Abstract
Objectives. We determined the factors associated with hepatitis C (HCV) infection among rural Appalachian drug users.
Methods. This study included 394 injection drug users (IDUs) participating in a study of social networks and infectious disease risk in Appalachian Kentucky. Trained staff conducted HCV, HIV, and herpes simplex-2 virus (HSV-2) testing, and an interviewer-administered questionnaire measured self-reported risk behaviors and sociometric network characteristics.
Results. The prevalence of HCV infection was 54.6% among rural IDUs. Lifetime factors independently associated with HCV infection included HSV-2, injecting for 5 or more years, posttraumatic stress disorder, injection of cocaine, and injection of prescription opioids. Recent (past-6-month) correlates of HCV infection included sharing of syringes (adjusted odds ratio = 2.24; 95% confidence interval = 1.32, 3.82) and greater levels of eigenvector centrality in the drug network.
Conclusions. One factor emerged that was potentially unique to rural IDUs: the association between injection of prescription opioids and HCV infection. Therefore, preventing transition to injection, especially among prescription opioid users, may curb transmission, as will increased access to opioid maintenance treatment, novel treatments for cocaine dependence, and syringe exchange.
Almost 2% of US residents have antibodies to the hepatitis C virus (HCV). Because HCV is highly transmissible parenterally, injection drug use is an efficient mechanism for virus transmission.1 In a comprehensive meta-analysis examining HCV infection among injection drug users (IDUs), Hagan et al.2 reported prevalence rates worldwide. Among US injection drug users, HCV prevalence among treated drug users ranged from 27% in Chicago to 92.8% in New York City.2 An important finding was that in countries with limited resources, the prevalence was higher earlier in drug users’ injection careers, perhaps because of less access to drug treatment and harm reduction interventions such as syringe exchange.2
Injection drug use accounts for more than 40% of incident HCV cases annually,1 but there are other routes of transmission. These include receiving tainted blood transfusions,1 using illicit drugs by noninjection routes and sharing drug paraphernalia (e.g., intranasal use and sharing straws or smoking and sharing crack pipes),3,4 and sexual intercourse.1 Risk factors for HCV infection among injectors include less education5 and older age.6,7 Injection-related correlates include more frequent injection,5,6,8,9 longer injection career,5,6,8,10,11 “backloading” (transferring drug solution from one syringe to another via removal of the plunger),5 shooting gallery attendance,5,12 cocaine injection,8,10 and sharing syringes6,9,12–14 and other injection-related paraphernalia such as filtration cottons,13 cookers,9,12–14 and rinse water.9,13 These risk factors are similar to those for HIV transmission among IDUs; however, the prevalence of HCV infection is far greater than that of HIV, which has ultimately altered the course of the 2 epidemics.15
Several studies have examined the importance of social networks in disease transmission.16–18 Although most have focused on HIV rather than HCV infection, given the overlapping risk factors for HIV and HCV infection, parallels can be drawn. Oftentimes, individual-level risk factors do not adequately explain disease transmission, and the addition of network measures provides a much clearer picture of the potential for transmission. As noted by Borgatti,19 measures of degree and eigenvector centrality are particularly useful when examining network diffusion and, in particular, infectious disease transmission.
The previously cited work5–14 on risk factors for HCV infection was primarily completed in urban populations; however, there are stark differences between urban areas and Appalachian Kentucky. In addition to having extreme economic distress, Appalachian Kentucky has levels of morbidity and mortality found in less developed countries.20 In addition, little is known about injection drug use in the rural United States other than that it is becoming more prevalent with the emergence of nonmedical prescription drug use. For example, in a study conducted in Appalachia prior to the prescription drug epidemic, the prevalence of injection drug use was reported as negligible.21 However, among a cohort of 184 rural prescription drug users interviewed in 2004 and 2005, the prevalence of injection was more than 40%.22 Importantly, most of these IDUs were not injecting cocaine or heroin but prescription opioids such as OxyContin, which is not designed for parenteral use.22 A more recent study comparing rural and urban drug users found that the prevalence of prescription opioid injection was significantly greater in the rural areas.23 Preparation (e.g., crushing, dissolving) of these prescription opioids is required before injection, making the risk of HCV transmission similar for rural prescription opioid injectors as for heroin and cocaine injectors, via both infected syringes and other injection-related paraphernalia such as filtration cottons, cookers, and rinse water.
Few published studies have investigated the prevalence and correlates of HCV infection among rural residents in the era of prescription drug abuse. We therefore aimed to determine the prevalence of HCV infection and both the individual and network factors associated with HCV infection among a sample of rural IDUs.
METHODS
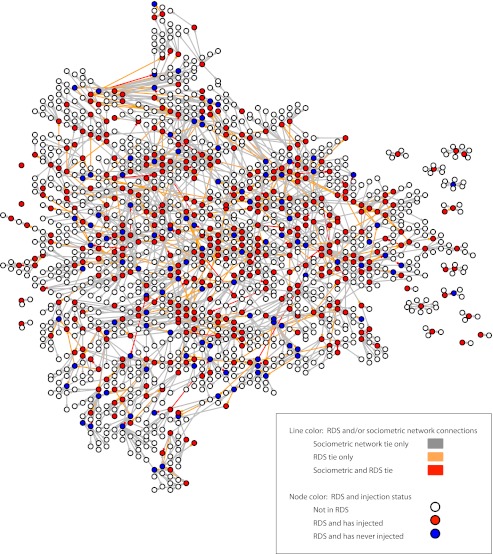
Study participants were enrolled in the Social Networks Among Appalachian People (SNAP) study, an epidemiological study examining social networks and infectious disease risk among rural Appalachian drug users. Participants were recruited through respondent-driven sampling between November 2008 and September 2010.24,25 Respondent-driven sampling is often used to access hidden populations, such as IDUs, and has been shown to be effective in recruiting rural drug users.26 Because we wanted to examine infectious disease risk behaviors, all of the seeds (i.e., the original participants, who in turn enlisted other participants) had a lifetime history of injection drug use. Once the seed IDUs completed their baseline interview, they were given 3 coupons and asked to recruit their drug-using peers (regardless of injection status). If the coupon was redeemed (i.e., their peer was eligible for the study and completed the baseline interview), the participant who distributed the coupon was given $10. A total of 107 seeds were needed to recruit 503 participants, and these 107 seeds resulted in 14 waves of recruitment. Figure 1 depicts the convergence between the respondent-driven sampling chains and the drug network. The sample did not reach equilibrium in terms of HCV prevalence. However, we were not attempting to calculate population estimates for HCV infection; rather, we were interested in the correlates of HCV infection in this specific sample of rural drug users. Whether equilibrium for HCV prevalence is reached or not does not affect the estimates of odds ratios for the regression models that are presented.27 To adjust for any additional biases that the respondent-driven sampling may have introduced, we adjusted all bivariate and multivariate regression models for the recruiter type (i.e., seed; not seed, not IDU; IDU, HCV negative; IDU, HCV positive). This captures differences in sampling (seed vs nonseed) as well as “homophily,” the tendency of individuals to recruit others like themselves, which if not corrected for can result in confidence intervals that are too narrow.
FIGURE 1—
Convergence of respondent-driven sampling (RDS) chains and drug network: Social Networks Among Appalachian People (SNAP) Study, 2008–2010.
Those eligible for participation were drug users aged 18 years or older who resided in Appalachian Kentucky and had used 1 of the following substances to get high in the prior 30 days: prescription opioids, cocaine, heroin, or methamphetamine. All participants received and signed statements of informed consent. Participants were compensated $50 for their time. A total of 503 participants completed the baseline interview; however, given the strong causal relationship between injection drug use and HCV infection,14 only those with a lifetime history of injection drug use (i.e., ever having injected any drug during one’s lifetime) were considered for inclusion in the analysis (n = 394). Two additional participants were excluded because their reported age of initiation of injection was greater than that of their actual age for a final sample size of 392 IDUs.
We used a name-generating questionnaire to determine with whom the participant had used drugs in the 6 months prior to the interview. Once we elicited the names and characteristics of network members, they were checked against other sources of information to confirm their identity. We then entered validated network members into a matrix to build the sociometric drug network and calculate the network measures with UCINet version 6.3 (Analytic Technologies, Harvard, MA). We used 3 measures of network position and cohesion in the current analysis. Degree centrality is a local centrality measure that takes into account the number of links to and from a person. As described by Wasserman and Faust,28 the degree centrality of actor i in a network of g actors is the sum of i’s direct ties to the g – 1 other actors in the network. To adjust for the effect of network size, we used a normalized degree centrality measure, in which actor i’s centrality score was divided by the maximum number of possible connections in the overall network. This yielded a proportion ranging from 0 (no connectivity) to 1 (complete connectivity). Eigenvector centrality extends the notion of degree centrality to take into account second-order connections.29 In other words, a node’s eigenvector centrality is dependent, in part, on the centrality of neighboring nodes. We used normalized eigenvector centrality for analysis. A k-core is a subset of the network in which each node within the k-core is connected to at least k other people.28,30 Previous research has demonstrated that certain k-core configurations can facilitate disease transmission.16
Trained staff tested participants for antibodies to HCV using the Home Access test (Home Access Health Corporation, Hoffman Estates, IL), which uses a third-generation enzyme immunoassay on dried blood spot specimens collected by finger-stick. Those tested were asked to return for their results approximately 2 weeks later. If they did not return for their results in person, participants were informed of results by telephone. Given the overlap in some risk factors for disease transmission, we also conducted rapid tests for HIV (OraQuick, OraSure Technologies, PA) and herpes simplex-2 virus (HSV-2; Biokit USA Inc, Lexington, MA). Pre- and posttest counseling was provided in accordance with Centers for Disease Control and Prevention guidelines, and all participants were provided with their test results. Those testing positive for HCV, HIV, or HSV-2 were referred to local community resources for further testing and treatment.
The questionnaire was administered by the interviewer, and responses were entered directly onto a touch screen laptop enabled with computer-assisted personal interviewing software (Questionnaire Development System, Nova Research Company, Bethesda, MD). All interviewers were residents of the target area, received extensive training in interviewing, and were certified as HIV counselors. The dependent variable of interest was a positive HCV test. We grouped independent variables in terms of lifetime and current (past-6-month) behaviors so as to differentiate those factors that were potentially associated with viral acquisition (lifetime) from those that could be targets for prevention of further transmission (current). Current variables included injection and injection-related risk behaviors such as receptive syringe or other equipment sharing, straw sharing, and syringe source. Lifetime variables included the following: sociodemographic indicators (age, race, gender, education, income, and employment [full-time or part-time]), drug use, HIV and HSV-2 infection, sexual history, and psychiatric disorders (major depressive disorder, generalized anxiety disorder, posttraumatic stress disorder [PTSD], and antisocial personality disorder). These were assessed by trained staff using the Mini International Neuropsychiatric Interview, version 5.0.31 These particular psychiatric diagnoses were measured because of their strong correlation with substance abuse, including prescription drug abuse.32–35 Those meeting the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)36 criteria for any of the these psychiatric diagnoses were provided with written information on community mental health resources.
We completed statistical analyses in 2 stages. First, we conducted a series of χ2 and Wilcoxon rank-sum tests for all categorical and continuous variables, respectively, to determine their association with HCV serostatus. Since we were examining 2 levels of data, individuals nested within social networks, we used a variance component model to determine whether HCV prevalence differed across the drug network components. The model was not significant, however, so we tested those variables for which P < .1 in a multivariable logistic regression model with robust standard errors. To account for the interdependence of the outcome with participant recruitment, we also adjusted all models for the recruiter type (seed; not seed, not IDU; IDU, HCV negative; IDU, HCV positive). We used a stepwise, forward elimination process until only those variables significant at the P < .05 level were included in the model. Although race only approached significance (P < .1), we retained it in the model because it did not change the estimates appreciably.
RESULTS
More than half of the participants were male (58.9%), had at least a high school education (56.6%), were non-Hispanic White (93.9%), and had a median age of 31 years (interquartile range = 26–38). Lifetime use of prescription opioids (OxyContin, illicit methadone, or hydrocodone) was far more common that that of heroin and methamphetamine. Among the 392 IDUs included in the analysis, prevalence of HCV infection was 54.8%; only 31.2% of those testing positive were aware of their serostatus. None of the IDUs tested positive for HIV, and the prevalence of HSV-2 was 12.5%. Most IDUs initiated injection with prescription opioids (61.7%), and 40.9% had begun injecting only in the past 5 years. Injection with prescription opioids was common (88.7% lifetime, 68.4% in past 6 months). Table 1 presents comparisons of both lifetime and current factors for those with and without antibodies to HCV.
TABLE 1—
Drug Use and Psychosocial Characteristics of 392 Rural Injection Drug Users, by HCV Status: Social Networks Among Appalachian People (SNAP) Study, 2008–2010
| HCV Positive (n = 215), No. (%) or Median (IQR) | HCV Negative (n = 177), No. (%) or Median (IQR) | P | |
| Demographics | |||
| Male gender | 131 (60.9) | 100 (56.5) | .375 |
| Age, y | 32 (27–38) | 30 (25–37) | .112 |
| White (vs other race/ethnicity) | 206 (95.8) | 162 (91.5) | .078 |
| Education, y | 12 (9–12) | 12 (10.8–12) | .099 |
| Income, $ | 700 (400–1450) | 700 (300–1200) | .339 |
| Employed (vs unemployed/underemployed) | 88 (50.3) | 127 (58.5) | .103 |
| DSM-IV psychiatric disorders | |||
| Major depressive disorder | 62 (28.8) | 48 (27.1) | .706 |
| Generalized anxiety disorder | 65 (30.2) | 48 (27.1) | .498 |
| Posttraumatic stress disorder | 23 (10.7) | 35 (19.8) | .012 |
| Antisocial personality disorder | 65 (30.2) | 70 (39.5) | .053 |
| History of methadone treatment | 31 (14.4) | 15 (8.5) | .069 |
| HIV | 0 | 0 | … |
| HSV-2 | 33 (15.3) | 16 (9.0) | .06 |
| Lifetime risk behaviors | |||
| Injection of prescription opioids | 197 (91.6) | 149 (84.2) | .023 |
| Injection of cocaine | 167 (77.7) | 104 (58.8) | < .001 |
| Injection of heroin | 62 (28.8) | 33 (18.6) | .019 |
| Injection of methamphetamine | 24 (11.2) | 15 (8.5) | .376 |
| Years injecting (continuous) | 7 (3–12) | 4 (1–9) | < .001 |
| Years injecting (categorical) | .001 | ||
| ≥ 1 | 28 (13.0) | 47 (26.5) | |
| 1.1–2 | 21 (9.8) | 20 (11.3) | |
| 2.1–3 | 14 (6.5) | 17 (9.6) | |
| 3.1–5 | 25 (11.6) | 25 (14.1) | |
| ≥ 5 | 127 (59.1) | 68 (38.4) | |
| Tattoo(s) or body piercing | 196 (91.2) | 157 (88.7) | .418 |
| Blood transfusion(s) | 22 (10.2) | 23 (13.0) | .393 |
| No. of sex partners | 20 (10–45) | 15 (8–32) | .036 |
| Lifetime substance use | |||
| Illicit methadone | 206 (95.8) | 174 (98.3) | .154 |
| OxyContin | 213 (99.1) | 170 (96.0) | .047 |
| Hydrocodone | 210 (97.7) | 171 (96.6) | .525 |
| Benzodiazepines | 205 (95.3) | 174 (98.3) | .104 |
| Cocaine | 206 (95.8) | 170 (96.0) | .908 |
| Heroin | 95 (44.2) | 66 (37.3) | .167 |
| Methamphetamine | 97 (45.1) | 84 (47.5) | .644 |
| Alcohol | 214 (99.5) | 176 (99.4) | .89 |
| Marijuana | 208 (96.7) | 174 (98.3) | .329 |
| Current risk behaviors (prior 6 mo) | |||
| Injection drug use | 169 (78.6) | 118 (66.7) | .008 |
| Straw sharing | 169 (78.6) | 150 (84.7) | .12 |
| Syringe sharing | 65 (30.2) | 27 (15.2) | < .001 |
| Cottons/cookers/rinse water sharing | 90 (41.9) | 46 (26.0) | .001 |
| Syringe source | .203 | ||
| Pharmacy | 9 (5.3) | 2 (1.7) | |
| Drug dealer | 33 (19.3) | 18 (15.0) | |
| Friends/family | 65 (38.0) | 60 (5.0) | |
| Diabetic | 58 (33.9) | 37 (3.8) | |
| Other | 6 (3.5) | 3 (2.5) | |
| Drug network characteristics | |||
| Degree centralitya | 0.40 (0.20–0.80) | 0.40 (0.20–0.60) | .071 |
| Eigenvector centralityb | 0.05 (0.001–1.40) | 0.02 (0.000002–0.60) | .012 |
| k-corenessc | 2 (1–2) | 1 (1–2) | .161 |
Note. DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; HCV = hepatitis C virus; HSV-2 = herpes simplex-2 virus; IQR = interquartile range.
Degree centrality takes into account the number of links to and from a person.
Eigenvector centrality extends the notion of degree centrality to take into account second-order connections.
k-coreness is a subset of the network in which each node within the k-core is connected to at least k other people.
Two forms of lifetime substance injection were independently associated with HCV seropositivity: injecting prescription opioids (adjusted odds ratio [AOR] = 2.22; 95% confidence interval [CI] = 1.13, 4.35) and injecting cocaine (AOR = 2.13; 95% CI = 1.31, 3.45; Table 2). Duration of injection was also an independent correlate of HCV infection. Compared with those who had been injecting for a year or less, those who had been injecting for 5 or more years had 3 times the odds of being HCV positive, but meeting the DSM-IV criteria for PTSD was correlated with a 65% reduction in the odds of being HCV positive (AOR = 0.35; 95% CI = 0.19, 0.64). Coinfection with HSV-2 was independently associated with HCV infection: those who had antibodies to HSV-2 were twice as likely as those without antibodies to also be HCV positive (AOR = 2.39; 95% CI = 1.13, 5.04), even after adjustment for all other variables in the model and the recruiter type.
TABLE 2—
Factors Independently Associated With Hepatitis C Virus Seropositivity: Social Networks Among Appalachian People (SNAP) Study, 2008–2010
| Odds Ratioa (95% CI) | Adjusted Odds Ratiob (95% CI) | |
| Lifetime demographic factors | ||
| White (vs other race/ethnicity) | 2.16c (0.90, 5.21) | 2.22 (0.92, 5.35) |
| Education in years | 0.99 (0.98, 1.00) | … |
| DSM-IV psychiatric disorders | ||
| PTSD | 0.45* (0.25, 0.80) | 0.35** (0.19, 0.64) |
| ASPD | 0.69c (0.45, 1.06) | … |
| Methadone treatment | 1.76 (0.89, 3.48) | … |
| HSV-2 | 1.94c (1.07, 3.71) | 2.39* (1.13, 5.04) |
| Lifetime injection risk behaviors | ||
| Prescription opioid injection | 1.72c (0.91, 3.24) | 2.22* (1.13, 4.35) |
| Cocaine injection | 2.30** (1.47, 3.69) | 2.13** (1.31, 3.45) |
| Heroin injection | 1.68c (1.03, 2.75) | … |
| Years injecting (continuous) | 1.04* (1.01, 1.08) | … |
| Years injecting (categorical) | ||
| < 1 (Ref) | 1.00 | 1.00 |
| 1–2 | 1.92 (0.85, 4.32) | 1.55 (0.65, 3.73) |
| 1–3 | 1.50 (0.60, 3.74) | 1.40 (0.54, 3.60) |
| 1–5 | 1.90c (0.91, 3.95) | 1.55 (0.70, 3.41) |
| ≥ 5 | 3.51** (2.00, 6.18) | 3.08** (1.67, 5.66) |
| No. of sex partners (continuous) | 1.00 (0.99, 1.00) | … |
| OxyContin use | 3.53 (0.76, 16.1) | … |
| Current (prior 6 mo) factors | ||
| Injection risk behaviors | ||
| Syringe sharing | 2.26** (1.34, 3.08) | 2.24** (1.32, 3.82) |
| Cottons/cookers/water sharing | 1.84** (1.18, 2.87) | … |
| Drug network characteristics | ||
| Degree centralityd | 1.63c (0.98, 2.72) | … |
| Eigenvector centralitye | 1.07** (1.02, 1.12) | 1.07** (1.02, 1.12) |
Note. ASPD = antisocial personality disorder; CI = confidence interval; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; HSV-2 = herpes simplex-2 virus; PTSD = posttraumatic stress disorder.
Adjusted for recruiter characteristics.
Adjusted for recruiter characteristics and all other variables in the model.
P value approached significance, and so variable was included in multivariable logistic regression model.
Degree centrality takes into account the number of links to and from a person.
Eigenvector centrality extends the notion of degree centrality to take into account second-order connections.
*P < .05; **P < .01.
IDUs who reported sharing syringes in the 6 months prior to the baseline interview were more than twice as likely as those who did not report syringe sharing to be HCV positive (AOR = 2.26; 95% CI = 1.34, 3.08; Table 2). Greater eigenvector centrality was also significantly associated with HCV infection, even after adjustment for syringe sharing and recruiter type (AOR = 1.07; 95% CI = 1.02, 1.12).
Given the finding that both a lifetime history of prescription opioid injection and of cocaine injection were independently associated with HCV infection, we conducted a series of logistic regression analyses adjusted for recruiter type in an attempt to differentiate these 2 types of injectors (Table 3). Cocaine injectors were significantly more likely than noncocaine injectors to have a longer duration of injection. Specifically, those injecting for 3.1 to 5 years or for 5 or more years were more likely than those who had been injecting for less than a year to be cocaine injectors, whereas duration of injection was not associated with lifetime prescription opioid injection. Similar syringe-related risk behaviors were observed with both the prescription opioid and cocaine injectors, whereas cocaine injectors appeared to be more central and to have more reach to other drug users within the drug network.
TABLE 3—
Risk Profiles for Lifetime Prescription Opioid Injectors and Lifetime Cocaine Injectors: Social Networks Among Appalachian People (SNAP) Study, 2008–2010
| Prescription Opioid Injectors, ORa (95% CI) | Cocaine Injectors, ORa (95% CI) | |
| Injection risk behaviors | ||
| Years injecting (categorical) | ||
| < 1 (Ref) | 1.00 | 1.00 |
| 1–2 | 1.15 (0.26, 4.99) | 2.16 (0.99, 4.72) |
| 1–3 | 2.76 (0.33, 22.9) | 1.72 (0.71, 4.15) |
| 1–5 | 0.52 (0.16, 1.63) | 3.61** (1.62, 8.04) |
| ≥ 5 | 0.54 (0.21, 1.41) | 4.85** (2.70, 8.71) |
| Syringe sharing (prior 6 mo) | 6.87** (1.61, 29.4) | 2.26** (1.26, 4.07) |
| Cottons/cookers/water sharing (prior 6 mo) | 7.66** (2.34, 25.1) | 2.21** (1.34, 3.65) |
| Drug network characteristics | ||
| Degree centralityb | 1.77 (0.62, 5.06) | 3.15** (1.63, 6.10) |
| Eigenvector centralityc | 1.11* (1.01, 1.23) | 1.05* (1.00, 1.12) |
| k-corenessd | 1.53* (1.07, 2.20) | 1.56** (1.20, 2.03) |
Note. CI = confidence interval; OR = odds ratio.
Adjusted for recruiter characteristics.
Degree centrality takes into account the number of links to and from a person.
Eigenvector centrality extends the notion of degree centrality to take into account second-order connections.
k-coreness is a subset of the network in which each node within the k-core is connected to at least k other people.
*P < .05; **P < .01.
DISCUSSION
In this study of the prevalence of HCV infection in a cohort of rural IDUs, we found an independent association between injection of prescription opioids and HCV infection. The prevalence of hepatitis C (> 50%) in this cohort of rural Appalachian IDUs was far greater than that of the general population (< 2%)37 and within the range of rates reported among urban IDUs in the United States (27%–92.8%).2 The high prevalence of HCV infection in this area of Appalachia, known to have extreme health disparities, causes concern for several reasons. First, assessment and treatment of HCV infection has already been shown to be limited,38 especially among drug users.39 The availability of specialized medical care, such as the treatment of chronic HCV, is markedly limited in Appalachian Kentucky compared with more urban areas.40 Second, these rural IDUs are likely to be at increased risk for HIV given the common risk factors for transmission of both viruses.15,41 Third, resources such as syringe exchange and opioid maintenance treatment, known to decrease the risks associated with syringe-related disease transmission,42,43 are not widely available in this region, if at all. Hagan et al. found that HCV acquisition can occur earlier among rural IDUs than their urban counterparts, given their limited access to harm reduction, assessment, and treatment resources.2 This clearly poses a barrier to preventing additional infections in a timely manner.
Among the current factors examined, sharing syringes was independently associated with HCV infection. It is known that HCV is highly transmissible via injection drug use44; however, IDUs continue to share injection equipment. Our findings of a significant association between syringe sharing and prevalent HCV infection are in agreement with those found among IDUs in San Francisco,6 as well as in longitudinal studies of incident HCV infections.12,14 In this particular cohort, access to sterile syringes is lacking. The major sources of syringes are family members or friends (42.2%), diabetics (32.5%), or syringe dealers (17.5%). Fewer than 4% of participants regularly purchased sterile syringes from pharmacies. Ideally, increased access to syringe exchange programs (SEPs) would be the one viable option for preventing additional infections.
IDUs with greater eigenvector centrality were also more likely to be HCV positive. Since those with greater eigenvector centrality have more ties to drug users who themselves have additional ties to other drug users, this finding has great implications for infectious disease transmission. Eigenvector centrality was highlighted by Borgatti19 as an important measure of the potential for transmission. This was further demonstrated by Bell et al.45 in their simulations of the potential for disease flow through networks, as eigenvector centrality was highly correlated with HIV transmission. Although greater eigenvector centrality is associated with increased levels of disease transmission, greater levels of eigenvector centrality may actually be useful when employing network-based interventions since the network may facilitate dissemination of information as well as disease. Therefore, interventions aimed at reducing risk behaviors may be most effectively implemented through network members who have the most reach to other members of the network. It is evident that current individually focused methodologies have had a negligible effect on HCV transmission46; however, network- or peer-based interventions have been employed among several high-risk groups, often with encouraging results.47–49
When we modeled lifetime risk behaviors, several factors emerged as being significantly associated with HCV infection. Duration of injection was highly predictive of being HCV positive. When we examined duration as a categorical variable, only those who had been injecting for 5 or more years had significantly greater odds of being infected. Although the overall result indicating a positive association between longer duration of injection and HCV infection is in concordance with the extant research,5,10,11,50 many studies of HCV infection among IDUs demonstrate a significant association with duration of injection much earlier in IDUs’ injection career. For example, Diaz et al. found that injecting for a minimum of 3 years was associated with HCV infection,11 and Thorpe et al. reported that, compared with injecting a year or less, injecting a minimum of 1 to 4 years was significantly associated with being HCV positive.5 This finding may have more to do with the type of drug being injected. In our study, we found that those who were injecting prescription opioids were significantly earlier in their injection careers than those injecting cocaine. In fact, IDUs were about half as likely to be injecting for 3 or more years if they had injected prescription opioids. Therefore, there may be opportunities to intervene with prescription opioid injectors, including increased access to harm reduction programs such as SEPs and opioid maintenance treatment. Unfortunately, there are considerable financial constraints in many rural areas that may preclude investing in harm reduction programs. In addition, there is the potential for cultural opposition, which may ultimately impede efforts to reduce transmission. More studies similar to those employed by researchers in New York, Sydney, London, and Valencia, who examined what factors were associated with long-term IDUs’ maintenance of HCV-negative status,51 may also be a way in which to differentiate the modifiable risk factors for HCV infection in this population of rural IDUs.
One of the more interesting findings of this study was that injection of prescription opioids was associated with infection with HCV. This finding is akin to that of a 2007 study conducted with a similar population of rural Appalachian IDUs, in which self-reported HCV infection was significantly greater among opioid injectors.22 To further differentiate prescription opioid injectors from cocaine injectors, we also examined injection risk behaviors in the 2 groups. As mentioned in the previous paragraph, the most interesting finding was related to duration of injection. Prescription opioid injectors appear to be earlier in their injection careers, which is promising for preventing HCV transmission if intervention can occur shortly after injection initiation. Although there is scant data on rural injection drug use with prescription opioids in other areas of the country, there are data that suggest that prescription opioid abuse is a problem in rural America, not just rural Kentucky. For example, in one study of overdose decedents, Hall et al.52 noted that alternative routes of administration such as injection drug use were more prevalent for deaths involving prescription opioids. Similar findings have been reported in New Mexico,53 West Virginia,54 and southwest Virginia.55 Thus, it appears that prescription opioid injection may not be unique to Appalachian Kentucky, and these results therefore have implications for HCV transmission in other rural areas.
Finally, although the prevalence of HCV infection in this cohort was high, none of the participants were HIV positive. This is surprising given the greater potential for HIV–HCV coinfection, especially among IDUs. However, studies in rural Appalachian Kentucky suggest there is very little endemic HIV infection.56 Many of the IDUs in the current study were socially isolated, which may also play a role in the lack of HIV infection.56 Those who were HSV-2 positive, however, were more than twice as likely as those without HSV-2 to be HCV positive. This indicates potentially greater levels of both sexual risk behaviors and injection-related risk behaviors in this sample. Unfortunately, HIV may yet become epidemic in this region, as research suggests that HCV infection may be sentinel for HIV.15,57 Presently, public health professionals are in a unique position given the low prevalence of HIV in this area, especially among IDUs, who are at most risk. Consequently, interventions aimed at curbing HCV transmission will have the added advantage of potentially preventing HIV transmission.
Meeting the DSM-IV criteria for PTSD decreased the odds of HCV infection by 65% in this sample of rural IDUs. This finding contrasts with other studies that found significantly greater levels of HCV infection among those with severe mental illness58,59 and PTSD in particular.60–62 It is possible that in this specific population, IDUs exhibiting symptoms of PTSD were more withdrawn from other drug users within the drug network and therefore not as engaged in risk behaviors associated with HCV transmission, such as sharing syringes.
Limitations
There are limitations to this study that warrant mention. We measured only exposure to the hepatitis C virus as opposed to active or chronic infection. In addition, data are cross-sectional and thus no conclusions can be made regarding the directionality of the reported associations.
Conclusions
Despite the limitations, these data are highly novel because they describe the factors associated with prevalent HCV infection among IDUs in an economically distressed rural area in the United States. The results provide evidence that injecting drug use, and sharing syringes in particular, is associated with HCV infection regardless of the drug that is injected. The findings also demonstrate that this particular population of IDUs are different from urban IDUs in that they are primarily injecting prescription opioids rather than heroin. However, given the limited resources in this area and surrounding regions, as well as the stigma associated with injection drug use, interventions such as SEPs that are aimed at reducing these risky behaviors are not likely to be implemented without significant efforts to lower the cultural opposition and increase the allocation of funding for such programs. Furthermore, substance abuse treatment such as methadone—shown to reduce engagement in injection risk behaviors42—is not readily accessible in Appalachian Kentucky, and office-based treatment with buprenorphine pharmacotherapy, although more available, is costly, which limits access. Given the alternative of having hundreds, if not thousands, of HCV infections in this area, establishment of SEPs and additional substance abuse treatment programs should be considered a necessity for Appalachian Kentucky, as the costs for treatment of chronic HCV infection (when indicated) are much higher than those of preventive efforts such as SEP and opioid maintenance treatment.
Acknowledgments
Funding for this study was provided by the National Institutes of Health, National Institute on Drug Abuse (NIDA; grant R01DA024598 to J. R. Havens). Additional funding was provided to S. D. W. Frost via grants from NIDA (R03DA24998) and the National Institute of Nursing Research (R21NR10961), as well as a Royal Society Wolfson Research Merit Award.
Human Participant Protection
The study was approved by the University of Kentucky institutional review board.
References
- 1.Alter MJ. The epidemiology of acute and chronic hepatitis C. Clin Liver Dis. 1997;1(3):559–568,vi–vii [DOI] [PubMed] [Google Scholar]
- 2.Hagan H, Pouget ER, Des Jarlais DC, Lelutiu-Weinberger C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am J Epidemiol. 2008;168(10):1099–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aaron S, McMahon JM, Milano Det al. Intranasal transmission of hepatitis C virus: virological and clinical evidence. Clin Infect Dis. 2008;47(7):931–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neaigus A, Gyarmathy VA, Zhao M, Miller M, Friedman SR, Des Jarlais DC. Sexual and other noninjection risks for HBV and HCV seroconversions among noninjecting heroin users. J Infect Dis. 2007;195(7):1052–1061 [DOI] [PubMed] [Google Scholar]
- 5.Thorpe LE, Ouellet LJ, Levy JR, Williams IT, Monterroso ER. Hepatitis C virus infection: prevalence, risk factors, and prevention opportunities among young injection drug users in Chicago, 1997–1999. J Infect Dis. 2000;182(6):1588–1594 [DOI] [PubMed] [Google Scholar]
- 6.Hahn JA, Page-Shafer K, Lum PJ, Ochoa K, Moss AR. Hepatitis C virus infection and needle exchange use among young injection drug users in San Francisco. Hepatology. 2001;34(1):180–187 [DOI] [PubMed] [Google Scholar]
- 7.Miller CL, Johnston C, Spittal PMet al. Opportunities for prevention: hepatitis C prevalence and incidence in a cohort of young injection drug users. Hepatology. 2002;36(3):737–742 [DOI] [PubMed] [Google Scholar]
- 8.Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health. 1996;86(5):655–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorpe LE, Ouellet LJ, Hershow Ret al. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. Am J Epidemiol. 2002;155(7):645–653 [DOI] [PubMed] [Google Scholar]
- 10.Miller ME, Pierre RB, Plummer MH, Shah DJ. Hepatitis B-associated nephrotic syndrome in Jamaican children. Ann Trop Paediatr. 2002;22(3):261–266 [DOI] [PubMed] [Google Scholar]
- 11.Diaz T, Des Jarlais DC, Vlahov Det al. Factors associated with prevalent hepatitis C: differences among young adult injection drug users in lower and upper Manhattan, New York City. Am J Public Health. 2001;91(1):23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villano SA, Vlahov D, Nelson KE, Lyles CM, Cohn S, Thomas DL. Incidence and risk factors for hepatitis C among injection drug users in Baltimore, Maryland. J Clin Microbiol. 1997;35(12):3274–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagan H, Pouget ER, Williams ITet al. Attribution of hepatitis C virus seroconversion risk in young injection drug users in 5 US cities. J Infect Dis. 2010;201(3):378–385 [DOI] [PubMed] [Google Scholar]
- 14.Hagan H, Thiede H, Des Jarlais DC. Hepatitis C virus infection among injection drug users: survival analysis of time to seroconversion. Epidemiology. 2004;15(5):543–549 [DOI] [PubMed] [Google Scholar]
- 15.Vickerman P, Hickman M, May M, Kretzschmar M, Wiessing L. Can hepatitis C virus prevalence be used as a measure of injection-related human immunodeficiency virus risk in populations of injecting drug users? An ecological analysis. Addiction. 2010;105(2):311–318 [DOI] [PubMed] [Google Scholar]
- 16.Friedman SR, Neaigus A, Jose Bet al. Sociometric risk networks and risk for HIV infection. Am J Public Health. 1997;87(8):1289–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothenberg R. Maintenance of endemicity in urban environments: a hypothesis linking risk, network structure and geography. Sex Transm Infect. 2007;83(1):10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman SR, Kottiri BJ, Neaigus A, Curtis R, Vermund SH, Des Jarlais DC. Network-related mechanisms may help explain long-term HIV-1 seroprevalence levels that remain high but do not approach population-group saturation. Am J Epidemiol. 2000;152(10):913–922 [DOI] [PubMed] [Google Scholar]
- 19.Borgatti SP. Centrality and AIDS. Connections. 1995;18(1):112–115 [Google Scholar]
- 20.Murray CJ, Kulkarni S, Ezzati M. Eight Americas: new perspectives on US health disparities. Am J Prev Med. 2005;29(5 suppl 1):4–10 [DOI] [PubMed] [Google Scholar]
- 21.Leukefeld CG, Logan TK, Farabee D, Clayton R. Drug use and AIDS: estimating injection prevalence in a rural state. Subst Use Misuse. 2002;37(5–7):767–782 [DOI] [PubMed] [Google Scholar]
- 22.Havens JR, Walker R, Leukefeld CG. Prevalence of opioid analgesic injection among rural nonmedical opioid analgesic users. Drug Alcohol Depend. 2007;87(1):98–102 [DOI] [PubMed] [Google Scholar]
- 23.Young AM, Havens JR. Transition from first illicit drug use to first injection drug use among rural Appalachian drug users: a cross-sectional comparison and retrospective survival analysis. Addiction. 2012;107(3):587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heckathorn D. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl. 1997;44(2):174–199 [Google Scholar]
- 25.Heckathorn D. Respondent-driven sampling, II: deriving valid population estimates from chain-referral samples of hidden populations. Soc Probl. 2002;49(1):11–34 [Google Scholar]
- 26.Wang J, Falck RS, Li L, Rahman A, Carlson RG. Respondent-driven sampling in the recruitment of illicit stimulant drug users in a rural setting: findings and technical issues. Addict Behav. 2007;32(5):924–937 [DOI] [PubMed] [Google Scholar]
- 27.Scott AJ, Wild CJ. Fitting logistic models under case-control or choice based sampling. J R Stat Soc [Ser A]. 1986;48(2):170–182 [Google Scholar]
- 28.Wasserman S, Faust K. Social Network Analysis. New York, NY: Cambridge University Press; 1994 [Google Scholar]
- 29.Freeman LC. Centrality in social networks conceptual clarification. Soc Networks. 1978–1979;1(3):215–239 [Google Scholar]
- 30.Seidman S. Network structure and minimum degree. Soc Networks. 1983;5(3):269–287 [Google Scholar]
- 31.Sheehan DV, Lecrubier Y, Sheehan KHet al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33, quiz 34–57 [PubMed] [Google Scholar]
- 32.Boscarino JA, Rukstalis M, Hoffman SNet al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776–1782 [DOI] [PubMed] [Google Scholar]
- 33.Liebschutz JM, Saitz R, Weiss RDet al. Clinical factors associated with prescription drug use disorder in urban primary care patients with chronic pain. J Pain. 2010;11(11):1047–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martins SS, Fenton MC, Keyes KM, Blanco C, Zhu H, Storr CL. Mood and anxiety disorders and their association with non-medical prescription opioid use and prescription opioid-use disorder: longitudinal evidence from the National Epidemiologic Study on Alcohol and Related Conditions. Psychol Med. 2012;42(6):1261–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall MT, Howard MO, McCabe SE. Prescription drug misuse among antisocial youths. J Stud Alcohol Drugs. 2010;71(6):917–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC; American Psychiatric Association; 1994 [Google Scholar]
- 37.Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002;36(5 suppl 1):S30–S34 [DOI] [PubMed] [Google Scholar]
- 38.Grebely J, Raffa JD, Lai Cet al. Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner city residents. J Viral Hepat. 2009;16(5):352–358 [DOI] [PubMed] [Google Scholar]
- 39.Grebely J, Genoway KA, Raffa JDet al. Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug Alcohol Depend. 2008;93(1–2):141–147 [DOI] [PubMed] [Google Scholar]
- 40.Ferrer RL. Pursuing equity: contact with primary care and specialist clinicians by demographics, insurance, and health status. Ann Fam Med. 2007;5(6):492–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim AY, Chung RT. Coinfection with HIV-1 and HCV—a one-two punch. Gastroenterology. 2009;137(3):795–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorensen JL, Copeland AL. Drug abuse treatment as an HIV prevention strategy: a review. Drug Alcohol Depend. 2000;59(1):17–31 [DOI] [PubMed] [Google Scholar]
- 43.Van Den Berg C, Smit C, Van Brussel G, Coutinho R, Prins M. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction. 2007;102(9):1454–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13(17):2436–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bell DC, Atkinson JS, Carlson JW. Centrality measures for disease transmission networks. Soc Networks. 1999;21(1):1–21 [Google Scholar]
- 46.Friedman SR, Mateau-Gelabert P, Sandoval M, Hagan H, DesJarlais DC. Positive deviance control-case life history: a method to develop grounded hypotheses about successful long-term avoidance of infection. BMC Public Health. 2008;8:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Latkin CA, Sherman S, Knowlton A. HIV prevention among drug users: outcome of a network-oriented peer outreach intervention. Health Psychol. 2003;22(4):332–339 [DOI] [PubMed] [Google Scholar]
- 48.Latkin CA, Donnell D, Metzger Det al. The efficacy of a network intervention to reduce HIV risk behaviors among drug users and risk partners in Chiang Mai, Thailand and Philadelphia, USA. Soc Sci Med. 2009;68(4):740–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medley A, Kennedy C, O’Reilly K, Sweat M. Effectiveness of peer education interventions for HIV prevention in developing countries: a systematic review and meta-analysis. AIDS Educ Prev. 2009;21(3):181–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garfein RS, Doherty MC, Monterroso ER, Thomas DL, Nelson KE, Vlahov D. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(suppl 1):S11–S19 [DOI] [PubMed] [Google Scholar]
- 51.Mateu-Gelabert P, Treloar C, Calatayud VAet al. How can hepatitis C be prevented in the long term? Int J Drug Policy. 2007;18(5):338–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hall AJ, Logan JE, Toblin RLet al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613–2620 [DOI] [PubMed] [Google Scholar]
- 53.Mueller MR, Shah NG, Landen MG. Unintentional prescription drug overdose deaths in New Mexico, 1994–2003. Am J Prev Med. 2006;30(5):423–429 [DOI] [PubMed] [Google Scholar]
- 54.Paulozzi LJ, Logan JE, Hall AJ, McKinstry E, Kaplan JA, Crosby AE. A comparison of drug overdose deaths involving methadone and other opioid analgesics in West Virginia. Addiction. 2009;104(9):1541–1548 [DOI] [PubMed] [Google Scholar]
- 55.Wunsch MJ, Nakamoto K, Behonick G, Massello W. Opioid deaths in rural Virginia: a description of the high prevalence of accidental fatalities involving prescribed medications. Am J Addict. 2009;18(1):5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oser CB, Smiley McDonald HM, Havens JR, Leukefeld CG, Webster JM, Cosentino-Boehm AL. Lack of HIV seropositivity among a group of rural probationers: explanatory factors. J Rural Health. 2006;22(3):273–275 [DOI] [PubMed] [Google Scholar]
- 57.Des Jarlais DC, Arasteh K, McKnight C, Hagan H, Perlman D, Friedman SR. Using hepatitis C virus and herpes simplex virus-2 to track HIV among injecting drug users in New York City. Drug Alcohol Depend. 2009;101(1–2):88–91 [DOI] [PubMed] [Google Scholar]
- 58.Rosenberg SD, Goodman LA, Osher FCet al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenberg SD, Swanson JW, Wolford GLet al. The five-site health and risk study of blood-borne infections among persons with severe mental illness. Psychiatr Serv. 2003;54(6):827–835 [DOI] [PubMed] [Google Scholar]
- 60.el-Serag HB, Kunik M, Richardson P, Rabeneck L. Psychiatric disorders among veterans with hepatitis C infection. Gastroenterology. 2002;123(2):476–482 [DOI] [PubMed] [Google Scholar]
- 61.Nguyen HA, Miller AI, Dieperink Eet al. Spectrum of disease in US veteran patients with hepatitis C. Am J Gastroenterol. 2002;97(7):1813–1820 [DOI] [PubMed] [Google Scholar]
- 62.Lehman CL, Cheung RC. Depression, anxiety, post-traumatic stress, and alcohol-related problems among veterans with chronic hepatitis C. Am J Gastroenterol. 2002;97(10):2640–2646 [DOI] [PubMed] [Google Scholar]