnosed with colorectal cancer, and 50 370 people died as a result of the disease in the same year.2 Over the past 30 years, there have been significant advances in the prevention of colorectal cancer, with reductions in mortality rates due predominantly to improvements in screening and early cancer detection.
One of the primary goals of colorectal cancer screening is to reduce mortality by promoting early detection of the disease. Methods used to detect colorectal cancer also aid physicians in the identification and removal of adenomas, which can give rise to colorectal cancer.3 However, because of the unequal distribution of social and economic resources in our society, knowledge about prevention and access to treatments for colorectal cancer is not universal but, rather, is unevenly distributed along the typical social cleavages of race, class, and gender. Thus, social inequalities in colorectal cancer outcomes remain remarkably evident even in an era of successful prevention and treatment strategies.4
To gain a more thorough understanding of how existing social inequalities have slowed the decline in mortality attributable to colorectal cancer, we used the “fundamental cause” hypothesis to analyze almost 40 years of US death certificate data. This theoretical construct, first put forth by Link and Phelan,5 stems from the observation that adverse social conditions are repeatedly associated with higher levels of mortality in distinctly different eras and settings.5–9 According to the hypothesis, the association between socioeconomic status (SES) and mortality endures because access to resources such as knowledge, money, power, prestige, and beneficial social connections influences the extent to which people are able to avoid disease and death as well as harness protective factors that can be used to reduce morbidity and mortality.
The fundamental cause hypothesis further predicts that as individuals learn how to better prevent or treat diseases, benefits stemming from these newfound abilities will not be distributed uniformly throughout a population. Instead, they will be realized to a greater extent by those who are less likely to face discrimination and stigma and are more likely to have access to socioeconomic resources such as education, money, and information,7 thus resulting in health disparities along common social divisions such as SES and race. According to the hypothesis, more advantaged individuals, relative to their less advantaged counterparts, are poised to disproportionately gain from new health-enhancing capabilities, which may translate to earlier and more rapid reductions in mortality rates.
We examined SES inequalities in colorectal cancer mortality in light of major advances in preventing or delaying death, advances predominantly due to improvements in screening and associated policy recommendations. Although colorectal cancer has been surgically treated for more than a century, an emphasis on the prevention of colorectal cancer through widespread screening has become routine only in the past 30 years. In July 1980, the American Cancer Society (ACS) first published recommendations for colorectal cancer screening.10 In 1997, the US Multi-Society Task Force (MSTF), assembled by the US Agency for Health Care Policy Research in conjunction with the American Gastroenterological Association, published its first guidelines for screening for colorectal cancer.11
The MSTF guidelines recommended that everyone with risk factors such as age (≥ 50 years), family or personal history of colorectal cancer, history of inflammatory bowel disease, chronic ulcerative colitis, adenomatous polyposis, juvenile polyposis, and hereditary nonpolyposis colorectal cancer be screened. Furthermore, following a positive screen, physicians should conduct a diagnostic evaluation of the colon and rectum, use recommended treatments (including the removal of adenomatous polyps), and consider follow-up surveillance after treatment. In 1997, influenced by MSTF’s recommendations, ACS revised its 1980 guidelines to include recommendations stratified by level of risk of developing colorectal cancer.11 Since then, both ACS and MSTF have issued updates on a regular basis.12
The 1997 ACS guidelines recommended that all individuals at an average level of risk begin colorectal cancer screening at the age of 50 years. Individuals at moderate risk, based on a personal or family diagnosis of gastrointestinal adenomatous polyps or colorectal cancer, were recommended to initiate screening at the time of onset, the age of 40 years, or 10 years before the youngest case in the family, whichever was earlier. High-risk individuals with hereditary predispositions to colorectal cancer or a personal diagnosis of inflammatory bowel disease were recommended to initiate screening at puberty, at the age of 21 years, or 8 to 15 years after the onset of inflammatory bowel disease, depending on their individual risk factors.11
According to the fundamental cause hypothesis, developments in colorectal cancer screening, such as clearly stated, evidence-based guidelines and their widespread dissemination, will benefit people of high SES more than their low-SES counterparts, thereby creating new health disparities or exacerbating existing disparities over time. Specifically, we expected individuals living in high-SES locales to benefit from recent developments in colorectal cancer screening, beginning with the release of the first colorectal cancer screening recommendations by ACS. Furthermore, given that socioeconomic inequalities are reproduced and often accentuated over time, we expected the association between SES and colorectal cancer mortality to increase over time.
METHODS
We combined population-level death certificate data for the United States with yearly population estimates, both of which were originally obtained from the National Center for Health Statistics, to calculate age-, gender-, and race-specific colorectal cancer mortality rates for 99% of counties in the continental United States for the period 1968 to 2005.13 The compressed mortality files made available by the Centers for Disease Control and Prevention contain aggregate mortality and population count data by race, gender, and age group for every county in every year. We used International Classification of Diseases (ICD) category codes (ICD-8 and ICD-9: 153–154; ICD-10: C18–C21) to identify colorectal cancer deaths during the study period.
Mortality Rates
We calculated mortality rates in each county and time period by dividing the number of individuals in each age-, gender-, and race-specific group who died from colorectal cancer by the corresponding population. To adjust for gender and race, we summed these rates using standardized population weights of 0.15 for Blacks, 0.85 for Whites, and 0.50 for men and women. Changing these weights increases variance but does not affect trends over time.
For graphical analyses, we present race-, gender-, and age-adjusted mortality rates by year. We present trends over time separated into yearly SES tertiles (3 groups, each containing 33% of the observations each year). We were able to include 99% of US counties (n = 3110) in our analyses after excluding 11 counties because their boundaries had changed during the study period. We used the World Health Organization standard population to standardize mortality rates. We excluded data from individuals younger than 35 years given that mortality from colorectal cancer before age 35 years is extremely rare. We also excluded data from the racial group labeled as “other,” which comprised a heterogeneous mix of individuals not racially identified as Black or White. Our decision to exclude this group stemmed from its small numbers and lack of comparability over the time period of interest.
Socioeconomic Status Measure
The county-level SES measure, an aggregate index composed of data from 4 US decennial censuses, includes information about the proportion of individuals in each county with less than 9 years of education, more than 12 years of education, white-collar occupations, indoor plumbing, or telephone access in their homes. We used factor analysis to derive a unifactoral SES measure that we standardized, to simplify interpretation, so that a 1-unit change referred to a 1 standard deviation change in SES.14,15 The data for intercensal years were linearly interpolated.
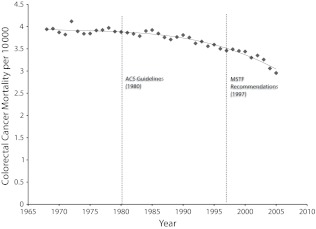
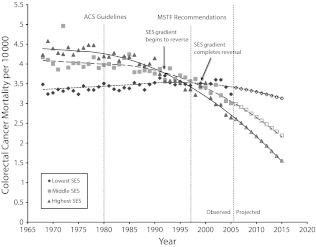
Results are presented in descriptive form and in multiple regression analyses. The descriptive results provided in Figure 1 show yearly changes in age-adjusted mortality; piecewise linear regression analyses (with results presented as B values) were used to assess changes in mortality rates over time. Our trend estimates can be interpreted as rates of change in yearly mortality rates per 10 000 individuals aged 35 years or older. To provide further context, Table 1 shows average mortality rates separated by SES tertiles. Finally, to allow an estimation of the size of future inequalities, Figure 2 shows results in which observed trends from 1980 to 2005 are used to project forward 10 years.
FIGURE 1—
Age-, race-, and gender-adjusted colorectal cancer mortality rates (per 10 000 individuals aged ≥ 35 years) and trends (solid line) before and after publication of the American Cancer Society (ACS) guidelines and US Multi-Society Task Force (MSTF) recommendations: United States, 1968–2005.
TABLE 1—
Yearly Colorectal Cancer Mortality Rate Ratios, by Socioeconomic Status (SES) Tertile and Policy Date: United States, 1968–2005
| SES Tertile |
|||
| Year | Low | Middle | High |
| 1968 | 3.49 | 4.18 | 4.24 |
| 1969 | 3.24 | 4.11 | 4.59 |
| 1970 | 3.27 | 4.01 | 4.40 |
| 1971 | 3.36 | 3.86 | 4.29 |
| 1972 | 3.31 | 4.98 | 4.26 |
| 1973 | 3.38 | 3.91 | 4.43 |
| 1974 | 3.32 | 4.03 | 4.24 |
| 1975 | 3.23 | 4.03 | 4.36 |
| 1976 | 3.44 | 3.93 | 4.42 |
| 1977 | 3.35 | 4.01 | 4.49 |
| 1978 | 3.34 | 4.18 | 4.49 |
| 1979 | 3.42 | 3.90 | 4.15 |
| 1980 | 3.51 | 3.98 | 4.20 |
| 1981 | 3.45 | 4.01 | 4.19 |
| 1982 | 3.36 | 3.91 | 4.29 |
| 1983 | 3.33 | 3.99 | 4.09 |
| 1984 | 3.40 | 4.16 | 4.20 |
| 1985 | 3.38 | 4.25 | 4.18 |
| 1986 | 3.52 | 3.80 | 4.24 |
| 1987 | 3.48 | 3.81 | 4.00 |
| 1988 | 3.34 | 3.84 | 3.97 |
| 1989 | 3.48 | 3.82 | 4.01 |
| 1990 | 3.53 | 3.76 | 4.15 |
| 1991 | 3.65 | 3.88 | 3.75 |
| 1992 | 3.70 | 3.60 | 3.56 |
| 1993 | 3.53 | 3.89 | 3.58 |
| 1994 | 3.48 | 3.63 | 3.57 |
| 1995 | 3.56 | 3.72 | 3.52 |
| 1996 | 3.49 | 3.66 | 3.35 |
| 1997 | 3.46 | 3.56 | 3.36 |
| 1998 | 3.63 | 3.62 | 3.22 |
| 1999 | 3.51 | 3.40 | 3.44 |
| 2000 | 3.39 | 3.41 | 3.52 |
| 2001 | 3.50 | 3.26 | 3.14 |
| 2002 | 3.60 | 3.47 | 2.98 |
| 2003 | 3.60 | 3.21 | 2.97 |
| 2004 | 3.42 | 3.06 | 2.71 |
| 2005 | 3.23 | 3.00 | 2.64 |
Note. The American Cancer Society recommendations and guidelines were made public in 1980; the US Multi-Society Task Force provided its first guidelines in 1997. Initially colorectal cancer mortality rates were substantially higher among those living in high-SES counties (top 33%) than among those living in low-SES counties (bottom 33%); by 1991, however, these rates were lower than those among residents of middle-SES counties, and by 1995 they surpassed rates among those living in low-SES counties. Middle-SES counties experienced the same, if slower, reductions and surpassed low-SES counties in 1998.
FIGURE 2—
Socioeconomic status (SES) gradients in age-, race-, and gender-adjusted colorectal cancer mortality rates and trends for each SES tertile before and after publication of the American Cancer Society (ACS) guidelines and US Multi-Society Task Force (MSTF) recommendations: United States, 1968–2005, and projections to 2015.
In our multivariate analyses, we relied on negative binomial regression models to estimate mortality rate ratios in which standard errors were adjusted for clustering at the county level. This approach is appropriate when analyzing count data and is particularly effective when overdispersion in the data is evident.15 In the multivariate context, mortality rates are seen as dependent on a set of observed variables shared by individuals within each county and during each year or period. We used robust Huber–White standard errors and included controls for county-level clustering of rates over time.
For ease of interpretation in our multivariate analyses, we constructed periods consisting of 5-year groups to allow us to better understand both the onset of change after 1980 and the progression of that change over time. When we tested the sensitivity of our results by reanalyzing the results without these groupings, the findings yielded the same conclusions as the analyses presented here. In the multivariate analyses, we include controls for age (10-year groups), race (Black and White), gender, year (in 5-year groups), and urbanicity (proportion of the population living in an urban area with more than 50 000 residents). We estimated the extent to which the relationship between SES and colorectal cancer mortality changed over time by interacting SES with year.
RESULTS
Figure 1 provides annual US colorectal cancer mortality rates from 1968 to 2005, standardized to the World Health Organization’s standard population. As the figure illustrates, little change in mortality rates occurs prior to 1980. We estimate this slope as being nonsignificant from 1968 to 1979 at B = −0.001 (95% confidence interval [CI] = −0.006, 0.004) deaths per 10 000 per year. Colorectal cancer mortality began to decline in 1980 at a rate of B = −0.022 (95% CI = −0.020, −0.024) deaths per 10 000 per year. After the MSTF change in recommendations in 1997, the rate of decrease in mortality more than doubled to −0.062 (95% CI = −0.058, −0.069) deaths per 10 000 per year.
Figure 2 presents separate age-, race-, and gender-adjusted mortality rates for 3 groups stratified according to county-level SES (low, medium, high). The figure reveals 3 critical findings. First, before 1980, SES was strongly related to colorectal cancer mortality such that the death rate in high-SES counties was significantly higher than the rate in low-SES counties.
Second, although both the highest and middle SES tertiles experienced reductions in mortality between 1980 and 1997, death rates within the highest SES tertile declined at a significantly faster pace (−0.055 deaths per 10 000 per year; 95% CI = −0.051, −0.058) than rates within the middle (−0.025; 95% CI = −0.030, −0.021) and lowest (0.011; 95% CI = 0.008, 0.014) SES tertiles. After the first (1997) MSTF recommendations, this pace quickened substantially in all 3 groups, with those in higher SES counties still seeing the largest reductions in mortality rates (high SES: B = −0.099; 95% CI = −0.108, −0.091; middle SES: B = −0.071; 95% CI = −0.064, −0.078; low SES: B = −0.019; 95% CI = −0.009, −0.029).
Third, although counties in the high-SES category had the highest colorectal mortality rates in 1980, cause-specific death rates had fallen so quickly that high-SES counties were reporting lower colorectal mortality than middle-SES counties by 1993 and lower mortality than low-SES counties by 1998. The rate of mortality reduction among counties in the high-SES category continued unabated until 2005 (the final year for which data were analyzed in this study), at which time high-SES counties experienced lower death rates from colorectal cancer (2.64 per 10 000 per year; 95% CI = 2.61, 2.67) than either middle-SES (3.00; 95% CI = 2.96, 3.05) or low-SES (3.23; 95% CI = 3.17, 3.30) counties. In summary, Figure 2 clearly illustrates a complete reversal of the directionality characterized by the SES–colorectal cancer mortality gradient whereby high SES, which was once associated with the greatest risk of death owing to this specific cause, now served as a protective factor against colorectal cancer mortality.
Table 1 presents annual mortality rates for each SES tertile and depicts the gap between the publication of the first colorectal cancer screening guidelines in 1980 and the proceeding decline in mortality. The table demonstrates the annual changes in colorectal cancer mortality rates as experienced by each group. All 3 SES groups experienced their highest annual mortality rate at least 5 years after the introduction of the 1980 colorectal cancer screening guidelines. That is, the colorectal cancer mortality rate for the high-SES tertile continued to increase until 1985, when the mortality rate for this particular group reached the highest annual rate in our data (4.24 per 10 000). The remaining 2 groups experienced similar increases in mortality after 1980. The low-SES group reached its highest colorectal cancer mortality rate in 1992 (3.70), and the highest rate for the middle-SES group was in 1985 (4.25).
Table 2 presents the results of a negative binomial regression analysis that formally tested whether and to what extent the association between SES and colorectal cancer mortality changed over the period of observation (1968–2005). In this analysis, we incorporated SES by 5-year-period interaction terms and tested their significance while controlling for age, gender, race, and urbanicity. Age and gender were related to mortality as expected, with older age and male gender being associated with significantly higher risks of death from colorectal cancer (P < .05). Similarly, Black race was predictive of excessive colorectal mortality, as reflected in the death rate ratio among Blacks (mortality rate ratio = 1.22; 95% CI = 1.20, 1.24), representing a 22% mortality excess compared to the mortality rate among Whites (3.67 per 10 000 from 1968–2005). Colorectal cancer rates were slightly but not significantly higher in urban than in nonurban counties.
TABLE 2—
Negative Binomial Regression Estimates of Colorectal Cancer Mortality Rate Ratios: United States, 1968–2005
| Individual or Study Variable | Mortality Rate Ratio (95% CI) |
| Age group, y | |
| 35–44 (Ref) | 1.00 |
| 45–54 | 3.97 (3.91, 4.03) |
| 55–64 | 11.99 (11.79, 12.19) |
| 65–74 | 27.40 (26.89, 27.91) |
| 75–84 | 52.82 (51.81, 53.85) |
| ≥ 85 | 90.27 (88.56, 92.02) |
| Urbanicity | 1.03 (0.99, 1.08) |
| Black | 1.22 (1.20, 1.24) |
| Male | 1.37 (1.37, 1.38) |
| Time period | |
| 1968–1970 (Ref) | 1.00 |
| 1971–1975 | 0.98 (0.97, 0.99) |
| 1976–1980 | 0.98 (0.97, 1.00) |
| 1981–1985 | 0.95 (0.93, 0.97) |
| 1986–1990 | 0.92 (0.90, 0.94) |
| 1991–1995 | 0.88 (0.86, 0.90) |
| 1996–2000 | 0.82 (0.80, 0.85) |
| 2001–2005 | 0.75 (0.72, 0.77) |
| SES | 1.14 (1.12, 1.16) |
| SES × time period | |
| 1968–1970 (Ref) | 1.00 |
| 1971–1975 | 0.99 (0.98, 1.00) |
| 1976–1980 | 0.98 (0.97, 0.99) |
| 1981–1985 | 0.96 (0.95, 0.97) |
| 1986–1990 | 0.93 (0.92, 0.94) |
| 1991–1995 | 0.89 (0.88, 0.90) |
| 1996–2000 | 0.87 (0.86, 0.88) |
| 2001–2005 | 0.84 (0.83, 0.86) |
Note. CI = confidence interval; SES = socioeconomic status.
Figure 2 contains projections of colorectal cancer mortality rates by SES tertiles through 2015 that were constructed on the basis of the regression results presented in Table 2. These projections suggest that the relationship between SES and death from colorectal cancer is likely to become even more pronounced between 2006 and 2015. We predict that by 2015, the colorectal mortality rate in low-SES counties (3.13 per 10 000 per year; 95% CI = 3.06, 3.20) is likely to be approximately twice that of high-SES counties (1.54 per 10 000 per year; 95% CI = 1.51, 1.57). This is equivalent to an absolute difference of 1.59 (95% CI = 1.51, 1.67) deaths per 10 000 per year.
DISCUSSION
Colorectal cancer mortality is on the decline in the United States.16 Although the association between SES and colorectal cancer mortality persists, there has been a remarkable reversal in the direction of influence. What was once thought of as a disease of the affluent is now a medical condition whose burden is disproportionately shouldered by the poor.4 In this study—the first, to our knowledge, that has attempted to explain this surprising turn of events regarding the relationship between SES and colorectal cancer mortality—we used a fundamental cause approach to analyze almost 40 years of US death certificate data in an effort to illustrate the important role that policy recommendations play in the establishment and entrenchment of social inequalities in health over time.
Our study highlights the role of policy recommendations in starting both a process of broad reductions in colorectal cancer and a gradient in colorectal cancer mortality. However, because of the dynamic nature of medical research, social structures, and informational dynamics, this process is complex and includes a number of individual, cultural, and structural factors. A recent review relating SES to colorectal cancer outcomes suggests that substantial, multidimensional SES effects persist that include lifestyle behaviors along with medical treatments.4 The World Cancer Research Fund has identified a number of risk factors that are themselves distributed according to socioeconomic factors such as physical activity and body size.17
Moreover, the social gradient cannot be fully attributed to stage of disease, type of operation, specialization of surgeon, or whether the surgery was curative because the direct effect of SES on mortality persists after adjustment for such factors.18 Thus, further research is needed that focuses on the complex role that SES plays in conjunction with different types of individual and other contextual factors.
Limitations
It was necessary to use aggregate as opposed to individual measures of SES in this study, because indicators that are consistent across both time and place for such an extended period of time were not readily available for death certificate data. Consequently, we were not able to focus on the individual-level characteristics that may help to better explain some of the observed relationships between colorectal cancer mortality and SES.
However, it is also true that important aspects of risk and protection are contextually driven, and to the extent that this is so contextual measures carry distinct advantages. In fact, the fundamental cause hypothesis specifically addresses this issue by arguing that it is not individual-level micro-processes that account for the remarkably consistent social patterning of death and disease, but the knowledge, power, prestige, and social connections stemming from macro-level stratification processes that enable individuals to take advantage of new, health-beneficial knowledge.5 Still, an optimal data set would include both individual and contextual measures of mortality risk relating to SES.
In addition, although our data allowed us to observe trends in colorectal cancer in relation to national-level policies, they did not include county-level public policies and priorities, access to preventive programs, and quality of human resources. Our findings suggest that a more finely grained analysis of policy uptake in counties would be a useful avenue for future research. Another potential limitation is the use of death certificate data that may be influenced by changes in disease classification over time. However, analyses conducted by Anderson et al. revealed high levels of comparability across different colorectal cancer categorization schemes (ICD-9 and ICD-10).19 Moreover, these changes would lead us to expect sharp discontinuities in the years in which the classification schemes changed, which we did not observe (Figure 1). In light of these limitations, we offer some conclusions that are in keeping with our findings.
Conclusions
The fundamental cause hypothesis argues that the health gradient in all-cause mortality arises because developments in prevention and treatment of disease are not distributed equally throughout societies.5,6,8 Although several tests of Link and Phelan’s theory exist,8,15,20–22 none to our knowledge have considered the importance of socioeconomic status in the sociohistorical context of changing knowledge of risk and protection concerning colorectal cancer.
Our findings support the fundamental cause hypothesis with respect to its relevance to colorectal cancer mortality in 2 ways. First, we showed that people living in low-SES counties were slower than those in middle- and high-SES counties to experience declines in colorectal cancer mortality after the introduction and widespread dissemination of preventative information and screening policies. Indeed, low-SES counties did not experience any decline in colorectal cancer mortality until 20 years after publication of the first colorectal cancer screening guidelines in 1980. Second, we illustrated that the rate of decrease in colorectal mortality after the release of the ACS guidelines was significantly more rapid among residents of high-SES counties than among residents of low- and middle-SES counties.
These results suggest that we are currently in an early phase of social inequalities in colorectal cancer mortality that may, if left alone, grow substantially over time. Our projections should be interpreted with extreme caution, however. We should be especially careful in understanding subpopulations in this way (such as is done for the different SES groupings here). Still, barring unforeseen events that might modify changes in rates over time, 10-year projections can be reasonably accurate estimates of what we might expect to observe. As such, lacking a concerted effort to reduce this momentum, even greater disparities in colorectal mortality according to SES category could become apparent in only a few short years. To contextualize these projections, colorectal mortality among residents of high-SES counties will be as low as 1.54 deaths per 10 000 population per year by 2015, a third of the rate among those who were living in high-SES counties in 1980.
Our results are in agreement with and supported by 2 recent studies of colorectal cancer mortality. Soneji et al.23 showed that racial inequalities in colorectal cancer are prominent and, since the 1980s, have become exacerbated in that rates among Whites (declining at least since 1980) have declined more rapidly than rates among Blacks (which began declining in the 1990s after first rising). These findings cohere with fundamental cause theory because they show that a disadvantaged group (Blacks) was less likely to benefit from new knowledge and technology than a more advantaged one (Whites).
In another study, Gorey et al.24 found inequalities in colorectal cancer due to socioeconomic factors in San Francisco, California, but not in Toronto, Ontario, and they argued that this was due to differences between the 2 cities in health care access. Their findings are critically important in interpreting our results because they underscore that the source of the emerging SES colorectal cancer mortality disparities can plausibly be related to policies that we create and practices that we adopt. Specifically, the findings of Gorey et al. indicate that consistent race- and SES-specific health inequalities are more likely to be attributable to the consistency of the policies and practices that place groups at a disadvantage than to anything inherent to the individuals within these groups. As such, the main implication of our findings is that when health-enhancing information is created and distributed, there needs to be a careful, thoughtful plan to develop policies and implement practices that prevent the emergence of health disparities in access to this information.
Acknowledgments
We thank the Robert Wood Johnson Foundation Health and Society Scholars program for its financial support. We also thank the Centers for Disease Control and Prevention for its financial support of this article through Public Health Dissertation Research funding (grant 1R36SH000004-01). Finally, we thank the Canada/US Fulbright Program for its support.
We thank Debbie S. Barrington, PhD, MPH, for her contribution and assistance with early versions of this article.
Note. The contents of the article are solely the responsibility of the authors and do not necessarily represent the official views of Centers for Disease Control and Prevention.
Human Participant Protection
No protocol approval was needed for this study because no human participants were involved.
References
- 1.American Cancer Society. Colorectal cancer facts and figures. Available at: http://www.cancer.org/Research/CancerFactsFigures/ColorectalCancerFactsFigures/index. Accessed September 5, 2012.
- 2.Altekruse S, Kosary C, Krapcho Met al. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010 [Google Scholar]
- 3.Byers T, Levin B, Rothenberger D, Dodd G, Smith R. American Cancer Society guidelines for screening and surveillance for early detection of colorectal polyps and cancer: update 1997. CA Cancer J Clin. 1997;47(3):154–160 [DOI] [PubMed] [Google Scholar]
- 4.Aarts MJ, Lemmens VEPP, Louwman MWJ, Kunst AE, Coebergh JWW. Socioeconomic status and changing inequalities in colorectal cancer? A review of the associations with risk, treatment and outcome. Eur J Cancer. 2010;46(15):2681–2695 [DOI] [PubMed] [Google Scholar]
- 5.Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;35(Extra Issue):80–94 [PubMed] [Google Scholar]
- 6.Link BG, Phelan J, Miech R, Westin EL. The resources that matter: fundamental social causes of health disparities and the challenge of intelligence. J Health Soc Behav. 2008;49(1):72–91 [DOI] [PubMed] [Google Scholar]
- 7.Phelan J, Link BG. Controlling disease and creating disparities: a fundamental cause perspective. J Gerontol Psychol Sci Soc Sci. 2005;60(Special Issue 2):27–33 [DOI] [PubMed] [Google Scholar]
- 8.Phelan JC, Link BG, Diez-Roux A, Kawachi I, Levin B. “Fundamental causes” of social inequalities in mortality: a test of the theory. J Health Soc Behav. 2004;45(3):265–285 [DOI] [PubMed] [Google Scholar]
- 9.Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities. J Health Soc Behav. 2010;51(suppl 1):S28–S40 [DOI] [PubMed] [Google Scholar]
- 10.Eddy D. Guidelines for the Cancer-Related Checkup: Recommendations and Rationale. Atlanta, GA: American Cancer Society; 1980 [Google Scholar]
- 11.Winawer S, Fletcher R, Miller L, Godlee F, Stolar M, Mulrow C. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112(2):594–642 [DOI] [PubMed] [Google Scholar]
- 12.Smith R, von Eschenbach A, Wender Ret al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers: Also: update 2001—testing for early lung cancer detection. CA Cancer J Clin. 2001;51(1):38–75 [DOI] [PubMed] [Google Scholar]
- 13. Compressed Mortality File, 1968–2005 [machine readable data file and documentation, CD-ROM Series 20-1A, 2E, 2N]. Hyattsville, MD: National Center for Health Statistics; 2007.
- 14.Singh G, Miller B, Hankey B. Changing area socioeconomic patterns in US cancer mortality, 1950–1998: part II—lung and colorectal cancers. J Natl Cancer Inst. 2002;94(12):916–925 [DOI] [PubMed] [Google Scholar]
- 15.Rubin MS, Colen CG, Link BG. Examination of inequalities in HIV/AIDS mortality in the United States from a fundamental cause perspective. Am J Public Health. 2010;100(6):1053–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards BK, Ward E, Kohler Bet al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Cancer Research Fund. Food, nutrition, physical activity, and the prevention of colorectal cancer. Available at: http://www.dietandcancerreport.org/. Accessed September 5, 2012.
- 18.Frederiksen BL, Osler M, Harling H, Ladelund S, Jorgensen T. Do patient characteristics, disease, or treatment explain social inequality in survival from colorectal cancer? Soc Sci Med. 2009;69(7):1107–1115 [DOI] [PubMed] [Google Scholar]
- 19.Anderson R, Miniño A, Hoyert D, Rosenberg H. Comparability of cause of death between ICD-9 and ICD10: preliminary estimates. Natl Vital Stat Rep. 2001;49(2):1–32 [PubMed] [Google Scholar]
- 20.Link BG, Northridge ME, Phelan J, Ganz ML. Social epidemiology and the fundamental cause concept: on the structuring of effective cancer screens by socioeconomic status. Milbank Q. 1998;76(3):375–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Link BG, Phelan J. The social shaping of health and smoking. Drug Alcohol Depend. 2009;104(suppl 1):S6–S10 [DOI] [PubMed] [Google Scholar]
- 22.Chang VW, Lauderdale DS. Fundamental cause theory, technological innovation, and health disparities: the case of cholesterol in the era of statins. J Health Soc Behav. 2009;50(3):245–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soneji S, Iyer S, Armstrong K, Asch D. Racial disparities in stage-specific colorectal cancer mortality: 1960–2005. Am J Public Health. 2010;100(10):1912–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorey K, Luginaah I, Bartfay Eet al. Effects of socioeconomic status on colon cancer treatment accessibility and survival in Toronto, Ontario, and San Francisco, California, 1996–2006. Am J Public Health. 2011;101(1):112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]